Abstract
Venetoclax (ABT-199), a specific inhibitor of the anti-apoptotic protein Bcl-2, is currently in phase I clinical trials for multiple myeloma. Results suggest that venetoclax is only active in a small cohort of patients therefore we wanted to determine its efficacy when used in combination. Combining venetoclax with melphalan or carfilzomib produced additive or better cell death in 4 of the 5 cell lines tested. The most striking results were seen with dexamethasone. Co-treatment of human myeloma cell lines and primary patient samples, with dexamethasone and venetoclax significantly increased cell death over venetoclax alone in 4 of the 5 cell lines, and in all patient samples tested. The mechanism by which this occurs is an increase in the expression of both Bcl-2 and Bim upon addition of dexamethasone. This results in alterations in Bim binding to anti-apoptotic proteins. Dexamethasone shifts Bim binding towards Bcl-2 resulting in increased sensitivity to venetoclax. These data suggest that knowledge of drug-induced alterations of Bim binding patterns may help inform better combination drug regimens. Furthermore, the data indicate combining this novel therapeutic with dexamethasone could be an effective therapy for a broader range of patients than would be predicted by single agent activity.
Keywords: Bcl-2, apoptosis, plasma cell dyscrasia
Introduction
Enormous advances in available therapeutics for the treatment of multiple myeloma have been made within the last 15 years. With FDA approval of IMiDs, proteasome inhibitors, and HDAC inhibitors, 5-year survival rates are now over 47%.1 However despite these advances most patients will relapse and develop drug resistant disease. Ultimately new therapeutic approaches used in combination with the current active agents must be developed.
The Bcl-2 family of proteins regulates the mitochondrial apoptotic pathway and represents a novel target. The Bcl-2 family can be divided into four categories: the anti-apoptotic proteins (Mcl-1, Bcl-2, Bcl-xL, Bcl-w, and Bfl-1), the pro-apoptotic “sensitizer” BH3-only proteins (Noxa, Bad, Bmf, Bik), the pro-apoptotic “activator” BH3-only proteins (Bim, Bid, and Puma), and the pro-apoptotic effector proteins (Bak and Bax).2–6 Activator BH3-only proteins bind to the effector proteins, Bak and Bax triggering them to oligomerize at the mitochondria and induce mitochondrial outer membrane permeabilization (MOMP) resulting in cytochrome c release and apoptosis.2–6 Anti-apoptotic proteins function by sequestering the activator proteins thereby preventing them from activating the effector proteins. The BH3-only “sensitizer” proteins are unable to activate Bak and Bax, and function by binding to the anti-apoptotic proteins leading to the release of the activator proteins and thus apoptosis.2–6
ABT-737, or the orally bioavailable ABT-263 (Navitoclax), are small molecule antagonists that mimic the binding of the BH3 domain of Bad to the anti-apoptotic proteins Bcl-2, Bcl-xL, and Bcl-w, thereby releasing any bound pro-apoptotic proteins to induce apoptosis.7, 8 While initial results from clinical trials in Bcl-2-dependent hematologic malignancies with navitoclax were promising, the efficacy is limited by dose-dependent thrombocytopenia.9–12 In an effort to circumvent this adverse effect caused by the inhibition of Bcl-xL, navitoclax has been re-engineered to specifically target Bcl-2. The new orally bioavailable compound, venetoclax, demonstrated minimal effects on platelets, both in vitro and ex vivo.13 Pre-clinical studies have demonstrated strong activity in cell lines, patient samples, and mouse xenograft models from Bcl-2 dependent malignancies such as chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML).13, 14
Additionally, potent cell killing was seen in disease subsets of non-Hodgkin’s lymphoma (NHL) and a subset of multiple myeloma [t(11;14)].13, 15 Given the promising pre-clinical data it is not surprising that single agent and combination venetoclax clinical trials are now underway for CLL, AML, NHL, and relapsed refractory multiple myeloma.
We have previously reported on a method of predicting sensitivity of myeloma cell lines and patient samples to the Bcl-2/xL inhibitor ABT-737, based on the binding pattern of pro-apoptotic protein Bim to anti-apoptotic proteins Mcl-1, Bcl-xL, and Bcl-2.16 In Mcl-1-dependent myeloma cells, Bim is primarily associated with Mcl-1, and are insensitive to ABT-737. In contrast, in myeloma cells that are co-dependent on Mcl-1 and Bcl-2/xL for survival, Bim is either predominantly associated with Bcl-2/xL or when it is released from Bcl-2/xL it can not bind to Mcl-1 because of the presence of the Mcl-1 inhibitor Noxa. As the adverse events associated with navitoclax limit its utility in the treatment of multiple myeloma, we sought to investigate the applicability of this method to venetoclax as well as determine its efficacy in a broad range of cell lines and patient samples alone and in combination with standard myeloma therapies.
Materials and Methods
Cell lines
Multiple myeloma cell line RPMI8226 (8226) was purchased from the American Type Culture Collection (ATCC, Manassas, VA). MM.1s cell line was obtained from Dr. Steven Rosen (Northwestern University, Chicago, IL), KMS11 and KMS18 cell lines were provided by Dr. P. Leif Bergsagel (Mayo Clinic, Scottsdale, AZ) and OPM2 by Nizar Bahlis (University of Calgary). Cells were maintained on supplemented RPMI-1640 media, as previously described.17
Reagents
Propidium iodide (PI), Melphalan (Mel), and Dexamethasone (Dex) were purchased from Sigma-Aldrich (St Louis, MO); Annexin-V–fluorescein isothiocyanate (FITC) was purchased from Biovision (Palo Alto, CA). Carfilzomib was generously provided by Onyx Pharmaceuticals and Venetoclax by AbbVie.
Apoptosis Assays
Cell death was measured by Annexin V-FITC and PI staining as previously described.18
Antibodies
The following primary antibodies were used for Western blot: mouse anti-Noxa mAb (Abcam, Cambridge, MA); rabbit anti-Bim pAb (EMD Millipore, Temecula, CA); rabbit anti-Mcl-1 pAb (Enzo Life Sciences, Farmingdale, NY); rabbit anti-Bcl-xL pAb (Cell Signaling Technology, Danvers, MA); rabbit anti-Bcl-2 pAb (Cell Signaling Technology); mouse anti-β-actin mAb (Sigma-Aldrich). For co-immunoprecipitation the following primary antibodies were used: mouse anti-Mcl-1 mAb (BD Biosciences, San Jose, CA); hamster anti-Bcl-2 mAb (BD Biosciences); mouse anti-Bcl-xL mAb (7B2.5).19 For Western blotting the following secondary antibodies were used: anti-mouse IgG1-HRP conjugate (Santa Cruz Biotechnology, Dallas, TX); ECL rabbit IgG-HRP linked whole antibody (from donkey; GE Healthcare Life Sciences, Piscataway, NJ). The secondary antibody used for Co-IP was provided in the Exacta- Cruz™ C Kit (Santa Cruz Biotechnology).
Western Blot Analysis
Western blotting was performed using standard techniques as previously described.17
Co-immunoprecipitation Studies
Immunoprecipitation experiments were performed using the Exacta- Cruz™ C Kit (Santa Cruz Biotechnology) following the manufacturer’s instructions as previously described.17
siRNAs
Small interfering RNAs (siRNAs) were purchased from Dharmacon (GE Life Sciences). ON-TARGETplus SMART pool siRNA against Noxa (PMAIP1) and the siCONTROL non-targeting siRNA [si(−)] were used per manufacturer’s protocol.
Real-time PCR
Real-time was performed as previously described,17 using Applied Biosystems High Capacity cDNA Reverse Transcription Kit (Life Technologies, Grand Island, NY) and amplified using the TaqMan Gene Expression Master Mix (Life Technologies) on the 7500 Fast Real-Time PCR System following the manufacturer’s protocol.
Patient Sample Processing
All samples were collecting following an Emory University Institutional Review Board-approved protocol as previously described.16
Results
Venetoclax is ineffective at inducing apoptosis in multiple myeloma cell lines
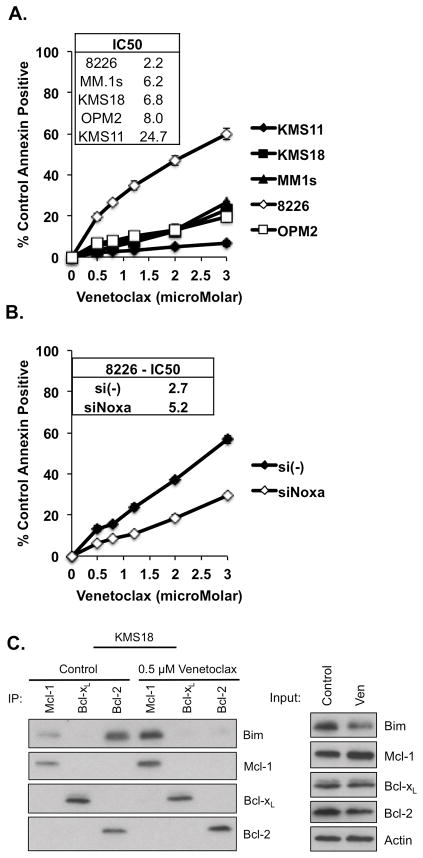
In order to determine the activity of venetoclax in multiple myeloma, we treated five human myeloma cell lines with increasing concentrations of venetoclax and examined the induction of apoptosis after 24 hours. Consistent with previous findings,15 only one cell line tested, RPMI8226 (8226), showed an appreciable dose response, although this line was relatively insensitive, with an IC50 of 2.2 μM (Fig. 1A). The remaining cell lines were resistant to the drug, with IC50s ranging from 6.2 μM for MM.1s to 24.7 μM for KMS11.
Figure 1. Multiple myeloma cell lines are insensitive to venetoclax.
A. KMS11, KMS18, MM.1s, 8226, and OPM2 were treated for 24 h with indicated concentrations of venetoclax. B. 8226 cells were transfected with the indicated siRNA. 12 h post transfection cells were treated with venetoclax for 24 h. C. KMS18 was treated with venetoclax for 24 h and protein lysates were prepared and subjected to co-immunoprecipitation with anti-Mcl-1, anti-Bcl-xL, and anti-Bcl-2 antibodies. Resulting protein complexes were subjected to Western blot analysis using anti- Mcl-1, Bcl-xL, and Bcl-2. Additionally, 20 μg of the protein lysates were subjected to Western blotting using the above antibodies to determine the protein input for each treatment. Cell viability in A. and B. was determined by flow cytometry following annexin-V-FITC/PI staining, Annexin = Annexin-V. The data in A and B are presented as the means (±SE) of 3 independent experiments. The Western blot in C is representative of four experiments.
Previous work from our lab has demonstrated that the sensitivity of 8226 cells to the Bcl-2/Bcl-xL inhibitor ABT-737 was due to the high expression of Noxa in this cell line.16 Therefore we wanted to determine if Noxa played a similar role in the response of 8226 to venetoclax. We transfected 8226 cells with either control or Noxa siRNAs prior to treatment with venetoclax. We were able to silence approximately half of the Noxa expression (Fig. S1), and in doing so, protected the cells from venetoclax-induced apoptosis (Fig. 1B).
The insensitivity observed with venetoclax in KMS18 was unexpected, as we have previously published data demonstrating the efficacy of ABT-737 at killing this line as well as evidence that this sensitivity was due to Bim binding to Bcl-2.16 To further investigate this lack of response to venetoclax, we performed co-immunoprecipitation studies on KMS18 cells pre- and post-treatment (Fig. 1C). Consistent with our previous results, Bim-binding in KMS18 cells is concentrated on Bcl-2 and Mcl-1. Upon treatment with venetoclax, Bim is released from Bcl-2, however it is redistributed to Mcl-1. Therefore the released Bim is not available to activate Bak and Bax and initiate apoptosis.
Venetoclax is active in combination with therapeutic agents commonly used in the treatment of multiple myeloma
As treatment of myeloma with a single agent is rare, we wanted to investigate the ability of venetoclax to affect the responses of myeloma cell lines to agents commonly used in myeloma treatment, such as melphalan, carfilzomib, and dexamethasone. We performed venetoclax dose curves similar to those presented in Figure 1, in the presence or absence of a single concentration of the additional myeloma drug.
Melphalan is a DNA alkylating agent used in the treatment of multiple myeloma and other cancers.20 In all cases, the addition of a single concentration of melphalan resulted in a left shift of the response curve (Fig. S2A). In 8226, KMS11, and OPM2 cell lines this shift resulted in a parallel response curve, which is indicative of an additive interaction. In contrast, in KMS18 and MM.1s cells, especially at the lower end of the dose curves, the change in the slope of the curve indicates a greater than additive interaction.
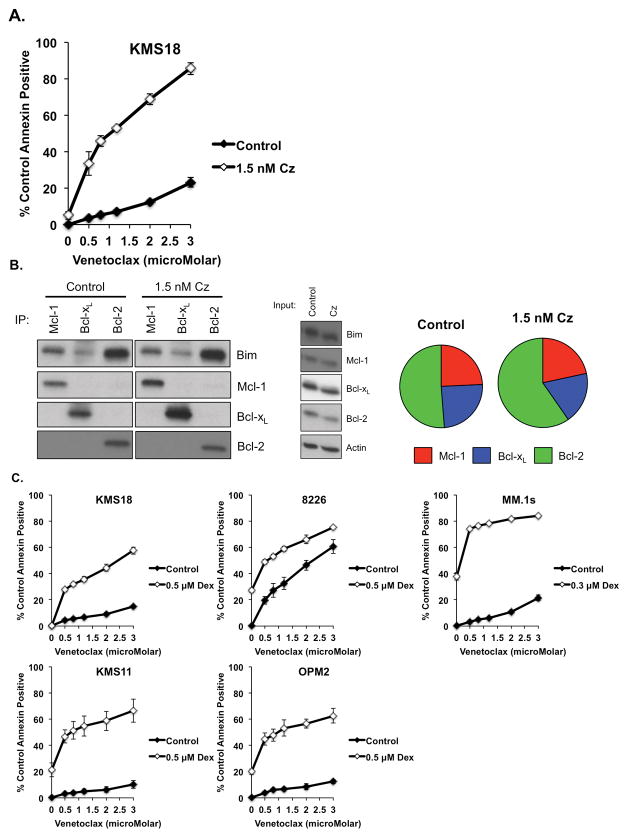
Carfilzomib is a highly active second generation proteasome inhibitor used in the treatment of relapse and refractory myeloma.21 Combining carfilzomib with venetoclax produced a greater than additive response in only one of the cell lines we tested, KMS18 (Fig. 2A and Fig. S2B). Proteasome inhibitors are known to induce the Mcl-1 inhibitor Noxa22 and we have observed this in myeloma cell lines with carfilzomib (manuscript in revision). Induction of Noxa would be expected to result in displacement of Bim from Mcl-1. To test this possibility, we co-immunoprecipitated Mcl-1, Bcl-xL, and Bcl-2 using monoclonal antibodies 24 h following treatment with carfilzomib and determined the distribution of Bim. As seen in Fig. 2B, nearly half of all the bound Bim is found on Bcl-2. Addition of carfilzomib results in a shift in the distribution of Bim from both Bcl-xL and Mcl-1 to Bcl-2. This should make these cells more Bcl-2 dependent and therefore more sensitive to venetoclax. In order to verify that this mechanism is unique to KMS18, we co-immunoprecipitated Mcl-1, Bcl-xL, and Bcl-2 in MM.1s following 24 h carfilzomib treatment and found no change in the Bim-Bcl-2 binding (Fig. S3).
Figure 2. Venetoclax is highly active in combination therapy.
KMS18, 8226, MM.1s, KMS11, and OPM2 were treated with indicated concentrations of venetoclax alone or in combination with the indicated concentration of A. Carfilzomib, or C. Dexamethasone for 24 h. B. KMS18 was treated with Carfilzomib for 24 h and protein lysates subjected to input analysis and co-immunoprecipitation as described in Figure 1. Densitometry measurements of the Bim bound to Mcl-1, Bcl-xL, and Bcl-2 under each treatment condition is represented as pie charts and the size of the pie is proportional to the total amount of bound Bim present under each treatment, relative to the control (1.0). Carfilzomib = 1.05. The data in A and C are presented as the means (±SE) of 3 independent experiments. The blot in B is representative of three experiments and densitometry average of two experiments.
Dexamethasone is a glucocorticoid steroid used in combination with multiple therapeutic agents in multiple myeloma. Co-treatment of myeloma cell lines with venetoclax and dexamethasone produced the greatest response among the combinations tested with greater than additive responses seen in 4 of the 5 cell lines tested (Fig. 2C), the exception being 8226. The largest increases in response to the combination were seen at the low end of the dose curves, suggesting that dexamethasone is making cells exquisitely Bcl-2 dependent.
Cell death induced by the combination of Venetoclax and Dexamethasone is mediated by changes in expression of the Bcl-2 family
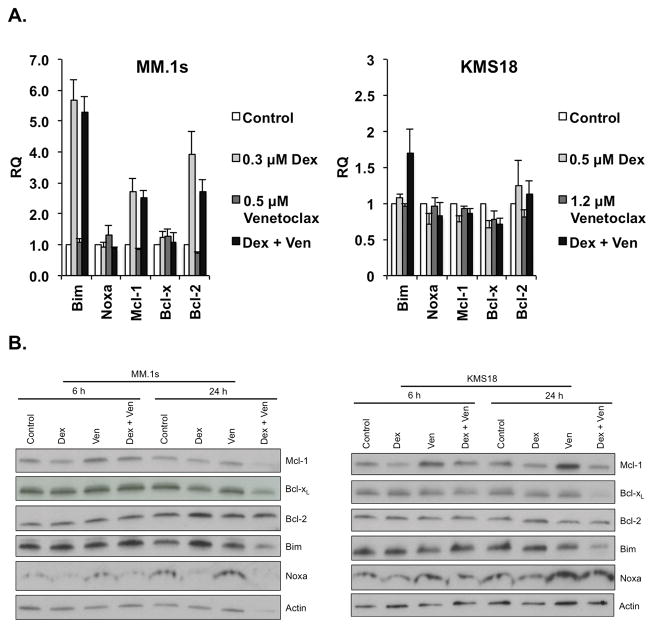
Given the striking apoptotic response generated by the co-treatment of myeloma cells with venetoclax and dexamethasone, we next sought to determine the mechanism. MM.1s and KMS18 cells were treated with two concentrations of venetoclax and a single concentration of dexamethasone alone or in combination for 24 h. For each condition, cells were collected for RNA-extraction and subjected to Real-time PCR. Changes in the mRNA levels of 5 members of the Bcl-2 family were examined (Fig. 3A and S4). Common to both cells lines, treatment with dexamethasone; either alone or in combination with venetoclax, increased the mRNA levels of Bim. Additionally an increase in Bcl-2 was observed in MM.1s. However, venetoclax alone resulted in minimal changes.
Figure 3. Dexamethasone synergizes with venetoclax through the induction of Bcl-2 and Bim.
A. MM.1s and KMS18 were treated with the indicated concentrations of venetoclax (Ven) and dexamethasone alone or in combination for 24 h. Real-time PCR was performed on cDNA generated from RNA isolated from control and treated samples. The data are presented as the means (±SE) of 3 independent experiments. B. Protein lysates were obtained from MM.1s and KMS18 6 and 24 h following treatment in A. Twenty μg of proteins were subjected to Western blotting and membranes probed with anti-Mcl-1, anti-Bcl-xL, anti-Bcl-2, anti-Bim, anti-Noxa, and anti-β-Actin antibodies. Blots are representative of two experiments.
In order to determine if these changes in mRNA levels translated into changes in protein expression Western blot analysis was performed. While the changes seen in protein expression were not to the same magnitude as those seen in mRNA, Bim and Bcl-2 protein levels were increased in response to dexamethasone and Bcl-xL decreased in response to co-treatment (Fig. 3B). In addition we noticed increased expression of both Noxa and Mcl-1 in response to venetoclax treatment in KMS18 cells. Together these events suggest that dexamethasone shifts cells to a more Bcl-2-dependent state.
Dexamethasone treatment induces alterations in the binding pattern of Bim to anti-apoptotic proteins results in increased Bcl-2 dependence
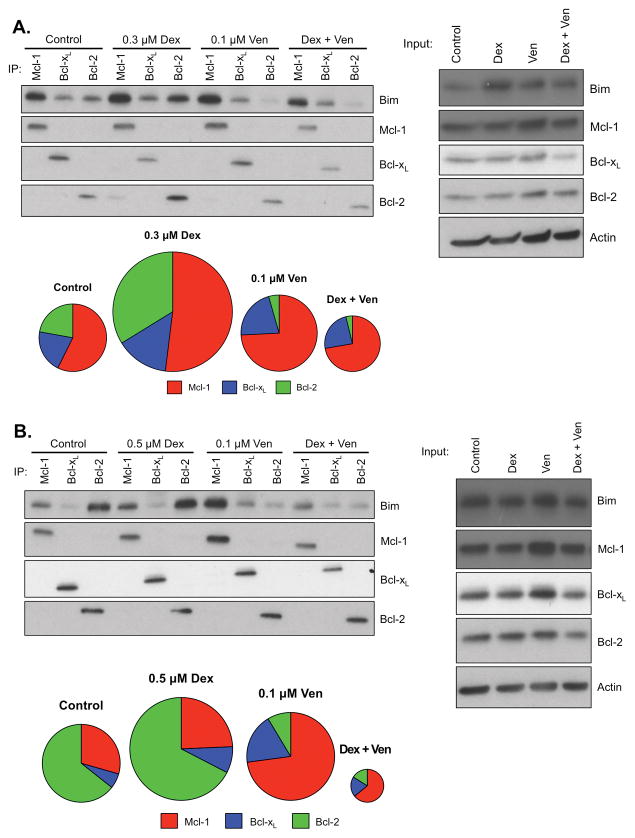
The pattern of Bim binding to anti-apoptotic Bcl-2 proteins is a better predictor of sensitivity than expression levels.16 Therefore, MM.1s (Fig. 4A) and KMS18 (Fig. 4B) cell lines were treated with venetoclax and dexamethasone alone or in combination, then lysed and co-immunoprecipitated with antibodies against Mcl-1, Bcl-2, and Bcl-xL. The relative levels of Bim bound to each anti-apoptotic under each treatment condition was then determined. Consistent with our previous findings, the control pattern of Bim binding in MM.1s and KMS18 differed, however alterations in the pattern due to drug treatment were similar. As shown above in Figure 1C, treatment with venetoclax alone leads to release of Bim from Bcl-2 and subsequent increased Bim bound to Mcl-1. Interestingly, treatment with dexamethasone in both cell lines resulted in increased binding of Bim to all three anti-apoptotic proteins, but with the most striking increase in Bcl-2 binding. Therefore upon co-treatment, a greater amount of Bim is released from Bcl-2 than in cells treated with venetoclax alone. These findings extend the data from Fig. 3, demonstrating that the dexamethasone-induced increase in Bim expression results in higher levels of apoptotic priming and an increase in the proportion of Bim bound to Bcl-2. In contrast, venetoclax has little effect on the amount of bound Bim, but drastically changes the distribution towards a Mcl-1 dependent phenotype. The combination treatment results in a reduction of the total bound Bim to levels that are similar (MM.1s) or less than (KMS18) control levels due to the release of nearly all the Bcl-2 bound Bim. Since the total bound Bim is released, the data suggest it has been released to activate Bax/Bak.
Figure 4. Co-treatment of MM.1s and KMS18 cell lines with venetoclax and dexamethasone induces changes in the pattern of Bim binding to anti-apoptotic proteins, resulting in apoptosis.
Lysates were obtained from A. MM.1s or B. KMS18 and protein subjected to co-immunoprecipitation and input Western blot analysis as described in Fig. 1. Densitometry measurements of the Bim bound to Mcl-1, Bcl-xL, and Bcl-2 under each treatment condition, as described in Fig. 2. A. MM.1s control = 1.00, Dex = 1.48, Venetoclax = 1.07, Combination = 0.89. B. KMS18 control = 1.00, Dex = 1.2, Venetoclax = 1.09, Combination = 0.65. Blots are representative of at least four experiments, densitometry average of two experiments.
Plasma cells from myeloma patients that are insensitive to Venetoclax alone become highly sensitive with the addition of low dose dexamethasone
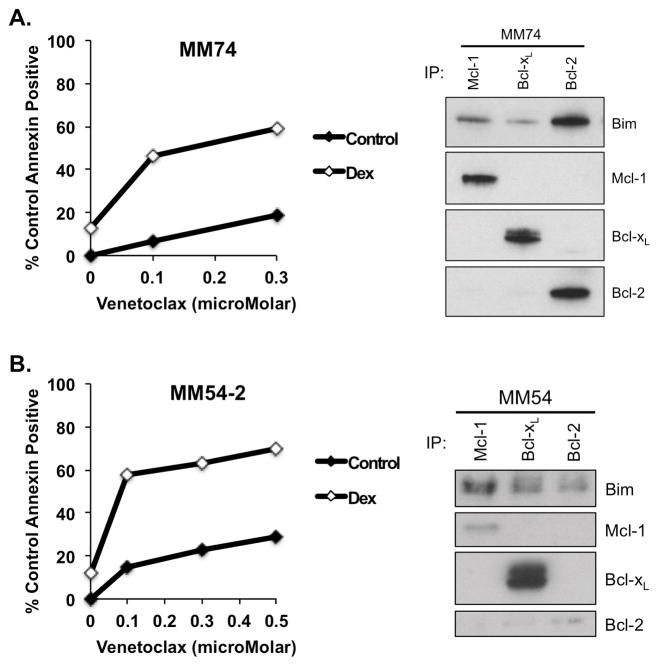
In order to determine if the response seen in myeloma cell lines could be reproduced in patient samples, we performed dose curves with venetoclax as a single agent or in combination with dexamethasone on cells isolated from bone marrow aspirates donated by multiple myeloma or plasma cell leukemia patients with varying genetic backgrounds (Table 1). In each sample tested, the addition of dexamethasone to venetoclax treatment decreased the IC50 over venetoclax alone, with fold changes ranging from 1.3 to 25 (Table 2). Figure 5 contains dose curves and co-immunoprecipitation data from two representative samples, chosen for their similarity to commonly used myeloma cell lines. MM74 had a Bim binding pattern and dose response very similar to that of the cell line KMS18, while MM54-2 is similar to MM.1s.
Table 1.
Patient Sample Diagnostics
| Sample | Sex | Age | Disease | ISS Stage | CTG | FISH | Prior Lines | LEN Ref | BZ Ref | CZ Ref | POM Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| MM68 | M | 74 | MM | ND | Complex karyotype | + IgH; + 1q; trisomy 3, 7; monosomy 13 | 5 | Y | Y | Y | N |
|
|
|||||||||||
| MM65 | M | 80 | sPCL | ND | Complex karyotype | del 1p; + 1q; + 14q; trisomy 9 | 5 | Y | Y | N | Y |
|
|
|||||||||||
| MM64 | F | 68 | MM | 1 | 46, XX | Negative | 1 | N | N | N | N |
|
|
|||||||||||
| MM74 | M | 50 | MM | 1 | 46, XY | t(4;14); + IgH; del 13q; trisomy 3; monosomy 7, 13 | 0 | N | N | N | N |
|
|
|||||||||||
| MM61 | M | 63 | pPCL | 3 | t(2;8) t(11;14) |
t(11;14) | 0 | N | N | N | N |
|
|
|||||||||||
| MM66 | M | 60 | MM | ND | Complex karyotype | + IgH; tetrasomy 1; trisomy 3, 7, 9; del 1p; del 13 | 4 | Y | Y | N | Y |
|
|
|||||||||||
| MM62 | F | 69 | MM | 3 | del (16) | t(4;14); + IgH; del 13 | 0 | N | N | N | N |
|
|
|||||||||||
| MM63 | F | 52 | MM | 1 | 46, XX | + IgH; + 1q; monosomy 13, 16 | 0 | N | N | N | N |
|
|
|||||||||||
| MM54-2 | M | 68 | MM | 1 | Complex karyotype | +7, 9, 11 | 2 | N | N | N | N |
Abbreviations: MM, multiple myeloma; sPCL, secondary plasma cell leukemia; pPCL, primary plasma cell leukemia; ND, not determined; CTG, cytogenetics; +, gain of; del, deletion of; LEN, lenalidomide; BZ, bortezomib; CZ, carfilzomib; POM, pomalidomide; Ref, refractory; Y, yes; N, no
Table 2.
IC50 (μM) of Plasma Cells +/− Dexamethasone
| Sample | Venetoclax | Ven + Dex | Fold Change |
|---|---|---|---|
|
|
|||
| MM68 | 1.4 | 1.1 | 1.3 |
|
|
|||
| MM65 | 2.5 | 1.4 | 1.79 |
|
|
|||
| MM64 | 2.2 | 0.98 | 2.24 |
|
|
|||
| MM74 | 1.4 | 0.24 | 5.8 |
|
|
|||
| MM61 | 4.7 | 0.64 | 7.34 |
|
|
|||
| MM66 | 3.3 | 0.38 | 8.68 |
|
|
|||
| MM62 | 4.2 | 0.24 | 17.5 |
|
|
|||
| MM63 | 30.2 | 1.5 | 20.13 |
|
|
|||
| MM54-2 | 2.0 | 0.08 | 25 |
Figure 5. A synergistic response to the combination of venetoclax and dexamethasone in plasma cells from myeloma patients.
Two million total cells from buffy coats were re-suspended in medium for culture, while the remaining cells were subjected to CD138+ cell isolation. Cells were treated with the indicated concentrations of venetoclax either alone or in combination with 0.5 μM dexamethasone for 24 h. Apoptosis was determined by staining with antibodies against CD38, CD45, and Annexin-V-FITC. Lysates were obtained from isolated CD138+ cells and protein subjected to co-immunoprecipitation and Western blot analysis as described in Fig. 1.
Discussion
Here we have taken advantage of the role the Bcl-2 family of proteins play in regulating apoptosis, the fact they are commonly overexpressed in hematologic malignancies, and a method we generated to gauge the reliance of myeloma cells on anti-apoptotic proteins for survival in order to determine the efficacy of venetoclax in treating multiple myeloma. Previous research has shown venetoclax to be highly effective in the treatment of myeloma cell lines and patient samples that harbor t(11;14).15 In order to broaden the pool of patients who may benefit from this drug, we chose non-t(11;14) cell lines for our study.
We tested the responses of 5 commonly used myeloma cell lines to treatment with venetoclax and found none to be sensitive. Interestingly, we have previously shown 3 of these cell lines to be sensitive to ABT-737, suggesting a co-dependence on Mcl-1 and Bcl-xL over Mcl-1 and Bcl-2. Upon further investigation, we found that treatment with venetoclax resulted in loss of Bim binding to Bcl-2, as is expected, but also a gain in Bim on Mcl-1. This suggests that at least some of the Mcl-1 in these cells is unoccupied by BH3-only proteins and acts a sink for the Bim released from Bcl-2 by venetoclax, thereby preventing apoptosis. This appears to be the case in 4 of the 5 cell lines. 8226 cells express high levels of Noxa, which, along with Bim can bind Mcl-1, leaving no unoccupied anti-apoptotic protein to neutralize the Bim that is released from Bcl-2 after treatment. These results suggest venetoclax may not be effective as a single agent but could be useful combined with agents that induce Noxa or Bim or alterations in Bim binding. Indeed, when we combined venetoclax with melphalan, carfilzomib or dexamethasone we saw at least additive responses with all cell lines. Importantly, in cell lines where combinations produced greater than additive responses, sub-lethal doses were sufficient to induced apoptosis.
As mentioned above, carfilzomib treatment results in increased Noxa expression, and we believed should produce a great response in combination with venetoclax. However, we only saw a greater than additive response in one cell line, KMS18. In the cells treated with low dose carfilzomib, Bim released from Mcl-1 and Bcl-xL is re-bound by Bcl-2, leaving only a small amount available to activate Bax and Bak, and suggesting saturation of Bcl-2 with Bim. When combined, venetoclax prevents the Bim released from Mcl-1 and Bcl-xL from binding Bcl-2 and also results in the loss of Bim from Bcl-2, thereby leading to a greater than additive response. Additionally, the greater amount of Bim bound to Bcl-2 under control conditions in KMS18 cells significantly contributes to this response.16
Dexamethasone has been shown to induce Bim expression in pediatric acute lymphoblastic leukemia primary patient samples,23 and produced the most striking response when combined with venetoclax in our myeloma cell lines. Evaluation of changes in expression of Bcl-2 family genes in response to dexamethasone and venetoclax alone or in combination revealed that most of the changes occur in response to dexamethasone alone, with minimal changes in response to venetoclax alone. However, when we examined changes in the expression pattern of Bcl-2 family members in response to the combination of dexamethasone and venetoclax, we see alterations with as low as 0.1 μM venetoclax, again suggesting that very little venetoclax is required to produce significant responses when it is combined with the proper therapeutic.
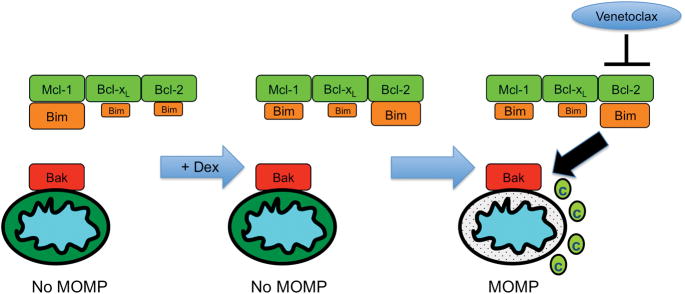
Multiple myeloma requires Mcl-1 for survival and therefore is more likely to be Mcl-1-primed, meaning Mcl-1 is bound by pro-death activator proteins, such as Bim. In Mcl-1 dependent cells there is much less Bim bound to Bcl-2, and when it gets released either it is not enough to activate Bax/Bak and induce MOMP, such as with MM.1s, or there is enough unoccupied Mcl-1 and Bcl-xL that the activators released from Bcl-2 can be re-bound by the other anti-apoptotic proteins, as with KMS18.4, 16 Under these conditions, a second agent is usually required to push the cell over the apoptotic threshold and induce cell death. The subset of multiple myeloma that is sensitive to venetoclax as a single agent harbors the CCND1 translocation t(11;14), which correlates with increased Bcl-2-priming.15, 24 Our co-immunoprecipitation studies revealed that treatment with dexamethasone results in increased Bim expression as well as shifting Bim binding patterns, such that MM.1s and KMS18 become Bcl-2 dependent (Figure 6). This dependence closely resembles the Bcl-2 priming seen with the CCND1 translocation, thereby rendering cells once resistant to venetoclax exquisitely sensitive. These data provide a striking example of how therapeutic agents can influence mitochondrial priming as was recently demonstrated with dynamic BH3 profiling.25 While those studies revealed how overall change in profiling could predict cellular responses, we have shown how specific changes in priming by a therapeutic agent (carfilzomib or dexamethasone) result in potent combination therapies.
Figure 6.
Model of Dexamethasone-induced Bcl-2 priming.
When considering treatment combinations it is important to determine the lowest dose of each drug required to obtain a significant response, as the ultimate goal for testing combinations in the lab is to translate them to a clinical setting. We tested the sensitivity of plasma cells from nine patients with either myeloma or plasma cell leukemia for sensitivity to venetoclax alone and in combination with low dose dexamethasone. The addition of dexamethasone drastically reduced the concentration of venetoclax required to achieve the IC50 in all samples tested. This trend was not dependent on disease sub-type as the there appears to be no cytogenetic or FISH diagnosis common to all of the patients whose samples we tested. Furthermore the correlation between our cell line data and patient samples suggests a combination regimen of venetoclax and dexamethasone may translate successfully to the clinic. Interestingly, MM61 was obtained at diagnosis and even though the plasma cell leukemia was shown to be positive for t(11;14) the cells were resistant to venetoclax, suggesting that patients with this sub-type may not universally respond to this drug. However, the addition of low dose dexamethasone significantly sensitized these cells to venetoclax. This should allow for an expansion of the use of venetoclax beyond patients with t(11;14) myeloma. Thus far; only patients with this subset have responded to venetoclax as a single agent and even within these patients, responses have not been uniform.26 A trial combining venetoclax with bortezomib and dexamethasone in relapsed/refractory myeloma is currently underway (ClinicalTrials.gov Identifier NCT01794507).
Supplementary Material
Real-time PCR was performed on cDNA isolated from si(−) and siNoxa transfected 8226 cells and analyzed for Noxa expression.
A. KMS18, 8226, MM.1s, KMS11, and OPM2 were treated with the indicated concentration of Melphalan for 24 h. B. 8226, MM.1s, KMS11, and OPM2 were treated with the indicated concentration of Carfilzomib for 24 h.
MM.1s was treated with Carfilzomib for 24 h and protein lysates subjected to input analysis and co-immunoprecipitation as described in Figure 1. Densitometry measurements of the Bim bound to Mcl-1, Bcl-xL, and Bcl-2 under each treatment condition is represented as pie charts and the size of the pie is proportional to the total amount of bound Bim present under each treatment, relative to the control (1.0). Carfilzomib = 1.06.
MM.1s and KMS18 were treated with the indicated concentrations of venetoclax and dexamethasone alone or in combination for 24 h. Real-time PCR was performed on cDNA generated from RNA isolated from control and treated samples. The data are presented as the means (±SE) of 3 independent experiments.
References
- 1.Cancer Facts & Figures. Ameriican Cancer Society; 2015. [Google Scholar]
- 2.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006 May;9(5):351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010 Feb 12;37(3):299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davids MS, Letai A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. J Clin Oncol. 2012 Sep 1;30(25):3127–3135. doi: 10.1200/JCO.2011.37.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuroda J, Taniwaki M. Involvement of BH3-only proteins in hematologic malignancies. Crit Rev Oncol Hematol. 2009 Aug;71(2):89–101. doi: 10.1016/j.critrevonc.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010 Sep;11(9):621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 7.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005 Jun 2;435(7042):677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 8.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008 May 1;68(9):3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 9.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007 Jan;117(1):112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Gaizo Moore V, Schlis KD, Sallan SE, Armstrong SA, Letai A. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood. 2008 Feb 15;111(4):2300–2309. doi: 10.1182/blood-2007-06-098012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hann CL, Daniel VC, Sugar EA, Dobromilskaya I, Murphy SC, Cope L, et al. Therapeutic efficacy of ABT-737, a selective inhibitor of BCL-2, in small cell lung cancer. Cancer Res. 2008 Apr 1;68(7):2321–2328. doi: 10.1158/0008-5472.CAN-07-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoenwaelder SM, Jarman KE, Gardiner EE, Hua M, Qiao J, White MJ, et al. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood. 2011 Aug 11;118(6):1663–1674. doi: 10.1182/blood-2011-04-347849. [DOI] [PubMed] [Google Scholar]
- 13.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013 Feb;19(2):202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 14.Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014 Mar;4(3):362–375. doi: 10.1158/2159-8290.CD-13-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Touzeau C, Dousset C, Le Gouill S, Sampath D, Leverson JD, Souers AJ, et al. The Bcl-2 specific BH3 mimetic ABT-199: a promising targeted therapy for t(11;14) multiple myeloma. Leukemia. 2014 Jan;28(1):210–212. doi: 10.1038/leu.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morales AA, Kurtoglu M, Matulis SM, Liu J, Siefker D, Gutman DM, et al. Distribution of Bim determines Mcl-1 dependence or codependence with Bcl-xL/Bcl-2 in Mcl-1-expressing myeloma cells. Blood. 2011 Aug 4;118(5):1329–1339. doi: 10.1182/blood-2011-01-327197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morales AA, Gutman D, Lee KP, Boise LH. BH3-only proteins Noxa, Bmf, and Bim are necessary for arsenic trioxide-induced cell death in myeloma. Blood. 2008 May 15;111(10):5152–5162. doi: 10.1182/blood-2007-10-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grad JM, Bahlis NJ, Reis I, Oshiro MM, Dalton WS, Boise LH. Ascorbic acid enhances arsenic trioxide-induced cytotoxicity in multiple myeloma cells. Blood. 2001 Aug 1;98(3):805–813. doi: 10.1182/blood.v98.3.805. [DOI] [PubMed] [Google Scholar]
- 19.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995 Jul;3(1):87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 20.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996 Jul 11;335(2):91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 21.Gupta VA, Nooka AK, Lonial S, Boise LH. Clinical potential of carfilzomib in the treatment of relapsed and refractory multiple myeloma. Blood & Lymphatic Cancer: Targets & Therapy. 2013;3:41. [Google Scholar]
- 22.Fennell DA, Chacko A, Mutti L. BCL-2 family regulation by the 20S proteasome inhibitor bortezomib. Oncogene. 2008 Feb 21;27(9):1189–1197. doi: 10.1038/sj.onc.1210744. [DOI] [PubMed] [Google Scholar]
- 23.Jing D, Bhadri VA, Beck D, Thoms JA, Yakob NA, Wong JW, et al. Opposing regulation of BIM and BCL2 controls glucocorticoid-induced apoptosis of pediatric acute lymphoblastic leukemia cells. Blood. 2015 Jan 8;125(2):273–283. doi: 10.1182/blood-2014-05-576470. [DOI] [PubMed] [Google Scholar]
- 24.Touzeau C, Ryan J, Guerriero JL, Moreau P, Chonghaile TN, Le Gouill S, et al. BH3-profiling identifies heterogeneous dependency on Bcl-2 family members in Multiple Myeloma and predicts sensitivity to BH3 mimetics. Leukemia. 2015 Jul 15; doi: 10.1038/leu.2015.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montero J, Sarosiek KA, DeAngelo JD, Maertens O, Ryan J, Ercan D, et al. Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell. 2015 Feb 26;160(5):977–989. doi: 10.1016/j.cell.2015.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar S, Vij R, Kaufman JL, Mikhael J, Facon T, Moreau P, et al. Phase I interim safety and efficacy of venetoclax (ABT-199/GDC-0199) monotherapy for relapsed/refractory (R/R) multiple myeloma (MM) J Clin Oncol. 2015;33(Suppl):abstr 8576. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Real-time PCR was performed on cDNA isolated from si(−) and siNoxa transfected 8226 cells and analyzed for Noxa expression.
A. KMS18, 8226, MM.1s, KMS11, and OPM2 were treated with the indicated concentration of Melphalan for 24 h. B. 8226, MM.1s, KMS11, and OPM2 were treated with the indicated concentration of Carfilzomib for 24 h.
MM.1s was treated with Carfilzomib for 24 h and protein lysates subjected to input analysis and co-immunoprecipitation as described in Figure 1. Densitometry measurements of the Bim bound to Mcl-1, Bcl-xL, and Bcl-2 under each treatment condition is represented as pie charts and the size of the pie is proportional to the total amount of bound Bim present under each treatment, relative to the control (1.0). Carfilzomib = 1.06.
MM.1s and KMS18 were treated with the indicated concentrations of venetoclax and dexamethasone alone or in combination for 24 h. Real-time PCR was performed on cDNA generated from RNA isolated from control and treated samples. The data are presented as the means (±SE) of 3 independent experiments.