Abstract
BACKGROUND
In the Carotid Revascularization Endarterectomy versus Stenting Trial, we found no significant difference between the stenting group and the endarterectomy group with respect to the primary composite end point of stroke, myocardial infarction, or death during the periprocedural period or any subsequent ipsilateral stroke during 4 years of follow-up. We now extend the results to 10 years.
METHODS
Among patients with carotid-artery stenosis who had been randomly assigned to stenting or endarterectomy, we evaluated outcomes every 6 months for up to 10 years at 117 centers. In addition to assessing the primary composite end point, we assessed the primary end point for the long-term extension study, which was ipsilateral stroke after the periprocedural period.
RESULTS
Among 2502 patients, there was no significant difference in the rate of the primary composite end point between the stenting group (11.8%; 95% confidence interval [CI], 9.1 to 14.8) and the endarterectomy group (9.9%; 95% CI, 7.9 to 12.2) over 10 years of follow-up (hazard ratio, 1.10; 95% CI, 0.83 to 1.44). With respect to the primary long-term end point, postprocedural ipsilateral stroke over the 10-year follow-up occurred in 6.9% (95% CI, 4.4 to 9.7) of the patients in the stenting group and in 5.6% (95% CI, 3.7 to 7.6) of those in the endarterectomy group; the rates did not differ significantly between the groups (hazard ratio, 0.99; 95% CI, 0.64 to 1.52). No significant between-group differences with respect to either end point were detected when symptomatic patients and asymptomatic patients were analyzed separately.
CONCLUSIONS
Over 10 years of follow-up, we did not find a significant difference between patients who underwent stenting and those who underwent endarterectomy with respect to the risk of periprocedural stroke, myocardial infarction, or death and subsequent ipsilateral stroke. The rate of postprocedural ipsilateral stroke also did not differ between groups. (Funded by the National Institutes of Health and Abbott Vascular Solutions; CREST ClinicalTrials.gov number, NCT00004732.)
We previously reported the outcomes up to 4 years in the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST).1 No significant difference was shown between patients assigned to stenting and those assigned to endarterectomy with respect to the composite primary end point of periprocedural stroke, myocardial infarction, or death and subsequent ipsilateral stroke. At baseline, the mean age of the patients was 69 years, and at that age the average life expectancy is 15 years for men and 17 years for women.2 As such, long-term treatment differences should be central to treatment decisions. We now report whether the outcomes after stenting and endarterectomy differed over 10 years of follow-up.
METHODS
STUDY DESIGN
The study design and methods have been reported previously.3 CREST was a randomized, controlled trial with blinded end-point adjudication. The protocol, which is available with the full text of this article at NEJM.org, was amended to include assessment of treatment differences over 10 years of follow-up. Approval of the protocol was obtained by the ethics review board at each participating center. The patients provided written informed reconsent for extended follow-up up to 10 years.
The authors designed the study, gathered and analyzed the data, wrote the manuscript, and made the decision to submit the manuscript for publication. The authors vouch for the completeness and accuracy of the data and analyses and attest to the fidelity of this report to the study protocol. Abbott Vascular Solutions contributed devices and funding but did not participate in the design of the study or in the preparation or review of the manuscript. Abbott Vascular Solutions assisted with site monitoring, including monitoring of all Canadian sites.
CENTERS AND PATIENTS
Patients were recruited at 117 centers in the United States and Canada. Certification was achieved by 477 surgeons and 224 interventionists.4 Eligible patients could have symptomatic or asymptomatic carotid stenosis. Patients were considered to be symptomatic if they had had a transient ischemic attack, amaurosis fugax, or minor nondisabling stroke involving the ipsilateral carotid artery within 180 days before randomization; eligibility criteria for symptomatic patients included stenosis of 50% or more of the diameter of the artery on angiography, 70% or more on ultrasonography, or 70% or more on computed tomographic angiography or magnetic resonance angiography if the stenosis on ultrasonography was 50 to 69%. For asymptomatic patients, eligibility criteria included stenosis of 60% or more on angiography, 70% or more on ultrasonography, or 80% or more on computed tomographic angiography or magnetic resonance angiography if the stenosis on ultrasonography was 50 to 69%. The eligibility criteria have been published previously3 and are described in the protocol.
TREATMENT
Stenting and endarterectomy were performed according to published guidelines.4–6 Dual anti-platelet treatment was initiated before stenting. The RX Acculink stent and, whenever feasible, the RX Accunet device to capture and remove emboli (“embolic protection” device) were used. After stenting, patients received one or two 325-mg doses of aspirin daily for 30 days and either clopidogrel, at a dose of 75 mg daily, or ticlopidine, at a dose of 250 mg twice daily, for 4 weeks. Patients assigned to endarterectomy received 325 mg of aspirin daily before the procedure. All the patients received aspirin at a dose of 80 to 325 mg after 4 weeks and medical therapy that was consistent with the current standard of care.7
ASCERTAINMENT OF END POINTS
Examination of patients was performed annually and included the administration of the Transient Ischemic Attack (TIA)–Stroke Questionnaire8 and ultrasonography of the carotid artery. A telephone interview, which included the administration of the TIA–Stroke Questionnaire, was performed at the 6-month point between the visits, as well as annually for patients who were unable to attend the annual visit.
The primary end point of the trial was a composite of any stroke, myocardial infarction, or death during the periprocedural period or ipsilateral stroke up to 10 years after randomization. We defined the periprocedural period as the period from randomization to 30 days after the procedure for patients who underwent the procedure within 30 days after randomization (i.e., per-protocol treatment). For patients who did not undergo the procedure within 30 days, we defined the periprocedural period as the period from randomization to 36 days after randomization. We chose 36 days because that was the median time after randomization for all patients receiving their treatment beyond 30 days. The primary end point for the 10-year follow-up study was ipsilateral stroke that occurred after 36 days after randomization among patients who had had no periprocedural event.
Study committees whose members were unaware of the treatment assignments adjudicated stroke and myocardial infarction. Stroke was defined as an acute neurologic event with symptoms and signs, lasting for 24 hours or more, that were consistent with focal cerebral ischemia. Stroke was defined as major on the basis of a review of clinical data gathered at the time of stroke assessment or if the National Institutes of Health Stroke Scale score9 was 9 points or higher (on a scale ranging from 0, indicating no deficit, to 42, consistent with quadriplegia and coma) after 90 days.10 Strokes were otherwise considered to be minor. Myocardial infarction was defined by a creatine kinase MB or troponin level that was twice the upper limit of the normal range or higher, in addition to either chest pain or symptoms that were consistent with ischemia or electrocardiographic evidence of ischemia.11
Time to restenosis was defined as the time from the procedure to either ipsilateral revascularization or the detection of stenosis of 70 to 99% or occlusion on an ultrasonographic examination performed annually after stenting or endarterectomy, with the degree of stenosis determined by the norms of the local ultrasonographic laboratory. Control of risk factors was assessed at annual visits. Treatment goals for the patients included a low-density lipoprotein cholesterol level of less than 100 mg per deciliter (2.60 mmol per liter), a systolic blood pressure of less than 140 mm Hg, a nonfasting glucose level of less than 200 mg per deciliter (11.1 mmol per liter), and discontinuation of smoking.
STATISTICAL ANALYSIS
All the analyses except time to restenosis were performed on an intention-to-treat basis; time to restenosis was evaluated in a per-protocol analysis that included patients who received their assigned treatment within 30 days after randomization. Long-term outcomes were assessed with the use of standard survival-analysis techniques including Kaplan–Meier survival curves and Cox proportional-hazards models with adjustment for age, sex, and symptomatic status. The assessment of differences in the rate of postprocedural ipsilateral stroke was performed with the use of similar approaches, but the analysis was restricted to patients who did not have a composite end-point event during the periprocedural period.
Event rates were calculated by means of Kaplan–Meier estimation. Because of the relatively small number of events, the 95% confidence intervals for the Kaplan–Meier event rates were estimated with the use of bootstrap techniques with 10,000 replications, and the 2.5th and 97.5th percentiles of the sample distribution are reported as the confidence intervals. The ratio of event rates with stenting to event rates with endarterectomy was estimated annually. The confidence intervals for the annual ratios were also assessed with the use of bootstrap methods. We determined whether there were temporal changes in the treatment effect for the two primary end points by means of pairwise assessment of differences in the relative risk estimated at annual intervals, with the P value calculated by bootstrap methods. Analyses of all strokes, nonipsilateral strokes, and life expectancy were not prespecified. Life-table approaches were used to calculate life expectancy according to sex and symptomatic status at 55, 65, and 75 years of age for the comparison with the general U.S. population (Fig. S1A, S1B, and S1C in the Supplementary Appendix, available at NEJM.org).
RESULTS
STUDY POPULATION AND TREATMENTS
From 2000 through 2008, a total of 2502 patients underwent randomization. The median follow-up was 7.4 years. The characteristics of the two groups at baseline were similar, except with respect to dyslipidemia (82.9% in the stenting group vs. 85.8% in the endarterectomy group, P = 0.05) (Table S1 in the Supplementary Appendix). A diagram showing the randomization and follow-up of the study patients is provided in Figure S2 in the Supplementary Appendix.
Consent for the long-term follow-up was obtained from 1607 patients and was not obtained from 895 patients. Of the 895 patients who did not give consent for long-term follow-up, 195 declined to participate, 276 withdrew from the study, 187 died, 76 completed the initial study before the initiation of long-term follow-up, and 161 had a primary composite end-point event (Table 1, and Fig. S2 in the Supplementary Appendix). Patients who provided consent were significantly more likely than patients who declined to provide consent to have asymptomatic stenosis or dyslipidemia but were significantly less likely to have diabetes or to be current smokers (Table 1).
Table 1.
Characteristics of the Study Population at Baseline, Overall and According to Provision of Informed Consent for Long-term Follow-up.*
| Characteristic | All Patients (N = 2502) |
Consented (N = 1607) |
Did Not Consent (N = 195) |
Consent Not Attempted (N = 700) |
|---|---|---|---|---|
| Age (yr) | 69.0±8.9 | 68.3±8.3 | 69.2±9.3 | 70.7±9.7 |
| Assigned to stenting (%) | 50.4 | 51.0 | 50.3 | 49.3 |
| Male sex (%) | 65.2 | 66.5 | 60.5 | 63.4 |
| White race (%)† | 93.2 | 94.5 | 91.8 | 90.7 |
| Asymptomatic (%) | 47.2 | 52.5 | 36.9 | 37.9 |
| Risk factor (%) | ||||
| Hypertension | 85.9 | 84.7 | 86.1 | 88.7 |
| Diabetes | 30.5 | 28.8 | 36.6 | 32.8 |
| Dyslipidemia | 84.4 | 87.0 | 83.0 | 78.6 |
| Current smoker | 26.3 | 24.8 | 34.4 | 27.5 |
| Prior cardiovascular disease or CABG | 45.0 | 43.3 | 43.6 | 49.4 |
| Severe stenosis (%)‡ | 86.0 | 86.8 | 83.6 | 84.9 |
Plus–minus values are means ±SD. There were no significant between-group differences in the characteristics at baseline, except for asymptomatic stenosis (P<0.001), diabetes (P = 0.02), and current smoking (P = 0.004). P values were calculated by the chi-square test, with the exception of a t-test for age, for the difference in the baseline rate between patients who consented and those who did not consent. CABG denotes coronary-artery bypass grafting.
Race was self-reported.
Severe stenosis was defined as stenosis of at least 70% of the diameter of the artery.
During the periprocedural period, the rate of the primary composite end point of stroke, death, or myocardial infarction did not differ significantly between the stenting group and the endarterectomy group (5.2% and 4.5%, respectively; P = 0.38), although the rates of the individual components differed significantly. There were more periprocedural strokes in the stenting group than in the endarterectomy group (4.1% vs. 2.3%, P = 0.01), although there were significantly fewer periprocedural myocardial infarctions in the stenting group than in the endarterectomy group (1.1% vs. 2.3%, P = 0.03) (Table 2).
Table 2.
Primary End Point, Components of the Primary End Point, and Other Events, According to Treatment Group.*
| End Point | Periprocedural Period plus 10-Yr Follow-up |
Periprocedural Period Only |
Postprocedural Period Only |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Events |
Rate (95% CI) |
Hazard Ratio (95% CI) |
P Value | No. of Events |
Rate (95% CI) |
Hazard Ratio (95% CI) |
P Value | No. of Events |
Rate (95% CI) |
Hazard Ratio (95% CI) |
P Value | |
| % | % | % | ||||||||||
| Primary composite end point | ||||||||||||
| Stenting | 108 | 11.8 (9.1–14.8) |
1.10 (0.83–1.44) |
0.51 | 66 | 5.2 (4.0–6.4) |
1.18 (0.82–1.68) |
0.38 | — | — | — | — |
| Endarterectomy | 97 | 9.9 (7.9–12.2) |
56 | 4.5 (3.3–5.7) |
— | — | — | — | ||||
| Stroke or periprocedural death | ||||||||||||
| Stenting | 98 | 11.0 (8.5–13.9) |
1.37 (1.01–1.86) |
0.04 | 55 | 4.4 (3.2–5.6) |
1.90 (1.21–2.98) |
0.005 | — | — | — | — |
| Endarterectomy | 71 | 7.9 (5.9–10.0) |
29 | 2.3 (1.5–3.1) |
— | — | — | — | ||||
| Myocardial infarction | ||||||||||||
| Stenting | — | — | — | — | 14 | 1.1 (0.5–1.7) |
0.50 (0.26–0.94) |
0.03 | — | — | — | — |
| Endarterectomy | — | — | — | — | 28 | 2.3 (1.5–3.1) |
— | — | — | — | ||
| Stroke | ||||||||||||
| Total | ||||||||||||
| Stenting | 95 | 10.8 (8.3–13.7) |
1.33 (0.98–1.80) |
0.07 | 52 | 4.1 (2.9–5.3) |
1.79 (1.14–2.82) |
0.01 | 42 | 6.9 (4.4–9.7) |
0.99 (0.64–1.52) |
0.96 |
| Endarterectomy | 71 | 7.9 (5.9–10.0) |
29 | 2.3 (1.5–3.1) |
41 | 5.6 (3.7–7.6) |
||||||
| Major | ||||||||||||
| Stenting | 24 | 3.5 (1.8–5.6) |
1.53 (0.80–2.92) |
0.19 | 11 | 0.9 (0.3–1.5) |
1.35 (0.54–3.36) |
0.52 | 12 | 2.7 (1.0–4.8) |
1.91 (0.71–5.10) |
0.20 |
| Endarterectomy | 15 | 2.0 (0.9–3.5) |
8 | 0.6 (0.2–1.0) |
6 | 1.1 (0.2–2.4) |
||||||
| Minor | ||||||||||||
| Stenting | 71 | 7.4 (5.4–9.5) |
1.23 (0.87–1.74) |
0.25 | 41 | 3.2 (2.2–4.2) |
1.95 (1.15–3.30) |
0.01 | 30 | 4.2 (2.5–6.3) |
0.83 (0.51–1.34) |
0.44 |
| Endarterectomy | 57 | 6.2 (4.5–8.0) |
21 | 1.7 (0.9–2.5) |
35 | 4.5 (3.0–6.2) |
||||||
The periprocedural period was defined, according to the study protocol, as the 30-day period after the procedure (for all patients who underwent the assigned procedure within 30 days after randomization) or the 36-day period after randomization (for all patients who did not undergo the assigned procedure within 30 days after randomization). The analysis of the periprocedural period plus 10-year follow-up was based on data for all the patients. The postprocedural-only period excluded patients who had a composite end-point event during the periprocedural period or who withdrew during the periprocedural period; the number of periprocedural events plus the number of postprocedural events therefore do not add up to the total. The primary end point was a composite of stroke, myocardial infarction, or death from any cause during the periprocedural period or ipsilateral stroke within 10 years after randomization. One patient in the stenting group and one in the endarterectomy group had a stroke after a periprocedural myocardial infarction; these two strokes are not included in the stroke end point in the postprocedural-only period. Among the patients assigned to endarterectomy, one patient had a minor stroke during the periprocedural period and a major stroke during the postprocedural period and is therefore included in the stroke end point for both major stroke and minor stroke. Hazard ratios are for the 10-year period and were adjusted for age, symptomatic status, and sex. P values were calculated on the basis of the significance of the hazard ratio (per the study protocol). Patients may have had more than one event (e.g., fatal stroke was counted as both a death and a stroke, patients may have had an ipsilateral stroke followed by a nonipsilateral stroke, and patients may have had a minor stroke followed by a major stroke).
PRIMARY END POINTS
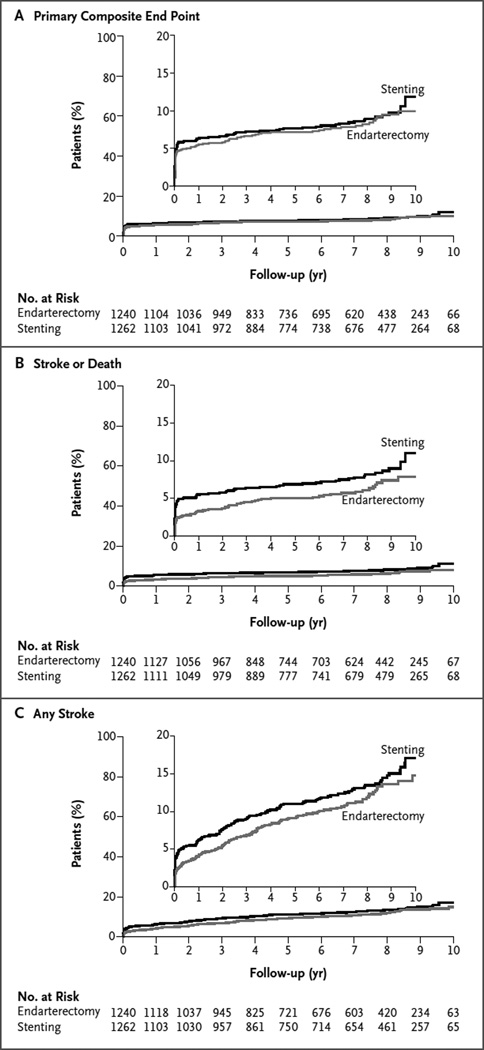
The 10-year risk of the primary composite end point (any stroke, myocardial infarction, or death during the periprocedural period or ipsilateral stroke thereafter) did not differ significantly between the stenting group and the endarterectomy group (hazard ratio in the stenting group, 1.10; 95% confidence interval [CI], 0.83 to 1.44; P = 0.51) (Table 2 and Fig. 1A). At 10 years, the event rates were 11.8% (95% CI, 9.1 to 14.8) in the stenting group and 9.9% (95% CI, 7.9 to 12.2) in the endarterectomy group (Table 2). There were also no significant differences between the stenting group and the endarterectomy group at any other year of follow-up between 1 and 9 years (Table S2 in the Supplementary Appendix).
Figure 1. Cumulative Proportions of Patients with the Primary Composite End Point, Stroke or Death, and Any Stroke, According to Treatment Group.
Shown are Kaplan–Meier estimates of event rates for the primary end point (a composite of stroke, myocardial infarction, or death from any cause during the periprocedural period or ipsilateral stroke within 10 years after randomization) (Panel A), any stroke or death during the periprocedural period or ipsilateral stroke afterward (Panel B), and any stroke (regardless of relationship with the target artery) (Panel C). The curves are shown separately for patients who were randomly assigned to carotid-artery stenting and those who were randomly assigned to carotid endarterectomy. Insets show the same data on an enlarged y axis.
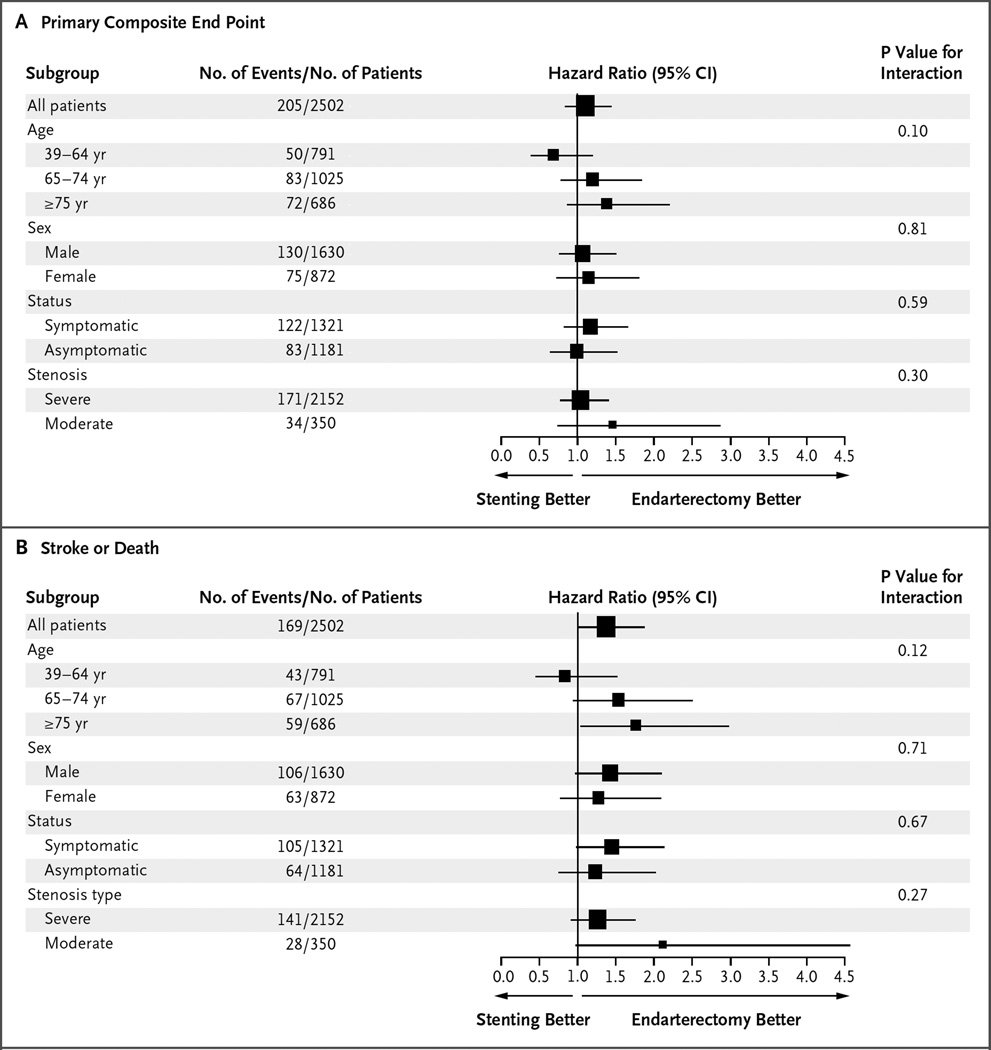
There were no significant treatment differences according to symptomatic status (hazard ratio among asymptomatic patients in the stenting group, 0.99; 95% CI, 0.64 to 1.52; hazard ratio among symptomatic patients in the stenting group, 1.17; 95% CI, 0.82 to 1.66; P = 0.59 for interaction) (Fig. 2, and Table S3 and Fig. S3 in the Supplementary Appendix). The P values for interaction with respect to the primary composite end point were as follows: 0.10 for the interaction between treatment and age, 0.81 for the interaction between treatment and sex, and 0.30 for the interaction between treatment and degree of stenosis; the corresponding P values with respect to the end point of stroke or death were 0.12, 0.71, and 0.27 (Fig. 2).
Figure 2. Subgroup Analyses of the Primary Composite End Point and the End Point of Stroke or Death.
Hazard ratios and associated 95% confidence intervals are shown for the primary composite end point of any stroke, death, or myocardial infarction during the periprocedural period plus ipsilateral stroke within 10 years after randomization (Panel A) and for any stroke or death during the periprocedural period plus ipsilateral stroke within 10 years after randomization (Panel B). Severe stenosis was defined as stenosis of at least 70% of the diameter of the artery, and moderate stenosis as less than 70%. The sizes of the boxes are proportional to the numbers of patients in the strata, and horizontal lines indicate 95% confidence intervals.
There were no significant differences in the rate of the primary long-term end point — post-procedural ipsilateral stroke over the 10-year follow-up — between the stenting group and the endarterectomy group (6.9% [95% CI, 4.4 to 9.7] and 5.6% [95% CI, 3.7 to 7.6], respectively; hazard ratio, 0.99; 95% CI, 0.64 to 1.52) (Table 2). There were 83 postprocedural strokes, and the risk was similar between patients assigned to stenting (42 events) and those assigned to endarterectomy (41 events). During the postprocedural period, there were nominally more major strokes among patients assigned to stenting than among those assigned to endarterectomy (12 events and 6 events, respectively); however, this difference was not significant (hazard ratio, 1.91; 95% CI, 0.71 to 5.10; P = 0.20). In the stenting group, the rate of stroke at 5 years was 2.5% (95% CI, 1.2 to 3.7) among symptomatic patients and 2.5% (95% CI, 1.1 to 3.8) among asymptomatic patients; the rates in the endarterectomy group were 2.7% (95% CI, 1.9 to 4.9) among symptomatic patients and 2.7% (95% CI, 1.8 to 4.9) among asymptomatic patients (Table S3 in the Supplementary Appendix).
SECONDARY ANALYSES
Stroke or Death
The risk of periprocedural stroke or death and subsequent ipsilateral stroke was 37% higher in the stenting group than in the endarterectomy group (hazard ratio, 1.37; 95% CI, 1.01 to 1.86; P = 0.04) (Table 2 and Fig. 1B). The advantage for endarterectomy was due primarily to differences in the rates of periprocedural events (Table 2). Graphs of the event rates stratified according to symptomatic status are provided in Figures S3, S4, and S5 in the Supplementary Appendix. Event rates for all stroke and for strokes that were ipsilateral or nonipsilateral to the study artery are shown, respectively, in Figure 1C and in Table S4 in the Supplementary Appendix.
Restenosis
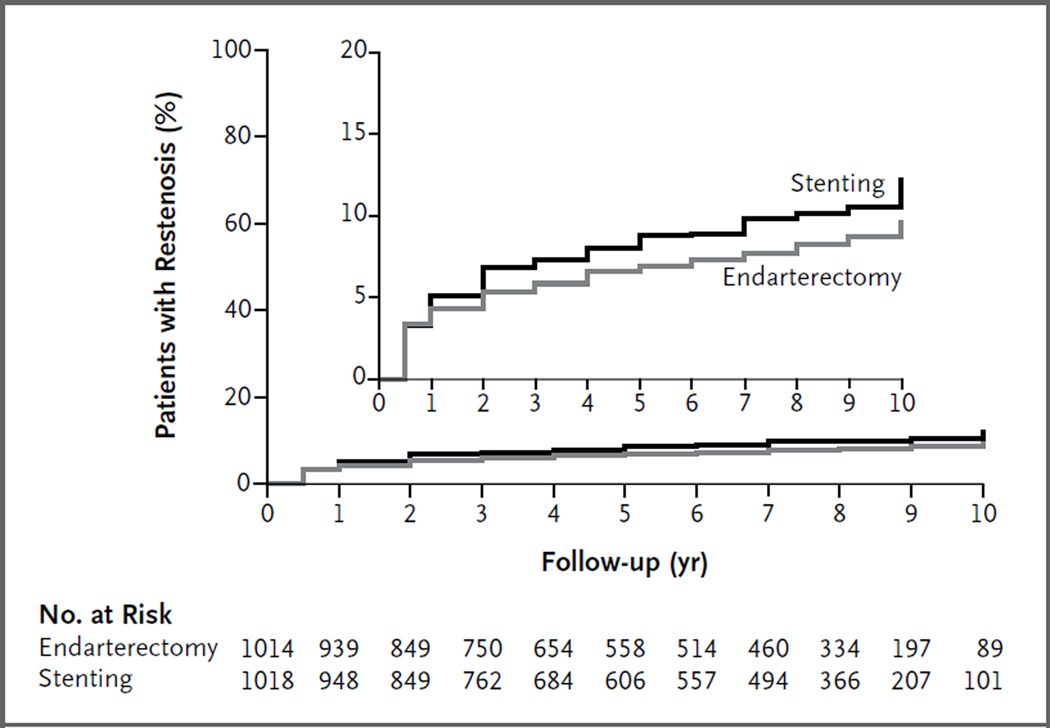
No significant difference between the two treatment groups was observed in the percentage of patients who had restenosis or underwent revascularization. Restenosis occurred or revascularization was performed in 12.2% of the patients treated with stenting and in 9.7% of those treated with endarterectomy (hazard ratio, 1.24; 95% CI, 0.91 to 1.70) (Fig. 3).
Figure 3. Estimated Rate of Restenosis.
Restenosis was defined by the first observation of stenosis of 70% or more of the diameter of the artery on duplex ultrasonography or by ipsilateral revascularization. Ultrasonography was performed at annual visits, and therefore a restenosis reported at year 1 was observed at the 1-year clinic visit (which was performed within a 30-day window). The inset shows the same data on an enlarged y axis.
Difference in Risk-Factor Control
We observed significant improvements from baseline in the control of most risk factors across the follow-up, with control of lipid levels increasing from 59.2% of patients at baseline to 80.7% at 120 months, control of blood pressure increasing from 51.6% to 60.5%, glucose control increasing from 74.8% to 80.2%, and smoking cessation increasing from 74.3% to 86.9% (Table S5 in the Supplementary Appendix). Significant treatment differences were limited to glucose control at 72 months (81.5% in the stenting group vs. 74.8% in the endarterectomy group, P = 0.03) and to smoking cessation at 12 months (76.9% vs. 81.0%, P = 0.03), at 48 months (78.1% vs. 86.3%, P = 0.002), and at 96 months (81.5% vs. 86.7%, P = 0.05).
LIFE EXPECTANCY FOR STUDY PATIENTS AND THE GENERAL U.S. POPULATION
Among persons 65 years of age, life expectancy was estimated to be 18 years for men and 20 years for women in the United States.12 Life expectancy was estimated to be approximately 2 years shorter for men and 1 year shorter for women in CREST, regardless of symptomatic or asymptomatic status, than for men and women in the general U.S. population (Fig. S1A, S1B, and S1C in the Supplementary Appendix).
DISCUSSION
Because life expectancy has increased in the elderly and has been similar for the patients in CREST,12 results regarding long-term outcomes after stenting and endarterectomy are needed to guide treatment decisions. In CREST, including up to 10 years of follow-up, we did not find significant differences in the primary composite end point of periprocedural stroke, myocardial infarction, or death and subsequent ipsilateral stroke between patients — including both men and women and both those with symptomatic and those with asymptomatic carotid stenosis — who underwent carotid-artery stenting and those who underwent carotid endarterectomy. In addition, there were no significant differences in the durability of the procedures as assessed by the primary long-term end point of postprocedural ipsilateral stroke. We did not detect significant between-group differences in either the primary composite end point or the primary long-term end point when the end points were analyzed according to symptomatic status, age, sex, or degree of stenosis.
The absolute rates were less than 7% across the postprocedural stroke end points in our trial, a finding that contrasts with results of previous randomized trials of surgical and medical treatments in symptomatic patients13,14 and asymptomatic patients.15,16 For example, in the stenting group, the 10-year estimated rate of postprocedural stroke was 6.9% among both symptomatic patients and asymptomatic patients. In comparison, the rate of any postprocedural stroke at 5 years was 22.3% after endarterectomy in the North American Symptomatic Carotid Endarterectomy Trial13 and 16.9% at 10 years in the Asymptomatic Carotid Surgery Trial.16 In addition, the postprocedural rates of ipsilateral-vessel and nonipsilateral-vessel stroke were strikingly similar regardless of the revascularization approach, a finding that implies that successful revascularization attenuates the contribution of ipsilateral carotid artery disease to the risk of stroke.
Over the 10-year follow-up, patients who were assigned to stenting had a higher risk of stroke or death than did those assigned to endarterectomy. However, no significant difference was detected in the rate of postprocedural stroke between the stenting group and the endarterectomy group. Hence, the higher risks of stroke or death and of stroke at 10 years appear to be attributable to the periprocedural differences in risk that were reported previously.1 With respect to stenting, proximal embolic protection and mesh-covered stents are under development.17,18 These and other anticipated advancements may lower the rates of periprocedural stroke. With respect to endarterectomy, advances in preoperative cardiac evaluation, anesthesia, and quality improvement may also reduce the risk of perioperative complications.19
In an analysis of symptomatic patients and asymptomatic patients separately, we found no significant differences according to treatment group in the primary composite end point or in the rate of postprocedural stroke over the 10-year follow-up. The rates of postprocedural ipsilateral stroke and all postprocedural stroke were strikingly similar in asymptomatic patients and symptomatic patients at 5 years and at 10 years, regardless of the revascularization method. This finding implies that symptomatic status is of relevance in the context of periprocedural risk but ceases to be a useful characterization of patients at 5 years and 10 years after revascularization.
The long-term follow-up results of several other randomized trials comparing stenting with endarterectomy have been reported.20–22 In the International Carotid Stenting Study (ICSS),22 patients were followed for a median of 4.2 years for the primary end point of fatal or disabling stroke in any vascular territory. The 5-year cumulative risk observed in ICSS was 6.4% in the stenting group and 6.5% in the endarterectomy group (hazard ratio, 1.06; 95% CI, 0.72 to 1.57; P = 0.77) — a result similar to that in CREST. In the Asymptomatic Carotid Trial (ACT I), the results of which are also reported in the Journal,23 carotid-artery stenting was noninferior to carotid endarterectomy with respect to the primary composite end point of death, stroke, and myocardial infarction within 30 days after the procedure plus ipsilateral stroke within 1 year after the procedure, and there was no significant difference in the composite end point in an analysis that included up to 5 years of follow-up.
The long-term results of CREST may help guide the treatment of patients with carotid artery disease. Emphasis should be given to reducing periprocedural risk with both stenting and endarterectomy. In the case of stenting, more than half the ipsilateral-vessel strokes over a 10-year period occurred within the first month. Nonetheless, at centers with experienced interventionists and surgeons who have verifiable good outcomes, as verified during certification in CREST,4 the rates of periprocedural complications were relatively low with stenting and with endarterectomy. Both procedures were associated with rates of stroke that were less than 7% over a 10-year period. Decision making is more challenging at centers where interventional and surgical expertise cannot be verified. Several studies of administrative databases24 have shown higher rates of periprocedural stroke or death after stenting than those reported in CREST and ICSS.22
Advances in the long-term treatment of atherosclerosis, combined with longer life expectancy, mean that reassessment of the role of carotid artery intervention in patients with asymptomatic carotid-artery stenosis and in those with milder but symptomatic carotid-artery stenosis is warranted. Although, by design, CREST did not assess a parallel medical group, the ongoing Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial (CREST-2; Clinical Trials.gov number, NCT02089217) is addressing the question of the relative benefits of revascularization over nonrevascularization in asymptomatic patients in the context of modern intensive medical therapy. The European Carotid Surgery Trial 2 (ECST-2; Current Controlled Trials number, ISRCTN97744893) is also comparing revascularization with nonrevascularization in asymptomatic patients and includes symptomatic patients who have been deemed at low risk for stroke.
In conclusion, the long-term follow-up results of CREST did not show significant differences between carotid-artery stenting and carotid endarterectomy with respect to the primary composite end point of periprocedural stroke, myocardial infarction, or death and postprocedural ipsilateral stroke over a time period that was appropriate for elderly asymptomatic patients and symptomatic patients with severe carotid artery disease. In addition, there was no evidence of a significant difference in the long-term durability of stenting and endarterectomy to prevent stroke during the postprocedural period. Restenosis was infrequent after either procedure.
Supplementary Material
Acknowledgments
Supported by a grant (U01 NS038384-11) from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health and by Abbott Vascular Solutions (formerly Guidant), including donations of Accunet and Acculink systems to CREST centers in Canada and to CREST centers in the United States that were at Veterans Affairs sites. Support from Abbott Vascular Systems was equivalent to approximately 15% of the total study cost.
We thank the patients and their families for participation in this study; and the site principal investigators, who are listed in the Supplementary Appendix.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Brott TG, Hobson RW, II, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Office of the Chief Actuary (U.S.) Actuarial life table: period life table. Baltimore: Social Security Administration; 2011. https://www.ssa.gov/oact/STATS/table4c6.html. [Google Scholar]
- 3.Sheffet AJ, Roubin G, Howard G, et al. Design of the Carotid Revascularization Endarterectomy vs. Stenting Trial (CREST) Int J Stroke. 2010;5:40–46. doi: 10.1111/j.1747-4949.2009.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopkins LN, Roubin GS, Chakhtoura EY, et al. The Carotid Revascularization Endarterectomy versus Stenting Trial: credentialing of interventionalists and final results of lead-in phase. J Stroke Cerebrovasc Dis. 2010;19:153–162. doi: 10.1016/j.jstrokecerebrovasdis.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hobson RW., II CREST (Carotid Revascularization Endarterectomy versus Stent Trial): background, design, and current status. Semin Vasc Surg. 2000;13:139–143. [PubMed] [Google Scholar]
- 6.Moore WS, Vescera CL, Robertson JT, Baker WH, Howard VJ, Toole JF. Selection process for surgeons in the Asymptomatic Carotid Atherosclerosis Study. Stroke. 1991;22:1353–1357. doi: 10.1161/01.str.22.11.1353. [DOI] [PubMed] [Google Scholar]
- 7.Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754–3832. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meschia JF, Brott TG, Chukwudelunzu FE, et al. Verifying the stroke-free phenotype by structured telephone interview. Stroke. 2000;31:1076–1080. doi: 10.1161/01.str.31.5.1076. [DOI] [PubMed] [Google Scholar]
- 9.Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 10.Hill MD, Brooks W, Mackey A, et al. Stroke after carotid stenting and endarterectomy in the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) Circulation. 2012;126:3054–3061. doi: 10.1161/CIRCULATIONAHA.112.120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rautaharju PM, MacInnis PJ, Warren JW, Wolf HK, Rykers PM, Calhoun HP. Methodology of ECG interpretation in the Dalhousie program; NOVACODE ECG classification procedures for clinical trials and population health surveys. Methods Inf Med. 1990;29:362–374. [PubMed] [Google Scholar]
- 12.Arias E. United States life tables, 2010. Nat Vital Stat Rep. 2014;63:1–63. [PubMed] [Google Scholar]
- 13.Barnett HJM, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med. 1998;339:1415–1425. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 14.European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351:1379–1387. [PubMed] [Google Scholar]
- 15.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 16.Halliday A, Harrison M, Hayter E, et al. 10-Year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet. 2010;376:1074–1084. doi: 10.1016/S0140-6736(10)61197-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giri J, Parikh SA, Kennedy KF, et al. Proximal versus distal embolic protection for carotid artery stenting: a national cardiovascular data registry analysis. JACC Cardiovasc Interv. 2015;8:609–615. doi: 10.1016/j.jcin.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Schofer J, Musiałek P, Bijuklic K, et al. A prospective, multicenter study of a novel mesh-covered carotid stent: the CGuard CARENET Trial (Carotid Embolic Protection Using MicroNet) JACC Cardiovasc Interv. 2015;8:1229–1234. doi: 10.1016/j.jcin.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Cronenwett JL, Kraiss LW, Cambria RP. The Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg. 2012;55:1529–1537. doi: 10.1016/j.jvs.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572–1579. doi: 10.1056/NEJMoa0708028. [DOI] [PubMed] [Google Scholar]
- 21.Mas JL, Trinquart L, Leys D, et al. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008;7:885–892. doi: 10.1016/S1474-4422(08)70195-9. [DOI] [PubMed] [Google Scholar]
- 22.Bonati LH, Dobson J, Featherstone RL, et al. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial. Lancet. 2015;385:529–538. doi: 10.1016/S0140-6736(14)61184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenfield K, Matsumura JS, Chaturvedi S, et al. Randomized trial of stent versus surgery for asymptomatic carotid stenosis. N Engl J Med. 2016;374:1011–1020. doi: 10.1056/NEJMoa1515706. [DOI] [PubMed] [Google Scholar]
- 24.Jalbert JJ, Nguyen LL, Gerhard-Herman MD, et al. Outcomes after carotid artery stenting in Medicare beneficiaries, 2005 to 2009. JAMA Neurol. 2015;72:276–286. doi: 10.1001/jamaneurol.2014.3638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.