Abstract
Mycobacterium tuberculosis can maintain a nonreplicating persistent state in the host for decades, but must maintain the ability to efficiently reactivate and produce active disease to survive and spread in a population. Among the enzymes expressed during this dormancy is alanine dehydrogenase, which converts pyruvate to alanine, and glyoxylate to glycine concurrent with the oxidation of NADH to NAD. It is involved in the metabolic remodeling of M. tuberculosis through its possible interactions with both the glyoxylate and methylcitrate cycle. Both mRNA levels and enzymatic activities of isocitrate lyase, the first enzyme of the glyoxylate cycle, and alanine dehydrogenase increased during entry into nonreplicating persistence, while the gene and activity for the second enzyme of the glyoxylate cycle, malate synthase were not. This could suggest a shift in carbon flow away from the glyoxylate cycle and instead through alanine dehydrogenase. Expression of ald was also induced in vitro by other persistence-inducing stresses such as nitric oxide, and was expressed at high levels in vivo during the initial lung infection in mice. Enzyme activity was maintained during extended hypoxia even after transcription levels decreased. An ald knockout mutant of M. tuberculosis showed no reduction in anaerobic survival in vitro, but resulted in a significant lag in the resumption of growth after reoxygenation. During reactivation the ald mutant had an altered NADH/NAD ratio, and alanine dehydrogenase is proposed to maintain the optimal NADH/NAD ratio during anaerobiosis in preparation of eventual regrowth, and during the initial response during reoxygenation.
Introduction
Mycobacterium tuberculosis is able to persist in humans for decades producing latent (asymptomatic) tuberculosis. Sequestered in granulomas, bacteria survive by entering a nonreplicating persistent state [1,2]. Reactivation followed by active disease can occur, with the risk estimated at 5–10% over a lifetime. Tuberculous granulomas can be hypoxic in humans, maintaining oxygen levels that prevent M. tuberculosis from replicating [2–5]. Since M. tuberculosis is an obligate aerobe, hypoxia presents many challenges including energy production and redox balancing.
During adaptation to decreasing oxygen levels, M. tuberculosis undergoes extensive metabolic remodeling which allows it to survive anaerobiosis. For example the bacteria express the less energy efficient cytochrome bd oxidase which has high affinity for oxygen, and shunts electron flow through the non-proton pumping type II NADH dehydrogenase [6]. A decreasing flow of electrons due to the lack of oxygen is associated with an increase in the concentration of reduced cofactors, such as NADH [2,7,8]. Nitrate can be utilized as an alternative electron acceptor in place of oxygen [9,10]. Changes that occur during reactivation, when oxygen levels increase to a level sufficient to support replication of M. tuberculosis, are largely unknown.
One of the genes upregulated in response to hypoxia is ald encoding alanine dehydrogenase (Ald), which is essential for the utilization of alanine as a nitrogen source [11]. Unlike Ald of some other bacteria, Ald of M. tuberculosis is a multispecific enzyme using either glyoxylate or pyruvate as substrates [11]. It catalyzes the reduction of pyruvate to alanine with pyruvate reductive aminase activity (PvRA), or glyoxylate to glycine with glyoxylate reductive aminase activity (GxRA) coupled with the oxidation of NADH to NAD. In the reverse direction it oxidizes alanine to pyruvate (ALD), but does not use glycine as a substrate [11]. In vivo alanine dehydrogenase enzymes have been shown to catalyze the production of alanine or glycine [12,13].
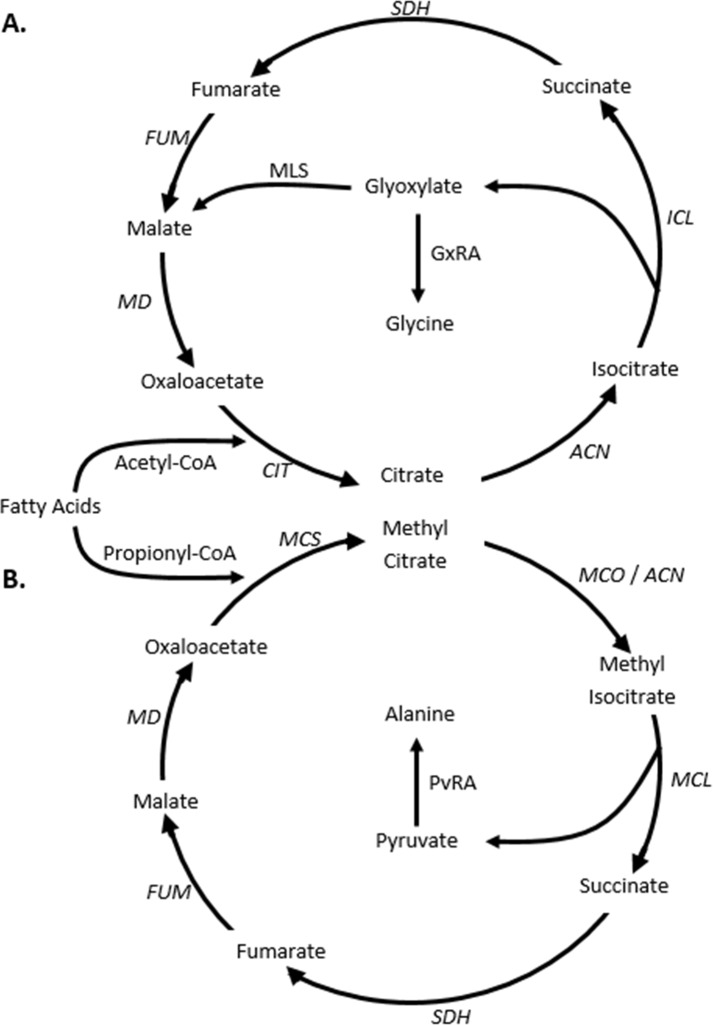
It has been proposed that alanine dehydrogenase plays a role in maintaining redox balance during shiftdown of M. tuberculosis to the NRP state [1,14]. Decreasing oxygen results in a shift in the NADH/NAD ratio towards NADH. The two substrates for alanine dehydrogenase, pyruvate and glyoxylate, would be supplied by isocitrate lyase, the first unique enzyme of the glyoxylate and the methylcitrate cycles (Fig 1). The anaplerotic glyoxylate pathway is used to synthesize C4 compounds from the acetyl-CoA produced during β-oxidation of fatty acids, which are the main sources of energy for M. tuberculosis in vivo (reviewed in [15]). The methylcitrate cycle is utilized during metabolism of odd-chain fatty acids and cholesterol, where propionyl-CoA is produced [16] (Fig 1). In M. tuberculosis isocitrate lyase is a multispecific enzyme as it serves also as the 2-methylisocitrate lyase which converts methylisocitrate to pyruvate and succinate [17]. The interaction of alanine dehydrogenase with either of these two cycles is uncharacterized.
Fig 1. Interaction of Ald with two metabolic pathways.
Even-chain fatty acids are degraded to acetyl-CoA while odd-chain fatty acids also produce propionyl-CoA. Acetyl-CoA is oxidized by the (A) glyoxylate cycle and propionyl-CoA by the (B) methylcitrate cycle. Enzyme activities are indicated in italics. ACN–aconitase; ICL–isocitrate lyase; MLS–malate synthase; MD–malate dehydrogenase; CIT–citrate synthase; SDH–succinate dehydrogenase; FUM–fumarase; MCD–methylcitrate dehydratase; MCL methylisocitrate lyase; MCS–methylcitrate synthase; GxRA–glycine dehydrogenase; PvRA–alanine dehydrogenase. ICL and MCL activities are catalyzed by the same isocitrate lyase enzyme. GxRA and PvRA activities are produced by the same alanine dehydrogenase.
Here alanine dehydrogenase and its interaction with the glyoxylate cycle were investigated. After the initial induction of both isocitrate lyase and alanine dehydrogenase, the former was down regulated while the latter was further upregulated. The late expression of Ald, and a defect in regrowth of an Ald mutant of M. tuberculosis suggested a role for Ald during reaeration. It is proposed that Ald is involved in maintaining the optimal NADH/NAD ratio not only during dormancy, but also during reactivation when oxygen levels increase enough to support regrowth of M. tuberculosis.
Results
Expression of genes and enzymes of the glyoxylate cycle
Alanine dehydrogenase, encoded by ald, has glyoxylate reductive aminase activity (GxRA), which could constitute a branch from the glyoxylate cycle [1,14] (Fig 1). The enzymes unique to the glyoxylate cycle are the two isocitrate lyases (Icl), encoded by icl1 and icl2, and malate synthase encoded by glcB. To determine how alanine dehydrogenase of M. tuberculosis H37Rv interacts with this cycle the expression of these genes was determined in in vitro and in vivo models.
We used the Wayne model as the in vitro model for dormancy [18]. Growth of M. tuberculosis cultures in sealed tubes results in gradual depletion of oxygen from the medium, giving rise to first a microaerobic nonreplicating persistent phase 1 (NRP-1) followed by the anaerobic NRP-2. In the latter stage M. tuberculosis can survive for extended periods of time.
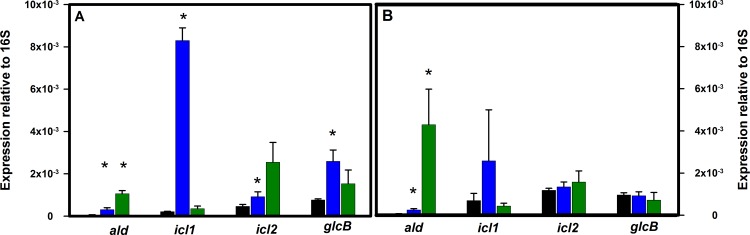
Expression of ald in the Wayne model was induced 5-fold in microaerobic NRP-1, but the highest expression was in anaerobic NRP-2 (16-fold induction relative to mid-log aerobic growing cultures) (Fig 2A). icl1 was strongly induced in NRP-1 (39-fold) but expression then decreased in NRP-2. icl2 showed a similar pattern to ald with the highest induction in NRP-2. glcB was induced 3-fold in NRP-1.
Fig 2. Expression of genes involved in the glyoxylate cycle.
Total RNA from M. tuberculosis cultures in either aerobic; microaerobic NRP-1 or anaerobic NRP-2 was extracted and quantitation of transcripts performed. (A) H37Rv. (B) Erdman. Black bars–aerobic cultures. Blue bars–NRP-1, and green bars–NRP-2. RNA levels are expressed relative to the stable 16S rRNA. The standard deviation is shown. Asterisks indicate statistical significance (p<0.05) of the comparison with the aerobic samples.
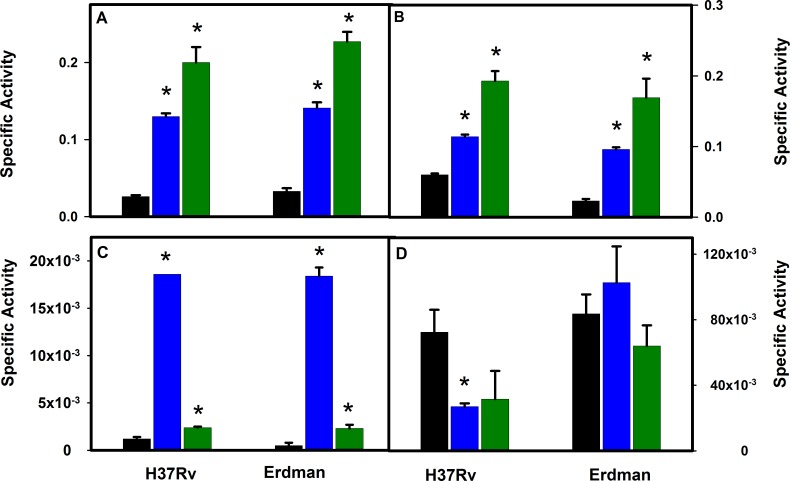
To verify that transcription levels reflected protein levels, enzyme activities were measured in aerobic, NRP-1 and NRP-2 cultures (Fig 3). Both GxRA and PvRA activities were induced by hypoxia. GxRA was induced 5-fold and PvRA 2-fold in NRP-1, while GxRA increased 8-fold, and PvRA 3-fold in NRP-2. Both showed peak activity in NRP-2 and, although activity levels were not identical, they closely reflected transcription changes. Isocitrate lyase was most active in NRP-1 (16-fold induction), which also closely mirrored transcription changes. Malate synthase levels decreased, which could suggest an additional mechanism of regulation.
Fig 3. Enzyme Activities in M. tuberculosis H37Rv and Erdman.
A: Glyoxylate reductive aminase activity. B: pyruvate reductive aminase activity. C:isocitrate lyase activity. D: malate synthase activity. Black bars are aerobic cultures, blue bars are NRP-1 and green bars are NRP-2. All units are μmoles/min/mg protein. The standard deviation is shown. Asterisks indicate statistical significance (p<0.05) of the comparison with the aerobic samples.
The icl2 gene of M. tuberculosis H37Rv produces an inactive enzyme due to a frameshift mutation. To exclude the possibility that this altered the expression of other genes, transcription was measured in M. tuberculosis Erdman, which produces an active Icl2 enzyme (Fig 2B). Results with the Erdman strain were similar to those obtained with H37Rv. ald was induced 4-fold in NRP-1 and 64-fold in NRP-2 relative to aerobic growing cultures. GxRA and PvRA levels increased in NRP-1 (both 4-fold relative to aerobic growing cultures) and 7-fold in NRP-2 cultures (Fig 3). Activities of enzymes reflected the increase in transcription although GxRA and PvRA levels did not fully reflect the induction in transcription in NRP-2. icl1 was induced in NRP-1 (4-fold) however not to the extent observed in H37Rv, but then decreased in NRP-2 similar to H37Rv. The increase in isocitrate lyase activity in NRP-1 in the Erdman strain (35-fold) was similar to that seen in H37Rv. Activity then decreased, although not to the extent that was detected in H37Rv, indicating that Icl2 contributes little activity under these conditions. Malate synthase transcription was not induced in either NRP-1 or NRP-2, and enzyme activities remained relatively constant.
In conclusion, both RNA and enzyme levels of alanine dehydrogenase and isocitrate \\lyase were induced by hypoxia, while malate synthesis was unaffected or decreased. Isocitrate lyase showed the strongest induction during microaerobic NRP-1 and then declined, while alanine dehydrogenase peaked in anaerobic NRP-2.
Ald expression in vivo, and the response to nitric oxide exposure and hypoxia in vitro
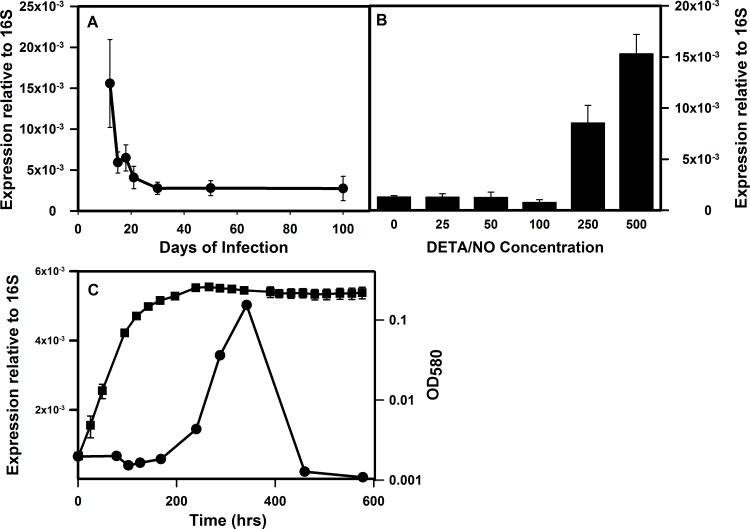
We had previously measured the expression of icl1 and glcB in the lungs of mice over the course of chronic infection [19]. Transcription of icl1 showed a peak in the initial stages of infection, and then decreased during the chronic stage, while glcB transcription declined following the initial infection. The expression of ald by M. tuberculosis in the lungs of mice was determined. Expression of ald was high during the initial stage of the infection, and then downregulated with progression of the infection (Fig 4A). The different expression pattern of ald during in vivo infection in comparison to that of in vitro models may reflect the lack of hypoxia in infected mouse lungs [2,3].
Fig 4. Transcriptional analysis of ald in vivo, and in response to stress conditions in vitro.
(A) Expression of ald in the lungs of mice. Lungs were harvested from mice at the indicated time points post-infection. Total RNA was extracted and quantitation of bacterial transcripts was performed. Shown is the mean (± standard deviation) of normalized mRNA copy numbers relative to 16S rRNA from three mice at each time point. (B) Expression after exposure to the NO donor DETA/NO. (C) Expression of ald during extended incubation in the Wayne model. Circles indicate expression of ald relative to the 16S RNA, and squares indicate the optical density of the culture at 580 nm.
In mice the production of nitric oxide is essential for the control of M. tuberculosis. Although its role in humans is less clear, it is produced by macrophages following infection with M. tuberculosis [20]. Many genes induced in response to hypoxia are also regulated by nitric oxide. Therefore the expression of ald during in vitro exposure to NO was tested. ald was strongly induced by the NO donor DETA/NO at levels above 100 mg/ml (Fig 4B).
The expression of ald during oxygen limitation was examined in more detail (Fig 4C). In the Wayne model cultures become microaerobic (NRP-1) by 100 hrs, and are anaerobic by 250 hrs (NRP-2). It was found that ald was fully induced by NRP-2 reaching a peak around 350 hrs in the Wayne model before declining.
Role of Ald in recovery from extended hypoxia
The survival of the ald deletion mutant RVW7 was analyzed during extended hypoxia in the Wayne model (S1 Fig). In comparison to wild type (WT) no difference in survival was detected. However it was noticed that RVW7 colonies were slower to appear on DOA plates, although they eventually reached numbers similar to wild type. This, along with the late expression of ald in the Wayne model, suggested a possible role for Ald in recovery, rather than entry into, hypoxic NRP.
However by 600 hr, ald transcription is low (Fig 4C). To determine whether enzyme activities were reflective of transcription changes, GxRA, and PvRA activities were determined at 350 and 600 hrs. Despite the decline in transcription of ald after 350 hrs, GxRA activity remained essential constant (0.15 U ± 0.06, at 350 hrs and 0.19 ± 0.09 after 600 hrs). PvRA activities also did not change significantly over this time frame (0.23 U ± 0.09 at 350 hrs, and 0.19 ± 0.03 at 600 hrs) suggesting Ald is stably maintained even after transcription decreases.
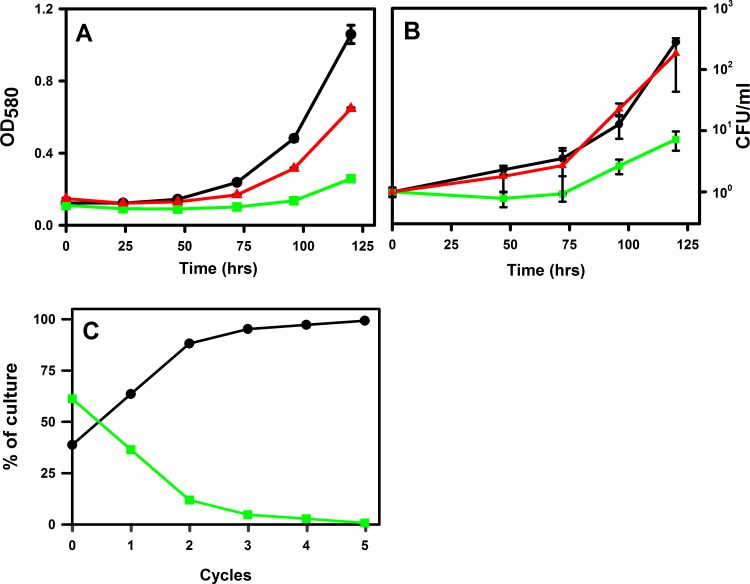
To test for a possible role during reaeration the growth of the ald mutant was analyzed. Cultures of wild type M. tuberculosis, RVW7 and the complemented mutant RVW7 pAld-Gen21 were grown in the Wayne model. There were no differences in growth rate and shiftdown curves between strains (data not shown). After 600 hrs (approximately 400 hrs of anaerobic conditions) cultures were opened and diluted 1:2 in fresh medium. Relative to wild type, the RVW7 mutant showed a reproducible and significant delay in growth as determined by optical density (Fig 5A). This was not seen in the complemented mutant. The delay was also observed when CFU’s were measured (Fig 5B).
Fig 5. Regrowth dynamic of M. tuberculosis during recovery from hypoxia.
Cultures were grown for 600 hrs in the Wayne model, diluted in fresh media and then grown with vigorous shaking. Growth was monitored by (A) optical density at 580 nm or (B) plating to determine CFUs. (C) Growth competition assay. WT and RVW7 were mixed in equal concentration and subjected to repeated cycles in the Wayne model. CFUs were determined after each cycle. WT–black circles. RVW7 –green squares. RVW7 pAld-Gen21 –red triangles.
The survival of RVW7 in competition with wild type was determined during several cycles of hypoxia. Both strains were grown together for 600 hrs in the Wayne model, plated to measure CFUs, and then diluted into fresh DTA for continued incubation. After growth in broth the culture was again inoculated into the Wayne model. In each passage the percentage of the ald mutant in the culture decreased while the WT strain increased (Fig 5C). After four passages the wild type strain constituted greater than 95% of the culture indicating a significant defect in reactivation in the ald mutant.
Role of Ald in redox balancing
A possible function for Ald during hypoxia is redox homeostasis [1]. With decreasing levels of oxygen M. tuberculosis experiences a shift in the NADH/NAD ratio towards elevated NADH [4,7,8,21]. Ald could perform a role in adjusting this ratio by the conversion of glyoxylate to glycine, and/or pyruvate to alanine. During fermentative growth of E. coli, this function is performed mainly by lactate dehydrogenase by converting pyruvate to lactate while oxidizing NADH to NAD. To determine whether Ald could maintain redox balance during anaerobiosis, the ability of the M. tuberculosis ald to complement a lactate dehydrogenase mutant (ldh) of E. coli was tested. The ald gene from M. tuberculosis was cloned under the control of the ldh promoter, integrated into the ldh locus of E. coli SZ194. As a negative control a hygromycin marker was integrated into ldh. SZ194 ldh::hyg did not grow anaerobically while SZ194 ldh::ald did (S2 Fig) suggesting Ald is able to perform NADH/NAD recycling during anaerobiosis.
Physiological factors during reaeration
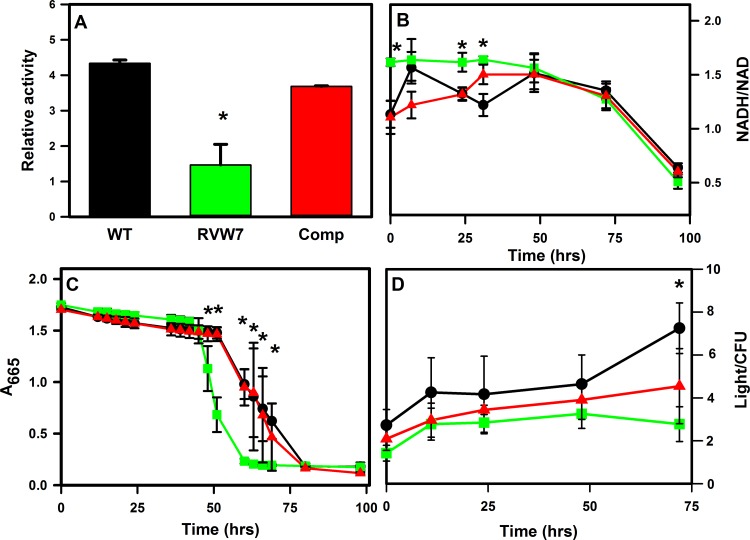
We propose a role for Ald in maintaining the NADH/NAD ratio of M. tuberculosis during recovery from hypoxia when oxygen levels increased enough to trigger exit from the NRP state. We analyzed physiological factors during reaeration by determining the metabolic activity of RVW7 after extended hypoxia. First we tested the reducing power of NRP-2 cultures of M. tuberculosis using the electron acceptor XTT. A significant decrease in reducing power was observed in the ald mutant compared to wild type and the complemented mutant (Fig 6A). This difference was not seen with aerobic cultures with these strains (data not shown).
Fig 6. Physiological parameters of M. tuberculosis during reaeration.
(A) Metabolic activity after extended hypoxia. Reducing activity was determined with XTT. Data are shown in arbitrary units. (B) The ratio of NADH to NAD. (C) Oxygen consumption was measured by methylene blue decolorization. (D) ATP levels in arbitrary units normalized to cell number. Black circles–WT, green squares–RVW7, red triangles–RVW7 pAld-Gen21. Asterisks indicate statistical significance (p<0.05) of the comparison of RVW7 with WT.
Next, the NADH/NAD ratio was determined in late NRP-2, and during reaeration in wild type and RVW7. At the end of the extended incubation in the Wayne model the NADH/NAD ratio was significantly lower for wild type than the ald mutant (Fig 6B). The ratio in WT remained lower in comparison to RVW7 during the lag phase. The ratio in wild type decreased when growth resumed, while in contrast the ratio in RVW7 was higher, but decreased towards that of wild type before growth began.
The NADH/NAD ratio is sensitive to oxygen levels so methylene blue was used to measure oxygen utilization during reaeration. Methylene blue was added to cultures of M. tuberculosis in NRP-2 to monitor oxygen levels measured during reactivation. Wild type cultures showed a lag phase characterized by an initial slow fading followed by rapid decolorization (Fig 6C). The ald mutant reproducibly decolorized the methylene blue more rapidly than WT, beginning after 42 hrs of incubation, and finishing by 60 hrs in comparison to 51 hrs and 80 hrs respectively for WT. No difference was seen with aerobically grown cultures (data not shown). This increase in oxygen utilization preceded bacterial replication in all strains.
A change in the NADH/NAD ratio could also have an effect on ATP levels. ATP levels were determined during re-growth and were similar in WT and the RVW7 mutant after extended hypoxia (Fig 6D). There was no significant difference in ATP levels in the first 50 hrs before resumption of growth, which occurred earlier for WT than RVW7.
Discussion
In response to decreasing oxygen, M. tuberculosis induces a set of dormancy genes that enable this obligate aerobe to adapt to, and survive low oxygen conditions. Included in this set, but often unrecognized, are genes functioning not for anaerobic survival, but to initiate recovery leading to eventual growth. These gene products perform important functions as M. tuberculosis is transitioning out of dormancy, and preparing to induce new protein synthesis.
The expression of Ald in M. tuberculosis is similar to that seen with some of the germination proteins of Bacillus subtilis. Transcription of the gerA operon, for example, is upregulated 2.5 hrs after induction of sporulation, and remains high but begins to decrease at the end of sporulation [22,23]. The protein is maintained in the mature spore, and is required to initiate germination in the presence of alanine. We show that ald transcription is upregulated in M. tuberculosis late in NRP-2 and then decreases as the cells enter the general shutdown characteristic of dormancy. Although transcription of ald decreases the protein is stably maintained so that the cell will be ready to reactivate when oxygen levels increase.
Alanine dehydrogenase is a multispecific enzyme [11] and part of the core transcriptome, a set of conserved genes important for virulence. A series of new inhibitors of Ald were shown to be lethal to dormant cultures of M. tuberculosis verifying the great potential of this target [24]. And Ald has been implicated in resistance of M. tuberculosis to the second-line antibiotic D-cycloserine used to treat drug resistant tuberculosis [25]. We now show that Ald also plays an unexpected role during reaeration since inactivation of ald resulted in a delayed recovery from dormancy (Fig 5).
Alanine dehydrogenase could play numerous roles during the recovery of M. tuberculosis. The production and/or accumulation of both alanine and glycine are a widely seen response to hypoxia with extensive examples from varied life forms including plants [26–28], protozoa [29,30] and mammals [31] suggesting this is an ancient mechanism used for dealing with hypoxia and reaeration. The production of alanine and glycine may be due to aminotransferases rather than Ald.
A role for Ald in carbon storage seems unlikely for M. tuberculosis, although the role of Ald in nitrogen utilization is known [11,32]. In plants, hypoxia due to waterlogging, leads to the accumulation of intracellular alanine, glycine, and succinate for the storage of carbon and nitrogen that are rapidly utilized after reoxygenation [26–28].
The oxidation of pyruvate in the TCA cycle requires oxygen. Ald could reduce the accumulation of intracellular pyruvate thus reducing the demand for the limiting levels of oxygen [26,33]. Lower levels of pyruvate would also reduce the buildup of glycolytic intermediates that are toxic to M. tuberculosis during hypoxia [34].
Both glycine and alanine protect against hypoxic injury in many types of cells and tissues [35,36]. Glycine provides protection during hypoxia and reaeration in rat livers presumably by inhibiting oxidative stress and lipid peroxidation [37].
We propose that the main role of Ald during hypoxia and recover is to maintain the redox balance of M. tuberculosis. NAD is the principal oxidant in the cell and essential for many reactions, so NADH must be rapidly oxidized to prevent inhibition of important processes. The ratio between NAD and NADH is tightly regulated by redundant systems, and in M. tuberculosis is generally between 1:3 and 1:10 [38–41]. When oxygen levels decrease or aerobic respiration is blocked by NO the ratio of NAD to NADH shifts [4,7,21]. This shift is also seen in M. tuberculosis in the lungs of mice [39] indicating that redox stress is present in vivo.
The addition of alanine, glycine, glyoxylate or pyruvate to the medium did not decrease the recovery time of the M. tuberculosis mutant after hypoxia suggesting the main function of Ald during reaeration is not the production or detoxification of these compounds (data not shown).
We propose that the ratio of NAD to NADH plays a role in regulating recovery of M. tuberculosis from hypoxia, and may prevent growth until the ratio achieves a certain level. It may also serve as the signal indicating to the cell the optimal time to transition from a nonreplicating persistent state to active growth. Indeed the ald mutant was unable to maintain the optimal ratio during anaerobic stress leaving it unprepared for recovery until the NADH/NAD was corrected. The NADH/NAD ratio remained relatively constant for approximately 48 hrs in WT after the oxygen levels increased to a level sufficient to support the growth of M. tuberculosis (Fig 6B). As oxygen was then utilized (Fig 6C), the ratio further shifted to a range that allowed growth to resume. However, at the end of NRP-2, the ratio was significantly higher in RVW7 suggesting a role for Ald in shifting the ratio towards the more oxidized form. The ald mutant is unable to decrease the ratio in the first 50 hours of reaeration and must utilize oxygen for this function which results in a delay in growth resumption. This proposal is supported by the observation that a DosR mutant which also had an altered NADH/NAD ratio, also showed a delay in recovering from extended hypoxia [21]. This would not solve all the redox problems raised by lack of oxygen, and there are undoubtedly other mechanisms at work.
In the absence of a final electron acceptor, fermentation can be used by M. tuberculosis to produce ATP [2,4,8]. Alanine dehydrogenase converts pyruvate to alanine while recycling NADH to NAD. In the ald mutant there was a difference in this ratio, with higher NADH levels in late NRP-2 relative to wild type (Fig 6). Ald was able to fulfill a redox balancing role as shown in a ldhA E. coli mutant by restoring anaerobic growth (S2 Fig). M. tuberculosis does not grow anaerobically but an alanine dehydrogenase mutant of the related bacteria M. smegmatis lost the ability to grow anaerobically suggesting a role for Ald in redox balancing [42].
Both intracellular and extracellular concentrations of alanine and glycine increased during exposure of M. tuberculosis to hypoxia [8]. There is evidence of increased levels of alanine in the granulomas of guinea pigs infected with M. tuberculosis [43]. Levels increased as the infection progressed and hypoxia increased. The mechanism of these changes is still unknown as is the possible involvement of Ald.
Alanine dehydrogenase may also play a role in redox balancing during entry into the nonreplicating persistent state by interacting with the glyoxylate and/or methylcitrate cycle. A main source of carbon and energy for M. tuberculosis in vivo is fatty acids. These can be utilized to produce energy through the TCA cycle or, for biosynthesis, by the glyoxylate cycle. It was originally proposed that the importance of isocitrate lyase in vivo was due to its role in the glyoxylate cycle [44]. However it has also been recognized that it has additional roles since this enzyme is involved in multiple pathways [45]. It functions as a methylisocitrate lyase making it a key enzyme of the methylcitrate cycle that is involved in the utilization of cholesterol and fatty acids with an odd number of carbons [17]. We suggest an additional role for isocitrate lyase as the first enzyme in a branch point from the glyoxylate and methylcitrate cycles involving alanine dehydrogenase (Fig 1).
In microaerobic NRP-1, isocitrate lyase was induced by M. tuberculosis, but not malate synthase suggesting that during microaerobic conditions carbon flow increases through alanine dehydrogenase. Activation of the glyoxylate cycle during hypoxia has been detected by isotopic labeling, but while intracellular levels of succinate increased 6.5-fold relative to aerobic conditions, malate levels increased only 1.4-fold [8,46]. Glyoxylate from the glyoxylate cycle and pyruvate, produced from the methylcitrate cycle and glycolysis, would be the substrates for alanine dehydrogenase. Isotope labeling experiments indicated that during hypoxia most of the succinate produced and excreted from the cell was from the reductive branch of the TCA cycle, but a significant amount was produced from the glyoxylate cycle [4].
During active growth of M. tuberculosis, the key enzymes of the glyoxylate and methylcitrate cycles were optimized for energy and biomass production. Isocitrate lyase, methylisocitrate lyase and malate synthase activities were high while alanine dehydrogenase activities were low. In response to conditions that inhibit aerobic respiration and replication, such as hypoxia or nitric oxide, isocitrate lyase is induced along with alanine dehydrogenase. This has been proposed to play a role in redox balancing during entry into the NRP state [1], and would explain the upregulation of isocitrate lyase without a similar change in malate synthase enzyme levels. As oxygen levels continue to decrease the final shift in metabolism would be to the production of enzymes needed for later reactivation. Isocitrate lyase expression would be downregulated and alanine dehydrogenase further upregulated. This conclusion is in line with our previous identification of three respiratory states of M. tuberculosis [6] and labeling experiments [4].
The tubercle was long thought to be a static structure preventing the escape of M. tuberculosis. Now the granuloma is seen as a more dynamic structure with ongoing interactions with the bacilli [47,48]. There may be ongoing cycles of increased oxygen or nitric oxide concentrations that produce nonreplicating states interspersed by growth. The alanine dehydrogenase may play a role during these cycles regulating when it is to the bacteria’s advantage to replicate, and when it is safer to remain in a dormant state. Thus the ability to rapidly initiate growth upon reaeration is essential for the survival of M. tuberculosis.
Materials and Methods
Growth conditions
M. tuberculosis H37Rv and Erdman strains were from the culture collection of this laboratory. The M. tuberculosis Δald mutant RVW7 and its complement were constructed previously [11]. Mycobacterial cultures were grown at 37°C in Dubos Tween-albumin broth (DTA) (Difco, Detroit, Mich). DTA consists of 0.05% (w/v) tryptone, 15 mM asparagine, 153 μM Tween-80, 7 mM KH2PO4, 18 mM Na2HPO4, 189 μM ferric ammonium citrate, 83 μM MgSO4, 5 μM CaCl2, 348 nM ZnSO4, 400 nM CuSO4, 15 mM NaCl, 0.5% bovine serum albumin fraction V and 42 mM dextrose.
Aerobic cultures were incubated on a model G24 rotary shaker-incubator (New Brunswick Scientific, Edison, N.J.). For hypoxic cultures (Wayne model) slow magnetic stirring in sealed tubes with a headspace ratio of 0.5 was used [18]. After approximately 67 hrs of incubation cultures entered microaerobic NRP-1, and at 200 hrs fully anaerobic NRP-2 was reached. Growth was monitored by following optical density at 580 nm.
DETA/NO was used as the nitric oxide source and conditions were as previously described [19]. All antibiotics and chemicals were from Sigma (St. Louis, MO).
Construction of E. coli strain expressing M. tuberculosis ald
To create the ald expressing strain, SZ194, a ldhA pflB strain of E. coli, provided by Lonnie Ingram was used [49]. The alanine dehydrogenase gene from M. tuberculosis was amplified using primers p204 and p207 (S1 Table). This was cloned into the plasmid pLOI4215 using SpeI and NsiI so that ald was controlled by the E. coli ldhA promoter [49]. The resulting plasmid pLOI-AldC was electroporated into SZ194 that also carried the plasmid pKD46. The Red recombinase, expressed from pKD64, resulted in the recombination of ald into the ldhA Site of SZ194. The presence of the M. tuberculosis ald in the ldhA site was verified by Southern blot. For a control strain a hygromycin marker was also inserted into ldhA of SZ194. The insertion of ald was verified by PCR. Expression of Ald was verified by Western analysis as previously described [11].
For anaerobic growth E. coli strains were inoculated into M9 with glucose, and grown in completely filled, sealed tubes. Nitrate when used was at 50 mM.
Assay of enzyme activities
M. tuberculosis was grown aerobically to either mid log phase with an approximate OD580 of 0.4, to NRP-1 at 115 hrs, or NRP-2 after 250 hrs, in the Wayne model. Cell extracts were prepared using a Mini-Bead Beater (Bio Spec Products, Bartlesville, OK) as previously described [11].
Pyruvate and glyoxylate reductive aminase assay
The assay for either pyruvate (PvRA) or glyoxylate (GxRA) reductive aminase activity was based on the oxidation of NADH [11]. Approximately 10 μg of cell extract protein was added to a 1 ml UV transparent cuvette containing 100 mM phosphate buffer. Ammonium sulphate to 400 mM and the substrate (pH 6.4) to 50 mM were added. The reactions were started with the addition of NADH to 80 μM, and measured by the rate of decrease of A340 on a Libra S32PC spectrophotometer (Biochrome, Cambridge, UK). A control reaction without ammonium sulphate was included to measure background, and an extinction coefficient of 6220 M-1 cm-1 was used [50]. Units are μmoles/min/mg protein.
Isocitrate lyase
Isocitrate lyase activity was measured as the amount of glyoxylate produced in the presence of phenylhydrazine [51]. The conditions were as previously reported [19]. A control reaction without isocitrate was included to measure background. Units are μmoles/min/mg protein.
Malate synthase
The formation of free CoA-SH from acetyl-CoA upon its condensation with glyoxylate was measured [52]. 50 μg of cell extract protein was added to 20 mM Tricine pH 8.0, and 1 mM sodium glyoxylate (pH 6.4). The reaction was started with the addition to 1 mM of acetyl-CoA. At 5 min intervals 50 μl samples were removed and added to 950 μl of 2.5 mM dithiobis nitrobenzoic acid. A control reaction without glyoxylate was included to measure background. The absorbance at 412 nm was measured and an extinction coefficient of 14150 M-1 cm-1was used [53]. Units are μmoles/min/mg protein.
Mouse infections
Mouse infections were as reported [19]. 24 C57BL/6 female mice at 8–10 weeks of age (Trudeau Institute) were used for experiments. All procedures involving live animals were performed in the BSL-3 facility in Trudeau Institute in accordance with the Guide for Care and Use of Laboratory Animals of the National Institutes of Health, and individual procedures were approved by the Trudeau Institute Institutional Animal Care and Use Committee. Mice were infected with ~75 CFU of mid-log phase M. tuberculosis strain H37Rv (Trudeau Mycobacterial Culture Collection no. 102), grown in Proskauer Beck medium containing 0.05% Tween 80, using a Glas-Col airborne infection system, as described [54]. Usually, C57BL/6 mice live between 250 and 300 days post-infection with virulent M. tuberculosis without apparent signs of disease. Infected animals were carefully monitored during the course of the experiment for evidence of significant pain or extreme distress by the attending veterinarian. Animal technicians monitored feed consumption, fecal output, and behavior of the animal. Three mice were sacrificed by cervical dislocation on day 1 of infection to verify the inoculum in the mouse lungs. Lungs from three mice at days 12, 15, 18, 21, 30, 50 and 100 post-infection were harvested, snap-frozen in liquid nitrogen and kept at −80 °C until use.
RNA isolation from mouse tissue and quantitative PCR (qRT-PCR)
Mouse infections were as reported [19]. RNA from infected mouse lungs [54] was prepared as described previously. cDNA was produced as reported [11,19]. qRT-PCR on cDNA produced from broth cultures was performed with the Brilliant SYBR Green QPCR Master Mix kit (Stratagene, La Jolla, CA) using an ICycler (Bio-Rad, Hercules, CA) as reported [11]. With cDNA from animal tissue AmpliTaq Gold polymerase (Applied Biosystems) and a Mx400 thermal cycler (Stratagene, Santa Clara, CA) were used [19]. Gene specific ald primers and beacons are as described [55]. During mouse lung infection by M. tuberculosis or in the Wayne model, as levels of 16S rRNA correlated with bacterial CFU numbers [6,54,56].
Survival and recovery from the Wayne model
For survival during extended hypoxia, wild type and RVW7 were grown in the Wayne model. At intervals tubes were opened for plating on Dubos Oleic Albumin plates and then subsequently discarded.
To analyze recovery from extended hypoxia cultures were grown for 600 hrs (25 days) in the Wayne model. Each tube was then opened and 5 ml of culture was diluted with 5 ml of fresh DTA, followed by aerobic incubation with shaking. Growth was measured by monitoring the OD580 or by plating to determine CFUs.
For the competitive assay a spontaneous kanamycin resistant mutant was used as the wild type strain. This wild type at 1.25 x 106 cells/ml was mixed with an equal number of RVW7 cells (hygromycin resistant) in the Wayne model. After 600 hrs the tube was opened and aliquots plated on DOA with either kanamycin (50 μg/ml) or hygromycin (50 μg/ml). The culture was then diluted 1:1 in fresh DTA and grown to mid log phase before being used to set up the next cycle of growth in the Wayne model.
Metabolic activity
An assay measuring the reducing power of whole cells using menadione as the electron carrier and XTT as the electron acceptor was used [57]. Cultures were grown in the Wayne model for 25 days in DTA. 1 ml of culture was added to a cuvette along with 2,3-bis(2-methoxy-4-nitro-5-sulfenyl)-(2H)-tetrazolium-5-carboxanilide (XTT—to 600 μM) and menadione sodium bisulfate (to 60 μM). The absorbance at 470 nm indicating XTT reduction was determined at 30 min intervals for 3 hrs. The extinction coefficient of XTT is 15600 M-1 cm-1.
Determination of the NADH/NAD ratio
Triplicate samples were quickly harvested by centrifuging 2 ml for 2 min. Each pellet was resuspended in 200 μl of either 0.2 M HCl to isolate NAD, or 0.2 M NaOH to isolate NADH. They were then frozen at -80°C until analyzed. Samples were thawed and heated to 80°C for 20 min. They were then cooled and carefully neutralized with Tris buffered 0.2 M HCl or 0.2 M NaOH. It was important to make sure the pH did not go beyond ~7.0 during the neutralization stage since the preparation is based on the selective destruction of either NAD or NADH by pH [58]. Cell debris was pelleted by centrifugation, and the NAD or NADH solutions transferred to new tubes.
To determine NADH or NAD concentrations the nucleotide cycling assay was used [59]. 100 μl aliquots of a reaction buffer containing 167 mM Bicine pH 8.0, 17% ethanol, 7 mM EDTA, 0.7 mM thiazolyl blue tetrazolium bromide and 5.6 mM phenazine ethosulfate were added to a 96 well plate. 90 μl of each NAD or NADH sample was added. The reactions, which were done in triplicate, were started with the addition of 10 μl of alcohol dehydrogenase (5 units). The absorbance at 550 nM was measured at 30 sec intervals for 10 min by a Versa Max Microplate reader (Molecular Devices, Sunnyvale CA). The results were compared to a standard curve. All samples were measured at the same time.
ATP measurements
ATP was isolated from cultures based on published protocols [18,60]. Briefly 1 ml of washed cells was suspended in HEPES buffer, and an ATP cell extract made by heating at 80°C for 20 min. Triplicate samples consisting of 100 μl of the ATP cell extract was mixed with 50 μl of a solution of luciferin and luciferase, and the light output measured on a Turner model TD-20e Luminometer (Turner Designs, Sunnyvale, CA). Cell densities were determined by measuring CFUs by plating on DOA.
Methylene blue oxidation
Cultures were grown for 600 hrs in DTA in the Wayne model. Each tube was opened and vortexed to introduce air. Methylene blue (final concentration 31 μM) was added, and 8 ml of culture was transferred to small screw cap tubes which held approximately 8.5 ml. These tubes were sealed and wrapped in parafilm. Cultures were incubated at 37°C with constant mixing and the absorbance at 665 nm measured at intervals.
Ethics statement
Mouse studies were performed in accordance to the National Institute of Health guidelines using recommendation in the Guide for the Care and Use of laboratory Animals. The protocols used in this study were approved by the Institutional Animal Care and Use Committee of the Trudeau Institute. Animals were sacrificed by cervical dislocation, and all efforts were made to minimize suffering.
Supporting Information
Each strain was grown in the Wayne model and then plated at intervals. WT–circles. RVW7 –squares. The standard deviation is shown.
(TIF)
Wild type SZ194 –circles. SZ194 ldhA::Hyg with a hygromycin marker in the lactate dehydrogenase gene–squares. SZ194 Mtb ald–with the M. tuberculosis ald expressed from the E. coli ldhA promoter—triangles.
(TIF)
(PDF)
Acknowledgments
For the ldhA pflB strain of E. coli we thank Lonnie Ingram. For technical help we thank Sandy Sudberg, Lucia Modesti and Sylors Chem.
Data Availability
All relevant data are within the paper and its Supporting Information Files.
Funding Statement
This study was supported by grants from the Medical Research Services of the U.S. Department of Veterans Affairs (CDS) and from the NIAID (AI104615) and NHLBI (HL 106788) (MLG).
References
- 1.Wayne LG, Sohaskey CD (2001) Nonreplicating persistence of Mycobacterium tuberculosis. Annu Rev Microbiol. 55: 139–163. [DOI] [PubMed] [Google Scholar]
- 2.Boshoff HI, Barry CE (2005) Tuberculosis—metabolism and respiration in the absence of growth. Nat Rev Microbiol. 3: 70–80. [DOI] [PubMed] [Google Scholar]
- 3.Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, et al. (2008) Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 76: 2333–2340. 10.1128/IAI.01515-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe S, Zimmermann M, Goodwin MB, Sauer U, Barry CE, Boshoff HI (2011) Fumarate reductase activity maintains an energized membrane in anaerobic Mycobacterium tuberculosis. PLoS Pathog. 7: e1002287 10.1371/journal.ppat.1002287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haapanen JH, Kass I, Gensini G, Middlebrook G (1959) Studies on the gaseous content of tuberculous cavities. Am Rev Respir Dis. 80: 1–5. [DOI] [PubMed] [Google Scholar]
- 6.Shi L, Sohaskey CD, Kana BD, Dawes S, North RJ, Mizrahi V, et al. (2005) Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitroconditions affecting aerobic respiration. Proc Natl Acad Sci USA. 102: 15629–15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boshoff HI, Myers TG, Copp BR, McNeil MR, Wilson MA, Barry CE (2004) The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J Biol Chem. 279: 40174–40184. [DOI] [PubMed] [Google Scholar]
- 8.Eoh H, Rhee KY (2013) Multifunctional essentiality of succinate metabolism in adaptation to hypoxia in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 110: 6554–6559. 10.1073/pnas.1219375110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sohaskey CD, Wayne LG (2003) Role of narK2X and narGHJI in hypoxic upregulation of nitrate reduction by Mycobacterium tuberculosis. J Bacteriol. 185: 7247–7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sohaskey CD (2008) Nitrate enhances the survival of Mycobacterium tuberculosis during inhibition of respiration. J Bacteriol. 190: 2981–2986. 10.1128/JB.01857-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giffin MM, Modesti L, Raab RW, Wayne LG, Sohaskey CD (2012) ald of Mycobacterium tuberculosis encodes both the alanine dehydrogenase and the putative glycine dehydrogenase. J Bacteriol. 194: 1045–1054. 10.1128/JB.05914-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castric PA (1983) Hydrogen cyanide production by Pseudomonas aeruginosa at reduced oxygen levels. Can J Microbiol. 29: 1344–1349. [DOI] [PubMed] [Google Scholar]
- 13.Caballero FJ, Cárdenas J, Castillo F (1989) Purification and properties of L-alanine dehydrogenase of the phototrophic bacterium Rhodobacter capsulatus E1F1. J Bacteriol. 171: 3205–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wayne LG, Lin K-Y (1982) Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect Immun. 37: 1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muñoz-Elías EJ, McKinney JD (2006) Carbon metabolism of intracellular bacteria. Cell Microbiol. 8: 10–22. [DOI] [PubMed] [Google Scholar]
- 16.Muñoz-Elías EJ, Upton AM, Cherian J, McKinney JD (2006) Role of the methylcitrate cycle in Mycobacterium tuberculosis metabolism, intracellular growth, and virulence. Mol Microbiol. 60: 1109–1122. [DOI] [PubMed] [Google Scholar]
- 17.Gould TA, van de Langemheen H, Muñoz-Elías EJ, McKinney JD, Sacchettini JC (2006) Dual role of isocitrate lyase 1 in the glyoxylate and methylcitrate cycles in Mycobacterium tuberculosis. Mol Microbiol. 61: 940–947. [DOI] [PubMed] [Google Scholar]
- 18.Wayne LG, Hayes LG (1996) An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun. 64: 2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi L, Sohaskey CD, Pheiffer C, Parks M, McFadden J, North RJ, et al. (2010) Carbon flux rerouting during Mycobacterium tuberculosis growth arrest. Mol Microbiol. 78: 1199–1215. 10.1111/j.1365-2958.2010.07399.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung JY, Madan-Lala R, Georgieva M, Rengarajan J, Sohaskey CD, Bange FC, et al. (2013) The intracellular environment of human macrophages that produce nitric oxide promoters growth of mycobacteria. Infect Immun. 81: 3198–3209. 10.1128/IAI.00611-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leistikow RL, Morton RA, Bartek IL, Frimpong I, Wagner K, Voskuil MI (2010) The Mycobacterium tuberculosis DosR regulon assists in metabolic homeostasis and enables rapid recovery from nonrespiring dormancy. J Bacteriol. 192: 1662–1670. 10.1128/JB.00926-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feavers I, Foulkes J, Setlow B, Sun D, Nicholson W, Setlow P, et al. (1990) The regulation of transcription of the gerA spore germination operon of Bacillus subtilis. Mol Microbiol. 4: 275–282. [DOI] [PubMed] [Google Scholar]
- 23.Hinc K, Nagórska K, Iwanicki A, Wegrzyn G, Séror SJ, Obuchowski M (2006) Expression of Genes Coding for GerA and GerK Spore germination receptors is dependent on the protein phosphatase PrpE. J Bacteriol. 188: 4373–4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saxena S, Samala G, Sridevi JP, Devi PB, Yogeeswari P, Sriram D (2015) Design and development of novel Mycobacterium tuberculosis L-alanine dehydrogenase inhibitors. Eur J Med Chem. 92: 401–414. 10.1016/j.ejmech.2014.12.046 [DOI] [PubMed] [Google Scholar]
- 25.Desjardins CA, Cohen KA, Munsamy V, Abeel T, Maharaj K, Walker BJ, et al. (2016) Genomic and functional analyses of Mycobacterium tuberculosis strains implicate ald in D-cycloserine resistance. Nat Genet. 10.1038/ng.3548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocha M, Sodek L, Licausi F, Hameed MW, Dornelas MC, van Dongen JT (2010) Analysis of alanine aminotransferase in various organs of soybean (Glycine max) and in dependence of different nitrogen fertilizers during hypoxic stress. Amino Acids. 39: 1043–1053. 10.1007/s00726-010-0596-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rocha M, Licausi F, Araúkjo WL, Nunes-Nesi A, Sodek L, Fernie AR, et al. (2010) Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by waterlogging of Lotus japonicus. Plant Physiol. 152: 1501–1513. 10.1104/pp.109.150045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyashita Y, Good AG (2008) Contribution of the GABA shunt to hypoxia-induced alanine accumulation in roots of Arabidopsis thaliana. Plant Cell Physiol. 49: 92–102. [DOI] [PubMed] [Google Scholar]
- 29.Edwards MR, Gilroy FV, Jimenez BM, O'Sullivan WJ (1989) Alanine is a major end product of metabolism by Giardia lamblia: a proton nuclear magnetic resonance study. Mol Biochem Parasitol. 37: 19–26. [DOI] [PubMed] [Google Scholar]
- 30.Paget TA, Raynor MH, Shipp DWE, Lloyd D (1990) Giardia lamblia produces alanine anaerobically but not in the presence of oxygen. Mol Biochem Parasitol. 42: 63–68. [DOI] [PubMed] [Google Scholar]
- 31.Fourneau JP, Davy M, Clément M, Ransom M, Darmois F, Lamarche M (1974) Studies on the effects of piridoxilate, a glyoxylic acid derivative, on the mammalian system, heart and muscle under normal or deficient oxygen supply. Attempts towards a biochemical approach. Arzneimittelforschung. 24: 27–34. [PubMed] [Google Scholar]
- 32.Chen JM, Alexander DC, Behr MA, Liu J (2003) Mycobacterium bovis BCG vaccines exhibit defects in alanine and serine catabolism. Infect Immun. 71: 708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zabalza A, van Dongen JT, Froehlich A, Oliver SN, Faix B, Gupta KJ, et al. (2009) Regulation of respiration and fermentation to control the plant internal oxygen concentration. Plant Physiol. 149: 1087–1098. 10.1104/pp.108.129288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phong WY, Lin W, Rao SP, Dick T, Alonso S, Pethe K (2013) Characterization of phosphofructokinase activity in Mycobacterium tuberculosis reveals that a functional glycolytic carbon flow is necessary to limit the accumulation of toxic metabolic intermediates under hypoxia. PLOS One. 8: e56037 10.1371/journal.pone.0056037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin M, Zhong Z, Connor HD, Bunzendahl H, Finn WF, Rusyn I, et al. (2002) Protective effect of glycine on renal injury induced by ischemia-reperfusion in vivo. Am J Physiol Renal Physiol. 282: F417–F423. [DOI] [PubMed] [Google Scholar]
- 36.Zhang K, Weinberg JM, Venkatachalam MA, Dong Z (2003) Glycine protection of PC-12 cells against injury by ATP-depletion. Neurochem Res. 28: 893–901. [DOI] [PubMed] [Google Scholar]
- 37.Deters M, Strubelt O, Younes M (1997) Protection by glycine against hypoxia-reoxygenation induced hepatic injury. Res Commun Mol Pathol Pharmacol 97: 199–213. [PubMed] [Google Scholar]
- 38.Gopinathan KP, Sirsi M, Ramakrishnan T (1963) Nicotin-amide-adenine nucleotides of Mycobacterium tuberculosis H37Rv. Biochem J. 37: 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boshoff HI, Xu X, Tahlan K, Dowd CS, Pethe K, Camacho LR, et al. (2008) Biosynthesis and recycling of nicotinamide cofactors in Mycobacterium tuberculosis: an essential role for NAD in non-replicating persistence. J Biol Chem. 283: 19329–19341. 10.1074/jbc.M800694200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh R, Manjunatha U, Boshoff HI, Ha YH, Niyomrattanakit P, Ledwidge R, et al. (2008) PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science. 322: 1392–1395. 10.1126/science.1164571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao SPS, Alonso S, Rand L, Dick T, Pethe K (2008) The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 105: 11945–11950. 10.1073/pnas.0711697105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng Z, Cáceres NE, Sarath G, Barletta RG (2002) Mycobacterium smegmatis L-alanine dehydrogenase (Ald) is required for proficient utilization of alanine as a sole nitrogen source and sustained anaerobic growth. J Bacteriol. 184: 5001–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Somashekar BS, Amin AG, Rithner CD, Troudt J, Basaraba R, Izzo A, et al. (2011) Metabolic profiling of lung granuloma in Mycobacterium tuberculosis infected guinea pigs: ex vivo 1H magic angle spinning NMR studies. J Proteome Res. 10: 4186–4195. [DOI] [PubMed] [Google Scholar]
- 44.McKinney JD, Höner zu Bentrup KH, Muñoz-Elías EJ, Miczak A, Chen B, Chan WT, et al. (2000) Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature. 406: 735–738. [DOI] [PubMed] [Google Scholar]
- 45.van Schaik EJ, Tom M, Woods DE (2009) Burkholderia pseudomallei isocitrate lyase is a persistence factor in pulmonary melioidosis: implications for the development of isocitrate lyase inhibitors as novel antimicrobials. Infect Immun. 77: 4275–4283. 10.1128/IAI.00609-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hartman T, Weinrick B, Vilchèze C, Berny M, Tufariello J, Cook GM, et al. (2014) Succinate dehydrogenase is the regulator of respiration in Mycobacterium tuberculosis. PLoS Pathog. 10: e1004510 10.1371/journal.ppat.1004510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramakrishnan L (2012) Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. 12: 352–366. 10.1038/nri3211 [DOI] [PubMed] [Google Scholar]
- 48.Ehlers S, Schaible UE (2013) The granuloma in tuberculosis: dynamics of a host-pathogen collusion. Front Immunol. 3: 411 10.3389/fimmu.2012.00411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Jantama K, Moore JC, Shanmugam KT, Ingram LO (2007) Production of L-alanine by metabolically engineered Escherichia coli. Appl Microbiol Biotechnol. 77: 355–366. [DOI] [PubMed] [Google Scholar]
- 50.Goldman DS (1959) Enzyme systems in the mycobacteria. VII. Purification, properties and mechanism of action of the alanine dehydrogenase. Biochim Biophys Acta. 34: 527–539. [DOI] [PubMed] [Google Scholar]
- 51.Hoyt JC, Johnson KE, Reeves HC (1991) Purification and characterization of Acinetobacter calcoaceticus isocitrate lyase. J Bacteriol. 173: 6844–6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith CV, Huang C, Miczak A, Russell DG, Sacchettini JC, Höner zu Bentrup K (2003) Biochemical and structural studies of malate synthase from Mycobacterium tuberculosis. J Biol Chem. 278: 1735–1743. [DOI] [PubMed] [Google Scholar]
- 53.Aitken A, Learmonth M (2002) Estimation of disulfide bonds using Ellman's reagent In: Walker JM, editors. The Protein Protocols Handbook. Totowa, NJ: Humana Press; pp. 595–596. [Google Scholar]
- 54.Shi L, Jung Y-J, Tyagi S, Gennaro ML, North R (2003) Expression of Th1-mediated immunity in mouse lungs induces a Mycobacterium tuberculosis transcription pattern characteristic of nonreplicating persistence. Proc Natl Acad Sci USA. 100: 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davidow A, Kanauiia GV, Shi L, Kaviar J, Guo X, Sung N, et al. (2005) Antibody profiles characteristic of Mycobacterium tuberculosis infection state. Infect Immun. 73: 6846–6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DesJardin LE, Hayes LG, Sohaskey CD, Wayne LG, Eisenach KD (2001) Microaerophilic induction of the alpha-crystallin chaperone protein homologues (hspX) mRNA of Mycobacterium tuberculosis. J Bacteriol. 183: 5311–5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Archuleta RJ, Hoppes PY, Primm TP (2005) Mycobacterium avium enters a state of metabolic dormancy in response to starvation. Tuberculosis (Edinb). 85: 147–158. [DOI] [PubMed] [Google Scholar]
- 58.London J, Knight M (1966) Concentrations of nicotinamide nucleotide coenzymes in micro-organisms. J Gen Microbiol. 44: 241–254. [DOI] [PubMed] [Google Scholar]
- 59.Leonardo MR, Dailly Y, Clark DP (1996) Role of NAD in Regulating the adhE Gene of Escherichia coli. J Bacteriol. 178: 6013–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sohaskey CD, Voskuil MI (2015) In vitro models that utilize hypoxia to induce non-replicating persistence in mycobacteria In: Parish T, Roberts DM, editors. Mycobacteria Protocols. New York: Springer Science; pp. 201–213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Each strain was grown in the Wayne model and then plated at intervals. WT–circles. RVW7 –squares. The standard deviation is shown.
(TIF)
Wild type SZ194 –circles. SZ194 ldhA::Hyg with a hygromycin marker in the lactate dehydrogenase gene–squares. SZ194 Mtb ald–with the M. tuberculosis ald expressed from the E. coli ldhA promoter—triangles.
(TIF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information Files.