Abstract
The aim of this paper is to identify and investigate an endophytic fungus (strain 28) that was isolated from Houttuynia cordata Thunb, a famous and widely-used Traditional Chinese Medicine. Based on morphological methods and a phylogenetic analysis of ITS sequences, this strain was identified as Chaetomium globosum. An antifungal activity bioassay demonstrated that the crude ethyl acetate (EtOAc) extracts of strain 28 had a wide antifungal spectrum and strong antimicrobial activity, particularly against Exserohilum turcicum (Pass.) Leonard et Suggs, Botrytis cinerea persoon and Botrytis cinerea Pers. ex Fr. Furthermore, the fermentation conditions, extraction method and the heat stability of antifungal substances from strain 28 were also studied. The results showed that optimal antifungal activity can be obtained with the following parameters: using potato dextrose broth (PDB) as the base culture medium, fermentation for 4–8 d (initial pH: 7.5), followed by extraction with EtOAc. The extract was stable at temperatures up to 80 °C. This is the first report on the isolation of endophytic C. globosum from H. cordata to identify potential alternative biocontrol agents that could provide new opportunities for practical applications involving H. cordata.
Keywords: Endophytic fungi, Houttuynia cordata Thunb., Chaetomium globosum, Antifungal activity, Process parameter
Introduction
Endophytic fungi are considered as novel sources for bioactive compounds that have important applications in the fields of agriculture and medicine. These fungi may possess the potential to produce bioactive compounds, and this potential to produce bioactive compounds is equal or similar to that of their host.1, 2, 3 Studies have shown positive symbiosis between endophytic fungi and their hosts. For example, the secondary metabolites of endophytic fungi can facilitate the growth of their host plants directly or indirectly and improve host resistance to abiotic or biotic stresses.4 Substantial renewed attention is now being paid to endophytic fungi, which have inhibitory activity toward pathogenic fungi5 and play an indispensable role in fungal biocontrol.6 Recently, an endophytic fungal strain was isolated by Santiago et al.7 from the Cinnamomum mollissimum plant. One bioactivity of a metabolite from this plant, which was identified as a polyketide, was the efficient killing of the fungal pathogen Aspergillus niger (IC50 1.56 μg mL−1).7
Conventional chemical fungicides have been shown to be harmful to the environment and to public health,8 and they can cause pathogens to become resistant when used for a long time.9 By contrast, extracts from natural products showed advantages with regards to environmental protection and human health. For example, Yin et al.10 suggested that an ethyl acetate (EtOAc) extract of Trichoderma harzianum fermented broth can control the tomato gray mold caused by Botrytis cinerea, without fungicide resistance and in an environmentally friendly manner. In addition, secondary metabolites have been a continuous source of new lead compounds and chemical entities in the fields of agriculture and medicine. For instance, multiple fungal metabolites, such as 12β-hydroxy-13α-methoxyverruculogen TR-2, fumitremorgin B, verruculogen, and helvolic acid and others from an endophytic fungus (strain Aspergillus fumigatus LN-4) exhibited high antifungal activities against multifarious phytopathogenic fungi.11
Houttuynia cordata Thunb. is a significant plant resource to screen for endophytic fungi that could produce specific metabolic products for use as biofungicides. In fact, a great number of medicinal plants have been used in China to cure various diseases for a long time, and abundant plant use experience has been accumulated. In Traditional Chinese Medicine (TCM), H. cordata has been used to cure inflammation, bronchitis infections of the upper respiratory cavity, coughs, arthritis, conjunctivitis and infections of the urethral channel because of its antifungal, antiviral, anti-mutagenic, anti-oxidative, and antileukemic activities and for its immunity-increasing effects. Nevertheless, little published work has focused on testing endophytic fungi from H. cordata. Consequently, our group is interested in these fungi and in particular in those with antifungal activity.
Based on metabolic studies about using endophytic fungi as biofungicides,12 we isolated an endophytic fungal strain from fresh, symptomless H. cordata tissues that were collected from Sichuan province, southwest China. We primarily investigated their antifungal activity and best processing parameters to lay a foundation for the further use of effective biocontrol agents.
Materials and methods
Isolation of the endophytic fungus
Source organism: The endophytic fungus (strain 28) was isolated from fresh, intact H. cordata, which was collected from Yaan City, southwest China and identified by Professor Wu Wei. The above site has a subtropical humid monsoon climate with abundant rainfall. Strain 28 was then stored in a refrigerator at 4 °C.
The isolation procedure was based on the article13, 14 with minor modifications: Healthy samples were collected in plastic bags and placed in an icebox at once, and then they were transported to the laboratory within a maximum of 48 h. The sample surfaces were washed with running water to remove soil and then sterilized as follows: the samples were rinsed with sterile distilled water 3 times; they were submerged in 75% ethanol for 1 min and rinsed 5 times with sterile water, followed by 0.1% HgCl2 for 1 min; and lastly, they were rinsed with sterile distilled water 5 more times. The sample surfaces were dried with sterile blotting paper. The surface-sterilized tubers were cut into 0.5 cm-long segments, and the center of the sterilized leaves was cut into 10 mm × 10 mm pieces with sterile blades. The tissues (4 segments or pieces per plate) were placed on potato dextrose agar (PDA) medium (containing more than 30 μg mL−1 streptomycin and ampicillin) and incubated at 28 °C in the dark for 5–7 d. Three replicate plates were used for each test. The colonies were examined periodically, and the endophytic fungal hyphae that emerged from the segments were transferred onto fresh PDA medium. Transfers were not repeated until a pure culture was obtained according to the colony morphology. To validate the effect of surface disinfection, uncut tissues that were sterilized according to the same steps were placed on PDA medium and incubated under the same conditions as the control. In addition, the last drift lotion from the surface-sterilization process (1–2 drops) was added to the PDA culture medium and coated evenly as another control. Finally, we examined the effect of disinfection after checking whether colonies had formed.
Morphological characterization
Strain 28 was incubated on PDA medium at 28 °C for 7 d. A morphological characterization was then used to identify the strain using a combination of the fungal characteristics in parallel with those in the literature15 including the mycelia, sporangium, and spore morphology as well as the external form of the colony.
Molecular identification, ITS sequence analysis16
After a strain 28 subculture was grown in an Erlenmeyer flask (250 mL) containing 150 mL of potato dextrose broth (PDB) (200 g L−1 potato and 20 g L−1 dextrose, initial pH 6.6–7.0) at 130 revolutions per minute (rev min−1) for 7 d at 26 °C, the mycelia and broth were separated by vacuum suction filtration. The mycelia were then scraped from the filter and ground into powder with the aid of liquid nitrogen. Genomic DNA was extracted by CTAB method.17 The quality and integrity of the DNA were estimated by electrophoresis on a 1% (w/v) agarose gel. The ITS1-5.8SrRNA-ITS2 region, which was the target rDNA region, was amplified using primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′).18 Each 25 μL reaction contained 2.5 mM 10× buffer (with Mg2+), 2.0 mM (10 μmol L−1) dNTPs, 2.0 mM (5 μmol L−1) of each primer, 1.0 mM (3 U) Taq DNA polymerase, and 1 mM genomic DNA. Polymerase chain reaction (PCR) amplifications were performed in a thermal cycler (ABI2720, USA) with an initial denaturing step at 94 °C for 5 min, followed by 35 amplification cycles of 94 °C for 30 s, 60 °C for 30 s, 72 °C for 60 s and a final extension step of 72 °C for 10 min. The amplification products were separated by electrophoresis on a 1% (w/v) agarose gel at 100 V for 30 min in 1× TAE buffer and stained with ethidium bromide (0.5 μg mL−1). The purified PCR product was then sent to Shanghai Sangon Biological Engineering Technology & Services Co. Ltd. for sequencing. The sequence was submitted to the National Centre for Biotechnology Information (NCBI) GenBank and analyzed by BLAST.
Culture conditions during fermentation
Strain 28 was stored in the refrigerator, and then it was incubated on PDA medium at 28 °C for 6–7 d. A fungus plug (4 mm diameter) of mycelial inoculum was cut from the margin of actively growing strain 28 colonies and transferred into an Erlenmeyer flask (250 mL) containing 150 mL of fermentation medium (4 plugs per flask). Under base culture conditions, the strains were incubated in PDB at 26 °C for 7 d and initial pH values of 6.6–7.0. The rotation rate of the incubator was fixed at 130 rev min−1. The culture conditions were varied on the basis of the experimental design.
Metabolite extraction
Fungal mycelia were separated from the culture broth by vacuum filtration and discarded after the fermentation procedures. The filtrate was collected and concentrated in as little as 10% of the original volume (v/v) in a vacuum rotary evaporator at 40–45 °C, then extracted three times at the liquid-liquid partition with EtOAc solvent 1:1 (v/v). The resulting crude extracts were collected and concentrated to dryness in a vacuum rotary evaporator at 40–45 °C, then dissolved into 3 mL of acetone per 150 mL of initial culture volume, followed by filtration through 0.22 μm Millipore filters to obtain the EtOAc-extracted solution.
Antifungal activity bioassay
The antifungal activity of the crude metabolites was determined by mycelial growth methods19 against the following 15 species of plant pathogens: Fusarium graminearum Sehw. (B1 for short), Alternaria solani (Ell. et Mart.) Sorauer (B2), Phytophthora capsici Leonian (B3), Fusarium oxysporum f. sp. niveum (B4), Sclerotinia sclerotiorum (Lib.) de Bary (B5), Exserohilum turcicum (Pass.) Leonard et Suggs (B6), Aphanomyces raphani Kendrick (B7), Puccinia zoysiae Diet. (B8), Fusarium moniliforme Sheld. (B9), Alternaria alternata (Fries) Keissler (B10), Botrytis cinerea persoon (B11), Alternaria solani Sorauer (B12), Thielaviopsis basicola (Berk. et Br.) Ferraris (B13), Botrytis cinerea Pers. ex Fr. (B14), and Bipolaris maydis (Nisikado et Miyake) Shoeml (B15). All the pathogens above were provided by the laboratory of phytopathology at Sichuan Agricultural University, China. The pathogens were activated from dormant states. After that, the fungus plugs (4 mm diameter) of 15 pathogenic fungi (B1–B15) from the margin of actively growing colonies were placed in the center of PDA medium plates containing 10% of the EtOAc-extracted solution (v/v). In addition, extract-free acetone (10% in medium) was used as a control. The experiments were performed in triplicate for each pathogenic fungus and control. Those plates then were incubated in the dark for 7 d, and the colony diameter was measured in two perpendicular directions.
Optimization of process parameters
To screen for the best processing parameters, the effects of the culture and the ferment disposal conditions on antifungal crude extract production were investigated using single-factor experiments. The inhibitory rate of the crude strain 28 extracts against B4 served as the indicator of antifungal activity. The base culture conditions and the crude extraction of the metabolites were completed according to the above method. Extract-free acetone was used as a negative control. All experiments were performed in triplicate.
Media Optimization: Strain 28 was inoculated into eight different liquid media (Gause No. 1 liquid medium, Czapek–Dox broth, Sabouraud's dextrose broth, BSE (0.5% succinate, 0.04% K2HPO4, 0.04% KH2PO4, 0.02% MgSO4·7H2O, 0.05% yeast extract; pH 6.5), Martin's broth, LB broth, medium A, and PDB) to select a suitable medium.
Initial pH test: Strain 28 was inoculated onto different PDB basic media at different initial pH values (initial pH values of 6.0, 6.3, 6.6, 6.9, 7.2 and 7.5).
Incubation time test: Ten conical flasks (250 mL), each of which contained 150 mL of the PDB basic medium, were incubated for 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 d.
Thermal stability of antifungal activity assay: Seven uniform-bore glass tubes with fine gradations were numbered as control keeping (CK) and 1–6. Three milliliter of the extracting solution was poured into the flasks, which were then heated in a water bath for 30 min at temperatures of 40 °C, 50 °C, 60 °C, 70 °C, 80 °C, 90 °C, and 100 °C, respectively.
Antifungal compound extraction: To investigate the effect of the extraction solvent on the fermentation broth, 5 different extraction solvents (petroleum ether, diethyl ether, n-butyl alcohol, EtOAc and alcohol) were chosen to extract the antifungal compounds.
Antifungal activity assay: We prepared enough 50-mL Erlenmeyer flasks so that each contained precisely 30 mL of PDB medium [including 10% of the extracted solution (v/v)] for each process parameter above. Next, this 30 mL mixture was poured into three Petri dishes per condition. A fungus plug (4 mm diameter) of mycelial inoculum was cut from the margin of actively growing B4 colonies as an indicator fungus, and it was placed in the center of the PDA medium of each dish. Having been inoculated, these dishes were wrapped with plastic wrap (which was purchased in the supermarket), and incubated in an incubator at 28 °C for 7 d. Finally, the suppression of B4 by fermented broth extract was measured in two perpendicular directions using a growth rate method.
Statistical analysis
The inhibitory rate of the crude strain 28 extracts against each fungus was calculated by using the following formula: antifungal rate = [(average colony diameter of control − average colony diameter of treatment)/average colony diameter of control] × 100.20 The colony diameter was measured in two perpendicular directions as described above.
All the results were expressed as the average ± standard deviation of triplicate determinations. An analysis of statistical significance was performed according to the one-way analysis of variance. Data analysis was performed with Microsoft Office Excel 2010 software.
Results and discussion
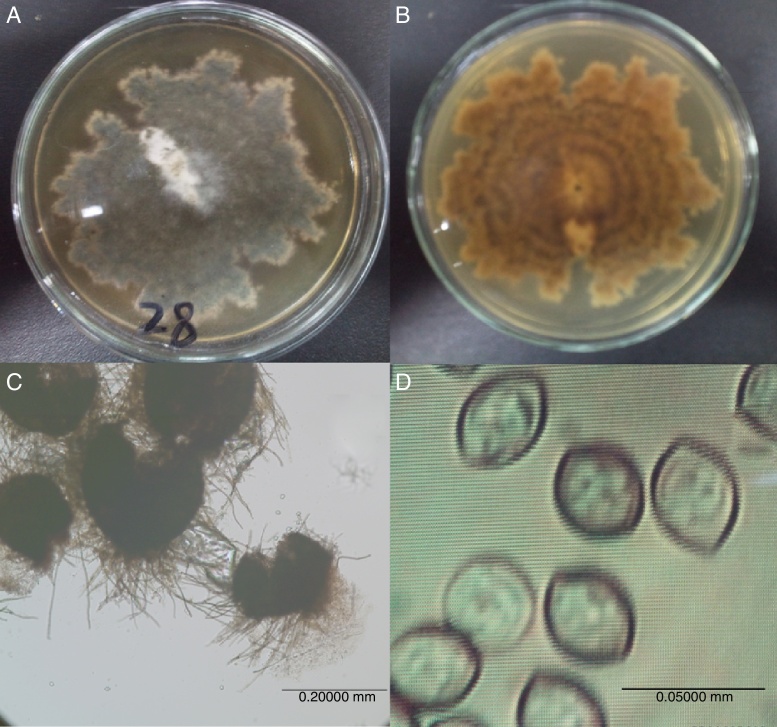
The strain 28 colonies (which grew on PDA plates at 28 °C for 6 d) were similarly flattened, black-gray, white and lobed on the edge, and they grew relatively slowly (Fig. 1A). The reverse sides of the colonies were dark yellow to aurantium (Fig. 1B), and the ascocarps were olive or green-yellow with ascomal hairs (Fig. 1C). Moreover, the spores were pale brown and lemon-shaped (Fig. 1D). Therefore, these characteristics corresponded well with those of Chaetomium globosum.15
Fig. 1.
The colony and microstructure of strain 28: colony front sides (A), colony back sides (B), ascocarps (C) and spore morphology (D). The colonial morphology was observed after growing on a PDA plate at 28 °C for 6 d, and the microstructure of the strain was observed under an Olympus BX 51 fluorescence microscope (OLYMPUS OPTICAL.CO.LTD. Tokyo, Japan) after growing for 7 d.
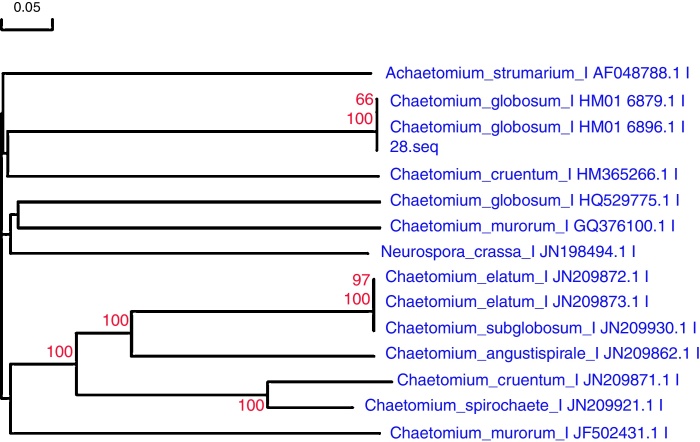
The amplified ITS1-5.8S rDNA-ITS2 region of the selected isolates (GenBank accession number: KF697163) was sequenced and compared with the ITS sequences of organisms represented in the NCBI GenBank database by using a BLAST search and DNAMAN to generate a phylogenetic tree (Fig. 2) by Neighbor-joining method. The sequences that showed E = 0.0 and the highest % similarity with the amplified sequences were included for alignment and bootstrapping using CLUSTALX. The phylogenetic relation of this isolate to related fungi is shown in Fig. 2. Thus, this strain was identified as C. globosum of Chaetomium sp. based on its morphological characteristics and the rDNA sequencing of its region data. Chaetomium sp. is cosmopolitan in distribution and it represents a considerable microorganismal resource.21
Fig. 2.
Cluster analysis of strain 28. The sequences that showed E = 0.0 and the highest % similarity with the amplified sequences were included for alignment and bootstrapping using CLUSTALX. This figure shows the phylogenetic relations of this isolate with related fungi. Thus, this strain was identified as Chaetomium globosum of Chaetomium sp. through the rDNA sequencing of ITS region data. This tree was constructed on the basis of the rDNA sequence (ITS1, 5.8S and ITS 2) by Neighbor-joining method. Bootstrap values >50% (1000 replicates) are shown at the branches. The bar indicates a 5% sequence divergence.
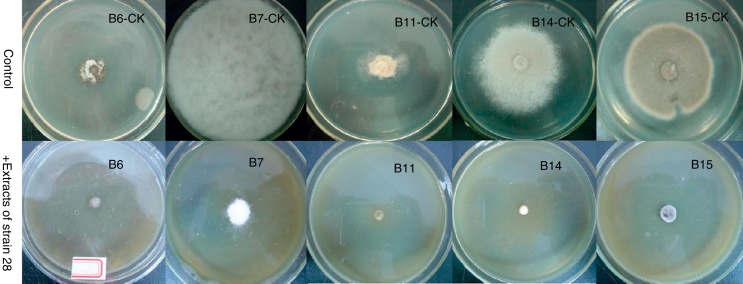
The results in Table 1 show that the crude EtOAc extracts of strain 28 could effectively inhibit 15 types of pathogenic fungi, especially B6, B11 and B14, the inhibition ratios of which were all 100.00% as well as B15 and B7 at 91.67 ± 0.24% and 86.18 ± 4.28%, respectively (Fig. 3). On the whole, the inhibition ratios for all the pathogenic fungi were above 50%, except for B13 and B12, which had inhibition ratios of 41.67 ± 1.27% and 45.93 ± 1.74%, respectively. This result shows that the crude extracts of strain 28 have broad-spectrum antifungal abilities. In 1954, Tveit22 suggested that C. globosum could control the wheat seedling blight caused by Helminthosporium victoriae. Abundant new reports have indicated that Chaetomium are interesting microbial antagonists that are used to control phytopathogens. There have been more than 200 compounds reported from this genus to date.11 Many of them are endophytic fungi from plants. Endophytic Chaetomium was reported to be antagonistic to the development of disease in wheat leaves caused by Pyrenophora tritici-repentis.23 A gliotoxin isolated from endophytic C. globosum that originated from Ginkgo biloba showed good antifungal activity against Fusarium graminearum.24 A novel cytotoxic agent that was derived from chlorinated azaphilone and named chaetomugilin was isolated from the cultures of C. globosum that originated from Ginkgo biloba.25 C. globosum EF18 endophytic fungus extract originating from Withania somnifera is reported to be significantly effective against Sclerotinia sclerotiorum.26
Table 1.
The inhibition of 15 types of pathogens by strain 28 fermentation filtrate in vitro.
| Pathogens | Average diameter with extract (cm)a | Control average diameter (cm)a | Inhibition ratio (%)a |
|---|---|---|---|
| B1 | 3.82 ± 0.22 | 8.62 ± 0.03 | 59.06 ± 2.66 |
| B2 | 2.83 ± 0.15 | 7.67 ± 0.12 | 67.44 ± 2.13 |
| B3 | 3.37 ± 0.23 | 8.67 ± 0.06 | 64.90 ± 2.76 |
| B4 | 3.62 ± 0.08 | 8.63 ± 0.06 | 61.68 ± 0.94 |
| B5 | 3.63 ± 0.12 | 8.27 ± 0.25 | 59.66 ± 1.49 |
| B6 | 0.50 ± 0.00 | 1.50 ± 0.20 | 100.00 ± 0.00 |
| B7 | 1.63 ± 0.35 | 8.70 ± 0.00 | 86.18 ± 4.28 |
| B8 | 3.73 ± 0.25 | 8.47 ± 0.32 | 59.41 ± 3.16 |
| B9 | 3.00 ± 0.10 | 8.70 ± 0.00 | 69.51 ± 1.22 |
| B10 | 2.80 ± 0.20 | 7.80 ± 0.26 | 68.49 ± 2.74 |
| B11 | 0.50 ± 0.00 | 3.20 ± 0.20 | 100.00 ± 0.00 |
| B12 | 3.60 ± 0.10 | 6.23 ± 0.25 | 45.93 ± 1.74 |
| B13 | 5.28 ± 0.10 | 8.70 ± 0.00 | 41.67 ± 1.27 |
| B14 | 0.50 ± 0.05 | 6.25 ± 0.48 | 100.00 ± 0.87 |
| B15 | 0.91 ± 0.01 | 5.38 ± 0.08 | 91.67 ± 0.24 |
Each value is the average ± standard deviation of triplicate determinations. Fusarium graminearum Sehw. (B1 for short), Alternaria solani (Ell. et Mart.) Sorauer (B2), Phytophthora capsici Leonian (B3), Fusarium oxysporum f. sp. niveum (B4), Sclerotinia sclerotiorum (Lib.) de Bary (B5), Exserohilum turcicum (Pass.) Leonard et Suggs (B6), Aphanomyces raphani Kendrick (B7), Puccinia zoysiae Diet. (B8), Fusarium moniliforme Sheld. (B9), Alternaria alternata (Fries) Keissler (B10), Botrytis cinerea persoon (B11), Alternaria solani Sorauer (B12), Thielaviopsis basicola (Berk. et Br.) Ferraris (B13), Botrytis cinerea Pers. ex Fr. (B14), and Bipolaris maydis (Nisikado et Miyake) Shoeml (B15).
Fig. 3.
Antifungal activity of culture extracts produced with Chaetomium globosum strain 28. Fungus plugs (4 mm diameter) of pathogenic fungi (B6, B7, B11, B14 and B15) from the margins of actively growing colonies were placed in the center of PDA medium containing the 10% EtOAc extracting solution of strain 28 (v/v). In addition, the extract-free acetone (10% in medium) was used as a control (B6-CK, B7-CK, B11-CK, B14-CK and B15-CK).
From a genetic standpoint, plant endophytic fungi are able to synthesize bioactive compounds that are equal or similar to those synthesized by their hosts, possibly as a result of horizontal gene transfer27 between endophytic fungi and their corresponding hosts. Furthermore, C. globosum is able to degrade lignin,28 which is beneficial to horizontal gene transfer. Other work has shown that a recombinant plasmid containing the TUB2-resistance gene conferred 300 times higher ressistance to carbendazim when transformed into a Chaetomium sp. protoplast using the PEG method.29 However, H. cordata shows potent antifungal activities as a TCM over a long period. Therefore, strain 28 might display effective antifungal properties as a result of horizontal gene transfer with its host.
Plant pathogens are harmful to the development of crops, and thus there have been many attempts to control them.30 However, abundant novel pathways or substances with efficient and environmentally friendly characteristics require further research because of the high-frequency heritable variation of pathogens with biotope changes and demands in agricultural development. Furthermore, endophytic fungi are becoming interesting sources of bioactive compounds including antifungals. Our work has shown that the crude EtOAc extracts of strain 28 were broad-spectrum and efficient in terms of their antifungal ability. Therefore, it is promising and interesting to study this fungus further and develop its utility value as a biocontrol agent.
We also discuss the optimization of process parameters using single-factor experiments. A large amount of endophytic fungal metabolites are obtained principally by means of liquid fermentation, in which culture conditions play a crucial role. Three variables (culture medium, initial pH and fermentation time) that significantly influence fungal growth were selected as the key culture condition factors prior to optimization.
Culture medium directly affects the biosynthesis and accumulation of secondary metabolites because it supplies nutrients and serves as a spatial carrier during the fermentation process. Table 2 indicates that there were significant differences (p < 0.05) among several culture media. Martin's broth displayed the most obvious ability to contribute to the accumulation of antifungal substances during strain 28 growths, and it possessed the maximum inhibition ratio (82.53% ± 1.95%) (p < 0.05).
Table 2.
The inhibition of B4 by strain 28 when cultivated in different liquid medium in vitro.
| Media | B4 |
|
|---|---|---|
| Average diameter (cm)a | Inhibition ratio (%)a | |
| Gause No. 1 liquid medium | – | – |
| Czapek–Dox broth | 8.80 ± 0.00 a* | 0.00 ± 0.00 f* |
| Sabouraud's dextrose broth | 2.53 ± 0.08 e | 75.50 ± 0.92 b |
| BSE | 5.52 ± 0.03 b | 39.56 ± 0.35 e |
| Martin's broth | 1.95 ± 0.23 f | 82.53 ± 1.95 a |
| LB broth | 4.02 ± 0.03 c | 57.63 ± 0.35 d |
| Medium A | 3.90 ± 0.17 cd | 59.04 ± 2.09 cd |
| PDB | 3.60 ± 0.23 d | 62.65 ± 2.76 c |
| Control | 8.80 ± 0.05 a | 0.00 ± 0.60 f |
Each value is the average ± standard deviation of triplicate determinations.
Values with the same letter are not significantly different (p < 0.05) according to the one-way analysis of variance (ANOVA).
The initial pH is widely recognized as a critical factor in fermentation because it can affect individual physiological phenomena, such as fungal growth, sporulation, and the production of enzymes, and it can influence enzyme stability,31 the surface charge of protoplasts and nutrient utilization efficiency.32 Therefore, research is needed to determine the optimal initial pH. The range of initial experimental pH values given here was based on preliminary tests. The results show obvious significant differences (p < 0.05) from pH 6.0–pH 7.5, and pH value of 6.9 is the most beneficial for the accumulation of antifungal substances and results in the highest inhibition ratio of 81.69% ± 1.81% (Table 3) in spite of showing no significant difference (p < 0.05) from that of pH 6.0–6.9.
Table 3.
The inhibition of Fusarium oxysporum f. sp. niveum by strain 28 when cultivated in liquid medium in vitro at different pH values.
| Initial pH |
Fusarium oxysporum f. sp. niveum |
|
|---|---|---|
| Average diameter (cm)a | Inhibition ratio (%)a | |
| 6.0 | 2.25 ± 0.28 cd* | 78.92 ± 3.35 ab* |
| 6.3 | 2.40 ± 0.20 cd | 77.11 ± 2.41 ab |
| 6.6 | 2.33 ± 0.13 cd | 77.95 ± 1.52 ab |
| 6.9 | 2.02 ± 0.16 d | 81.69 ± 1.81 c |
| 7.2 | 2.53 ± 0.06 c | 75.54 ± 0.70 b |
| 7.5 | 3.05 ± 0.15 b | 69.28 ± 1.94 a |
| Control | 8.80 ± 0.05 a | 0.00 ± 0.60 d |
Each value is the average ± standard deviation of triplicate determinations.
Values with the same letters are not significantly different (p < 0.05) according to the one-way ANOVA.
The inhibition ratio increased substantially (p < 0.05) from 3 d to 4 d of fermentation time, and then there was no significant difference from 4 d to 8 d, immediately followed by a significant decrease (p < 0.05) with an increase in the fermentation time (Table 4). Secondary metabolites (such as antibiotics) begin to be produced near the onset of the stationary growth phase of a microbial growth curve, where the specific growth rate is zero.33, 34 Therefore, the species and content of antifungal substances increased with increased fermentation time. Eventually, however, more and more fungal cell death occurred, usually because the cells burst open, which is also known as cell lysis, and this lysis resulted in reduced inhibition ratios after more than 8 d. To save time without compromising on the content of antifungal substance, 4 d of fermentation was chosen as a suitable time.
Table 4.
The antifungal ability of strain 28 when cultivated for different times in vitro.
| Days |
Fusarium oxysporum f. sp. niveum |
|
|---|---|---|
| Average diameter (cm)a | Inhibition ratio (%)a | |
| 3 | 8.60 ± 0.17 a* | 2.41 ± 2.09 d* |
| 4 | 3.82 ± 0.08 d | 60.04 ± 0.92 a |
| 5 | 3.63 ± 0.12 d | 62.25 ± 1.39 a |
| 6 | 3.60 ± 0.10 d | 62.65 ± 1.20 a |
| 7 | 3.77 ± 0.06 d | 60.64 ± 0.70 a |
| 8 | 3.92 ± 0.18 d | 58.84 ± 2.12 a |
| 9 | 4.65 ± 0.09 c | 50.00 ± 1.04 b |
| 10 | 4.75 ± 0.05 c | 48.80 ± 0.60 b |
| 11 | 5.35 ± 0.13 b | 41.57 ± 1.59 c |
| 12 | 5.58 ± 0.16 b | 38.76 ± 1.94 c |
| Control | 8.80 ± 0.05 a | 0.00 ± 0.60 d |
Each value is the average ± standard deviation of triplicate determinations.
Values with the same letter are not significantly different (p < 0.05) according to the one-way ANOVA.
Temperature treatments of the crude extracts over the course of concentration and solvent extraction is also critical apart from the culture conditions because some compounds are temperature-sensitive or have different solubilities at different temperatures. Therefore, the conditions of ferment disposal were optimized.
The thermal stability of extracts from fermentation broth should be investigated as much as possible to prevent thermal degradation.35 We tested the thermal stability of the extracts in a water bath at different temperatures. Table 5 lists the changes in antifungal activity against the indicator pathogen (B4) after the EtOAc extracted solution was heated for 30 min at temperatures of 40 °C, 50 °C, 60 °C, 70 °C, 80 °C, 90 °C, and 100 °C. The antimicrobial activity of the EtOAc extracting solution varied from 0.00 ± 0.00% to 55.12 ± 0.35%. There were significant differences (p < 0.05) above 70 °C, and the inhibition ratio decreased rapidly to 0 at 100 °C. Clearly, this result indicates that the antimicrobial substances from strain 28 were heat-stable after thermal treatment up to 80 °C. The antimicrobial activities of the extracts that were treated at temperatures higher than 80 °C declined sharply, possibly because of thermal degradation. It is notable that Zhao et al.36 drew a similar conclusion when the antibacterial metabolites of a marine fungus (NJ0104) were thermally stable up to 80 °C. Hence, there is a justification in that part of the antibacterial metabolites can maintain biological activity at least at marine temperatures.
Table 5.
Inhibition ratio of the antimicrobial substances in strain 28 when treated at different temperatures in vitro.
| Temperature (°C) |
Fusarium oxysporum f. sp. niveum |
|
|---|---|---|
| Average diameter (cm)a | Inhibition ratio (%)a | |
| 40 | 4.20 ± 0.26 de* | 54.88 ± 3.23 ab* |
| 50 | 4.18 ± 0.03 e | 55.12 ± 0.35 a |
| 60 | 4.38 ± 0.18 de | 52.68 ± 2.14 ab |
| 70 | 4.58 ± 0.08 cd | 50.24 ± 0.93 bc |
| 80 | 4.73 ± 0.08 c | 48.41 ± 0.93 c |
| 90 | 7.97 ± 0.15 b | 8.90 ± 1.86 d |
| 100 | 8.70 ± 0.00 a | 0.00 ± 0.00 e |
| Control | 8.70 ± 0.09 a | 0.00 ± 1.06 e |
Each value is the average ± standard deviation of triplicate determinations.
Values with the same letters are not significantly different (p < 0.05) according to the one-way ANOVA.
Bioactive antifungal compounds are extracted efficiently only by suitable extraction solvents because of their different polarity and solubility properties. There were significant differences (p < 0.05) among five extraction solvents (Table 6). EtOAc extract exhibited the strongest antifungal ability followed by diethyl ether and n-butyl alcohol extracts.
Table 6.
Inhibition ratio of the fermentation filtrates from strain 28 when extracted in different solvents in vitro.
| Extraction solvents |
Fusarium oxysporum f. sp. niveum |
|
|---|---|---|
| Average diameter (cm)a | Inhibition ratio (%)a | |
| Petroleum ether | 4.22 ± 0.26 b* | 55.18 ± 3.09 d* |
| Diethyl ether | 2.12 ± 0.06 d | 80.48 ± 0.70 b |
| N-butyl alcohol | 2.20 ± 0.26 cd | 79.52 ± 3.19 bc |
| Ethyl acetate | 1.91 ± 0.02 e | 83.01 ± 0.28 a |
| Alcohol | 2.27 ± 0.06 c | 78.67 ± 0.70 c |
| Control | 8.80 ± 0.05 a | 0.00 ± 0.60 e |
Each value is the average ± standard deviation of triplicate determinations.
Values with the same letter are not significantly different (p < 0.05) according to the one-way ANOVA.
On a more general note, there is also a need for further study on how to use secondary metabolites from strain 28 as biocontrol agents. For example, there is a lack of studies on direct antifungal effects in crops, and phytopathogenic fungus is one of the vital links to developing bio-antifungal agents. Previous studies only revealed the antifungal activity of the crude EtOAc extracts in spite of the activity of the single components. However, this investigative direction would lead to fascinating scientific research and practical application value because of the extract's obvious broad-spectrum antifungal ability. This article is also the first report on the isolation of endophytic C. globosum from H. cordata as a biocontrol agent. Moreover, preliminary data from process parameter optimization assays will provide good guiding significance for harvesting more secondary metabolites from strain 28. In addition, numerous secondary metabolites from multiple C. globosum strains have been reported and showed a variety of biological activities including cytotoxicity, antibacterial activity, phytotoxicity, elicitor activity and others.37 Therefore, studying this organism and its antifungal compounds further to develop practical applications will be a promising line of research for the future.
Author contributions
FP, ZQL and WW conceived and designed the experiment. ZQL, QC, and YWX participated in sample collection and the isolation of the strain. FP, ZQL and KH analyzed and interpreted the sequence data and identified the strain. In addition, the antifungal activity bioassay and optimization of process parameters including statistical analysis were performed by FP and ZQL. Moreover, FP, ZQL and WW drafted and revised the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was financial supported by the “211” Project Double-Support Plan of Sichuan Agricultural University (No. 00170705). We are grateful to the assistance of the laboratory of phytopathology of Sichuan agricultural University, China for providing plant pathogens.
Associate Editor: Eleni Gomes
References
- 1.Wani M.C., Taylor H.L., Wall M.E., Coggon P., McPhail A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 2.You X., Feng S., Luo S. Studies on a rhein-producing endophytic fungus isolated from Rheum palmatum L. Fitoterapia. 2013;85:161–168. doi: 10.1016/j.fitote.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Kusari S., Pandey S.P., Spiteller M. Untapped mutualistic paradigms linking host plant and endophytic fungal production of similar bioactive secondary metabolites. Phytochemistry. 2013;91:81–87. doi: 10.1016/j.phytochem.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Kuldau G., Bacon C. Clavicipitaceous endophytes: their ability to enhance resistance of grasses to multiple stresses. Biol Control. 2008;46:57–71. [Google Scholar]
- 5.Mishra A., Gond S., Kumar A., Sharma V., Verma S., Kharwar R.N. Springer Press; Netherlands: 2012. pp. 581–612. (Sourcing the fungal endophytes: a beneficial transaction of biodiversity, bioactive natural products, plant protection and nanotechnology: Microorganisms in sustainable agriculture and biotechnology). [Google Scholar]
- 6.Mejía L.C., Rojas E.I., Maynard Z. Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biol Control. 2008;46:4–14. [Google Scholar]
- 7.Santiago C., Fitchett C., Munro M.H., Jalil J., Santhanam J. Cytotoxic and antifungal activities of 5-Hydroxyramulosin, a compound produced by an endophytic fungus isolated from Cinnamomum mollisimum. Evid Based Complement Alternat Med. 2012;2012:689310. doi: 10.1155/2012/689310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller A.K., Bosgra S., Boon P.E., Voet H.V.D., Nielsen E., Ladefoged O. Probabilistic cumulative risk assessment of anti-androgenic pesticides in food. Food Chem Toxicol. 2009;47:2951–2962. doi: 10.1016/j.fct.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 9.Jensen B., Knudsen I.B., Jensen D. Biological seed treatment of cereals with fresh and long-term stored formulations of clonostachys rosea: biocontrol efficacy against Fusarium culmorum. Eur J Plant Pathol. 2000;106:233–242. [Google Scholar]
- 10.Yin G., Wang W., Sha S., Liu L., Yu X. Inhibition and control effects of the ethyl acetate extract of Trichoderma harzianum fermented broth against Botrytis cinerea. Afr J Microbiol Res. 2010;15:1647–1653. [Google Scholar]
- 11.Li X.J., Zhang Q., Zhang A.L., Gao J.M. Metabolites from Aspergillus fumigatus, an endophytic fungus associated with Melia azedarach, and their antifungal, antifeedant, and toxic activities. J Agric Food Chem. 2012;60:3424–3431. doi: 10.1021/jf300146n. [DOI] [PubMed] [Google Scholar]
- 12.Kumar S., Kaushik N. Metabolites of endophytic fungi as novel source of biofungicide: a review. Phytochem Rev. 2013:1–16. [Google Scholar]
- 13.Premalatha K., Kalra A. Molecular phylogenetic identification of endophytic fungi isolated from resinous and healthy wood of Aquilaria malaccensis, a red listed and highly exploited medicinal tree. Fungal Ecol. 2013;6:205–211. [Google Scholar]
- 14.Xu D., Xia X., Xu N., An L. Isolation and identification of a novel endophytic bacterial strain with antifungal activity from wild blueberry Vaccinium uliginosum. Ann Microbiol. 2007;57:673–676. [Google Scholar]
- 15.Dugan F.M. APS Press; Saint Paul, Minnesota, USA: 2006. The identification of fungi: an illustrated introduction with keys, glossary, and guide to literature. [Google Scholar]
- 16.Gherbawy Y., Voigt K. Springer press; Berlin, Germany: 2010. Molecular identification of fungi. [Google Scholar]
- 17.Porebski S., Bailey L.G., Baum B. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol Biol Rep. 1997;15:8–15. [Google Scholar]
- 18.White T.J., Bruns T., Lee S., Taylor J. Vol. 18. Academic Press; New York, USA: 1990. pp. 315–322. (Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications). [Google Scholar]
- 19.Fang Z.D. Agriculture Press; Beijing, China: 1998. (Research method of plant pathogen). [Google Scholar]
- 20.Yang X., Qiu D., Yang H., Liu Z., Zeng H., Yuan J. Antifungal activity of xenocoumacin 1 from Xenorhabdus nematophilus var. pekingensis against Phytophthora infestans. World J Microbiol Biotechnol. 2011;27:523–528. [Google Scholar]
- 21.Lee S., Hanlin R.T. Phylogenetic relationships of Chaetomium and similar genera based on ribosomal DNA sequences. Mycologia. 1999:434–442. [Google Scholar]
- 22.Tveit M., Moore M. Isolates of Chaetomium that protect oats from Helminthosporium victoriae. Phytopathology. 1954;44:686–689. [Google Scholar]
- 23.Istifadah N., McGee P.A. Endophytic Chaetomium globosum reduces development of tan spot in wheat caused by Pyrenophora tritici-repentis. Aust Plant Path. 2006;35:411–418. [Google Scholar]
- 24.Li H.Q., Li X.J., Wang Y.L. Antifungal metabolites from Chaetomium globosum, an endophytic fungus in Ginkgo biloba. Biochem Syst Ecol. 2011;39:876–879. [Google Scholar]
- 25.Qin J.C., Zhang Y.M., Gao J.M. Bioactive metabolites produced by Chaetomium globosum, an endophytic fungus isolated from Ginkgo biloba. Bioorg Med Chem Lett. 2009;19:1572–1574. doi: 10.1016/j.bmcl.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S., Kaushik N., Proksch P. Identification of antifungal principle in the solvent extract of an endophytic fungus Chaetomium globosum, from Withania somnifera. SpringerPlus. 2013;2:37. doi: 10.1186/2193-1801-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gophna U., Ron E.Z., Graur D. Bacterial type III secretion systems are ancient and evolved by multiple horizontal-transfer events. Gene. 2003;312:151–163. doi: 10.1016/s0378-1119(03)00612-7. [DOI] [PubMed] [Google Scholar]
- 28.Haider K., Trojanowski J. Decomposition of specifically 14C-labelled phenols and dehydropolymers of coniferyl alcohol as models for lignin degradation by soft and white rot fungi. Arch Microbiol. 1975;105:33–41. [Google Scholar]
- 29.Chi Y.J., Yang Q. Construction and identification of recombinant plasmid of TUB2 Gene and transformation into Chaetomium sp. High Technol Lett. 2003;13:34–40. [Google Scholar]
- 30.Arya A., Perelló A.E. CABI Publishing; Gottingen, Germany: 2010. Management of fungal plant pathogens. [Google Scholar]
- 31.Taragano V.M., Pilosof A.M.R. Application of Doehlert designs for water activity, pH, and fermentation time optimization for Aspergillus niger pectinolytic activities production in solid-state and submerged fermentation. Enzyme Microb Technol. 1999;25:411–419. [Google Scholar]
- 32.Grignon C., Sentenac H. pH and ionic conditions in the apoplast. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:103–128. [Google Scholar]
- 33.Buchanan R.L., Whiting R.C., Damert W.C. When is simple good enough: a comparison of the Gompertz, Baranyi, and three-phase linear models for fitting bacterial growth curves. Food Microbiol. 1997;14:313–326. [Google Scholar]
- 34.Madigan M.T., Martinko J.M. 11th ed. Prentice Hall International Inc.; 2006. Brock biology of microorganisms. [Google Scholar]
- 35.Chan C.H., Yusoff R., Ngoh G.C., Kung F.W.L. Microwave-assisted extractions of active ingredients from plants. J Chromatogr A. 2011;1218:6213–6225. doi: 10.1016/j.chroma.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 36.Zhao S., Li S., Liu H., Zhao Q., Wang J., Yan M. Screening of marine fungus from Nanji Island and activity of their metabolites against pathogenic Vibrio from Pseudosciaena crocea. Chin J Oceanol Limnol. 2012:1–11. [Google Scholar]
- 37.Zhang Q., Li H.Q., Zong S.C., Gao J.M., Zhang A.L. Chemical and bioactive diversities of the genus Chaetomium secondary metabolites. Mini Rev Med Chem. 2012;12:127–148. doi: 10.2174/138955712798995066. [DOI] [PubMed] [Google Scholar]