Abstract
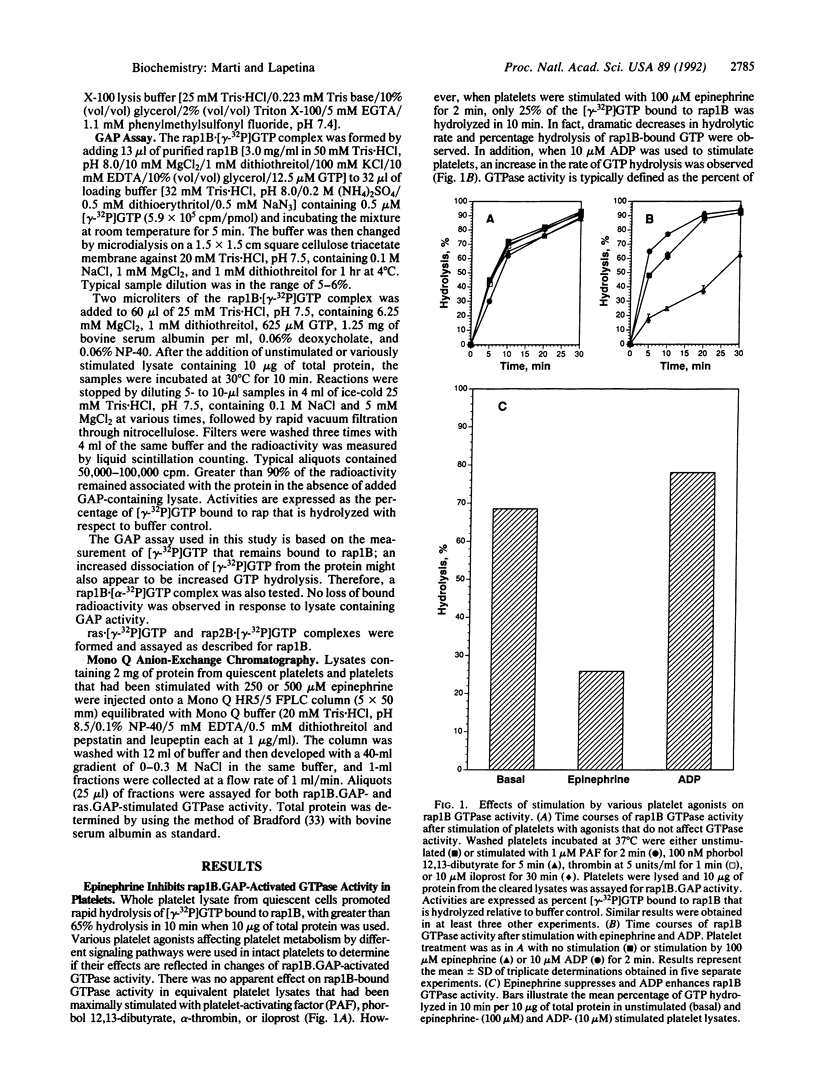
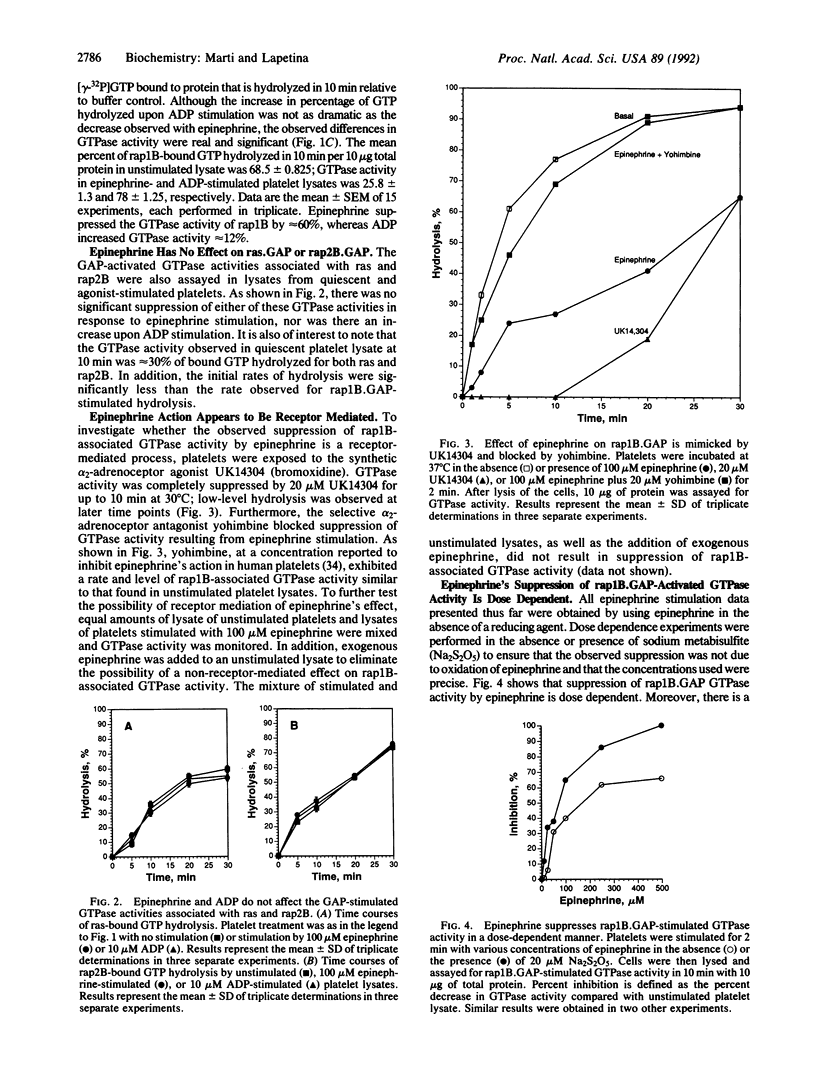
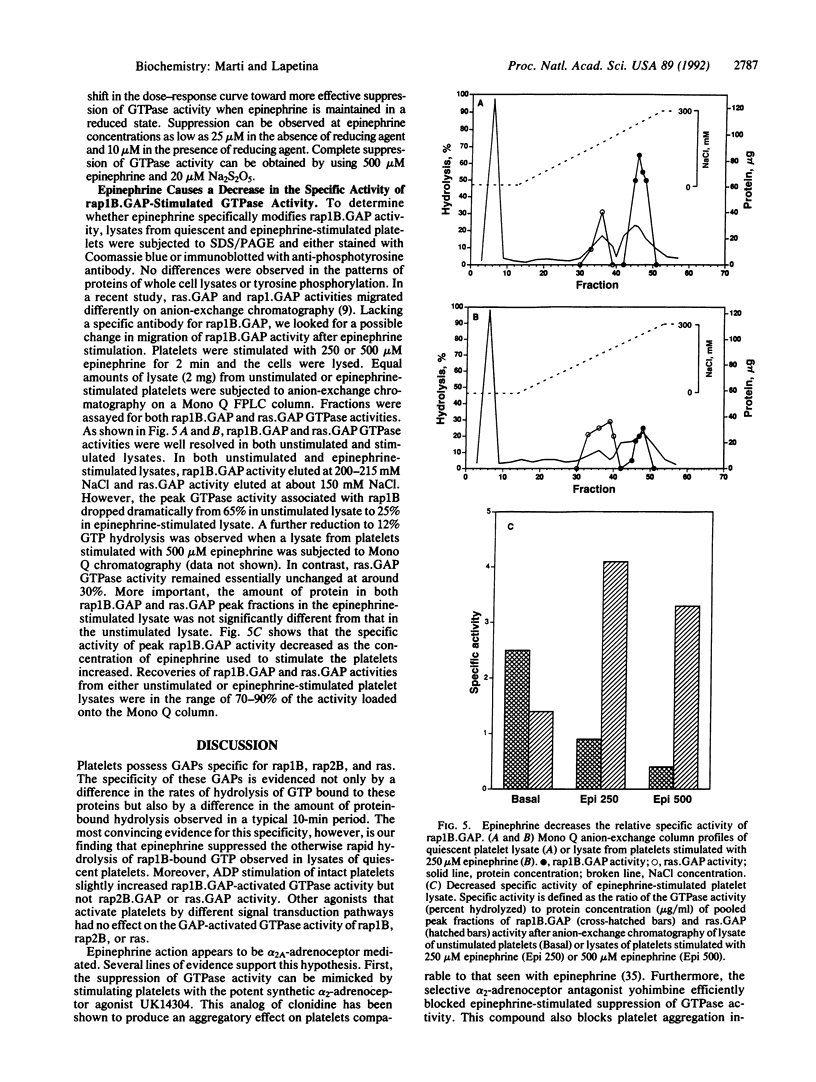
Lysate from quiescent platelets promotes rapid hydrolysis of [gamma-32P]GTP bound to rap1B. Various platelet agonists, including platelet-activating factor, phorbol 12,13-dibutyrate, alpha-thrombin, epinephrine, ADP, and iloprost, that affect platelet metabolism by different signal transduction pathways were used to stimulate intact platelets and study their effects on rap1B.GAP-activated GTPase activity (GAP, GTPase-activating protein). Only epinephrine was found to dramatically decrease not only the rate but also the amount of hydrolysis of rap1B-bound GTP activated by rap1B.GAP. This effect was dose dependent and occurred rapidly. The suppression of GTPase activity was specific for rap1B.GAP in that ras.GAP- and rap2B.GAP-activated GTPase activity were not affected by epinephrine stimulation. This effect appears to be mediated by the alpha 2-adrenergic receptor, as evidenced by a similar suppression of GTPase activity by stimulating platelets with the synthetic alpha 2-adrenergic receptor agonist UK14304 (bromoxidine). Furthermore, the selective alpha 2-adrenergic receptor antagonist yohimbine blocked the suppression of GTPase activity expressed in epinephrine-stimulated cell lysates. No apparent changes in the patterns of protein expression or tyrosine phosphorylation were observed. Although the migration characteristics upon anion-exchange chromatography of rap1B.GAP and ras.GAP activities were unaffected by epinephrine stimulation, the specific activity of rap1B.GAP was noticeably decreased with 250 and 500 microM epinephrine. These results suggest a possible role for rap1B and rap1B.GAP in epinephrine-stimulated signal transduction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adari H., Lowy D. R., Willumsen B. M., Der C. J., McCormick F. Guanosine triphosphatase activating protein (GAP) interacts with the p21 ras effector binding domain. Science. 1988 Apr 22;240(4851):518–521. doi: 10.1126/science.2833817. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burstein E. S., Linko-Stentz K., Lu Z. J., Macara I. G. Regulation of the GTPase activity of the ras-like protein p25rab3A. Evidence for a rab3A-specific GAP. J Biol Chem. 1991 Feb 15;266(5):2689–2692. [PubMed] [Google Scholar]
- Bylund D. B., Ray-Prenger C., Murphy T. J. Alpha-2A and alpha-2B adrenergic receptor subtypes: antagonist binding in tissues and cell lines containing only one subtype. J Pharmacol Exp Ther. 1988 May;245(2):600–607. [PubMed] [Google Scholar]
- Cerione R. A., Regan J. W., Nakata H., Codina J., Benovic J. L., Gierschik P., Somers R. L., Spiegel A. M., Birnbaumer L., Lefkowitz R. J. Functional reconstitution of the alpha 2-adrenergic receptor with guanine nucleotide regulatory proteins in phospholipid vesicles. J Biol Chem. 1986 Mar 15;261(8):3901–3909. [PubMed] [Google Scholar]
- Cotecchia S., Kobilka B. K., Daniel K. W., Nolan R. D., Lapetina E. Y., Caron M. G., Lefkowitz R. J., Regan J. W. Multiple second messenger pathways of alpha-adrenergic receptor subtypes expressed in eukaryotic cells. J Biol Chem. 1990 Jan 5;265(1):63–69. [PubMed] [Google Scholar]
- Crouch M. F., Lapetina E. G. A role for Gi in control of thrombin receptor-phospholipase C coupling in human platelets. J Biol Chem. 1988 Mar 5;263(7):3363–3371. [PubMed] [Google Scholar]
- Crouch M. F., Winegar D. A., Lapetina E. G. Epinephrine induces changes in the subcellular distribution of the inhibitory GTP-binding protein Gi alpha-2 and a 38-kDa phosphorylated protein in the human platelet. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1776–1780. doi: 10.1073/pnas.86.6.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer T. H., Gatling M. N., Lacal J. C., White G. C., 2nd rap1B, a cAMP-dependent protein kinase substrate, associates with the platelet cytoskeleton. J Biol Chem. 1990 Nov 15;265(32):19405–19408. [PubMed] [Google Scholar]
- Frech M., John J., Pizon V., Chardin P., Tavitian A., Clark R., McCormick F., Wittinghofer A. Inhibition of GTPase activating protein stimulation of Ras-p21 GTPase by the Krev-1 gene product. Science. 1990 Jul 13;249(4965):169–171. doi: 10.1126/science.2164710. [DOI] [PubMed] [Google Scholar]
- Garrett M. D., Major G. N., Totty N., Hall A. Purification and N-terminal sequence of the p21rho GTPase-activating protein, rho GAP. Biochem J. 1991 Jun 15;276(Pt 3):833–836. doi: 10.1042/bj2760833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B., Schaber M. D., Allard W. J., Sigal I. S., Scolnick E. M. Purification of ras GTPase activating protein from bovine brain. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5026–5030. doi: 10.1073/pnas.85.14.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J. A., Scrutton M. C. Interaction of selective alpha-adrenoceptor agonists and antagonists with human and rabbit blood platelets. Br J Pharmacol. 1980;71(1):121–134. doi: 10.1111/j.1476-5381.1980.tb10917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halenbeck R., Crosier W. J., Clark R., McCormick F., Koths K. Purification, characterization, and western blot analysis of human GTPase-activating protein from native and recombinant sources. J Biol Chem. 1990 Dec 15;265(35):21922–21928. [PubMed] [Google Scholar]
- Hata Y., Kikuchi A., Sasaki T., Schaber M. D., Gibbs J. B., Takai Y. Inhibition of the ras p21 GTPase-activating protein-stimulated GTPase activity of c-Ha-ras p21 by smg p21 having the same putative effector domain as ras p21s. J Biol Chem. 1990 May 5;265(13):7104–7107. [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Hsu C. Y., Knapp D. R., Halushka P. V. The effects of alpha adrenergic agents on human platelet aggregation. J Pharmacol Exp Ther. 1979 Mar;208(3):366–370. [PubMed] [Google Scholar]
- Jakobs K. H., Saur W., Schultz G. Reduction of adenylate cyclase activity in lysates of human platelets by the alpha-adrenergic component of epinephrine. J Cyclic Nucleotide Res. 1976 Nov-Dec;2(6):381–392. [PubMed] [Google Scholar]
- Kawata M., Kikuchi A., Hoshijima M., Yamamoto K., Hashimoto E., Yamamura H., Takai Y. Phosphorylation of smg p21, a ras p21-like GTP-binding protein, by cyclic AMP-dependent protein kinase in a cell-free system and in response to prostaglandin E1 in intact human platelets. J Biol Chem. 1989 Sep 15;264(26):15688–15695. [PubMed] [Google Scholar]
- Kikuchi A., Sasaki T., Araki S., Hata Y., Takai Y. Purification and characterization from bovine brain cytosol of two GTPase-activating proteins specific for smg p21, a GTP-binding protein having the same effector domain as c-ras p21s. J Biol Chem. 1989 Jun 5;264(16):9133–9136. [PubMed] [Google Scholar]
- Kitayama H., Sugimoto Y., Matsuzaki T., Ikawa Y., Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989 Jan 13;56(1):77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Lacal J. C., Reep B. R., Molina y Vedia L. A ras-related protein is phosphorylated and translocated by agonists that increase cAMP levels in human platelets. Proc Natl Acad Sci U S A. 1989 May;86(9):3131–3134. doi: 10.1073/pnas.86.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski E. R., Lacal J. C., Lapetina E. G. Agonist-induced phosphorylation of an immunologically ras-related protein in human erythroleukemia cells. Biochem Biophys Res Commun. 1989 Jun 30;161(3):972–978. doi: 10.1016/0006-291x(89)91338-7. [DOI] [PubMed] [Google Scholar]
- Macfarlane D. E., Stump D. C. Parallel observation of the occupancy of the alpha 2-adrenergic receptor in intact platelets and its ability to inhibit the adenylate cyclase. Mol Pharmacol. 1982 Nov;22(3):574–579. [PubMed] [Google Scholar]
- Michel M. C., Brass L. F., Williams A., Bokoch G. M., LaMorte V. J., Motulsky H. J. Alpha 2-adrenergic receptor stimulation mobilizes intracellular Ca2+ in human erythroleukemia cells. J Biol Chem. 1989 Mar 25;264(9):4986–4991. [PubMed] [Google Scholar]
- Morii N., Kawano K., Sekine A., Yamada T., Narumiya S. Purification of GTPase-activating protein specific for the rho gene products. J Biol Chem. 1991 Apr 25;266(12):7646–7650. [PubMed] [Google Scholar]
- North R. A., Williams J. T., Surprenant A., Christie M. J. Mu and delta receptors belong to a family of receptors that are coupled to potassium channels. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5487–5491. doi: 10.1073/pnas.84.15.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizon V., Lerosey I., Chardin P., Tavitian A. Nucleotide sequence of a human cDNA encoding a ras-related protein (rap1B). Nucleic Acids Res. 1988 Aug 11;16(15):7719–7719. doi: 10.1093/nar/16.15.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. G., Rubinfeld B., Evans T., McCormick F. Purification of a plasma membrane-associated GTPase-activating protein specific for rap1/Krev-1 from HL60 cells. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):239–243. doi: 10.1073/pnas.88.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B., Munemitsu S., Clark R., Conroy L., Watt K., Crosier W. J., McCormick F., Polakis P. Molecular cloning of a GTPase activating protein specific for the Krev-1 protein p21rap1. Cell. 1991 Jun 14;65(6):1033–1042. doi: 10.1016/0092-8674(91)90555-d. [DOI] [PubMed] [Google Scholar]
- Siess W., Lapetina E. G. Functional relationship between cyclic AMP-dependent protein phosphorylation and platelet inhibition. Biochem J. 1990 Nov 1;271(3):815–819. doi: 10.1042/bj2710815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siess W., Weber P. C., Lapetina E. G. Activation of phospholipase C is dissociated from arachidonate metabolism during platelet shape change induced by thrombin or platelet-activating factor. Epinephrine does not induce phospholipase C activation or platelet shape change. J Biol Chem. 1984 Jul 10;259(13):8286–8292. [PubMed] [Google Scholar]
- Siess W., Winegar D. A., Lapetina E. G. Rap1-B is phosphorylated by protein kinase A in intact human platelets. Biochem Biophys Res Commun. 1990 Jul 31;170(2):944–950. doi: 10.1016/0006-291x(90)92182-y. [DOI] [PubMed] [Google Scholar]
- Steen V. M., Tysnes O. B., Holmsen H. Synergism between thrombin and adrenaline (epinephrine) in human platelets. Marked potentiation of inositol phospholipid metabolism. Biochem J. 1988 Jul 15;253(2):581–586. doi: 10.1042/bj2530581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt J. D., Blair I. A., Cragoe E. J., Limbird L. E. Inhibitors of Na+/H+ exchange block epinephrine- and ADP-induced stimulation of human platelet phospholipase C by blockade of arachidonic acid release at a prior step. J Biol Chem. 1986 Jul 5;261(19):8660–8666. [PubMed] [Google Scholar]
- Trahey M., Wong G., Halenbeck R., Rubinfeld B., Martin G. A., Ladner M., Long C. M., Crosier W. J., Watt K., Koths K. Molecular cloning of two types of GAP complementary DNA from human placenta. Science. 1988 Dec 23;242(4886):1697–1700. doi: 10.1126/science.3201259. [DOI] [PubMed] [Google Scholar]
- Vogel U. S., Dixon R. A., Schaber M. D., Diehl R. E., Marshall M. S., Scolnick E. M., Sigal I. S., Gibbs J. B. Cloning of bovine GAP and its interaction with oncogenic ras p21. Nature. 1988 Sep 1;335(6185):90–93. doi: 10.1038/335090a0. [DOI] [PubMed] [Google Scholar]



