Abstract
Many marine and terrestrial clades show similar latitudinal gradients in species richness, but opposite gradients in range size—on land, ranges are the smallest in the tropics, whereas in the sea, ranges are the largest in the tropics. Therefore, richness gradients in marine and terrestrial systems do not arise from a shared latitudinal arrangement of species range sizes. Comparing terrestrial birds and marine bivalves, we find that gradients in range size are concordant at the level of genera. Here, both groups show a nested pattern in which narrow-ranging genera are confined to the tropics and broad-ranging genera extend across much of the gradient. We find that (i) genus range size and its variation with latitude is closely associated with per-genus species richness and (ii) broad-ranging genera contain more species both within and outside of the tropics when compared with tropical- or temperate-only genera. Within-genus species diversification thus promotes genus expansion to novel latitudes. Despite underlying differences in the species range-size gradients, species-rich genera are more likely to produce a descendant that extends its range relative to the ancestor's range. These results unify species richness gradients with those of genera, implying that birds and bivalves share similar latitudinal dynamics in net species diversification.
Keywords: Rapoport's rule, biogeography, latitudinal diversity gradient, range expansion, species–genus ratio
1. Introduction
Most terrestrial and marine taxonomic groups show latitudinal diversity gradients (LDGs) with maximum diversity in the tropics [1–6]. However, LDGs seem to be underlain by different configurations of geographical ranges in marine and terrestrial systems. Although range-size gradients vary with the spatial resolution, with the extent of the study system, and through time [7–9], species in terrestrial clades (e.g. birds, mammals, amphibians, insects, and trees) tend to have smaller latitudinal ranges in the tropics and increase towards mid-latitudes, at least across the Northern Hemisphere [10–15]. In contrast, latitudinal ranges in marine clades (e.g. teleost fish, corals, bivalves, and brachyuran crabs) tend to be broadest in the tropics and decline towards the poles [16–21]. Such contrasting range-size gradients challenge the idea that range-size distributions can account for LDGs [22], invalidating hypotheses that explain gradients in species richness by specific range-size dynamics [23–26] rather than by gradients in net species diversification [27,28].
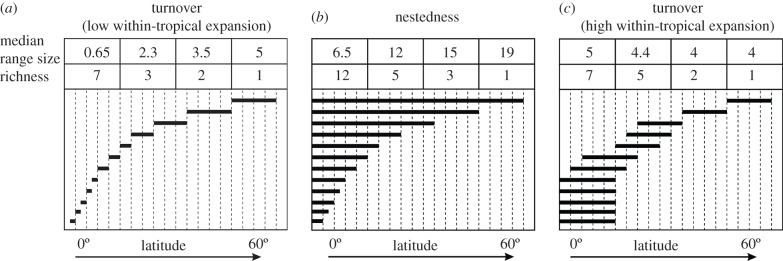
Alternative range configurations can link LDGs to gradients in range size (figure 1). A positive correlation between range size and latitude can arise in two ways. First, narrow-ranging taxa may be replaced by broad-ranging taxa towards high latitudes in the absence of a latitudinal gradient in range overlap (turnover, figure 1a): when applied to species, this pattern is known as Rapoport's rule [22]. Second, narrow-ranging taxa may be nested within the distributions of broad-ranging taxa (nestedness, figure 1b), with an increasing number of narrow-ranging taxa towards low latitudes [29]. In this scenario, high-latitude taxa either preferentially expand towards low latitudes, or low-latitude taxa originate in the tropics and differ in their ability to expand to high latitudes. In a third scenario, a decline in taxon richness towards the poles can occur when range size decreases with latitude (i.e. the inverse of Rapoport's rule), so that, e.g. numerous broad-ranging tropical taxa are replaced by a few narrow-ranging extratropical taxa owing to steeper environmental gradients and/or greater frequency of barriers at higher latitudes (figure 1c).
Figure 1.
Conceptual figure showing two different ways an increase in median range sizes towards high latitudes can be associated with more taxa in the tropics (turnover in a and nestedness in b), and one example whereby larger range sizes in the tropics are associated with more taxa in the tropics (c). Panel (a) is usually taken to represent the range dynamics underlying Rapoport's rule.
Here, we consider how two well-documented biogeographic systems that show an increase in species richness towards the tropics—terrestrial birds [30–33] and marine bivalves [34]—conform to these alternatives. To analyse the sources of variation in range-size gradients and their link to richness gradients, we extend our analyses to the next taxonomic level beyond species, that of genera. Genera in our focal systems represent a set of closely related, ecologically and morphologically similar species. Although genus range size is partly a function of factors that determine range limits of existing species, it is also related to within-genus species diversification whenever new species have the potential to enter new areas, generating a link between genus range-size gradients and species richness gradients [35,36].
Our analyses show that despite contrasting trends at the species level, genus range sizes tend to decrease towards the tropics in both marine bivalves and terrestrial birds. We show that genera with high species richness are more likely to attain broad ranges in both systems, and that this tendency produces nested range-size patterns and a proportional increase of species-rich genera towards higher latitudes, owing to a greater restriction of narrow-ranging genera to the tropics. In determining the species richness gradient, species-level differences in range dynamics between marine and terrestrial realms are outweighed by shared within-genus species diversification dynamics.
2. Material and methods
(a) Bivalves
We compiled 60 942 occurrence records of marine bivalves (5 903 species in 1 073 genera) occurring at depths less than 200 m from the literature and museum collections [34]. Species occurrences were resolved to a median latitudinal and longitudinal resolution of around 1°. We analysed the richness and range size of all marine bivalves globally, and in more detail within two oceanic transects flanking the New World (figure 2): the Eastern Pacific (1 003 species in 415 genera) and the Western Atlantic (890 species in 390 genera). These two transects are biogeographically distinct except at high latitudes, as indicated by the small proportion of shared species (15%). Both transects show almost constant sea-surface temperatures throughout the tropics and steep declines towards higher latitudes, but tropical temperatures are latitudinally more extensive in the Western Atlantic than in the Eastern Pacific (figure 3).
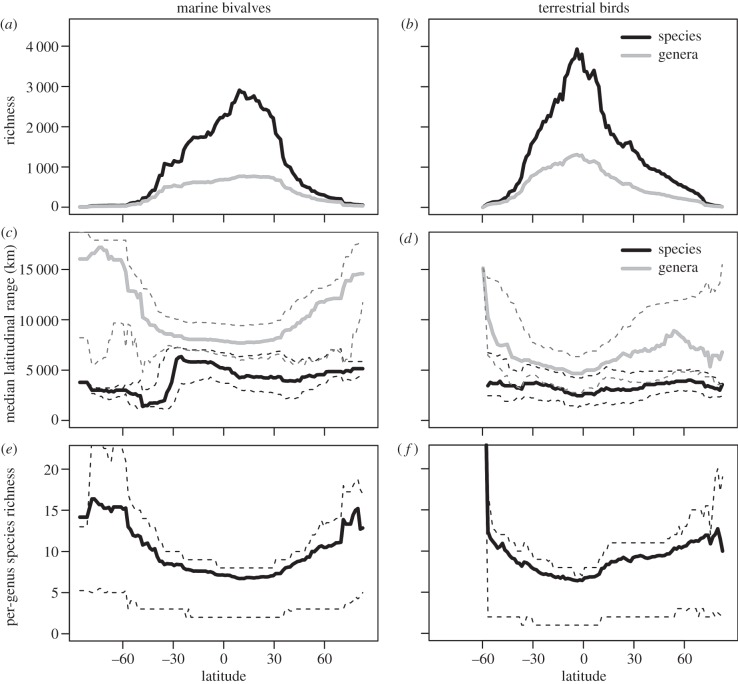
Figure 2.
(a,b) Latitudinal gradients in the richness of species and genera. (c,d) Latitudinal gradients in median species and genus latitudinal range size. (e,f) Latitudinal gradients in the mean number of species within genera (species–genus ratio) that are found at a given latitude (all species in each genus are tallied, not just the species at a given latitude). All figures are plotted at the resolution of 1° latitudinal bands. Dashed lines represent 25th and 75th percentiles of underlying range-size frequency distributions and per-genus richness frequency distributions. Latitudinal gradients in per-genus species richness are markedly congruent between bivalves and birds.
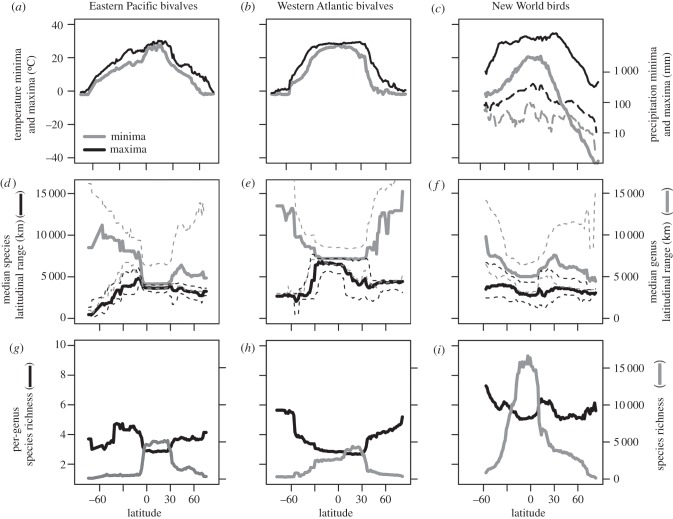
Figure 3.
(a–c) Latitudinal gradients in minimum (solid grey lines) and maximum temperature (solid black lines) averaged across 1° longitudinal cells (and in precipitation in the New World, dashed lines). (d–f) Latitudinal gradients in median species (black) and genus (grey) latitudinal range size. Dashed lines represent 25th and 75th percentiles of the underlying range-size frequency distributions. (g–i) Latitudinal gradients in mean per-genus species richness (black) and species richness (grey).
(b) Birds
We obtained breeding ranges for all 10 224 bird species (2 149 genera) recognized by the IOC (International Ornithological Congress) v. 3.3 [37]. These ranges were defined from published literature, sightings, and from museum records [38,39]. Ranges were compiled at a resolution of 1° × 1°, resulting in a total of 2 785 600 occurrences initially considered in our analysis. As with the bivalves, we studied both global and regional (New World, 4 216 species in 1 083 genera) patterns. The analysed range limits of bivalve and bird species are available at http://dx.doi.org/10.5061/dryad.p0q25.
(c) Comparability of genera
Classifications of bivalve species and genera are based on a combination of recent phylogenetic studies and traditional morphological approaches [40,41]. Morphologically defined bivalve genera have been shown to be highly correlated with molecularly defined units, and robustly capture macroecological variables such as geographical range size [40]. With respect to birds, the taxonomic ranks of both species and genera are clearly defined under IOC classifications [37], based upon both genetic and morphological characters. Species–genus ratios are similar between both groups at the global scale, suggesting comparability (species–genus ratio in bivalves = 5.5, in birds = 4.8).
Tropical bivalve species have strikingly broader ranges than tropical bird species. A taxonomic issue that could potentially bias this result would be if broad-ranging tropical bivalve species represent allopatric cryptic species. Although meta-analyses are not yet available, many genetically identified species prove to be morphologically separable on close examination, implying that modern morphology-based studies tend to capture most biological species [40,41]. Furthermore, cryptic species that have been discovered are not exclusively allopatric, and may be partially or fully nested within the geographical ranges of related species, even in the tropics [42,43]. Some tropical species show limited genetic divergence over vast distances in the Indo-West Pacific [44–46], and others have been broken into a few species, most with extensive latitudinal and longitudinal ranges [47,48].
(d) Analyses
We analysed latitudinal gradients in species and genus richness, species and genus range size, and mean per-genus species richness found within 1° latitudinal bands. We focused on 1° latitudinal bands rather than on equal-area grid-cells. This approach does not differentiate the effects of longitudinal area, heterogeneity in altitude, bathymetry, and other environmental factors affecting range size or richness at finer spatial scales, but it should be less sensitive to under-sampling of rare or endemic species.
We quantified the latitudinal range of a species as the latitudinal distance between a species' southern and northern range limits and ignored any gaps in the range. We obtained the latitudinal range of a genus by aggregating all species in the genus and taking the minimum and maximum latitudinal range limits of the aggregate. To assess the latitudinal gradient of range sizes, we determined the median range size for all species and genera that occurred within each 1° band (following the approach of Stevens [22]). Although alternative methods for assessing latitudinal gradients in range size based on range midpoints (i.e. calculating the median range size of species whose midpoints fall within a specific latitudinal band) are less influenced by spatial autocorrelation, Rapoport's rule relates to all species that intersect at a given region, not just to species whose midpoints fall within a given region. Midpoint-based methods do not assess how the frequency of narrow- or broad-ranging species changes across latitudes [49,50], and thus do not directly link overall species richness to latitude. To assess the relationship between latitude and median latitudinal range size, we use generalized least-squares regression [51], which minimizes spatial autocorrelation in the data (the correlation among the residuals mainly caused by the occurrence of the majority of species in multiple latitudinal bands). We distinguished the turnover and nestedness components in the total (Sorenson) dissimilarity in species and genus composition among latitudinal bands, and assessed whether species and genera showed different degrees of nestedness [52,53] (see the electronic supplementary material).
The range size of a genus is a function of the range sizes of its constituent species and the spatial separation among them. We evaluated the contribution of these components to genus range size by computing Spearman's rank correlations (ρ) between the latitudinal ranges of genera and two measures: median species range size and mean distance between latitudinal midpoints of congeneric species. We compared these correlations to a null expectation from randomized data to account for the non-independence of the variables (see the electronic supplementary material).
To assess whether the latitudinal gradient in genus range size is associated with the average number of species in a genus, we quantified the mean per-genus species richness in each 1° latitudinal band as the mean of the number of species that occurred anywhere within the range of each genus that occurred within the band. To quantify per-genus species richness for each climatic zone (here, considered as the tropics and extratropics, see the electronic supplementary material for definitions), we compared (i) the per-genus number of tropical-only species in tropical-only genera (TG in figure 4), (ii) the per-genus number of tropical-only species in genera that occur in both tropical and extratropical regions (bridge genera TSB in figure 4, [34]), (iii) the per-genus number of species endemic to the extratropics in bridge genera (ESB in figure 4), and (iv) the per-genus number of extratropical-only species in extratropical-only genera (EG in figure 4). Thus, these tests for within-region differences in diversification are not confounded by the global diversities of the bridge genera. We computed mean per-genus species richness with bootstrapped 95% confidence intervals sampled from the distribution of per-genus species richness for each of these four groups.
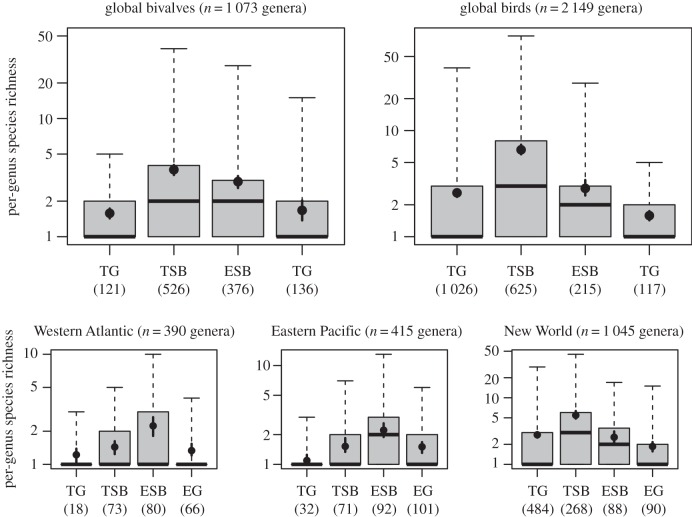
Figure 4.
Boxplots showing the link between the ability of genera to cross the tropical/temperate boundary and their species richness. Bridge genera have significantly more species within the tropics (TSB) than genera limited to the tropics (TG), and are significantly more species-rich within the extratropics (ESB) than genera limited to the extratropics (EG, Wilcoxon rank sum test, see text). Black points within the boxplots represent mean per-genus species richness with 95% bootstrapped confidence intervals. TG, tropical genera; TSB, tropical-only species of bridge genera; ESB, extratropical-only species of bridge genera; EG, extratropical genera. Numbers in parentheses show the number of genera in each category. The numbers of bridge genera differ between TSB and ESB because some bridge genera contain bridge species and tropical-only species but do not contain extratropical-only species.
3. Results
(a) Richness gradients
For both bivalves and birds, global species and genus richness are the highest in the tropics. In bivalves, species richness peaks slightly north of 0°, whereas genera show a shallower gradient from south to north (figure 2a). Bird richness peaks at approximately 0° latitude for both species and genera (figure 2b). Similar gradients in richness are seen in the New World datasets (figure 3g–i).
(b) Species range sizes
Species and genus ranges do not show simple monotonic trends with latitude at 1° resolution, but the first-order differences between birds and bivalves at the species level, and similarities at the genus level, are striking (figure 2c,d). At the global scale, median species range size in bivalves increases abruptly from the southernmost latitudes, to a maximum at the equator, followed by a decline in the Northern Hemisphere tropics, with range size remaining relatively constant to the northernmost latitudes (black line in figure 2c). However, in the Eastern Pacific and Western Atlantic, median species range sizes in bivalves are larger in the tropics than at higher latitudes in both hemispheres (figure 3d,e), resulting in a significantly negative relationship between range size and latitude (electronic supplementary material, table S1). Median species range size in birds tends to be the smallest at low latitudes (approx. 2 500 km), and the largest at mid-to-high latitudes (approx. 4 000 km), declining slightly at northernmost latitudes (figure 2d). In the New World, median range size peaks around 30°N (figure 3f). Despite the flatter latitudinal trends in bird species range size, bivalves and birds differ strongly in the shape of the species range-size gradient in the Southern Hemisphere (electronic supplementary material, figure S2). The differences are smaller in the Northern Hemisphere but the slopes of bivalve species remain significantly negative along the two transects, whereas the slopes of bird species are significantly positive in the Northern Hemisphere at global scales (electronic supplementary material, table S1). Analysis at a spatial resolution of 1° equal-area cells (rather than latitudinal bands) shows that median species ranges in birds are rather latitude-invariant [12], but the shape of the range-size gradient in birds still differs markedly from that in bivalves (electronic supplementary material, figure S1).
Bivalve species have few range limits within the tropics (electronic supplementary material, figure S3), in contrast to the range limits of bird species that cluster at major dispersal barriers, which may not only lie between provinces (electronic supplementary material, figure S3) but also within them (e.g. set by barriers associated with islands and mountains). The turnover pattern predominates at the species level (electronic supplementary material, figures S4 and S5), with narrow-ranging bird species at low latitudes replaced by broad-ranging bird species at high latitudes (similarly as in the configuration in figure 1a), and conversely, with broad-ranging bivalve species at low latitudes replaced by narrow-ranging bivalve species at high latitudes (similarly as in the configuration in figure 1c).
(c) Genus range sizes
Considering genera with more than one species, in bivalves genus range size does not correlate with median species range size (ρ = 0.2, n = 1 073 genera, p = 0.38), but in birds it does (ρ = 0.58, n = 2 149 genera, p < 0.0001; electronic supplementary material, figure S6). Genus range size correlates more strongly with the mean distance among midpoints of congeneric species in both bivalves (ρ = 0.62, p < 0.0001) and birds (ρ = 0.73, p < 0.0001; electronic supplementary material, figure S6). Therefore, although the contribution of species range sizes differs between the two groups, the geographical distance among congeneric species contributes more strongly to genus range size than does range size of the constituent species in both bivalves and birds.
Patterns of bird genera are more complex than those of bivalves, with median genus range size reaching a peak at intermediate latitudes in the Northern Hemisphere (figures 2d and 3f). However, the two groups show generally concordant patterns, with the smallest median ranges of both bivalve and bird genera occurring in the tropics (figure 2c,d). In both groups, broad-ranging genera span the tropics and extratropics (electronic supplementary material, figure S4). Nestedness rather than turnover thus accounts for the range-size gradients of genera (i.e. configuration in figure 1b), with narrow-ranging genera of bivalves and birds restricted to the tropics, and broad-ranging genera occurring both tropically and extratropically (electronic supplementary material, figure S4). In both groups, the contribution of nestedness to total dissimilarity in composition among latitudinal bands is significantly larger at the genus than at species level (electronic supplementary material, figure S5).
(d) Gradients in per-genus species richness
Genus range size correlates strongly with per-genus species richness in both bivalves and birds (ρ = 0.62, n = 1 073 bivalves; ρ = 0.73, n = 2 149 birds; p < 0.0001 in both groups). Species-rich genera become proportionally more frequent towards higher latitudes, even as species richness declines (figures 2e,f and 3g–i). This gradient underlies the nested distribution of genus ranges and their latitudinal increase in size: species-rich genera span a large latitudinal range, and species-poor genera are concentrated in the tropics. Bivalve and bird genera that span both tropical and temperate latitudes (i.e. bridge genera TSB and ESB in figure 4) are not only more species-rich overall than those restricted to either region, but they also tend to have significantly more species within the tropics than do genera confined to the tropics, and have significantly more species outside of the tropics than are genera restricted to the extratropics (figure 4; significance tests in the electronic supplementary material, table S2). Therefore, the ability of lineages to expand to different latitudes or climatic zones is positively related to their species richness even when the effect of geographical area is factored out.
4. Discussion
Our terrestrial–marine comparison of birds and bivalves starts with the observation that similar LDGs emerge from different gradients in range size at the species level (figure 1). Thus, models for the LDG predicated on relationships between species richness and geographical range size (or hypothesized correlates such as dispersal ability or environmental tolerances) cannot be general explanations. Although the species-level differences likely reflect contrasting geographical range dynamics in response to present-day and Pleistocene conditions that differ between the two systems, we hypothesize that genus-level patterns shared across the marine and terrestrial environments reflect commonalities in the propensity of species-rich genera to expand to new latitudes. Before considering this hypothesis in more detail, we first address the issue of why species range-size patterns differ between the marine and terrestrial realms.
(a) Species range sizes
The bird–bivalve discordance in species range-size gradients may reflect (i) marine-terrestrial differences in the current distribution of range-limiting conditions along the latitudinal gradients [54,55] and/or (ii) past history, notably the stronger effects of Pleistocene glaciations in the terrestrial compared with the marine system [56].
With respect to present-day conditions, the large tropical ranges of bivalve species and other marine groups can be related to weak tropical climatic gradients (i.e. isotherms can be tracked over broad latitudinal distances) and the lack of physical barriers in the tropics (figure 3a,b). Isotherm tracking in the tropics [20] both reverses the tendency of geographical ranges to increase towards latitudes with higher climatic variability (as postulated by one of the explanations for Rapoport's rule [22]) and explains the asymmetry in bivalve range-size gradients between the two hemispheres, because the seasonal differences between thermal minima and maxima are larger in the Northern Hemisphere than in the Southern Hemisphere [20,57]. Temperate species in the Northern Hemisphere accordingly tend to have broader climate envelopes than Southern Hemisphere equivalents, resulting in generally broader ranges in the Northern Hemisphere temperate zone. Terrestrial systems show steeper climatic latitudinal gradients and greater longitudinal heterogeneity in temperature and rainfall at individual latitudes (figure 3a–c), and dispersal distances and scales of connectivity tend to be smaller [58,59]. Elevation, moisture, water bodies, and other range-limiting barriers in terrestrial systems are absent from marine systems. In birds, tropical montane species on average tend to have narrower ranges than tropical lowland species [60]. Further, barriers in the tropics may be more efficient than in temperate areas owing to the low dispersal propensity [61], narrow environmental tolerances in less seasonal environments [55], and/or the higher environmental heterogeneity and smaller climatic overlap between elevations [62–64]. The expected consequence is a finer subdivision of the within-tropics geographical ranges in terrestrial systems.
Although the distribution and type of barriers can explain differences in range-size patterns between bivalves and birds, historical effects, notably the Pleistocene climate, may have affected present range sizes, with these effects stronger in terrestrial systems. First, climate fluctuations likely had a relatively minor effect on bivalve distributions. While numerous marine species coped with climate change by shifting latitudinally to track temperatures [65], many others persisted during Pleistocene cooling in high-latitude refugia [66–69], either at depths below potential ice damage or in relatively warm-water coastal embayments, before subsequent range expansion across latitudes or depths during warmer periods [70,71]. Although many marine population bottlenecks and some differentiation is attributable to Pleistocene sea-level drops and shifts in climate and circulation [72], speciation and global marine extinction was modest in the Pleistocene tropics compared to temperate extinction following the initiation of Pleistocene glacial cycles [73,74]. In sum, the broad species ranges in tropical bivalves seem to reflect gene flow across a shallow latitudinal gradient in temperature, with relatively minor lasting effects of Pleistocene climate.
On the other hand, Pleistocene climatic effects likely contribute to the narrow ranges of bird species in the tropics and larger ranges at more northerly temperate latitudes. First, the Pleistocene climate appears to have created multiple opportunities for bird speciation in the tropics (e.g. along the Andes, [75]). Again in contrast to bivalves, most bird species were evidently pushed out of the north-temperate regions by the Pleistocene ice sheets, followed by postglacial range expansions, creating large latitudinal ranges [76,77]. Pleistocene speciation events in temperate and boreal birds are largely along a west–east axis [78], which would not affect the latitudinal patterns we discuss here.
(b). Species richness and genus range sizes
The differences in the latitudinal patterns of species range sizes between bivalves (broad ranges in the tropics) and birds (broad ranges in temperate areas) do not persist to the genus level (figures 2c,d and 3d–f). Genus ranges in both groups are increasingly nested, with the narrow-ranging genera confined mainly to the tropics and species-rich genera more widely distributed than species-poor genera (electronic supplementary material, figure S4), such that species-rich genera increasingly predominate at higher latitudes, even as overall diversity declines (figures 2 and 3). Evidently, the geographical range sizes of constituent species (which may or may not be related to the range of environmental tolerances) are less important than the net production of species in promoting genus range expansion into novel latitudes. Factoring out total genus range size by analysing tropical and extratropical regions separately (figure 4) allows us to see that the broad ranges of many genera are linked to their propensity to diversify at the species level. A simple probabilistic model would hold that these more prolific diversifiers are more likely to contain species that extend their range (and hence that of the whole genus), or produce a descendant that extends its range relative to that of their ancestor's. In accord with this ‘speciation-pressure’ hypothesis, genus range sizes in both groups are influenced more strongly by the overall latitudinal separation among congeneric species than by the size of individual species ranges, thus frequently producing bridge genera (although median species range size also contributes significantly to genus range size in birds). More work is needed to determine whether bridge genera have higher speciation rates, or if their species have greater competitive or colonizing ability that impart lower extinction rates [34]. Flowering plants show similar diversity trends [79], and testing that group for bridge species patterns would be interesting: their sessile lifestyle and dispersal via many small propagules resembles bivalves, while their terrestrial habit is of course shared with birds.
Another mutually non-exclusive mechanism for both the nestedness pattern and for the tendency of broad-ranging genera to be species-rich can be driven by an association between niche diversity and geographical range. Sets of common and widely distributed resources can facilitate the origin and persistence of widespread species-rich genera, whereas sets of rare resources may be more likely restricted to one region, more often the tropics. This argument is most readily made for birds. For example, large-billed insectivore genera, which are species-poor, are concentrated in Central America (at 8–10° N) rather than in temperate North America (at 42–44° N), whereas small-billed bird genera, which tend to be species-rich, are about equally frequent in each region [80]. The absence of large-billed species and genera in the temperate zones may reflect the relative scarcity of large arthropods in this region [80,81]. In bivalves, the decline in functional diversity cannot be distinguished from a passive consequence of the latitudinal decline in species richness [82].
In summary, bivalves and birds show discordant latitudinal gradients in species range size that we suggest result from the biogeographic behaviour of clades during and after Pleistocene glaciations, and reflect general differences in environmental heterogeneity between marine and terrestrial systems [83,84]. The two groups share a latitudinal gradient in species and genus richness, mean per-genus species richness and genus range size, with higher latitudes increasingly enriched in broad-ranging and (globally) species-rich genera as diversity declines. These shared patterns at the genus level suggest that species richness gradients originate independently of species range-size gradients in both groups, and support a simple underlying process whereby genera with higher net species diversification are more likely to enter novel latitudes and climates than are species-poor genera. Evolutionary and biogeographic dynamics appear to be closely tied.
Supplementary Material
Acknowledgements
We thank the two anonymous reviewers, M. Foote, J. Leonard-Pingel, and A. Michelson for comments that helped us improve this manuscript, and Louis Hansen for help compiling the bird distributional dataset. We are grateful to the many malacologists who kindly provided taxonomic advice, assistance, and/or access to collections in their care. S.H. and S.K. thank the Alexander von Humboldt Foundation and T.C. Chamberlin Fellowship from the University of Chicago for support through postdoc fellowships. Finally, we thank the Danish National Research Foundation for its support of the Center for Macroecology, Evolution and Climate.
Data accessibility
The data with range limits for both groups are available at Data Dryad at http://dx.doi.org/10.5061/dryad.p0q25.
Authors' contributions
A.T., J.D.K., S.H., T.D.P., and D.J. designed the study; A.T. and J.D.K. performed analyses; C.R. and D.J. collected data; all authors wrote the first draft of the manuscript and contributed to interpretation of data and revisions.
Competing interests
We declare we have no competing interests.
Funding
We thank the DNRF (grant number DNRF96), NASA (EXOB08-0089), NSF (EAR-0922156, DEB-0919451), the Slovak VEGA Agency (0136-15), and the European Union's Horizon 2020 research and innovation programme under grant agreement No 643084 for funding support.
References
- 1.Hillebrand H. 2004. On the generality of the latitudinal diversity gradient. Am. Nat. 163, 192–211. ( 10.1086/381004) [DOI] [PubMed] [Google Scholar]
- 2.Davies RG, et al. 2007. Topography, energy and the global distribution of bird species richness. Proc. R. Soc. B 274, 1189–1197. ( 10.1098/rspb.2006.0061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diniz-Filho JAF, Fernando T, Rangel VB, Bini LM, Hawkins BA. 2007. Macroevolutionary dynamics in environmental space and the latitudinal diversity gradient in New World birds. Proc. R. Soc. B 274, 43–52. ( 10.1098/rspb.2006.3712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tittensor DP, Mora C, Jetz W, Lotze HK, Ricard D, Vanden Berghe E, Worm B. 2010. Global patterns and predictors of marine biodiversity across taxa. Nature 466, 1098–1101. ( 10.1038/nature09329) [DOI] [PubMed] [Google Scholar]
- 5.Romdal TS, Araujo MB, Rahbek C. 2012. Life on a tropical planet: niche conservatism and the global diversity gradient. Glob. Ecol. Biogeogr. 22, 344–350. ( 10.1111/j.1466-8238.2012.00786.x) [DOI] [Google Scholar]
- 6.Belmaker J, Jetz W. 2015. Relative roles of ecological and energetic constraints, diversification rates and region history on global species richness gradients. Ecol. Lett. 18, 563–571. ( 10.1111/ele.12438) [DOI] [PubMed] [Google Scholar]
- 7.Rahbek C. 2005. The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 8, 224–239. ( 10.1111/j.1461-0248.2004.00701.x) [DOI] [Google Scholar]
- 8.Ruggiero A, Werenkraut V. 2007. One-dimensional analyses of Rapoport's rule reviewed through meta-analysis. Glob. Ecol. Biogeogr. 16, 401–414. ( 10.1111/j.1466-8238.2006.00303.x) [DOI] [Google Scholar]
- 9.Veter NM, et al. 2013. Is Rapoport's rule a recent phenomenon? A deep time perspective on potential causal mechanisms. Biol. Lett. 9, 20130398 ( 10.1098/rsbl.2013.0398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arita H, Rodríguez P, Vásquez-Domínguez E. 2005. Continental and regional ranges of North American mammals: Rappoport's rule in real and null worlds. J. Biogeogr. 32, 961–971. ( 10.1111/j.1365-2699.2005.01276.x) [DOI] [Google Scholar]
- 11.Rohde K. 1996. Rapoport's rule is a local phenomenon and cannot explain latitudinal gradients in species diversity. Biodivers. Lett. 3, 10–13. ( 10.2307/2999704) [DOI] [Google Scholar]
- 12.Orme CDL, et al. 2006. Global patterns of geographic range size in birds. PLoS Biology 4, e208 ( 10.1371/journal.pbio.0040208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitton FJS, Purvis A, Orme CDL, Olalla-Tárraga MÁ. 2012. Understanding global patterns in amphibian geographic range size: does Rapoport rule? Glob. Ecol. Biogeogr. 21, 179–190. ( 10.1111/j.1466-8238.2011.00660.x) [DOI] [Google Scholar]
- 14.Swaegers J, Janssens SB, Ferreira S, Watts PC, Mergeay J, McPeek MA, Stoks R. 2014. Ecological and evolutionary drivers of range size in Coenagrion damselflies. J. Evol. Biol. 27, 2386–2395. ( 10.1111/jeb.12481) [DOI] [PubMed] [Google Scholar]
- 15.Morin X, Lechowicz MJ. 2011. Geographical and ecological patterns of range size in North American trees. Ecography 34, 738–750. ( 10.1111/j.1600-0587.2010.06854.x) [DOI] [Google Scholar]
- 16.Rohde K, Heap M, Heap D. 1993. Rapoport's rule does not apply to marine teleosts and cannot explain latitudinal gradients in species richness. Am. Nat. 142, 1–16. ( 10.1086/285526) [DOI] [Google Scholar]
- 17.Jones GP, Caley MJ, Munday PL. 2002. Rarity in coral reef fish communities. In Coral reef fishes: dynamics and diversity in a complex ecosystem (ed. Sale PF.), pp. 81–102. New York, NY: Academic Press. [Google Scholar]
- 18.Connolly SR, Bellwood DR, Hughes TP. 2003. Indo-Pacific biodiversity of coral reefs: deviations from a mid-domain model. Ecology 84, 2178–2190. ( 10.1890/02-0254) [DOI] [Google Scholar]
- 19.MacPherson E. 2003. Species range size distributions for some marine taxa in the Atlantic Ocean. Effect of latitude and depth. Biol. J. Linnean Soc. 80, 437–455. ( 10.1046/j.1095-8312.2003.00256.x) [DOI] [Google Scholar]
- 20.Tomašových A, Jablonski D, Berke SK, Krug AZ, Valentine JW. 2015. Nonlinear thermal gradients shape broad-scale patterns in geographic range size and can reverse Rapoport's rule. Glob. Ecol. Biogeogr. 24, 157–167. ( 10.1111/geb.12242) [DOI] [Google Scholar]
- 21.Papacostas KJ, Freestone AL. 2016. Latitudinal gradient in niche breadth of brachyuran crabs. Glob. Ecol. Biogeogr. 25, 207–217. ( 10.1111/geb.12400) [DOI] [Google Scholar]
- 22.Stevens GC. 1989. The latitudinal gradient in geographical range: How so many species coexist in the tropics. Am. Nat. 133, 240–256. ( 10.1086/284913) [DOI] [Google Scholar]
- 23.Kerr JT. 1999. Weak links: ‘Rapoport's rule’ and large-scale species richness patterns. Glob. Ecol. Biogeogr. 8, 47–54. ( 10.1046/j.1365-2699.1999.00315.x) [DOI] [Google Scholar]
- 24.Šizling AL, Storch D, Keil P. 2009. Rapoport's rule, species tolerances, and the latitudinal diversity gradient: geometric considerations. Ecology 90, 3575–3586. ( 10.1890/08-1129.1) [DOI] [PubMed] [Google Scholar]
- 25.Antonelli A, Zizka A, Silvestro D, Scharn R, Cascales-Miñana B, Bacon CD. 2015. An engine for global plant diversity: highest evolutionary turnover and emigration in the American tropics. Front. Genet. 6, 130 ( 10.3389/fgene.2015.00130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rolland J, Condamine FL, Beeravolu CR, Jiguet F, Morlon H. 2015. Dispersal is a major driver of the latitudinal diversity gradient of Carnivora. Glob. Ecol. Biogeogr. 24, 1059–1071. ( 10.1111/geb.12354) [DOI] [Google Scholar]
- 27.Cardillo M, Orme CDL, Owens IP. 2005. Testing for latitudinal bias in diversification rates: an example using New World birds. Ecology 86, 2278–2287. ( 10.1890/05-0112) [DOI] [Google Scholar]
- 28.Hurlbert AH, Stegen JC. 2014. On the processes generating latitudinal richness gradients: identifying diagnostic patterns and predictions. Front. Genet. 5, 420 ( 10.3389/fgene.2014.00420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hausdorf B. 2006. Latitudinal and altitudinal diversity patterns and Rapoport effects in north-west European land snails and their causes. Biol. J. Linn. Soc. 87, 309–323. ( 10.1111/j.1095-8312.2006.00580.x) [DOI] [Google Scholar]
- 30.Jetz W, Rahbek C. 2001. Geometric constraints explain much of the species richness pattern in African birds. Proc. Natl Acad. Sci. USA 98, 5661–5666. ( 10.1073/pnas.091100998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hawkins BA, Diniz-Filho JAF, Jaramillo CA, Soeller SA. 2007. Climate, niche conservatism, and the global bird diversity gradient. Am. Nat. 170, S16–S27. ( 10.1086/519009) [DOI] [PubMed] [Google Scholar]
- 32.Smith BT, Bryson RW, Houston DD, Klicka J. 2012. An asymmetry in niche conservatism contributes to the latitudinal species diversity gradient in New World vertebrates. Ecol. Lett. 15, 1318–1325. ( 10.1111/j.1461-0248.2012.01855.x) [DOI] [PubMed] [Google Scholar]
- 33.Kennedy JD, Wang Z, Weir JT, Rahbek C, Fjeldså J, Price TD. 2014. Into and out of the tropics: the generation of the latitudinal gradient among New World passerine birds. J. Biogeogr. 41, 1746–1757. ( 10.1111/jbi.12346) [DOI] [Google Scholar]
- 34.Jablonski D, Belanger CL, Berke SK, Huang S, Krug AZ, Roy K, Tomasovych A, Valentine JW. 2013. Out of the tropics, but how? Fossils, bridge species, and thermal ranges in the dynamics of the marine latitudinal diversity gradient. Proc. Natl Acad. Sci. USA 110, 10 487–10 494. ( 10.1073/pnas.1308997110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krug AZ, Jablonski D, Valentine JW. 2008. Species-genus ratios reflect a global history of diversification and range expansion in marine bivalves. Proc. R. Soc. B 275, 1117–1123. ( 10.1098/rspb.2007.1729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foote M. 2012. Evolutionary dynamics of taxonomic structure. Biol. Lett. 8, 135–138. ( 10.1098/rsbl.2011.0521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gill F, Donsker D. 2010. IOC World Bird List (v. 2.7.). See www.worldbirdnames.org.
- 38.Rahbek C, Graves GR. 2001. Multiscale assessment of patterns of avian species richness. Proc. Natl Acad. Sci. USA 98, 4534–4539. ( 10.1073/pnas.071034898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahbek C, Hansen LA, Fjelds J. 2012. One degree resolution database of the global distribution of birds. Denmark: Natural History Museum of Denmark, University of Copenhagen. [Google Scholar]
- 40.Jablonski D, Finarelli JA. 2009. Congruence of morphologically-defined genera with molecular phylogenies. Proc. Natl Acad. Sci. USA 106, 8262–8266. ( 10.1073/pnas.0902973106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bieler R, Mikkelsen PM, Giribet G. 2013. Bivalvia—a discussion of known unknowns. Am. Malac Bull 31, 123–133. ( 10.4003/006.031.0105) [DOI] [Google Scholar]
- 42.Bennett KF, Reed AJ, Lutz RA. 2011. DNA barcoding reveals Brachidontes (Bivalvia: Mytilidae) from two ecologically distinct intertidal habitats on Long Key, Florida Keys, are cryptic species, not ecotypes. Nautilus 125, 63–71. [Google Scholar]
- 43.González VL, Giribet G. 2012. A new cryptic species of carditid bivalve from the Gulf of California (Mollusca, Bivalvia, Archiheterodonta, Carditidae). Malacologia 55, 235–250. ( 10.4002/040.055.0205) [DOI] [Google Scholar]
- 44.Borsa P, Fauvelot C, Tiavouane J, Grulois D, Wabnitz C, Abdon Naguit MR, Andréfouët S. 2015. Distribution of Noah's giant clam, Tridacna noae. Mar. Biodivers. 45, 339–344. ( 10.1007/s12526-014-0265-9) [DOI] [Google Scholar]
- 45.Tëmkin I. 2010. Molecular phylogeny of pearl oysters and their relatives (Mollusca, Bivalvia, Pterioidea). BMC Evol. Biol. 10, 342 ( 10.1186/1471-2148-10-342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cunha RL, Blanc F, Bonhomme F, Arnaud-Haond S. 2011. Evolutionary patterns in pearl oysters of the genus Pinctada (Bivalvia: Pteriidae). Mar. Biotechnol. 13, 181–192. ( 10.1007/s10126-010-9278-y) [DOI] [PubMed] [Google Scholar]
- 47.Terranova M, Lo Brutto S, Arculeo M, Mitton J. 2007. A mitochondrial phylogeography of Brachidontes variabilis (Bivalvia: Mytilidae) reveals three cryptic species. J. Zool. Syst. Evol. Res. 45, 289–298. ( 10.1111/j.1439-0469.2007.00421.x) [DOI] [Google Scholar]
- 48.Lemer S, Buge B, Bernis A, Giribet G. 2014. First molecular phylogeny of the circumtropical bivalve family Pinnidae (Mollusca, Bivalvia): evidence for high levels of cryptic species diversity. Mol. Phylogenet. Evol. 75, 11–23. ( 10.1016/j.ympev.2014.02.008) [DOI] [PubMed] [Google Scholar]
- 49.Connolly SR. 2009. Macroecological theory and the analysis of species richness gradients. In Marine macroecology (eds Witman JD, Roy K), pp. 279–309. Chicago, IL: University of Chicago Press. [Google Scholar]
- 50.McCain CM, Knight BK. 2013. Elevational Rapoport's rule is not pervasive on mountains. Glob. Ecol. Biogeogr. 22, 750–759. ( 10.1111/geb.12014) [DOI] [Google Scholar]
- 51.Pinheiro JC, Bates D, DebRoy S, Sarkar D.2013. nlme: Linear and Nonlinear Mixed Effects Models. See http://CRAN.R-project.org/package=nlme .
- 52.Baselga A. 2012. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Glob. Ecol. Biogeogr. 21, 1223–1232. ( 10.1111/j.1466-8238.2011.00756.x) [DOI] [Google Scholar]
- 53.Baselga A, Orme CDL. 2012. betapart: an R package for the study of beta diversity. Methods Ecol. Evol. 3, 808–812. ( 10.1111/j.2041-210X.2012.00224.x) [DOI] [Google Scholar]
- 54.Sunday JM, Bates AE, Dulvy NK. 2012. Thermal tolerance and the global redistribution of animals. Nat. Clim. Change 2, 686–690. ( 10.1038/nclimate1539) [DOI] [Google Scholar]
- 55.Khaliq I, Hof C, Prinzinger R, Böhning-Gaese K, Pfenninger M. 2014. Global variation in thermal tolerances and vulnerability of endotherms to climate change. Proc. R. Soc. B 281, 20141097 ( 10.1098/rspb.2014.1097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Annan JD, Hargreaves JC. 2013. A new global reconstruction of temperature changes at the Last Glacial Maximum. Clim. Past 9, 367–376. ( 10.5194/cp-9-367-2013) [DOI] [Google Scholar]
- 57.Sunday JM, Bates AE, Dulvy NK. 2011. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. B 278, 1823–1830. ( 10.1098/rspb.2010.1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kinlan BP, Gaines SD. 2003. Propagule dispersal in marine and terrestrial environments: a community perspective. Ecology 84, 2007–2020. ( 10.1890/01-0622) [DOI] [Google Scholar]
- 59.Carr MH, Neigel JE, Estes JA, Andelman S, Warner RR, Largier JL. 2003. Comparing marine and terrestrial ecosystems: implications for the design of coastal marine reserves. Ecol. Appl. 13, 90–107. (doi:10.1890/1051-0761(2003)013[0090:CMATEI]2.0.CO;2) [Google Scholar]
- 60.Fjeldså J, Bowie RCK, Rahbek C. 2012. The role of mountain ranges in the diversification of birds. Annu. Rev. Ecol. Evol. Syst. 43, 249–265. ( 10.1146/annurev-ecolsys-102710-145113) [DOI] [Google Scholar]
- 61.Quintero I, Gonzalez-Caro S, Zalamea P-C, Cadena CD. 2014. Asynchrony of seasons: genetic differentiation associated with geographic variation in climatic seasonality and reproductive phenology. Am. Nat. 184, 352–363. ( 10.1086/677261) [DOI] [PubMed] [Google Scholar]
- 62.Ghalambor CK, Huey RB, Martin PR, Tewksbury JJ, Wang G. 2006. Are mountain passes higher in the tropics? Janzen's hypothesis revisited. Int. Comp. Biol. 46, 5–17. ( 10.1093/icb/icj003) [DOI] [PubMed] [Google Scholar]
- 63.Pigot AL, Owens IPF, Orme CDL. 2010. The environmental limits to geographic range expansion in birds. Ecol. Lett. 13, 705–715. ( 10.1111/j.1461-0248.2010.01462.x) [DOI] [PubMed] [Google Scholar]
- 64.Stein A, Gerstner K, Kreft H. 2014. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 17, 866–880. ( 10.1111/ele.12277) [DOI] [PubMed] [Google Scholar]
- 65.Valentine JW, Jablonski D. 1993. Fossil communities: compositional variation at many time scales. In Species diversity in ecological communities: historical and geographical perspectives (eds Ricklefs RE, Schluter D), pp. 341–349. Chicago, IL: University of Chicago Press. [Google Scholar]
- 66.Wares JP, Goldwater DS, Kong BY, Cunningham CW. 2002. Refuting a controversial case of a human-mediated marine species introduction. Ecol. Lett. 5, 577–584. ( 10.1046/j.1461-0248.2002.00359.x) [DOI] [Google Scholar]
- 67.Maggs CA, et al. 2008. Evaluating signatures of glacial refugia for North Atlantic benthic marine taxa. Ecology 89, S108–S122. ( 10.1890/08-0257.1) [DOI] [PubMed] [Google Scholar]
- 68.Marko PB, Hoffman JM, Emme SA, McGovern TM, Keever CC, Cox LN. 2010. The ‘Expansion–Contraction’ model of Pleistocene biogeography: rocky shores suffer a sea change? Mol. Ecol. 19, 146–169. ( 10.1111/j.1365-294X.2009.04417.x) [DOI] [PubMed] [Google Scholar]
- 69.Panova M, Mäkinen T, Fokin M, Andre C, Johannesson K. 2008. Microsatellite cross-species amplification in the genus Littorina and detection of null alleles in Littorina saxatilis. J. Moll. Stud. 74, 111–117. ( 10.1093/mollus/eym052) [DOI] [Google Scholar]
- 70.Roy K, Jablonski D, Valentine JW. 1995. Thermally anomalous assemblages revisited: patterns in the extraprovincial range shifts of Pleistocene marine mollusks. Geology 23, 1071–1074. () [DOI] [Google Scholar]
- 71.Tomašových A, Dominici S, Zuschin M, Merle D. 2014. Onshore–offshore gradient in metacommunity turnover emerges only over macroevolutionary time-scales. Proc. R. Soc. B 281, 20141533 ( 10.1098/rspb.2014.1533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kiessling W, Simpson C, Beck B, Mewis H, Pandolfi JM. 2012. Equatorial decline of reef corals during the last Pleistocene interglacial. Proc. Natl Acad. Sci. USA 109, 21 378–21 383. ( 10.1073/pnas.1214037110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Valentine JW, Jablonski D, Krug AZ, Roy K. 2008. Incumbency, diversity, and latitudinal gradients. Paleobiology 34, 169–178. ( 10.1666/0094-8373(2008)034%5B0169:IDALG%5D2.0.CO;2) [DOI] [Google Scholar]
- 74.Ludt WB, Rocha LA. 2015. Shifting seas: the impacts of Pleistocene sea-level fluctuations on the evolution of tropical marine taxa. J. Biogeogr. 42, 25–38. ( 10.1111/jbi.12416) [DOI] [Google Scholar]
- 75.Weir JT. 2006. Divergent timing and patterns of species accumulation in lowland and highland neotropical birds. Evolution 60, 842–855. ( 10.1111/j.0014-3820.2006.tb01161.x) [DOI] [PubMed] [Google Scholar]
- 76.Price TD, Helbig AJ, Richman AD. 1997. Evolution of breeding distributions in the Old World leaf warblers (Genus Phylloscopus). Evolution 51, 552–561. ( 10.2307/2411127) [DOI] [PubMed] [Google Scholar]
- 77.Hewitt G. 2000. The genetic legacy of the Quaternary ice ages. Nature 405, 907–913. ( 10.1038/35016000) [DOI] [PubMed] [Google Scholar]
- 78.Weir JT, Schluter D. 2004. Ice sheets promote speciation in boreal birds. Proc. R. Soc. Lond. B 271, 1881–1887. ( 10.1098/rspb.2004.2803) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fine PV. 2015. Ecological and evolutionary drivers of geographic variation in species diversity. Ann. Rev. Ecol. Evol. Syst. 46, 369–392. ( 10.1146/annurev-ecolsys-112414-054102) [DOI] [Google Scholar]
- 80.Schoener TW. 1971. Large-billed insectivorous birds: a precipitous diversity gradient. Condor 73, 154–161. ( 10.2307/1365836) [DOI] [Google Scholar]
- 81.Price TD, Mohan D, Tietze DT, Hooper DM, Orme CDL, Rasmussen P. 2011. Determinants of northerly range limits along the Himalayan bird diversity gradient. Am. Nat. 178, S97–S108. ( 10.1086/661926) [DOI] [PubMed] [Google Scholar]
- 82.Berke SK, Jablonski D, Krug AZ, Valentine JW. 2014. Origination and migration drive latitudinal gradients in marine functional diversity. PLoS ONE 9, e101494 ( 10.1371/journal.pone.0101494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Poloczanska ES, et al. 2013. Global imprint of climate change on marine life. Nature Climate Change 3, 919–925. ( 10.1038/nclimate1958) [DOI] [Google Scholar]
- 84.Burrows MT, et al. 2014. Geographical limits to species-range shifts are suggested by climate velocity. Nature 507, 492–495. ( 10.1038/nature12976) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data with range limits for both groups are available at Data Dryad at http://dx.doi.org/10.5061/dryad.p0q25.