Abstract
Coral spawning times have been linked to multiple environmental factors; however, to what extent these factors act as generalized cues across multiple species and large spatial scales is unknown. We used a unique dataset of coral spawning from 34 reefs in the Indian and Pacific Oceans to test if month of spawning and peak spawning month in assemblages of Acropora spp. can be predicted by sea surface temperature (SST), photosynthetically available radiation, wind speed, current speed, rainfall or sunset time. Contrary to the classic view that high mean SST initiates coral spawning, we found rapid increases in SST to be the best predictor in both cases (month of spawning: R2 = 0.73, peak: R2 = 0.62). Our findings suggest that a rapid increase in SST provides the dominant proximate cue for coral mass spawning over large geographical scales. We hypothesize that coral spawning is ultimately timed to ensure optimal fertilization success.
Keywords: phenology, reproduction, biogeography, macroecology, Acropora, Indo-Pacific
1. Introduction
Understanding the distribution of species diversity across the Earth is a fundamental challenge of ecology; however, the underlying processes that generate and maintain these patterns remain unresolved. One way forward is to move beyond an overwhelming focus on adults towards a more concerted effort to understand stages earlier in an organism's life history. Adults often need different resources and environmental conditions to thrive compared with earlier life stages and, therefore, our opportunity to identify important mechanisms is limited if we explore adult requirements alone [1–3]. Moreover, such knowledge is required to solve global conservation challenges more effectively, because we must ensure not only adult survival, but also successful reproduction and development, to prevent species sliding inexorably towards extinction.
Successful reproduction depends on timing. Reproductive events have evolved to occur at optimal times for the next generation to survive, often coinciding with the presence of food resources, the absence of predators, or favourable environmental conditions [4]. These conditions can be linked to seasonal changes, which might provide the proximate cue for reproduction to occur. Owing to its dependence on external conditions, phenology (defined as the interplay of life cycle events with environmental conditions) emerges as a key factor to determine species distributions [1]. Temperature is supported strongly as either a proximate cue or mechanistic driver of the timing of life-history events in a wide range of species, including insects [5], birds [6], fish [7] and plants [8]. However, these studies are concentrated in temperate regions and the extent to which seasonal temperature variations play a role in tropical phenology is speculated to be minimal owing to more muted seasonal variations [9,10], although this speculation remains untested.
Synchronized release of gametes for external fertilization in the water column is common among marine invertebrates with a sessile adult stage [11]; presumably because fertilization success is greatly diminished when small numbers of colonies spawn because of rapid gamete dilution in the water column [12,13]. Mass reproductive events, where multiple species release gametes or propagules simultaneously, are hypothesized to offer additional benefits, such as predator satiation [14,15]. Regardless of the selective advantage, disentangling the proximate cues and ultimate underlying mechanisms that control the timing of such events remains a significant challenge.
Reef-forming corals generate some of the most spectacular mass reproductive events in the world. During the annual ‘mass spawn’, defined by Willis et al. [16] as ‘the synchronous release of gametes by many species of corals, in one evening between dusk and midnight’ on the Great Barrier Reef (GBR; [17]), up to 30 species release gametes within hours on a single reef [16] and over 130 species spawn in the week following the full moon in October or November [16,18]. Individual colonies usually spawn only once each year on a seasonal cycle, and gamete maturity is often synchronous within colonies [18]. Seasonality of spawning appears to be strong, even in the equatorial tropics where annual variation in environmental conditions is traditionally considered to be relatively stable [19].
Despite three decades of study, the proximate cues to initiate coral spawning remain controversial. A wide range of environmental factors might play a role in reproductive timing and these may act at both a proximate and an ultimate (or mechanistic) level [14,16]. The most common hypothesis for corals states that environmental cues work at progressively finer scales to regulate: (i) the month of spawning, (ii) night of spawning (lunar cues, [20]) and (iii) the time of spawning (sunset) [21,22]. Previous analyses of the time of year have been limited to a handful of sites [23,24] and species [25–27] owing to the difficulty of collecting coral reproductive data across a large geographical area.
These limitations have led to inconsistencies in our understanding of the relationship between environmental conditions and spawning times. For instance, the evidence to support temperature as a cue is ambiguous, and there are indications that other environmental features could play a role [16,23,25,28]. Mendes & Woodley [25] noted an interaction between spawning times, temperature and monthly mean rainfall, suggesting that coral spawning events were timed to allow gametes to avoid decreased salinity and for larvae to benefit from the subsequent nutrient pulse. Alternatively, in the western Atlantic, spawning was predicted by the rate of change of solar insolation [28]. More recently, van Woesik [24] found the length of the reproductive season and wind speed were correlated, and suggested that spawning times had evolved to avoid high wind speeds that could reduce fertilization success and larval survival.
It is difficult to reconcile these disparate studies that often use only a small number of environmental variables, a limited number of sites or species, or pool species with very different life-history patterns. Moreover, competing hypotheses tend to be tested independently, which makes their relative importance difficult to assess. Consequently, conclusions are often based on weak statistical inference and generally applicable cues remain elusive.
Macroecological approaches, which search for generalizable explanations for ecological phenomena, offer a way to test whether hypothesized cues apply across multiple species and regions. Here, we determine the extent to which coral spawning months and peak spawning month can be predicted by environmental conditions at 34 reefs throughout the Indian and Pacific Oceans (‘Indo-Pacific’ throughout) for assemblages of Acropora—the most speciose coral genus (approx. 150 species, [29]). Specifically, we test the predictive capability of photosynthetically available radiation (PAR), sea surface temperature (SST), rainfall, wind speed, current speed and sunset time. All of these variables have been hypothesized previously to influence the month of coral spawning (see Methods for further details).
2. Methods
(a). Data collection for timing of spawning
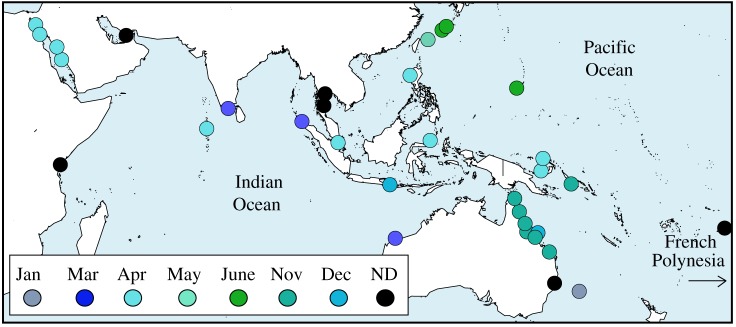
The Acropora genus dominates coral cover and abundance in almost all shallow water habitats throughout the Indo-Pacific and plays a key functional role in coral reef ecosystems of the region. In addition, Acropora species have very similar reproductive biology [30], and therefore, are expected to respond similarly to environmental cues. Acropora species are hermaphroditic broadcast spawners that usually have a single gametogenic cycle per year and egg size on release is remarkably consistent within the genus, ranging between 575 and 600 µm diameter (coraltraits.org). The reproductive stages of Acropora colonies were determined at 34 reefs throughout the Indo-Pacific between 2000 and 2012, encompassing a total of 99 Acropora species. Of these reefs, 28 were sampled on sufficient occasions to be confident of the presence or absence of spawning in each month throughout the year (figure 1 and electronic supplementary material, table S1). Peak spawning month could be reliably identified at 28 reefs (overlap of 22 reefs with season).
Figure 1.
Location of sampled reefs across the Indo-Pacific with month of peak spawning indicated by colour. ND, no data for peak spawning month, reef included in month of spawning model only. One reef in French Polynesia (17.56° S, 149.70° W, eastern Pacific) has been moved for plotting.
The reproductive condition of all Acropora colonies encountered during 40 min haphazard swims at each site was established by breaking small sections of coral branches (colonies generally recover in less than two weeks) to expose the developing oocytes. Three reproductive conditions were defined based on the colour of the oocytes following Baird et al. [30]: mature—oocytes pigmented; immature—oocytes white; empty—oocytes too small to see in the field or absent. Colonies with mature oocytes were assumed to have spawned in the calendar month they were sampled, colonies with immature oocytes were assumed to have spawned one month post-sampling [30]. These data generated a binary variable of ‘spawned’ or ‘not spawned’ for each species across months and reefs. We also determined a second binary variable of peak spawning, defined as the month in which the highest proportion of colonies were mature or data in a single month revealed that greater than 50% sampled coral colonies had mature oocytes.
(b). Environmental predictors
In total, eight environmental predictors were selected for inclusion in a predictive model. The monthly mean values of five environmental variables were considered potential predictors of spawning in the Acropora based on the reproductive synchrony literature: SST; rainfall; wind speed; current speed and difference in sunset time (electronic supplementary material, table S2). Although monthly means often represent plant phenology poorly [9], Acropora corals are constrained to spawn at a certain point of the lunar phase [30], so monthly means match the resolution of coral spawning season data. To capture cumulative energy, hypothesized to be a phenological cue for a variety of taxa, we followed van Woesik et al. [28] and summed PAR over the 10 months prior to the target month (electronic supplementary material, table S2). We also considered the rate of change in SST and PAR between months and normalized these values within reefs by subtracting the previous month from the target month, and dividing this by the range for that reef.
SST (°C), wind speed (10 m above surface) and rainfall rates in mm h−1 were obtained from the Tropical Rainfall Measuring Mission at a 0.25° (approx. 25 km) spatial resolution. Monthly current speed data in m s−1 were downloaded from Ocean Surface Current Analyses Real Time [31] at a spatial resolution of 1° (approx. 100 km). PAR data (einsteins m−2 day−1) were obtained from the moderate resolution imaging spectroradiometer aboard NASA's Aqua satellite. We used the monthly climatology for 2003–2014 at 4 km resolution. For further detail on environmental data, see electronic supplementary material, appendix S1.
Environmental variables were normalized and centred with a mean of zero and standard deviation of one to facilitate comparison and interpretation of regression coefficients (except mean SST, which was standardized within sites). Current speed was square root-transformed. Normalized variables were checked for multicollinearity using Pearson's correlations with a cut-off of greater than 0.6 or a variance inflation factor (VIF) greater than 2.5 within the initial GLM (see ‘Statistical analysis'), and visually inspected to rule out nonlinear relationships. In the case of collinear variables, one was removed on the basis that (i) it was collinear with the highest number of other variables or (ii) it was less interpretable biologically.
(c). Statistical analysis
To determine the extent to which environmental variables were able to predict months of Acropora spp. spawning, we fitted generalized linear models (GLMs) with a binomial error structure (spawned/not spawned) and logit link function. Initially, GLMs were fitted separately for each predictor variable to determine whether a linear or quadratic function best captured the relationship with spawning, i.e. greater than 3 ΔBayesian information criterion (ΔBIC). We generated a full GLM with the same error structure that included all environmental variables to test GLM assumptions. To ensure spawning predictors were generalizable, regardless of the time of year and geographical location, we included month and reef as random effects in a generalized linear mixed model (GLMM). This was necessary to account for non-independence of data, because our sampling design had a hierarchical structure, and the data were overdispersed.
We performed model selection on the GLMM by testing all combinations of variables, including biologically plausible interactions, after the steps above to find the best set of models as indicated by BIC. Models that were within 3 ΔBIC of the best model were averaged [32]. We calculated 95% confidence intervals (CIs) on full model coefficients with shrinkage for variables included within the averaged model and interpreted predictor variables with intervals that did not overlap zero as contributing significantly. Response plots were generated for each of these significant model-averaged variables. We report the marginal R2, which gives the proportion of variation in the data explained by the fixed effects alone, and conditional R2, which further includes the contribution of random effects [33].
The above-mentioned workflow was repeated for peak spawning month with two exceptions. Because our peak spawning data contained many samples with a value of zero, we used a complementary log–log link function to better capture the error structure of our data [32]. Second, reef was not included as a random effect, because one ‘success' only was available for each reef.
All analyses were performed in R v. 3.1.1 [34] with the lme4 v. 1.1–7 [35] and MuMIn v. 1.14.0 [36] packages.
3. Results
(a). Spatio-temporal pattern of spawning
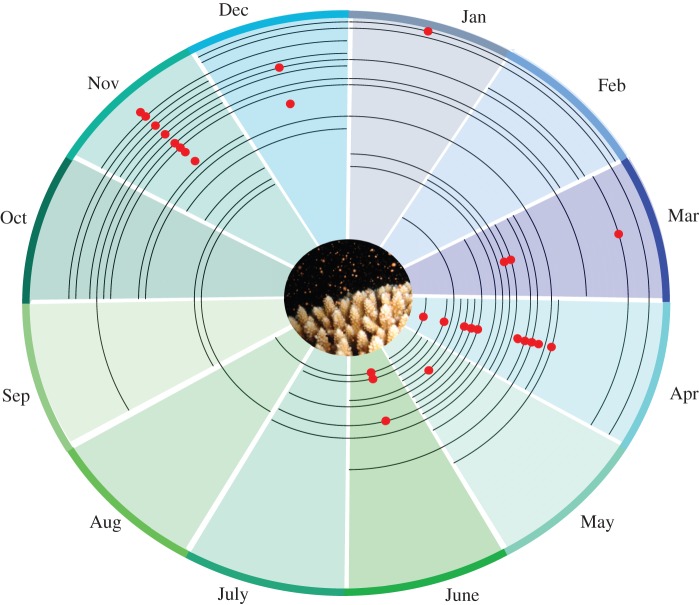
Number of months during which Acropora spp. assemblages were spawning was highly variable, ranging from two to nine months across the geographical extent. The highest number of spawning months was found in Thailand, which was split across two seasons. The shortest spawning durations were in Indonesia, New Caledonia and the Red Sea. A clear separation in the month of spawning was evident between the Northern and Southern Hemispheres, because spawning tended to occur from spring to early summer in each hemisphere, and peak spawning was concentrated in April and November, respectively (figure 2).
Figure 2.
Months of spawning (black line) and peak spawning month (red dot) for all reefs. Reefs are ordered by latitude: outer line is the most southerly reef, inner line is the most northerly reef. Inset photo is a spawning Acropora colony (photo credit: A. Chelliah).
Peak spawning occurred in regional clusters. Throughout the majority of the Indo-Australian Archipelago (excluding the Solomon Islands), the Andaman Sea and across the Indian Ocean and in the Red Sea, the peak month of spawning was in March or April (figure 1). In the northwestern Pacific, including Japan, peak spawning was in June (figure 1). In the south and central Pacific, including the GBR, peak spawning was predominantly in November (figure 1). In the western Pacific, peak spawning appears to occur later into the spring season at sites progressively further away from the equator, e.g. the Philippines into Japan and along the east coast of Australia to Lord Howe Island (figure 1).
(b). Environmental predictability of coral spawning
Relative change in PAR had a strong collinear effect in the model (VIF > 2.5) and was removed from further analysis. It was not highly correlated with any one variable, as indicated by the Pearson correlation coefficients, and therefore, its effect was unclear (electronic supplementary material, table S3). To ensure the importance of this variable was not hidden by correlations with other variables, we created a model with PAR relative change as a single predictor. This model was not significantly better than the intercept only model. Model assumptions were met for the GLM. Wind speed was best fitted by a quadratic function, whereas all other terms were best represented with linear functions.
(i). Spawning months
SST relative change and monthly mean wind speed were selected in each of the two best models; only one model included monthly mean SST. These two models were subsequently averaged because they could not be differentiated (i.e. were less than 3 ΔBIC apart). Relative change in SST contributed positively and most strongly to the averaged model (β = 1.65; 95% CI lower = 1.16, upper = 2.13; table 1). Specifically, the probability of spawning was high in months when SST had risen rapidly (figure 3a). Spawning was also associated with intermediate wind speed (βlinear = −19.11; 95% CI lowerlinear = −31.97, upperlinear = −6.26; βquadratic = −17.91; 95% CI lowerquadratic = −28.74, upperquadratic = −7.08; table 1 and figure 3b). By contrast, the contribution of monthly mean SST to the averaged model was unclear, with CIs that overlapped zero (β = 0.07; 95% CI lower = −0.26, upper = 0.40; table 1), which we interpreted as indicating SST monthly mean did not contribute significantly. The best model, which included relative change in SST, monthly mean SST and monthly mean wind speed, explained 55% of the variation in the data with environmental predictors alone, and 73% when reef and month were included as random effects (marginal R2 = 0.55, conditional R2 = 0.73). The actual (as opposed to relative) magnitude of the change in SST is shown in electronic supplementary material, figure S3.
Table 1.
Model-averaged coefficients (with shrinkage) and 95% confidence intervals (CIs). Coefficients with CIs that do not overlap zero are italicized.
| β coefficient | lower 95% CI | upper 95% CI | |
|---|---|---|---|
| spawning season | |||
| (intercept) | −1.47 | −2.35 | −0.6 |
| wind speed | −19.11 | −31.97 | −6.26 |
| wind speed 2 | −17.91 | −28.74 | −7.08 |
| SST change | 1.65 | 1.16 | 2.13 |
| SST monthly mean | 0.07 | −0.26 | 0.4 |
| peak spawning month | |||
| (intercept) | −4.25 | −5.54 | −2.96 |
| SST change | 1.72 | 1.04 | 2.4 |
| current speed | −0.24 | −0.83 | 0.36 |
Figure 3.
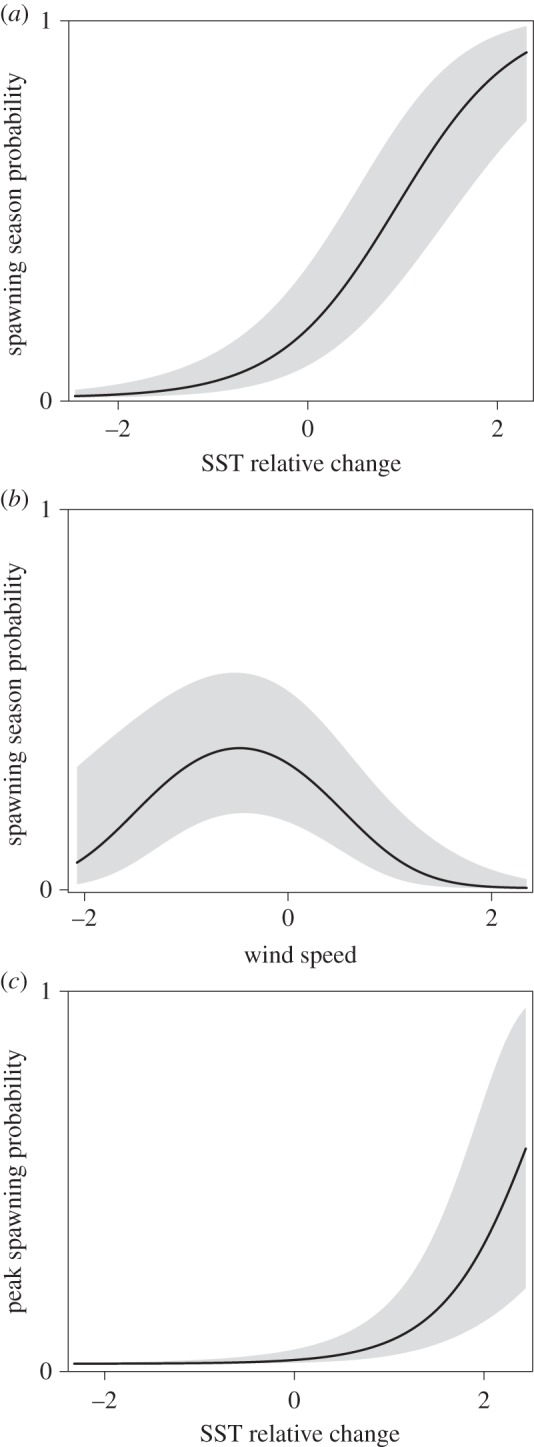
Environmental conditions that predict (a,b) month of spawning and (c) month of peak spawning, in an averaged set of generalized mixed-effect models. Partial coefficients for variables with 95% CIs that do not overlap zero in an averaged model are shown. Models for month of spawning (a,b) used a logit link function, whereas models for peak spawning (c) used a complementary log–log link function.
Regardless of environmental conditions, spawning was most likely in the months of April and May, and least likely in the months of August and September, indicated by the offset from the intercept of the random effect groups (electronic supplementary material, figure S1a). In addition, there was a higher baseline likelihood of spawning at three reefs, two of which were located in Thailand where spawning occurred in six or seven different months, and one in the Solitary Islands on the eastern coast of Australia where spawning occurred every month from January to April (electronic supplementary material, figure S1b).
(ii). Peak spawning month
SST relative change was selected for both models in the averaged set, and one model also included monthly mean current speed. Relative change in SST contributed strongly to the averaged model (β = 1.72; 95% CI lower = 1.04, upper = 2.40; table 1). Similar to the month of spawning model, the probability of spawning was high in months when SST had risen rapidly (figure 3c). Current speed was also included in the model-averaged set but was weak, and the direction of the relationship was unclear, with 95% CIs that overlapped zero (β = −0.24; 95% CI lower = −0.83, upper = 0.36; table 1), indicating a lack of significant contribution to the averaged model. The best model, which included both variables, explained 44% of the variation in the data with the environmental predictors alone, and 62% when reef and month were included as random effects (marginal R2 = 0.44, conditional R2 = 0.62). Regardless of environmental conditions, the random effect of month indicated that peak spawning was more likely in the months of April, June and November (electronic supplementary material, figure S2).
4. Discussion
Environmental conditions required to initiate and optimize reproductive events are an essential determinant of biodiversity. Understanding these requirements and the spatial pattern of phenological events is necessary to address global conservation challenges through highlighting potential vulnerabilities across species' life stages. Here, we show that spawning across 99 Acropora spp. (the most speciose coral genus) is synchronous over very large spatial scales throughout the Indo-Pacific, and all reefs in our dataset experienced multi-specific spawning events [30]. Month of spawning and peak month of spawning for Acropora coincided with the largest month-to-month increase in SST. Intermediate wind speeds also contributed to the prediction of spawning months, although the relationship was weak. Monthly mean SST, a critical determinant of adult coral distributions, was selected in the best model set for spawning; however, its relationship with reproductive timing was not significant. Overall, approximately half of the variation in the timing of coral spawning can be predicted by the environmental conditions tested here. Seasonal rapid increases in SST appear to be the dominant proximate cue on Indo-Pacific reefs for Acropora corals to spawn.
(a). Tropical phenology
Phenological events in tropical regions are perceived to be linked poorly to seasonal climate owing to muted seasonal variations [9,10]. For this reason, early discussions discredited the potential for SST to act as a proximate cue and doubted whether corals in these regions would engage in mass spawning events [14]. In contrast, our results show that temperature can act as a generalizable proximate cue for mass spawning events throughout the tropical Indo-Pacific. Therefore, tropical species appear to be more sensitive to small climate fluctuations than assumed previously.
Phenological events in the tropics have also been predicted to be more closely tied to biotic, rather than to abiotic cues, because mistiming would create fewer physiological issues, thus the selection for timing would be weak [9]. The absence of biotic factors from our analyses could explain the high conditional R2, which implies that additional variables related to specific months explain 18% of the variation in month of spawning and peak month. For instance, coral spawning times might have evolved to avoid times of highest predator abundance. Stomach content analyses during mass spawning on the GBR, Australia, showed that multiple fish species switched from an omnivorous diet to one predominantly of coral spawn [37,38]. Indeed, biotic interactions have been associated strongly with phenology in other species groups [39]. Unfortunately, there are no detailed data on predator abundance and diet switching across a large geographical extent that would allow us to test the importance of interactions for coral spawning directly.
(b). Proximate cues
Temperature was the foremost environmental variable thought to control seasonal patterns in the initial discussions on the phenomenon of coral mass spawning [12,14,16,40]. Strong evidence for a role of SST at a regional scale comes from the split spawning of corals in the central GBR [16]: at the same latitude and separated by only 60 km, species on inshore reefs spawned a month earlier than colonies of the same species at mid- and outer shelf reefs. These spawning peaks were correlated with SST that had warmed a month earlier closer to the mainland [22]. Our analyses confirm these original ideas, albeit with a focus on rates of temperature increase rather than monthly mean SST. Similarly, many terrestrial species respond to growing degree days, which is the integral of temperature over time, and is a stronger predictor of butterfly emergence than calendar date [41].
Conversely, more recent work had largely dismissed the role for temperature and focused instead on solar insolation—in our analysis captured by PAR [23,28]. The assumed mechanism in this latter work was that solar insolation mediated coral energy acquisition via symbiotic algae, leading to gamete production. Although we do not dismiss a contribution of solar-derived energy, we did not find PAR to be of predictive value for Indo-Pacific Acropora spp. spawning phenology.
One reason for this discrepancy could be, as noted by Penland et al. [23], that solar insolation covers a wide bandwidth (300–5000 nm) of which PAR is a relatively narrow component (360–700 nm). Therefore, solar insolation (PAR was not readily available at that time) might be a poor proxy for energy acquisition leading to premature conclusions. Another reason might lie in the samples used for these analyses. Penland et al. [23] had data from only four Pacific sites, whereas the sites included by van Woesik et al. [28] were in the Atlantic, which has undergone a different evolutionary history and contains an order of magnitude fewer hard coral species than the Indo-Pacific, with only 12 species included in that analysis. Similarly, rainfall was identified to interact with temperature as a cue in previous analyses [25], but this was for one species only and therefore, cannot provide evidence for rainfall as a cue that can be generalized across species and regions. Our comprehensive analysis across geographical space and species supports the original ideas, and suggests temperature is the major cue that initiates coral spawning.
Proximate cues for phenological events must be reliable and indicate onset of environmental or other conditions that will ensure optimum reproductive success if species are to evolve to detect and respond to them [9]. Light availability, of which PAR is a part, is dependent on water clarity and cloud cover, which local conditions such as river discharge can influence strongly [42]. As a result, PAR might provide a less reliable cue compared with the rate of SST rise. However, light is thought to be important on shorter timescales: for example, photoreceptors sensitive to the blue spectrum in moonlight are hypothesized to initiate night-time release of gametes during a particular lunar phase [43].
Absolute SST is also susceptible to fluctuations, albeit on longer decadal scales, which could curtail selection for corals that respond to this as a proximate cue. For instance, cyclical phenomena, such as the El Niño Southern Oscillation (ENSO), can warm sections of the Pacific Ocean more than 2°C approximately every 2–7 years [44]. Experimental and observational evidence suggest that Cnidaria, the phylum to which corals belong, have developmental programmes (e.g. gamete/larval release, settlement, metamorphosis) that coincide with sustained seasonal shifts in temperature [43]. This evidence fits with the idea that changes in temperature are better proximate cues than absolute temperatures. Thermoreceptors in Cnidaria are poorly understood [45] and might provide a fruitful avenue for research to identify underlying detection mechanisms for the proximate cue.
(c). Ultimate drivers and mechanism
The ultimate reason corals would evolve to spawn during the fastest rate of SST increase is more difficult to ascertain. Even in the most well-studied systems, such as reproductive phenology of the pied flycatcher (Ficedula hypoleuca), there are ongoing struggles to resolve mechanistic explanations underlying phenological timing [39]. To truly elucidate the mechanisms of phenology, detailed experiments across a broad biogeographic extent over evolutionary timescales are necessary. Climate change is providing a natural experiment along these lines in some cases [46], but long time series are still required to take advantage of this opportunity. Unfortunately, these do not exist for most organisms, and we must therefore speculate on the most plausible explanation given the data available.
Proposed mechanistic explanations underlying the proximate seasonal SST rise cue include synchronous spawning to maximize gamete density, plus a possible temperature effect on sperm motility. In addition to temperature averages, the accumulation of energy through periods of rising temperature might be an important driver of physiological processes related to reproduction: the onset of gametogenesis in corals has been linked experimentally to temperature [47].
However, the simplest possibility to consider is that the ultimate reason for synchronized gamete release is to ensure maximum chances for egg and sperm to come into contact, regardless of external conditions. In this case, multiple species would spawn simultaneously purely because they are similar physiologically and therefore constrained to recognize a relatively narrow suite of cues [14]. The trade-off associated with such multi-specific gamete release is an increased probability of unviable hybrids [12] and negative density-dependence [15]. However, it seems implausible that there has not also been selection for spawning times to coincide with environmental conditions that are optimal for fertilization and subsequent larval survival, particularly because fertilization is external, and therefore, exposed to the elements. Therefore, corals are likely to have been subjected to selection pressures that ensure conditions at the time of spawning will maximize fertilization success and larval survival. Our results show that absolute temperature during the greatest rate of SST rise varied greatly across the Indo-Pacific, but, in general, it was below the maximum and well above the minimum. This suggests either coral propagules are insensitive to absolute temperature, or that they are locally adapted to regional thermal regimes.
Evidence for local adaptation in early life stages of corals has been revealed in experimental situations, where larvae of the same species from populations at different latitudes respond differently to temperature treatments: larvae from lower latitude populations are more tolerant of higher temperatures [3]. High SST (2–4°C above ambient at time of spawning) is associated with an increased frequency of abnormal embryos, lower survival rates and greatly reduced fertilization success in Acropora species [3,48]. The small magnitude of intra-annual temperature fluctuations in the tropics means it is unlikely that the temperature during the month of fastest SST rise is 2–4°C below the annual maximum SST. However, a shift in temperature could provide a thermal safety margin to ensure corals can fertilize even when inter-decadal cycles, such as ENSO events, generate positive temperature anomalies.
Intermediate wind speed is likely to contribute through the generation of water movement that ensures horizontal advection of gametes but without inducing excessive vertical mixing (as would result from higher winds) that could take gametes deeper in the water column. Some agitation is required to quickly break apart egg and sperm cells that are released together in mucous-wrapped bundles from the adult corals [12]. It is particularly important to break up these bundles rapidly, because evidence suggests that self-fertilization is rarely successful compared with cross-fertilization among individuals of the same species [49]. Ultimately, the optimum fertilization success achieved from water movement and avoidance of maximum annual SST provides a plausible explanation for coral spawning phenology.
5. Conclusion
Seasonal rapid increase in SST has the potential to provide a proximate environmental cue to synchronize mass coral spawning phenology across the Indo-Pacific, with a secondary role for moderate winds. The use of temperature as a phenological cue is widespread across species; however, in this case, it is the shift in temperature, rather than the absolute SST, that initiates coral gamete release. While there is high confidence that SST will rise over the coming decades [50], if the difference in SST between months is maintained, the same month will continue to experience the greatest rise in SST. Under these conditions, the impact of climate change on coral reproductive timing might be minimal relative to species that rely on absolute temperatures, which have already experienced shifting phenologies [46]. Specific projections of intra-annual SST derivatives under climate change scenarios would provide further context in which to consider our results.
An alternative scenario in response to climate change is that the proximate cue of SST rise and the ultimate selective force might become decoupled [51]. Rising SST, shifted currents, altered nutrient distributions and increased frequencies of extreme events are projected for the oceans over the coming decades [50,52]. For corals that cannot adapt through behavioural plasticity or rapid evolution, decoupling is a real danger. If the month of greatest rise in SST remains stable while the optimum environmental conditions for fertilization and larval survival shift to a different month, then decoupling of proximate and ultimate drivers could lead to suboptimal population replenishment. Ultimately, this reproductive decline could further exacerbate the threat of climate change for corals and the wider reef ecosystem.
Supplementary Material
Supplementary Material
Acknowledgements
Thanks to K. A. Marske for helpful discussions and M.K. Borregaard for help with figures. The contents in this manuscript are solely the opinions of the authors and do not constitute a statement of policy, decision or position on behalf of NOAA or the US Government.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material, table S1.
Author contributions
A.H.B., J.A.M. and S.A.K. designed the study, A.E., J.R.G., A.G.B., A.H.B., M.L.B., J.B., S.P., R.v.H. and S.F.H. collected the data, S.A.K. performed the analyses, S.A.K., A.H.B. and J.A.M. wrote the manuscript, all authors contributed equally to editing the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
We are grateful for funding support from VILLUM FONDEN (S.A.K., grant no. 10114), the Danish National Research Foundation for support to the Center for Macroecology, Evolution and Climate (S.A.K., C.R., grant no. DNRF96), the Australian Research Council Centre of Excellence for Coral Reef Studies (A.G.B., S.A.K.), the European Research Commission Marie Curie Actions programme (J.A.M.), the King Abdullah University of Science and Technology (M.L.B.) and NOAA Coral Reef Conservation Program (S.F.H. and R.v.H.).
References
- 1.Chuine I. 2010. Why does phenology drive species distribution? Proc. R. Soc. B 365, 3149–3160. ( 10.1098/rstb.2010.0142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keith SA, Herbert RJH, Norton PA, Hawkins SJ, Newton AC. 2011. Individualistic species limitations of climate-induced range expansions generated by meso-scale dispersal barriers. Divers. Distrib. 17, 275–286. ( 10.1111/j.1472-4642.2010.00734.x) [DOI] [Google Scholar]
- 3.Woolsey ES, Keith SA, Byrne M, Schmidt-Roach S, Baird AH. 2015. Latitudinal variation in thermal tolerance thresholds of early life stages of corals. Coral Reefs 34, 471–478. ( 10.1007/s00338-014-1253-z) [DOI] [Google Scholar]
- 4.Forrest J, Miller-Rushing AJ. 2010. Toward a synthetic understanding of the role of phenology in ecology and evolution. Proc. R. Soc. B 365, 3101–3112. ( 10.1098/rstb.2010.0145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colinet H, Sinclair BJ, Vernon P, Renault D. 2015. Insects in fluctuating thermal environments. Annu. Rev. Entomol. 60, 123–140. ( 10.1146/annurev-ento-010814-021017) [DOI] [PubMed] [Google Scholar]
- 6.Visser ME, Holleman LJM, Caro SP. 2009. Temperature has a causal effect on avian timing of reproduction. Proc. R. Soc. B 276, 2323–2331. ( 10.1098/rspb.2009.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mooij WM, De Senerpont Domis LN, Hülsmann S. 2008. The impact of climate warming on water temperature, timing of hatching and young-of-the-year growth of fish in shallow lakes in the Netherlands. J. Sea Res. 60, 32–43. ( 10.1016/j.seares.2008.03.002) [DOI] [Google Scholar]
- 8.Badeck F-W, Bondeau A, Böttcher K, Doktor D, Lucht W, Schaber J, Sitch S. 2004. Responses of spring phenology to climate change. New Phytol. 162, 295–309. ( 10.1111/j.1469-8137.2004.01059.x) [DOI] [Google Scholar]
- 9.Pau S, Wolkovich EM, Cook BI, Davies TJ, Kraft NJB, Bolmgren K, Betancourt JL, Cleland EE. 2011. Predicting phenology by integrating ecology, evolution and climate science. Glob. Change Biol. 17, 3633–3643. ( 10.1111/j.1365-2486.2011.02515.x) [DOI] [Google Scholar]
- 10.Burrows MT, et al. 2011. The pace of shifting climate in marine and terrestrial ecosystems. Science 334, 652–655. ( 10.1126/science.1210288) [DOI] [PubMed] [Google Scholar]
- 11.Mercier A, Hamel J-F. 2010. Synchronized breeding events in sympatric marine invertebrates: role of behavior and fine temporal windows in maintaining reproductive isolation. Behav. Ecol. Sociobiol. 64, 1749–1765. ( 10.1007/s00265-010-0987-z) [DOI] [Google Scholar]
- 12.Oliver J, Babcock R. 1992. Aspects of the fertilization ecology of broadcast spawning corals: sperm dilution effects and in situ measurements of fertilization. Biol. Bull. 183, 409–417. ( 10.2307/1542017) [DOI] [PubMed] [Google Scholar]
- 13.Levitan DR, Fukami H, Jara J, Kline D, McGovern TM, McGhee KE, Swanson CA, Knowlton N, Bonhomme F. 2004. Mechanisms of reproductive isolation among sympatric broadcast-spawning corals of the Montastraea annularis species complex. Evolution 58, 308–323. ( 10.1554/02-700) [DOI] [PubMed] [Google Scholar]
- 14.Oliver JK, Babcock RC, Harrison PL, Willis BL. 1988. Geographic extent of mass coral spawning: clues to ultimate causal factors. Proc. Sixth Int. Coral Reef Symp. Australia 2, 803–810. [Google Scholar]
- 15.Kelly D, Sork VL. 2002. Mast seeding in perennial plants: why, how, where? Annu. Rev. Ecol. Evol. Syst. 33, 427–447. ( 10.1146/annurev.ecolsys.33.020602.095433) [DOI] [Google Scholar]
- 16.Willis BL, Babcock RC, Harrison PL, Oliver JK, Wallace CC. 1985. Patterns in the mass spawning of corals on the Great Barrier Reef from 1981 to 1984. Proc. Fifth Int. Coral Reef Congress Tahiti 4, 343–348. [Google Scholar]
- 17.Harrison RL, Babcock RC, Bull GD, Oliver JK, Wallace CC, Willis BL. 1984. Mass spawning in tropical reef corals. Science 223, 1186–1189. ( 10.1126/science.223.4641.1186) [DOI] [PubMed] [Google Scholar]
- 18.Harrison PL, Wallace CC. 1990. Reproduction, dispersal and recruitment of scleractinian corals. Ecosyst. World 25, 133–207. [Google Scholar]
- 19.Guest JR, Baird AH, Goh BPL, Chou LM. 2005. Seasonal reproduction in equatorial reef corals. Invert. Reprod. Dev. 48, 207–218. ( 10.1080/07924259.2005.9652186) [DOI] [Google Scholar]
- 20.Kaniewska P, Alon S, Karako-Lampert S, Hoegh-Guldberg O, Levy O. 2015. Signaling cascades and the importance of moonlight in coral broadcast mass spawning. eLife 4, e0991 ( 10.7554/eLife.09991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweeney AM, Boch CA, Johnsen S, Morse DE. 2011. Twilight spectral dynamics and the coral reef invertebrate spawning response. J. Exp. Biol. 214, 770–777. ( 10.1242/jeb.043406) [DOI] [PubMed] [Google Scholar]
- 22.Babcock RC, Bull GD, Harrison PL, Heyward AJ, Oliver JK, Wallace CC, Willis BL. 1986. Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar. Biol. 90, 379–394. ( 10.1007/BF00428562) [DOI] [Google Scholar]
- 23.Penland L, Kloulechad J, Idip D, Van Woesik R. 2004. Coral spawning in the western Pacific Ocean is related to solar insolation: evidence of multiple spawning events in Palau. Coral Reefs 23, 133–140. ( 10.1007/s00338-003-0362-x) [DOI] [Google Scholar]
- 24.Van Woesik R. 2010. Calm before the spawn: global coral spawning patterns are explained by regional wind fields. Proc. R. Soc. B 277, 715–722. ( 10.1098/rspb.2009.1524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendes JM, Woodley JD. 2002. Timing of reproduction in Montastraea annularis: relationship to environmental variables. Mar. Ecol. Prog. Ser. 227, 241–251. ( 10.3354/meps227241) [DOI] [Google Scholar]
- 26.Padilla-Gamino JL, Gates RD. 2012. Spawning dynamics in the Hawaiian reef-building coral Montipora capitata. Mar. Ecol. Prog. Ser. 449, 145–160. ( 10.3354/meps09530) [DOI] [Google Scholar]
- 27.Mangubhai S, Harrison PL. 2009. Extended breeding seasons and asynchronous spawning among equatorial reef corals in Kenya. Mar. Ecol. Prog. Ser. 374, 305–310. ( 10.3354/meps07910) [DOI] [Google Scholar]
- 28.Van Woesik R, Lacharmoise F, Köksal S. 2006. Annual cycles of solar insolation predict spawning times of Caribbean corals. Ecol. Lett. 9, 390–398. ( 10.1111/j.1461-0248.2006.00886.x) [DOI] [PubMed] [Google Scholar]
- 29.Wallace CC, Done BJ, Muir PR. 2012. Revision and catalogue of worldwide staghorn corals Acropora and Isopora (Scleractinia: Acroporidae) in the Museum of Tropical Queensland. Queensland, Australia: Queensland Museum. [Google Scholar]
- 30.Baird AH, Birrell CL, Hughes TP, McDonald A, Nojima S, Page CA, Pratchett MS, Yamasaki H. 2009. Latitudinal variation in reproductive synchrony in Acropora assemblages: Japan vs. Australia. Galaxea 11, 101–108. ( 10.3755/galaxea.11.101) [DOI] [Google Scholar]
- 31.Bonjean F, Lagerloef GSE. 2002. Diagnostic model and analysis of the surface currents in the tropical Pacific ocean. J. Phys. Oceanogr. 32, 2938–2954. () [DOI] [Google Scholar]
- 32.Bolker B. 2008. Modeling variance. In Ecological models and data in R (ed. Bolker B.), pp. 316–336. Princeton, NJ: Princeton University Press. [Google Scholar]
- 33.Nakagawa S, Schielzeth H. 2012. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 34.R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 35.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: linear mixed-effects models using S4 classes. R package version 1.1–7. See http://CRAN.R-project.org/package=lme4.
- 36.Barton K. 2015. MuMIn: multi-model inference. R package version 1.14.0. See http://CRAN.R-project.org/package=MuMIn.
- 37.Westneat M, Resing J. 1988. Predation on coral spawn by planktivorous fish. Coral Reefs 7, 89–92. ( 10.1007/BF00301646) [DOI] [Google Scholar]
- 38.Pratchett MS, Gust N, Goby G, Klanten SO. 2001. Consumption of coral propagules represents a significant trophic link between corals and reef fish. Coral Reefs 20, 13–17. ( 10.1007/s003380000113) [DOI] [Google Scholar]
- 39.Visser ME, Gienapp P, Husby A, Morrisey M, de la Hera I, Pulido F, Both C. 2015. Effects of spring temperatures on the strength of selection on timing of reproduction in a long-distance migratory bird. PLoS Biol. 13, e1002120 ( 10.1371/journal.pbio.1002120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliver JK, Willis BL. 1987. Coral-spawn slicks in the Great Barrier Reef: preliminary observations. Mar. Biol. 94, 521–529. ( 10.1007/BF00431398) [DOI] [Google Scholar]
- 41.Cayton HL, Haddad NM, Gross K, Diamond SE, Ries L. 2015. Do growing degree days predict phenology across butterfly species? Ecology 96, 1473–1479. ( 10.1890/15-0131.1) [DOI] [Google Scholar]
- 42.Fabricius KE, Logan M, Weeks S, Brodie J. 2014. The effects of river run-off on water clarity across the central Great Barrier Reef. Mar. Poll. Bull. 84, 191–200. ( 10.1016/j.marpolbul.2014.05.012) [DOI] [PubMed] [Google Scholar]
- 43.Bosch TCG, et al. 2014. How do environmental factors influence life cycles and development? An experimental framework for early-diverging metazoans. Bioessays 36, 1185–1194. ( 10.1002/bies.201400065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cobb KM, Charles CD, Cheng H, Edwards RL. 2003. El Niño/Southern Oscillation and tropical pacific climate during the last millennium. Nature 424, 271–276. ( 10.1038/nature01779) [DOI] [PubMed] [Google Scholar]
- 45.Hroudova M, Vojta P, Strnad H, Krejcik Z, Ridl J, Paces J, Vlcek C, Paces V. 2012. Diversity, phylogeny and expression patterns of Pou and six homeodomain transcription factors in hydrozoan jellyfish Craspedacusta sowerbyi. PLoS ONE 7, e36420 ( 10.1371/journal.pone.0036420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 47.Nozawa Y. 2012. Annual variation in the timing of coral spawning in a high-latitude environment: influence of temperature. Biol. Bull. 222, 192–202. [DOI] [PubMed] [Google Scholar]
- 48.Negri AP, Marshall PA, Heyward AJ. 2007. Differing effects of thermal stress on coral fertilization and early embryogenesis in four Indo Pacific species. Coral Reefs 26, 759–763. ( 10.1007/s00338-007-0258-2) [DOI] [Google Scholar]
- 49.Heyward AJ, Babcock RC. 1986. Self- and cross-fertilization in scleractinian corals. Mar. Biol. 90, 191–195. ( 10.1007/BF00569127) [DOI] [Google Scholar]
- 50.Collins M, et al. 2013. Long-term climate change: projections, commitments and irreversibility. In Climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM). Cambridge, UK: Cambridge University Press. [Google Scholar]
- 51.Visser ME, Caro SP, van Oers K, Schaper SV, Helm B. 2010. Phenology, seasonal timing and circannual rhythms: towards a unified framework. Proc. R. Soc. B 365, 3113–3127. ( 10.1098/rstb.2010.0111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai W, et al. 2014. Increasing frequency of extreme El Niño events due to greenhouse warming. Nat. Clim. Change 4, 111–116. ( 10.1038/nclimate2100) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material, table S1.