Abstract
The degree to which biological control is exercised compared to physical control of the organization of biogenic materials is a central theme in biomineralization. We show that the outlines of biogenic calcite domains with organic membranes are always of simple geometries, while without they are much more complex. Moreover, the mineral prisms enclosed within the organic membranes are frequently polycrystalline. In the prismatic layer of the mollusc shell, organic membranes display a dynamics in accordance with the von Neumann–Mullins and Lewis Laws for two-dimensional foam, emulsion and grain growth. Taken together with the facts that we found instances in which the crystals do not obey such laws, and that the same organic membrane pattern can be found even without the mineral infilling, our work indicates that it is the membranes, not the mineral prisms, that control the pattern, and the mineral enclosed within the organic membranes passively adjusts to the dynamics dictated by the latter.
Keywords: biomineralization, prismatic layer, mollusc
1. Introduction
In the biomaterials making up the shells of molluscs, the question of biological versus physical control has usually focused on the interactions between organic macromolecules and their influence on the mineral phase [1,2], crystal morphology and orientation [3–5]. At a microscopic level, the mutual relationships of the organic matrices and forming minerals have been studied in nacre [6,7], and other microstructural aggregates [8,9]. These studies have led to the proposals of models of self-organization for particular microstructures that help to explain their complex arrangement on simple physical grounds. This is the case of the interlamellar membranes of nacre [7] and the horizontal membranes filling cuttlebone chambers [10], both of whose patterning has been explained by liquid crystallization. Physical explanations have been recently provided for the calcitic prismatic layers of Pinna. These belong to a family of similar materials found in many representatives of the extensive order Pteriomorphia of the class Bivalvia, including pen-shells, pearl oysters, oysters, saddle oysters and some scallops. They are strictly termed of the calcitic columnar prismatic or CCP type and are formed by prismatic grains of calcite with regular polygonal outlines, which elongate and grow perpendicular to the shell surfaces. The mineral prisms are surrounded by relatively thick (0.5–3 µm) organic membranes. They constitute important functional materials acting in bending, their high degree of flexibility being provided by the organic envelopes. The growth in depth of the calcitic prisms of the outer layer of the pen shell Pinna nobilis has recently been explained by normal grain growth theory [11], according to which those prisms that initially exhibited lower transverse surface area or a lesser number of sides were more prone to be outcompeted during growth. In this view, the organic sheaths should passively adjust to the changes in dimensions of the prisms perpendicular to the growth axis [12]. Here, we have studied in detail the prismatic calcite layers of several molluscan taxa, particularly focusing on the irregularities observed during the evolution of the polygonal pattern. We conclude that when the organic network is considered, it is found to evolve according to the predictions of von Neumann–Mullins Law (which also applies to normal grain growth), therefore being comparable to a soap froth or emulsion. Conversely, several features of the calcite grains do not fit into the normal grain growth theory. The principal implication is that the organic network is responsible for the cellular pattern observed. This is a good example of the preponderance of the organic over the mineral phase.
2. Results and discussion
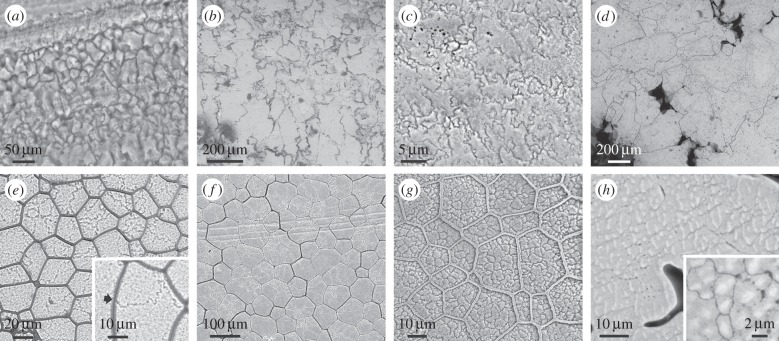
Several groups of molluscs gastropods [13–15], belemnoid cephalopods and a few bivalves [16,17], some brachiopods [18] and even vertebrate eggshells [19], secrete external calcitic shell layers organized into prisms or fibres that are not accompanied by any organic membranes. In all these cases, the calcitic crystalline domains grow perpendicular to the shell surfaces, and display very irregular outlines (figure 1a–c). These vary from irregular polygonal (figure 1a,b) to fully dendritic (figure 1c). There is good evidence that each domain is a single crystal [18,20] (electronic supplementary material, figure S1). Similar outlines are also found in abiogenic sedimentary calcite, e.g. speleothems [21,22] (figure 1d). The irregularity shown by these examples is in stark contrast to the prisms found when calcitic domains are surrounded by organic membranes.
Figure 1.
Biogenic (a–c) and abiogenic (d) calcite grains with complex morphologies, compared to the polycrystalline prismatic units of pteriomorph bivalves (e–h). (a) Tegula funebralis (gastropod). (b) Belemnoid rostrum (Oxfordian, Wittlesey, England). (c) Chama arcana (bivalve). (d) Speleothem (The Pyrenees, locality unknown). (e) Pinctada margaritifera. The inset is a detail of a triple junction between mineral grains. (f) Pinna nobilis. (g) Isognomon legumen. (h) Prismatic pearl of Pinna. The inset shows the organic pellicles which surround the crystalline domains. All surface views. Panels (e,g) have been slightly etched.
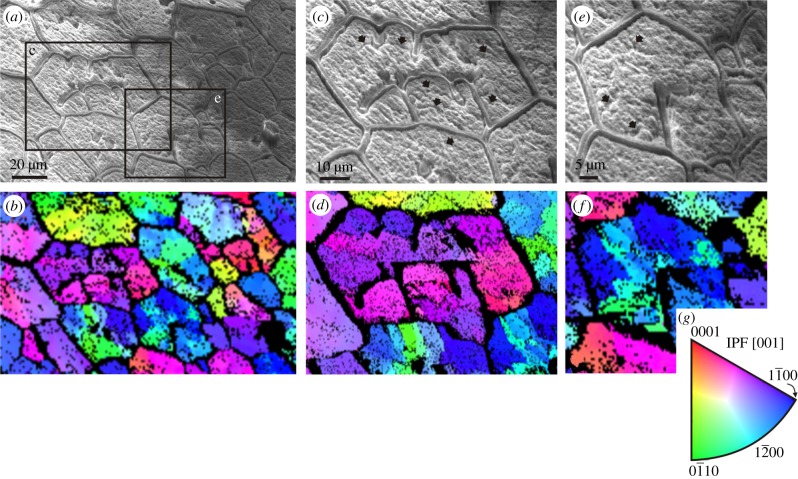
In the CCP layers of pteriomorphs, the mineral prisms can be a single crystal, or be polycrystalline. Evidence that the prisms contained within the organic membranes of some species of the pearl oyster Pinctada are polycrystalline is particularly clear. This is formally demonstrated with electron diffraction [23,24] (figure 2), but is also revealed by slight etching [25] (figure 1e). In Pinctada margaritifera prisms begin as single crystals with fluctuating crystallographic orientations, but soon begin to split progressively into different crystalline domains [24]. However, throughout this behaviour of the mineral phase, the organic membranes remain unaltered and the prisms develop with the prisms having a smaller surface area or fewer sides becoming preferentially extinguished (electronic supplementary material, figure S2). Except for rare instances of polycrystalline prisms (figure 1f), the prisms of Pinna are monocrystalline and retain consistent crystalline orientations throughout growth [24], such that it is in Pinna, impossible to differentiate prism dynamics from that of the organic membranes. Polycrystalline prisms have been observed in the CCP layers of oysters [26] and other pteriomorph bivalves (figure 1g) and, interestingly, are also commonly encountered in calcitic prismatic pearls attributed to Pinna (figure 1h). In all cases prism walls meet at 120°, and many walls are curved to meet this Plateau border condition. Nevertheless, this condition is not fulfilled for crystal domains meeting across prism boundaries (figures 1e, inset and 3e). Thus, judging from the cases in which the prisms are polycrystalline, organic membranes set the boundaries of mineral prisms and not the other way round.
Figure 2.
Electron back scatter diffraction maps of the internal surfaces of Pinctada margaritifera. Secondary electron images and corresponding orientation maps of a wide area (a,b) and two sub-areas indicated in a (c–f). The loose membranes are receding membranes. Same specimen as in figure 4a. Different colours indicate different crystal orientations (colour key provided in g). The individual cells contain many crystalline domains and the receding membranes act as boundaries between domains. Note also that the tips of membranes continue into contacts between domains. Upon etching, some crystal boundaries are evident in c,e (arrows).
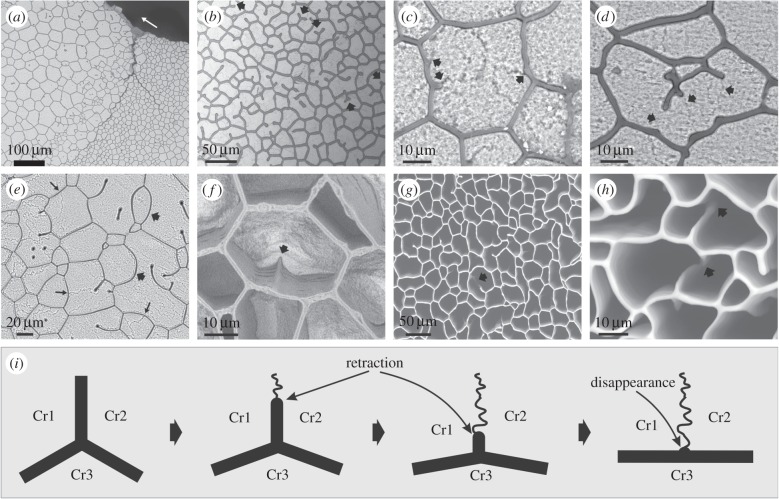
Examination of the internal shell surfaces of Pinctada reveals membranes running towards the prism interiors that incompletely divide a prism (figure 3a–f). Not infrequently membranes may even be isolated within prisms (figure 3b,e). Decalcification shows that these are receding membranes (figure 3f), which, in at least some instances, result from membranes formerly forming complete boundaries that have split and separated during growth (figure 3g,h). At a triple junction formed by an incomplete membrane with two other wall segments the dihedral angles tend to be similar (approx. 120°), although, in general, the angle between the other wall segments tends to increase with shortening of the receding membrane (figure 3c–e). When the receding membrane becomes very short or disappears, the angle between segments of the adjacent boundary membrane tends to the rectilinear (approx. 180°; figure 3c,d,f). With a reduction in the number of sides of a prism, many membranes become strongly curved (figure 3e). In all cases examined, the ends of the receding membranes coincide with domain boundaries of a prism, as revealed after slight etching (figures 2 and 3c–e). This is explicable as the two formerly separated crystalline domains progressively closing the gap left upon recession of the membrane. Note that when the process is complete, the crystals do not fulfil the Plateau Law any more, whereas this continues to hold for the membranes (figure 3c–e). This complete process is sketched in figure 3i.
Figure 3.
Instances of receding membranes in Pinctada margaritifera. (a) View of the transition of the normal cellular pattern to another pattern composed of wide cells with loose or incomplete membranes. The arrow points towards the shell interior. (b) General view of a similar pattern. Note the abundance of incomplete membranes forming triple junctions with the membrane walls. There are instances of incomplete trifurcate membranes within the cell interiors (arrows). (c,d) Close-up views of two cells with incomplete membranes, some of them about to disappear completely (c). The tips of some of them continue into wavy crystalline boundaries (arrows). (e) Particularly loose pattern. Some of the cells become particularly large (more than 100 µm). Note strongly curved walls (thick arrows). The normal pattern can be seen in the bottom left part of the image. The long thin arrows point towards triple junctions between mineral grains. (f) Semidecalcified specimen showing a receding membrane that finally disappears towards the internal shell surface. Note how the cell wall changes from angled (arrow) to flat with the disappearance of the loose membrane. (g) General view of a completely decalcified specimen showing abundant receding membranes. An instance of a residual nanomembrane that has survived decalcification is indicated (arrow). (h) Details of splitting membranes (arrows) in similarly decalcified specimens. (i) Sketch of the retraction and disappearance of a membrane at a triple junction (thick lines). The receding membrane leaves behind the contact between two crystalline domains (wavy line). With retraction of the membrane, the angle between the other two segments becomes wider, until they form a single flat membrane. Note that Plateau's Law fulfils only for membranes but not for crystalline domains (Cr1, Cr2, Cr3). Panels (a–h) are views of the internal shell (growth) surfaces.
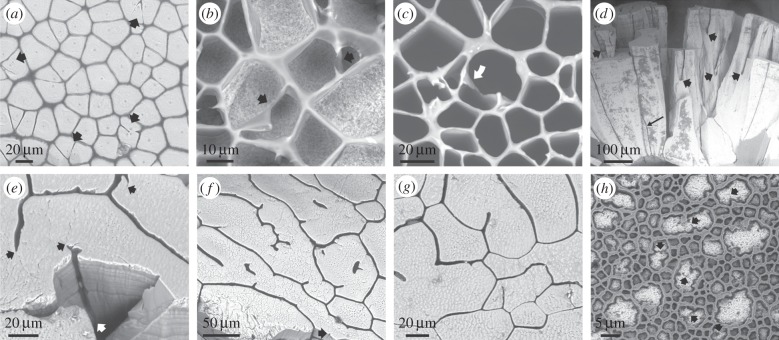
The reverse process, by which a new membrane extends to separate two crystalline domains previously in contact, is also observed. Observation of the external shell surface reveals the existence of prisms that contain two, three or, rarely, more crystalline domains (figure 4a). Incipient membranes may initially extend, or not, between these different domains. As the prismatic layer thickens, the membranes sometimes extend along the domain boundaries, until they may meet and fuse (figure 4b,c). In this way, an initial prism is divided into two, each of a single domain. Upon extension, the growing membrane deforms the parental membrane until producing a triple junction at or close to 120° (figure 4b). Calcitic prismatic pearls of Pinna are particularly interesting because, as usual, some prisms disappear, but, owing to the increase in surface area with pearl growth, prismatic units rather tend to split with the insertion of new organic membranes (figure 4d,e). The final pattern (figure 4e–g) tends to be much more irregular than in the Pinctada shell. Without exception, the tips of growing membranes continue into the boundaries between crystallographic domains (figure 4e–g), which are seen by the presence of nanometric membranes (figure 1h, inset). Some membranes may even begin in the interior of prisms, isolated from previous membranes, but always precisely at the boundaries between domains (figure 4f). Some particularly intricate and densely reticulated patterns are recorded in Pinc. margaritifera (figure 4h) in which, perhaps owing to the abundance of available organic matter, membranes intrude through the boundaries of the intraprismatic crystalline domains until almost all of them are surrounded by thick organic walls. The disappearance or production of membranes and, eventually, of new prisms brings about a local reshaping of the network topology, which clearly influences the sizes of crystalline domains adjacent to the intervening membranes. A model for either situation is depicted in figure 5. In order for the organic membranes locally to displace the crystalline domains they must include (presumably proteinic) components able to inhibit mineral growth. In summary, it is the organic membranes, and not the mineral domains, that produce the prismatic pattern typical of this biomineral composite. One further piece of evidence strengthens our conclusion: membranes can be found without accompanying mineral domains during shell regeneration (electronic supplementary material, figure S3a–c) or abnormal shell secretion (electronic supplementary material, figure S3d; see also [26]).
Figure 4.
Instances of advancing membranes in Pinctada margaritifera (a–c,h) and pearls of Pinna (d–g). (a) General aspect of the external shell surface, with the periostracum removed. Some prismatic units contain two or three crystals (arrows), which are partly fused. (b,c) Instances in which incomplete membranes meet and fuse during shell growth, thus delineating two different prismatic units. In (c), one of the incomplete membranes has rotated and stuck to the cell wall (arrow). (d) Radial fracture through a pearl of Pinna. During growth, some units wedge out (long thin arrow), but the pattern is dominated by the splitting of prismatic units owing to the initiation of organic membranes (thick arrows). (e) Oblique view of the fracture and surface of the same pearl as in (d). The membrane seen on the fracture surface initiates at the position of the white arrow. The tips of the membranes and of their minor branches consistently coincide with boundaries between crystalline domains (black arrows). (f,g) Two views of the growth surface of another pearl. As in (e), the tip of every membrane branch always continues into a nanocrystalline domain boundary. Isolated membranes appear within the cells interior in (f). The arrow in (f) points to the position of a very incipient subsidiary membrane. (h) Locally developed meshwork, possibly delineating individual crystalline domains (arrows point to some crystal boundaries). Panels (a–c) are views of the external shell surfaces, and (e–h) are views of the growth surfaces.
Figure 5.
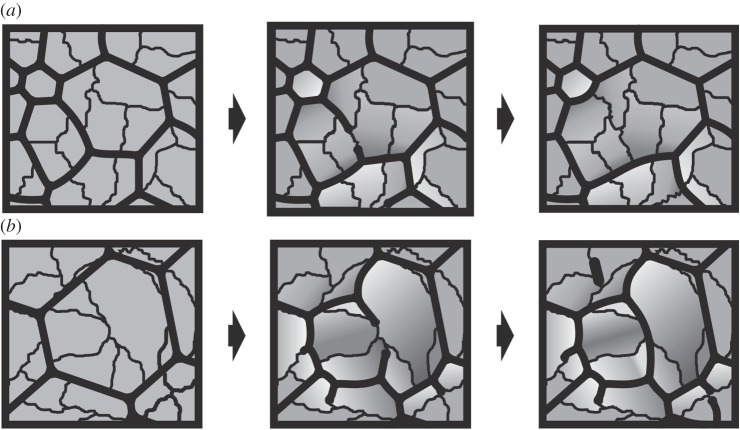
Model for the interaction between membranes and crystals in the CCP layer of bivalves. (a) Sequence for the disappearance of the membrane between two cells thus leading to their complete fusion onto a single one. Recession of the membrane causes the previously separated crystalline domains to come into contact. (b) Sequence for the division of cells owing to production of new membranes. These extend along the boundaries between crystalline domains. In the central cell, two membranes initiate at the opposite ends of a domain until they meet and fuse. Note how angles of triple junctions change with the origination or disappearance of membranes. Light grey indicates contracting crystalline areas and dark grey indicates expanding crystalline areas. See also figure 3i.
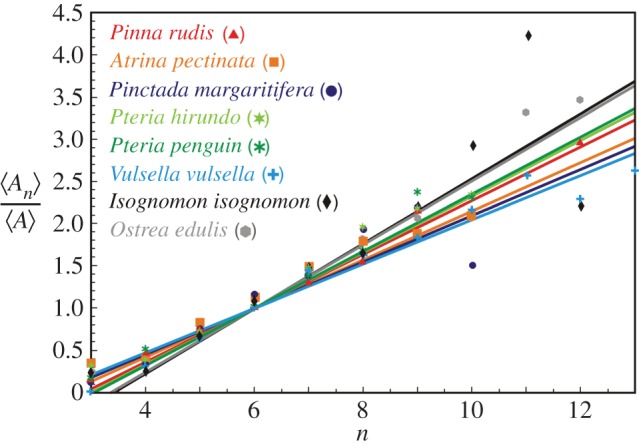
In a recent study on the calcitic prismatic layer of Pinna [11], the development of the prismatic biomineral aggregate was explained based on normal grain growth theory, by assuming that the increase of thickness of the layer is proportional to the passage of time. According to that hypothesis, the organic membranes, and not the mineral grains, are the passive elements [12]. We are proposing for the same biomaterials that, contrariwise, the main patterning agent is the development with time of the organic network. The organic material is made up of a two-dimensional fluid network within the extrapallial space between the shell and the soft body of the organism, otherwise filled with the extrapallial liquid. It has, therefore, the dynamics of a two-dimensional emulsion, which, like grain growth, belongs to the class of phenomena termed a ‘fluid foam’ or ‘cellular fluid’ that includes foams, emulsions, magnetic garnets and grain boundaries in crystals. This dynamics is governed by the von Neumann–Mullins topological Law [27,28], which states that those elements with a number of sides greater than six will grow at the expense of those that have less than six sides. We find concomitantly that the prismatic layers of distant molluscan species all obey the Lewis Law [29–31]; the average area of a polygon of n sides is proportional to n (figure 6).
Figure 6.
The Lewis Law 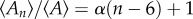 in the CCP layer of bivalves where n is the number of sides of a prism,
in the CCP layer of bivalves where n is the number of sides of a prism, 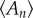 is the average area of prisms of n sides,
is the average area of prisms of n sides, 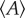 is the average area of prisms. The species analysed belong to different orders and superfamilies of the bivalve subclass Pteriomorphia: order Pteroida, superfamily Pinnoidea (Pinna rudis (gradient α = 0.32), Atrina pectinata (α = 0.29)), superfamily Pterioidea (Pinctada margaritifera (α = 0.27), Pteria hirundo (α= 0.33), Pteria penguin (α = 0.34), Vulsella vulsella (α = 0.26), Isognomon isognomon (α = 0.39)), order Ostreoida, superfamily Ostreoidea (Ostrea edulis (α = 0.38)). The relationship is linear for the common prisms with good statistics whose number of sides is close to six.
is the average area of prisms. The species analysed belong to different orders and superfamilies of the bivalve subclass Pteriomorphia: order Pteroida, superfamily Pinnoidea (Pinna rudis (gradient α = 0.32), Atrina pectinata (α = 0.29)), superfamily Pterioidea (Pinctada margaritifera (α = 0.27), Pteria hirundo (α= 0.33), Pteria penguin (α = 0.34), Vulsella vulsella (α = 0.26), Isognomon isognomon (α = 0.39)), order Ostreoida, superfamily Ostreoidea (Ostrea edulis (α = 0.38)). The relationship is linear for the common prisms with good statistics whose number of sides is close to six.
3. Conclusion
The mantle cells of the pearl oyster Pinctada radiata are in close proximity with the forming mineral prisms and the organic membranes [32]; that is, the extrapallial space thickness is nanometric. Let us perform some order-of-magnitude calculations to see how long it would take calcium and carbonate to diffuse across the extrapallial space, on the one hand, compared to how long it would take an initial homogeneous mixture of two immiscible phases to self-organize into structures of prism size, on the other. We have a layer—the extrapallial space—of height h ∼ 100 nm (10−7 m). Let us consider a structure that forms in this space—the prism size—of characteristic size d ∼ 10 µm (10−5 m). The diffusion time tD ∼ L2/D, where D is the diffusion constant. The surface-tension-driven velocity will scale as ∼γ/μ where γ is the surface tension and μ is the viscosity. This gives a timescale to compare against the diffusion time: a surface-tension-driven time tγ ∼ μ L/γ. Now let us compare: for CaCO3 crossing the extrapallial space, the diffusion constant is D ∼ 10−9 m2 s−1 (as for any small molecule or ion) and the diffusion time is tD ∼ 10−5 s. The surface-tension-driven time for the two phases to self-organize on a scale of d is μ d/γ, where the surface tension is γ ∼ 0.1 N m−1 (this is water-air, but surface tensions will be this or less) and the viscosity (of water) is 10−3 kg m s−1 so tγ = 10−7 s. Thus surface-tension-driven processes are much faster than diffusive processes, the unmixing of the initially homogeneous mixture is much faster than the diffusion time; so a self-organized mechanism is possible. Another possibility is that crystals and membranes are formed by direct deposition from the mantle cells. Given the relative sizes of prisms (tens of micrometres), membranes (1–3 µm) and mantle cells (5–10 µm) [32], every mantle cell must be secreting both organic and inorganic materials, depending on exactly which components it is in contact with. This contact is not permanent during shell secretion, because the mantle is periodically extruded and withdrawn. This implies that a recognition process must operate for mantle cells to continue the production of the prisms and membranes of the microstructure. Such a mechanism may explain how the new organic membranes extend exactly at the boundaries between crystalline domains: mantle cells must somehow be able to perceive the boundaries between the crystallographic domains and use them as signals to either extend previous or produce new membranes. This signal might well be the tiny organic pellicles trapped between crystallographic domains in Pinc. margaritifera previously defined [25] or recognized here in the pearls of Pinna (figure 1h, inset). The two mechanisms need not necessarily be competing hypotheses. During membrane production, the mantle cells only secrete, but do not influence the pattern of the organic network, which is only guided by physical laws. In a similar way, the crystals will also interact and compete for space, but only within the volumes set up by the organic cavities. In summary, besides physical constraints, there is a well-orchestrated subjacent cellular strategy. Finally, the fact that organic membranes advance, recede or remain stationary depends on the local availability of organic material. What is certain is that the membranes are very elastic and highly deformable. When we observe it under the microscope, the organic component is a solid, presumably owing to a solidification/polymerization process, currently unknown. The organic component we suppose is liquid when it is laid down; hence we assimilate it to an emulsion. A liquid precursor (PILP, polymer-induced liquid precursor, [33,34]) has been hypothesized to be generated by acidic biopolymers during biomineral formation [34,35]. Acidic proteins are usually occluded within biogenic calcite prisms (e.g. [36]), and there is good evidence for the occurrence of PILP-like intermediates during prism formation in Pinna [37]. The observations of internal surfaces and growth lines demonstrate that the organic membranes and the mineral prisms are always growing at the same level [26]. Therefore, the conditions for an emulsion between the organic and mineral (plus intracrystalline organic) phases to have developed could have been fulfilled during growth of the CCP layers.
CCP layers form extensive flanges or lamellae that act in bending upon closure of the shell, in order to provide a tight seal of the shell, effective against predators or water loss [38] (electronic supplementary material, figure S4). This ability to flex is provided by the deformability of the organic fraction [39]. CCP is possibly the richest in organic matter of all molluscan shell microstructures, with values around 10% (thermogravimetry data on Pinna nobilis; electronic supplementary material, figure S5). Because the metabolic cost of producing the organic fraction is much higher than that of the mineral component [40,41], this microstructure is particularly expensive. The organic membrane network evolves to lower the free energy of the system by reducing the area of the membranes. From the organismal viewpoint, this implies saving part of the energy required for its production.
This is a good example of how organisms make use of simple physical processes to adjust their physiological and adaptive requirements.
4. Material and methods
(a). Samples and sample preparation
Samples of Pinc. margaritifera (Pteriomorphia, Pteriidae) came from different sources, mainly Olango Island (Philippines) and French Caledonia. The prismatic pearls of Pinna (Pacific coast of Mexico) were provided by KCB Natural Pearls (San Francisco, CA, USA). All specimens are housed in the Department of Stratigraphy and Palaeontology, University of Granada (Spain). Partial or complete decalcification was carried out by immersion in 2–4% EDTA.
(b). Optical and scanning electron microscopy
For optical microscopy, we used a Nikon SMZ1000 binocular microscope equipped with a digital image acquisition system. Scanning electron microscopy (SEM) observations were carried out in a desktop SEM Phenom Pro (Department of Stratigraphy and Palaeontology, University of Granada) and in an Environmental SEM FEI Quanta 400 (CIC, University of Granada, Spain). Back scatter electron imaging was mainly used in order to enhance the contrast between the organic and mineral phases.
(c). Electron back scatter diffraction
Prior to analysis, samples of the internal surfaces of the shells of Pinc. margaritifera were cleaned with 5% NaOCl for 2 min. In order to expose crystal boundaries, they were subsequently etched with 4% EDTA for 2 min. In order to relate surface features to crystal distribution, samples were analysed unpolished. Since this technique is very sensitive to surface irregularities, the percentage of indexable patterns dropped drastically compared to polished samples, although the number of available data provided relevant information. To avoid excessive charging, samples were coated with a thickness of 2 nm of carbon in a Baltec MED 020 electron beam evaporator (CIC, University of Granada, Spain). Samples of Chama arcana (electronic supplementary material, figure S1) were analysed in a Zeiss Auriga Cross Beam work-station (operated at 20 kV) equipped with an Oxford Instruments Nordlysnano EBSD detector of the Centro de Instrumentación Científica (CIC), University of Granada, whereas samples of Pinc. margaritifera (figure 2) were analysed in an identical instrument of the CITIUS, University of Seville, under the same conditions.
(d). Thermogravimetry
The organic matter content of samples of the prismatic shell of Pinna nobilis (electronic supplementary material, figure S5) was determined using a thermogravimetric analyser (SHIMADZU TGA-50H; CIC, University of Granada) for a temperature range from 25°C to 950°C (rate 10°C min−1). Significant weight losses more than 200°C (when water loss is complete) and less than 600°C (when CaCO3 decomposition into CaO begins) are attributed to the combustion of organic matter.
(e). Numerical methods
We used SEM images covering areas approximately 600 × 400 µm of the prismatic layers of Atrina pectinata, Isognomon isognomon, Ostrea edulis, Pinc. margaritifera, Pinna rudis, Pteria hirundo, Pteria penguin and Vulsella vulsella. We processed the images using Mathematica. We binarized the images and performed a morphological component analysis to extract the connected image elements. From this, we obtained the areas and neighbour counts for the prisms, discounting those at the edges of images.
Supplementary Material
Acknowledgements
Special thanks are given to Isabel Sánchez-Almazo (Univ. Granada) for her help with scanning microscopy. Ana Vasiliu (IACT, Granada) kindly allowed the study of the Pinna pearls. We thank Stefan Hutzler and David Whyte (Trinity College Dublin) for advice on numerical analysis, and Silvana Cardoso (University of Cambridge) for discussions of fluid dynamics.
Data accessibility
Figures S1–S5 have been uploaded as part of the electronic supplementary material.
Authors' contributions
A.G.C., conception, design and coordination, acquisition of data, analysis and interpretation of data, drafting and revision of the article; E.M.-S., sample preparation, acquisition of data, analysis and interpretation of data, revision of the article. Both authors contributed equally to this study; E.M.H., acquisition of data, contribution of unpublished data, revision of the article for important intellectual content; J.H.E.C., critical revision of the article for important intellectual content; numerical analysis, fluid dynamics and condensed matter physics.
Competing interests
The authors have no competing financial interests.
Funding
This research was funded by projects CGL2013-48247-P (to A.G.C., E.M.-S. and E.M.H.) and FIS2013-48444-C2-2-P (to J.H.E.C.) of the Spanish Ministerio de Ciencia e Innovación, and RNM6433 of the Andalusian Consejería de Innovación Ciencia y Tecnología (to A.G.C., E.M.-S. and J.H.E.C.). A.G.C., E.M.-S. and J.H.E.C. also acknowledge the Research Group RNM363 (Junta de Andalucía).
References
- 1.Belcher AM, Wu XH, Christensen RJ, Hansma PK, Stucky GD, Morse DE. 1996. Control of crystal phase switching and orientation by soluble mollusc-shell proteins. Nature 381, 56–58. ( 10.1038/381056a0) [DOI] [Google Scholar]
- 2.Falini G, Albeck S, Weiner S, Addadi L. 1996. Control of aragonite or calcite polymorphism by mollusk shell macromolecules. Science 271, 67–69. ( 10.1126/science.271.5245.67) [DOI] [Google Scholar]
- 3.Mann S. 2001. Biomineralization. Principles and concepts in bioinorganic materials chemistry. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Weiner S, Dove PM. 2003. An overview of biomineralization processes and the problem of the vital effect. Rev. Mineral. Geochem. 54, 1–29. ( 10.2113/0540001) [DOI] [Google Scholar]
- 5.Cusack M, Freer A. 2008. Biomineralization: elemental and organic influence in carbonate systems. Chem. Rev. 108, 4433–4454. ( 10.1021/cr078270o) [DOI] [PubMed] [Google Scholar]
- 6.Nakahara H. 1991. Nacre formation in bivalve and gastropod molluscs. In Mechanisms and phylogeny of mineralization in biological systems (eds Suga S, Nakahara H), pp. 343–350. Berlin, Germany: Springer; ( 10.1007/978-4-431-68132-8_55) [DOI] [Google Scholar]
- 7.Cartwright JHE, Checa AG. 2007. The dynamics of nacre self-assembly. J. R. Soc. Interface 4, 491–504. ( 10.1098/rsif.2006.0188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harper EM, Checa AG, Rodríguez-Navarro AB. 2009. Organization and mode of secretion of the granular prismatic microstructure of Entodesma navicula (Bivalvia: Mollusca). Acta Zool. 90, 132–141. ( 10.1111/j.1463-6395.2008.00338.x) [DOI] [Google Scholar]
- 9.Checa AG, Harper EM. 2010. Spikey bivalves: intra-periostracal crystal growth in anomalodesmatans. Biol. Bull. 219, 231–248. [DOI] [PubMed] [Google Scholar]
- 10.Checa AG, Cartwright JHE, Sánchez-Almazo I, Andrade JP, Ruiz-Raya F. 2015. The cuttlefish Sepia officinalis (Sepiidae, Cephalopoda) constructs cuttlebone from a liquid-crystal precursor. Sci. Rep. 5, 11513 ( 10.1038/srep11513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayerlein B, Zaslansky P, Dauphin Y, Rack A, Fratzl P, Zlotnikov I. 2014. Self-similar mesostructure evolution of the growing mollusc shell reminiscent of thermodynamically driven grain growth. Nat. Mater. 13, 1102–1107. ( 10.1038/nmat4110) [DOI] [PubMed] [Google Scholar]
- 12.Sommerdijk NAJM, Cusack M. 2014. Crystals competing for space. Nat. Mater. 13, 1078–1079. ( 10.1038/nmat4147) [DOI] [PubMed] [Google Scholar]
- 13.Mutvei H. 1989. Structure of molluscan prismatic shell layers. In Origin, evolution and modern aspects of biomineralization in plants and animals (ed. Crick RE.), pp. 137–151. New York, NY: Plenum Press. [Google Scholar]
- 14.Fuchigami T, Sasaki T. 2005. The shell structure of the recent Patellogastropoda (Mollusca: Gastropoda). Paleontol. Res. 9, 143–168. ( 10.2517/prpsj.9.143) [DOI] [Google Scholar]
- 15.Taylor JD, Reid DG. 1990. Shell microstructure and mineralogy of the Littorinidae: ecological and evolutionary significance. Hydrobiologia 193, 199–215. ( 10.1007/BF00028077) [DOI] [Google Scholar]
- 16.Taylor JD, Kennedy WJ. 1969. The shell structure and mineralogy of Chama pellucida Broderip. Veliger 11, 391–398. [Google Scholar]
- 17.Harper EM. 1998. Calcite in chamid bivalves. J. Molluscan Stud. 64, 391–399. ( 10.1093/mollus/64.3.391) [DOI] [Google Scholar]
- 18.Goetz AJ, Steinmetz DR, Griesshaber E, Zaefferer S, Raabe D, Kelm K, Irsen S, Sehrbrock A, Schmahl WW. 2011. Interdigitating biocalcite dendrites form a 3-D jigsaw structure in brachiopod shells. Acta Biomater. 7, 2237–2243. ( 10.1016/j.actbio.2011.01.035) [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez-Navarro A, Kalin O, Nys Y, Garcia-Ruiz JM. 2002. Influence of the microstructure on the shell strength of eggs laid by hens of different ages. Br. Poultry Sci. 43, 395–403. ( 10.1080/00071660120103675) [DOI] [PubMed] [Google Scholar]
- 20.Pérez-Huerta A, Dauphin Y, Cuif JP, Cusack M. 2011. High resolution electron backscatter diffraction (EBSD) data from calcite biominerals in recent gastropod shells. Micron 42, 246–251. ( 10.1016/j.micron.2010.11.003) [DOI] [PubMed] [Google Scholar]
- 21.Fairchild IJ, Baker A. 2012. Speleothem science. From process to past environment. Chichester, UK: Wiley-Blackwell. [Google Scholar]
- 22.Perrin C, Prestimonaco L, Servelle G, Tilhac R, Maury M, Cabrol P. 2014. Aragonite-calcite speleothems: identifying original and diagenetic features. J. Sedim. Res. 84, 245–269. ( 10.2110/jsr.2014.17) [DOI] [Google Scholar]
- 23.Okumura T, Suzuki M, Nagasawa H, Kogure T. 2010. Characteristics of biogenic calcite in the prismatic layer of a pearl oyster, Pinctada fucata. Micron 41, 821–826. ( 10.1016/j.micron.2010.05.004) [DOI] [PubMed] [Google Scholar]
- 24.Checa AG, et al. 2013. Crystallographic orientation inhomogeneity and crystal splitting in biogenic calcite. J. R. Soc. Interface 10, 20130425 ( 10.1098/rsif.2013.0425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dauphin Y. 2003. Soluble organic matrices of the calcitic prismatic shell layers of two pteriomorphid bivalves Pinna nobilis and Pinctada margaritifera. J. Biol. Chem. 17, 15 168–15 177. ( 10.1074/jbc.M204375200) [DOI] [PubMed] [Google Scholar]
- 26.Checa AG, Rodríguez-Navarro AB, Esteban-Delgado FJ. 2005. The nature and formation of calcitic columnar prismatic shell layers in pteriomorphian bivalves. Biomaterials 26, 6404–6414. ( 10.1016/j.biomaterials.2005.04.016) [DOI] [PubMed] [Google Scholar]
- 27.Von Neumann J. 1952. Written discussion on a paper of C. S. Smith. In Metal interfaces (ed. Herring C.), pp. 108–110. Cleveland, OH: American Society of Metals. [Google Scholar]
- 28.Mullins WW. 1956. Two-dimensional motion of idealized grain boundaries. J. Appl. Phys. 27, 900–904. ( 10.1063/1.1722511) [DOI] [Google Scholar]
- 29.Lewis FT. 1931. A comparison between the mosaic of polygons in a film of artificial emulsion and the pattern of simple epithelium in surface view (cucumber epidermis and human amnion). Anat. Rec. 50, 235–265. ( 10.1002/ar.1090500303) [DOI] [Google Scholar]
- 30.Schliecker G. 2002. Structure and dynamics of cellular systems. Adv. Phys. 51, 1319–1378. ( 10.1080/00018730210140814) [DOI] [Google Scholar]
- 31.Cerisier P, Rahal S, Rivier N. 1996. Topological correlations in Bénard-Marangoni convective structures. Phys. Rev. E 54, 5086–5094. ( 10.1103/PhysRevE.54.5086) [DOI] [PubMed] [Google Scholar]
- 32.Nakahara H, Bevelander G. 1971. The formation and growth of the prismatic layer of Pinctada radiata. Calcif. Tiss. Res. 7, 31–45. ( 10.1007/BF02062591) [DOI] [PubMed] [Google Scholar]
- 33.Gower LB, Odom DJ. 2000. Deposition of calcium carbonate films by a polymer-induced liquid-precursor (PILP) process. J. Cryst. Growth 210, 719–734. ( 10.1016/S0022-0248(99)00749-6) [DOI] [Google Scholar]
- 34.Gower LB. 2008. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem. Rev. 108, 4551–4627. ( 10.1021/cr800443h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schenk AS, Zope H, Kim Y-Y, Kros A, Sommerdijk NAJM, Meldrum FC. 2012. Polymer-induced liquid precursor (PILP) phases of calcium carbonate formed in the presence of synthetic acidic polypeptides—relevance to biomineralization. Faraday Discuss. 159, 327–344. ( 10.1039/c2fd20063e) [DOI] [Google Scholar]
- 36.Marin F, Luquet G, Marie B, Medakovic D. 2008. Molluscan shell proteins: primary structure, origin, and evolution. Curr. Top. Dev. Biol. 80, 209–276. ( 10.1016/S0070-2153(07)80006-8) [DOI] [PubMed] [Google Scholar]
- 37.Wolf SE, Lieberwirth I, Natalio F, Bardeau J-F, Delorme N, Emmerling F, Barrea R, Kappl M, Marin F. 2012. Merging models of biomineralisation with concepts of nonclassical crystallisation: is a liquid amorphous precursor involved in the formation of the prismatic layer of the Mediterranean fan mussel Pinna nobilis? Faraday Discuss. 159, 433–448. ( 10.1039/c2fd20045g) [DOI] [Google Scholar]
- 38.Vermeij GJ. 1987. Evolution and escalation: an ecological history of life. Princeton, NJ: Princeton University Press. [Google Scholar]
- 39.Harper EM, Skelton PW. 1993. The Mesozoic marine revolution and epifaunal bivalves. Scripta Geol. Spec. Issue 2, 127–153. [Google Scholar]
- 40.Palmer AR. 1983. Relative cost of producing skeletal organic matrix versus calcification: evidence from marine mollusks. Mar. Biol. 75, 287–292. ( 10.1007/BF00406014) [DOI] [Google Scholar]
- 41.Palmer AR. 1992. Calcification in marine molluscs: how costly is it? Proc. Natl Acad. Sci. USA 89, 1379–1382. ( 10.1073/pnas.89.4.1379) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Figures S1–S5 have been uploaded as part of the electronic supplementary material.