Abstract
Female mating preferences can influence both intraspecific sexual selection and interspecific reproductive isolation, and have therefore been proposed to play a central role in speciation. Here, we investigate experimentally in the African cichlid fish Pundamilia nyererei if differences in male coloration between three para-allopatric populations (i.e. island populations with gene flow) of P. nyererei are predicted by differences in sexual selection by female mate choice between populations. Second, we investigate if female mating preferences are based on the same components of male coloration and go in the same direction when females choose among males of their own population, their own and other conspecific populations and a closely related para-allopatric sister-species, P. igneopinnis. Mate-choice experiments revealed that females of the three populations mated species-assortatively, that populations varied in their extent of population-assortative mating and that females chose among males of their own population based on different male colours. Females of different populations exerted directional intrapopulation sexual selection on different male colours, and these differences corresponded in two of the populations to the observed differences in male coloration between the populations. Our results suggest that differences in male coloration between populations of P. nyererei can be explained by divergent sexual selection and that population-assortative mating may directly result from intrapopulation sexual selection.
Keywords: mate choice, assortative mating, sexual selection, reproductive isolation, sensory drive, cichlid
1. Introduction
A broad range of animals has evolved a remarkably diverse set of secondary sexual signals that are used in mate choice both at the intra- and interspecific level [1]. Such sexual signals can diverge between populations and species, and thereby play an important role in the origin and maintenance of premating reproductive isolation, facilitating speciation and species coexistence [2–4]. Several mechanisms can drive signal divergence between populations, including mutation and random genetic drift [5,6], run-away sexual selection for novel or exaggerated signals [7,8], ecologically mediated sensory bias sexual selection (i.e. sensory drive [9]) and ecological selection (e.g. differential predation [10]) (reviewed in [11]). Sensory drive has been shown in many different animal groups, including birds [12], reptiles [13], insects [14] and fish [10]. Sensory drive suggests that habitat heterogeneity will cause divergent selection on sensory systems and/or signals associated with mate choice, and hence affect mating preferences, potentially facilitating speciation [9,15]. The strongest evidence for sensory drive in visual communication, showing a link between the divergence both in signal and visual system and reproductive isolation, comes from fish (stickleback [16] and cichlid [17]).
In haplochromine cichlid fish of the East African Lakes, which are known for their extensive diversification in secondary sexual and ecological traits [18], visual signals can be a target of sexual selection by female mate choice at both the intra- and interspecific level (i.e. within populations, and between populations and species; reviewed in [19]). It has been suggested that female choice for male coloration might play a key role in the evolution of reproductive isolation and speciation of African cichlids [20–23].
Cichlids in the genus Pundamilia have been intensively studied to test this hypothesis (reviewed in [24]). In the genus Pundamilia, it has been shown that reproductive isolation by female mate choice most likely arose as a consequence of a sensory drive divergence process [17]. The species divergence in male coloration and female mating preferences observed between the sister-species Pundamilia pundamilia (primarily blue nuptial coloration) and P. nyererei (primarily red and yellow) correlates with divergent underwater light regimes and divergent visual sensitivities. The two species are geographically fully sympatric at several islands in Lake Victoria, but within an island, they inhabit subtly different depth ranges with different light environments [25]. Behavioural mating preference and mate choice experiments have shown that the differences in colour between males of the two species are necessary and sufficient for species-assortative mating [26,27]. Pundamilia nyererei inhabits deeper waters with more red-shifted light conditions and has a more red-shifted retinal visual pigment composition than P. pundamilia [17,28,29]. The extent of differentiation in visual sensitivity to certain wavelengths of light and the strength of reproductive isolation in this species pair both vary with the extent of depth habitat differentiation between the species, such that they show reduced or no reproductive isolation where the difference in depth and in visual sensitivity is smaller or absent [17]. The extent of differentiation in depth habitat, visual sensitivity and reproductive isolation between the two species also covaries positively with water transparency at different islands [17]. In one of the two species, namely P. nyererei, the populations from turbid and clear water islands differ in the expression patterns of retinal visual pigments [28,29] and in some components of male coloration [30]. The coloration covaries positively with water transparency, such that the colours red and yellow are more saturated, shifted towards longer wavelengths (i.e. redder) and less variable (i.e. similar hue values) in populations that inhabit clear waters compared with populations from more turbid waters [30]. This correlates with a dramatic change in the environmental light spectrum from turbid to clear water islands [21,30]. It has been proposed that this might generate divergent selection between para-allopatric populations (i.e. island populations with gene flow) of P. nyererei and that the observed differences in colour between these conspecific populations are adaptations to different underwater light environments [30,31].
Here, we test if the observed divergence in components of male coloration between island populations of P. nyererei may be explained by intraspecific sexual selection that may be divergent between populations. Previous studies in two populations of P. nyererei have demonstrated directional sexual selection on male coloration, such that when females from a moderately turbid and a clear-water population could choose among two own-population males they both showed a preference for redder males, and additionally the females from the clear water population preferred deeper black and deeper yellow males [32,33]. We expand on these findings and test if possible population differences in intraspecific mating preferences for components of male coloration predicts interpopulation differences in coloration. We further investigate if interpopulation and interspecific mating preferences are based on the same components of male coloration and go in the same direction as intrapopulation sexual selection within the three populations of P. nyererei. Compared with the behavioural preference test studies of Maan et al. [32,33], we measured several more colours in males and investigated whether variation in male colours within and between populations predicts male mating success in real mate-choice experiments. We allowed females of three different island populations of P. nyererei to choose among two males each of their own and the other two populations, and among two males of a geographically nearby population of the closely related sister-species P. igneopinnis. We included males of the species P. igneopinnis in our experiment because it resembles P. nyererei in ecology, morphology and visual system [17], and the main difference between the two species is in male coloration and the structure of the habitats they inhabit [34,35]. Pundamilia igneopinnis tends to live at steeper sloping shores and whereas the male nuptial coloration of P. nyererei consists mainly of red, yellow and black that of P. igneopinnis consists almost completely of black with only small patches of yellow to red on the body but bright orange fins. Hence, we aimed additionally at testing if intraspecific mating preference for blacker males, previously demonstrated in P. nyererei [32,33], may result in heterospecific matings with the nearly black males of the para-allopatric P. igneopinnis.
2. Material and methods
(a). Study species
Pundamilia nyererei [36] and P. igneopinnis [35] are members of the mbipi (rock-dwelling) group of haplochromine cichlids endemic to Lake Victoria, East Africa. The populations of P. nyererei and P. igneopinnis inhabit rocky shores of islands in the southeastern parts of Lake Victoria [34]. We compared mate choice of females of para-allopatric populations [37] of P. nyererei from three different islands that vary in water transparency: Python island (moderately turbid water [21]), Makobe island and Senga point (clear water [21] and [38], respectively]). Senga point is a rocky outcrop at the tip of a peninsula. The population of P. igneopinnis comes from Igombe Island, situated halfway between Senga Point and Makobe Island. Populations from three of the four sites inhabit relatively deep (Python (0.5–6 m), Makobe (4–12 m), Igombe (2–5 m), Senga (1 m to more than 4 m)) and red-shifted (in terms of wavelengths of light) waters. We will refer hereafter in the figures and tables to the P. nyererei populations from Makobe as PNM, from Python as PNP, from Senga as PNS and P. igneopinnis as PII. The majority of haplochromine cichlids (including the species in our study) are maternal mouthbrooders and sexually dimorphic in a number of characters (e.g. size, colour and behaviour) [39].
Females in P. nyererei are, in general, cryptically yellowish brown with dark vertical bars on the flanks and show no strong variation in morphology or colour. Previous studies in Pundamilia suggest that males do not visually discriminate between females of own and other species based on colour or morphology [26,27]. In general, the males of P. nyererei are yellow between the bars on the lower flanks, but red around and above the lateral line. The dorsal, anal and caudal fins are orange to red and the pelvic fin is black. The males of the three populations of P. nyererei show some differences in hue, saturation and the extent of the different colours [30]. P. igneopinnis consists almost completely of black coloration with only small patches of yellow to red on the body but bright orange fins. A detailed description and representative photos depicting the differences in colour observed between the three populations of P. nyererei and of P. igneopinnis can be found in electronic supplementary material, figure S1.
(b). Housing
We used laboratory-bred (Fx) fish from large stock populations (approx. n = 100). Fish were raised and kept in groups containing both sexes of only one population. Tanks were part of a central recirculation system. The fish had no direct previous experience with individuals from other populations or species. Both the stock aquariums and the experimental aquarium had water temperature at 25 ± 2°C and a 12 : 12 h light/dark cycle.
(c). Experimental set-up
We used a full-contact, partial-partition mate-choice design conducted in a large octagonal aquarium (approx. 10 m and 5 m in outer and inner circumference, respectively, 0.8 m in width and 0.8 m in height), which contained for each experiment females of a single population of P. nyererei and eight males that held non-overlapping circular territories (1 m in diameter, 0.8 m in height). Each male was enclosed in a net cage with mesh sizes that allowed the smaller females to enter and leave the cage, while confining the larger males inside their territories. The cages included a PVC tube as a refuge to motivate territoriality. The position of the males in the cages was randomized in every replicate. A replicate always consisted of a unique combination of males. For all replicates (with one exception), we had two males (i.e. a male-pair) each of the three P. nyererei populations and of P. igneopinnis. For one replicate with females from Makobe, only one male of P. igneopinnis was available. For a detailed description on the experimental procedure and data collection, see electronic supplementary material.
The experiments with the females of Makobe were carried out in two different time periods: October to December 2008 and January to March 2012 (referred to hereafter in all tables as PNM_1 and PNM_2, respectively). The females of Senga were tested from March to May 2012, and the females of Python from June to September 2012.
(d). Parentage assignment
Five microsatellites (pPun05, pPun07, pPun17, pPun21, pPun32) were genotyped and used for the parentage assignment. A detailed genotyping protocol is described by Selz et al. [40]. On average, a total of 8 ± 3 (standard deviation, s.d.), that is, 34 ± 19% (s.d.) (electronic supplementary material, table S4), of the fry per clutch were genotyped, as well as all mothers and potential fathers. We repeated the PCR amplification and genotyping deliberately for all parents at least twice. Genotypes were visualized using the program GeneMarker and scored manually. The assignment of offspring and parents was performed using a parental-exclusion program running in Visual Basic for Excel (VITASSIGN V8-5.1 [41]). We allowed for up to two mismatches to assign a sire [41] (electronic supplementary material, table S4).
(e). Colour analysis
All males were photographed after each replicate was terminated (i.e. a female had spawned) under standard light conditions with the same SLR camera (Canon 60D) and 50 mm macro lens (Canon). The aperture was set to 10 and ISO to 100. In Photoshop (Adobe Systems Inc.), white balance was adjusted in each photo to the white standard (Kodak) that was attached to the front side of the cuvette. Afterwards, the photos were cropped to include only the fish's body including the eyes and the fins (except for the pelvic fins) to measure the different colours in ImageJ with the colour criteria defined in electronic supplementary material, table S5. Coloration is composed of a combination of hue (colour), saturation (chroma) and brightness [42], and we adjusted these three parameters accordingly in ImageJ for each colour component (electronic supplementary material, table S5). With the aid of an attached colour strip, the parameters to delineate the different colours were defined, whereby all white-balanced photos were screened.
Compared with previous studies on P. nyererei populations [32,33], we used slightly different and refined criteria for all three parameters (hue, saturation and brightness) of the different colours (electronic supplementary material, table S5). We divided the hue range of the colour ‘red’ defined in previous studies by Maan et al. [32,33] into three different ranges resulting in the colours ‘red’, ‘orange’ and ‘magenta’. A further colour component that we added was ‘blue’, and we used the same definition of ‘yellow’ and ‘black’ as did Maan et al. [32,33]. We calculated the percentage of the fish body covered by the different colours by dividing the number of pixels of each colour component by the total number of pixels (i.e. the whole body of the fish). The percentage of body coverage will hereafter be referred to as redscore, orangescore, yellowscore, bluescore, magentascore and blackscore. Colour analyses were done for all three population mate choice experiments except for those from the experimental period PNM_1. Colour analyses were not conducted on males from the PNM_1 experimental period, because the photos were not taken under standardized light conditions and camera settings.
(f). Statistical analysis
Statistical analyses were carried out in R (v. 3.2.2.). Generalized linear mixed-effects models (GLMMs) were used, in which the factor replicate and nested within it the factor spawning were included as random effects to account for the unique combination of males and the number of spawning in each replicate. All models were tested for overdispersion (none of the models were significantly overdispersed). The mate choice data were coded as 1 for males that sired a clutch and 0 for males that did not sire a clutch, and these binomial data were analysed with GLMM's with logistic link function using the R-package lme4. Model selection was performed with a backward approach (unless otherwise mentioned) using the drop-function in R, whereby explanatory variables with the highest p-value were removed as long as the model likelihood (AIC) increased.
Three separate principal component analyses (referred to as population-specific principal component analyses, PCAs) were done on all P. nyererei males from each of the three P. nyererei population female mate choice experiments to see if females of each of the three populations had a similar choice of male colour variation distributed similarly over males of the three populations of P. nyererei.
To test for intraspecific sexual selection in each population mate choice experiment, we used all male colour traits (after scaling each colourscore to the mean and standard deviation) as explanatory variables in a GLMM. We included all homotypic spawnings in the model to test if females showed sexual selection for certain colourscores between the homotypic male pairs. Backward model selection was used for the PNM and PNP mate choice experiments. However, for the population of PNS, the full model containing all six colourscores could not converge, and hence model selection was performed with a forward approach, whereby one variable at a time was first tested and then a second variable was added to the one-variable model with the lowest AIC. This procedure persisted until adding an extra variable would no longer increase the model likelihood (AIC). Furthermore, in the model selection of the PNP mate choice experiment, the model with the lowest AIC resulted in a model with four colour variables for which individual parameters could not uniquely be determined. We used a ‘forced’ approach, in which we removed one of the four variables, which resulted in models with only three of the four variables that each gave estimates for the individual parameters.
To test for population-assortative mating, we tested with a binomial test if females of each P. nyererei population significantly preferred to mate with homotypic males over heterotypic males of each of the other P. nyererei populations and over males of both of the other populations. Furthermore, to test if colourscores can predict the population-assortative mating patterns, we used the first two principal components derived from the population-specific PCAs as explanatory variables in a GLMM. Three separate PCAs (referred to as population-specific PCAs) were done on all P. nyererei males from each of the three P. nyererei population mate choice experiments. Based on scree plots and the percentage of variation explained, the first two principal components from each of these population-specific PCAs were used to plot the colour-space occupied by the three populations and used as explanatory variables in GLMMs.
To test for interspecific assortative mating we used a binomial test to test whether females of each P. nyererei population significantly preferred to mate with males of P. nyererei over males from P. igneopinnis.
3. Results
(a). Testing for intraspecific sexual selection
We tested for each population separately if females were consistent in their choice with regard to differences in phenotypic traits (i.e. colour) between the two homotypic males. Females of all three populations exerted intraspecific directional sexual selection on colour. The direction of this selection and the targeted male colours differed between populations.
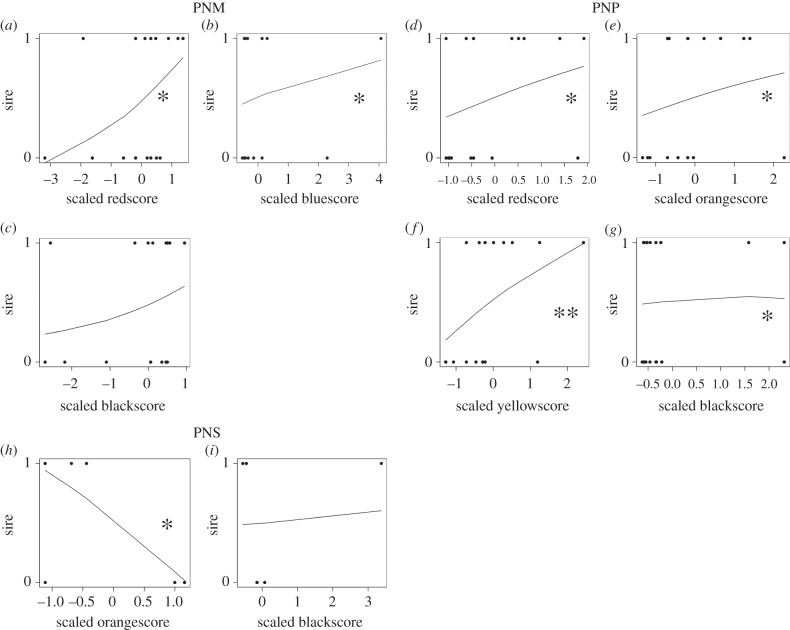
In the most likely model females from Makobe showed significant mating preferences for homotypic males with higher redscores (p = 0.016) and bluescores (p = 0.013), whereas blackscore explained a non-significant part of the variation in mating (p = 0.193; electronic supplementary material, table S1 and S2). Hence, females from Makobe preferred to mate with homotypic males with significantly higher red- and bluescores and non-significant higher blackscores (figure 1a–c).
Figure 1.
Results of the tests if females from the three populations of P. nyererei exerted directional intrapopulation sexual selection on different male colours. Fitted lines show predicted probabilities to be a sire based on the colourscores from the most likely GLMM model for each population mate choice experiment (PNM (a–c), PNP (d–g), PNS (h–i)). p-values derive from the most likely GLMM model explaining female mate choice except for PNP, where the p-values are derived from models with ‘forced’ backward selection (see Material and methods, electronic supplementary material, table S1). •p < 0.1, *p < 0.05, **p < 0.05.
For females from Python island the most likely model contained the explanatory variables red-, orange-, yellow- and blackscore (AIC = 27.5; electronic supplementary material, table S1 and S2). However, in this model, individual parameters could not uniquely be estimated most likely owing to the fact that the four variables are highly correlated (electronic supplementary material, table S2). Removal of one of the four variables (i.e. ‘forced’ backward selection) resulted in highly significant effects of all or most of the other explanatory variables with one exception (electronic supplementary material, table S2), but the model fit decreased (AIC range between 42.2 and 63.6; electronic supplementary material, table S1). Hence, Python females showed significant mating preferences for homotypic males with higher red-, orange-, yellow- and blackscores (figure 1d–g).
The most likely model could not be identified with a backward selection approach for females from Senga island, because the model containing all six variables did not converge. Instead, a forward selection approach yielded a most likely model containing the colourscores orange and black. Orangescore explained a significant fraction of the variation in mating (p = 0.025), whereas blackscore on its own did not (p = 0.178). Females from Senga showed mating preferences for homotypic males with significantly lower orangescores and non-significant higher blackscores (figure 1h–i).
(b). Population differences in Pundamilia nyererei male colour
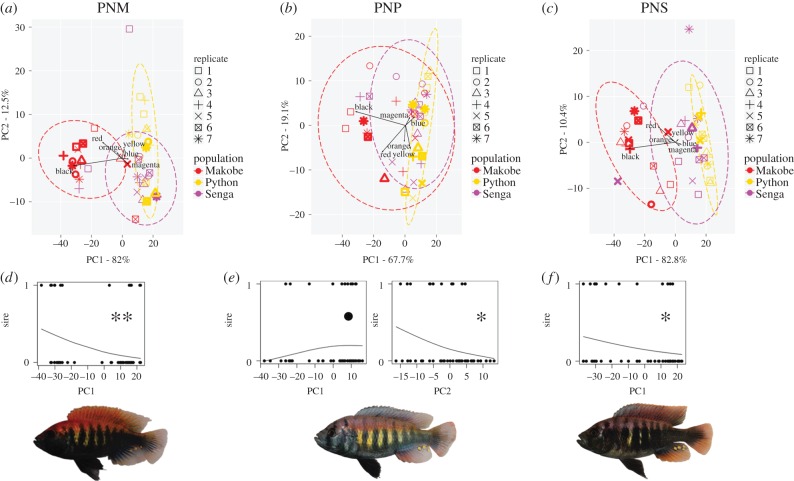
The PCA plots from the population-specific PCAs on the colour scores showed that females of all three populations were choosing among males from the three populations with similar colour variation (figure 2a–c). The PCA plots in all three mate choice experiments revealed that the Python population occupied a relatively unique part of the colour-space. The Makobe population also occupied a relatively unique part of the colour-space, but some of the males of the Makobe population showed an overlap with the Python population. The Senga population overlapped equally with both other populations.
Figure 2.
Population-specific PCA bi-plots on all P. nyererei males from each of the three P. nyererei population female mate choice experiments (PNM (a), PNP (b), PNS (c)). Sires in each female mate choice experiment are highlighted with bold symbols. The black arrows represent the colour variables in PCA space. Below are the GLMM results from the three female mate choice experiments (PNM (d), PNP (e), PNS (f)). The plots of the predicted probability to be a sire show that either one PC axis or both PC axes explained the positive population assortative mating in PNM and PNP and the negative assortative mating in PNS. Representative photos of a homotypic male are given below the probability plots for the female mate choice experiments (PNM (d), PNP (e), PNS (f)). •p < 0.1, *p< 0.05, **p < 0.05.
(c). Between population variation in the extent of population-assortative mating
Makobe and Python females showed significant tendencies towards population-assortative mating, albeit incomplete.
Trials with Makobe females were done in two different time periods (PNM_1 and PNM_2). During the first phase (PNM_1), in a total of nine replicates six Makobe females mated with a Makobe male and two each mated with a Python or Senga male. In the second phase (PNM_2), in a total of seven replicates, 13 Makobe females mated with a Makobe male, four with a Python male and one with a Senga male. The distribution of mating decisions of Makobe females did not differ significantly between the two phases (PNM_1 versus PNM_2, χ2-test, 6–2–2 versus 13–1–4, Chi = 2.37, p = 0.306); therefore, the datasets were combined. Makobe females showed a moderate tendency for population-assortative mating, choosing homotypic males in nineteen out of 28 spawnings (68% assortative, PNM versus PNP + PNS = 19 versus 9, p = 0.087). In a total of 16 replicates 19 Makobe females mated with Makobe males, six with Python males, and three with Senga males. Females from Makobe significantly preferred to mate with homotypic males over males from either Python or Senga (PNM versus PNP = 19 versus 6, p = 0.015; PNM versus PNS = 19 versus 3, p < 0.001). In the most likely GLMM model explaining female mate choice, females from Makobe showed significant mating preferences for males expressing more negative values on the principal component axis 1 (PC1) derived from the population-specific PCA (p < 0.001; electronic supplementary material, table S1 and S2 and figure 2d). Two of the three colours (redscore and blackscore, respectively) that are under positive directional sexual selection in males from Makobe have a strong negative loading on PC1 (redscore = −0.362, blackscore = −0.907; electronic supplementary material, table S3). Yet bluescore, which is also under positive directional sexual selection in males from Makobe, shows a weak positive loading on PC1 (bluescore = 0.128; electronic supplementary material, table S3).
Python females showed strong population-assortative mating, choosing homotypic males in 20 out of 23 spawnings (87% assortative). In a total of seven replicates, 20 Python females mated with a Python male and three with a Makobe male. None mated with a Senga male (PNP versus PNM = 20 versus 3, binomial test p < 0.001). For females from Python island, the most likely GLMM model explaining female mate choice contained the explanatory variables PC1 and PC2. Females from Python showed a trend of preferring mates with positive values on PC1 and showed significant mating preference for males with negative values on PC2 (PC1: p = 0.073, PC2: p = 0.001; electronic supplementary material, table S2 and figure 2e). Three of the four colours (red-, orange- and yellowscore, respectively) that are under positive directional sexual selection in males from Python have a strong negative loading on PC2 (redscore = −0.623, orangescore = −0.388, yellowscore = −0.547; electronic supplementary material, table S3). Yet blackscore, which is also under positive directional sexual selection in males from Python shows a positive loading on PC2 (blackscore = 0.305; electronic supplementary material, table S3).
Finally, Senga females mated randomly among homotypic and heterotypic males of the three P. nyererei populations and actually showed a tendency for negative assortative mating choosing homotypic males in seven out of 23 spawnings (29% assortative, PNS versus PNM + PNP = 7 versus 16, p = 0.093). In fact, they showed a weak, non-significant mating preference for males from Makobe. In a total of seven replicates, seven Senga females mated with Senga males, 13 with Makobe males and three with Python males (PNS versus PNM = 7 versus 13, p = 0.263; PNS versus PNP, 7 versus 3, p = 0.344). This is also reflected in the most likely GLMM model explaining female mate choice for females from Senga. Females from Senga showed significant mating preferences for males with negative values on PC1 (p = 0.013; electronic supplementary material table S3 and figure 2f). The two colourscores that load heavily negative on PC1 are red- and blackscore (redscore = −0.355, blackscore = −0.926; electronic supplementary material, table S3), which are the colours that most strongly distinguish between the Makobe population and the other two populations on the PCA bi-plots (figure 2c). Blackscore is under non-significant positive directional sexual selection in males from Senga, whereas orangescore is under significant negative directional sexual selection in males from Senga and has a very marginal negative loading on PC1 (orangescore = −0.07; electronic supplementary material, table S3).
(d). Species-assortative mating
Pundamilia nyererei females from all three populations showed strong to complete species-assortative mating, mating more often with males of P. nyererei than with P. igneopinnis (all: p < 0.001). Both Makobe and Python females showed complete species-assortative mating, exclusively mating with males of P. nyererei and never with males of P. igneopinnis (100% assortative). Senga females showed strong interspecific assortative mating, mating only once with a P. igneopinnis male (96% assortative).
4. Discussion
Sexual selection has been proposed to play an important role in speciation in animals [2–4]. It is often thought to contribute to speciation via the coevolution of male signals and female mating preference for those signals, resulting in behavioural isolation between divergent lineages [1,2,7,43]. In cichlid fish, male visual signals (i.e. nuptial coloration) have been shown to be a target of sexual selection by female mate choice both at the intra- and interspecific level [19], and female choice for male coloration has been suggested to promote speciation in African cichlids [20–23]. Yet it has rarely been tested whether interpopulation and interspecific assortative mating preferences might result from intrapopulation sexual selection [19,44].
Here we show in a mate-choice experiment first that female mate choice in three para-allopatric populations of the cichlid fish P. nyererei exerts directional sexual selection on different male colours. Second, we show that females of the three populations show variable levels of assortative mating among populations. Populations showed a continuum from negatively assortative mating (females from Senga, 29% homotypic matings), to a moderate tendency of population-assortative mating (females from Makobe, 68% homotypic matings) and finally a strong tendency of population-assortative mating (females from Python, 87% homotypic matings). Third, we show that females of all three populations mated species-assortatively, mating exclusively (Makobe and Python females, 100% assortative) or nearly so (Senga females, 96% assortative) with P. nyererei males rather than with males of the closely related, para-allopatric species P. igneopinnis. Fourth, in the two populations that showed population-assortative mating (i.e. Makobe and Python), the colours that are under directional sexual selection to some extent predicted population-assortative mating (i.e. loadings on the PC-axes).
Females of all three P. nyererei populations exerted directional sexual selection on components of male coloration, but females of different populations selected for different components. The differences in male coloration that we observed between populations correspond to some extent to the colours that we found to be under directional sexual selection within the populations of Makobe and Python. Specifically, females from Makobe preferred redder, blacker and bluer homotypic males and PC1, which significantly predicted the population-assortative mating of females from Makobe, had strong loadings in the same direction of red- and blackscore with low loadings of bluescore in the opposite direction. The females from Python preferred males with more red, orange, yellow and black and PC2, which predicted significantly the population-assortative mating of females from Python had strong loadings in the same direction for red-, yellow- and orangescore with minor loading for blackscore in the opposite direction. The intraspecific differences in directional sexual selection on certain male colours between two of the three populations (i.e. Makobe and Python) may partly explain the population-assortative mating in females from Makobe and Python and the differences in coloration between these populations.
Our colour-space analysis revealed that the three populations of P. nyererei occupy to various extents unique parts of colour-space. The distribution of males in the total colour-space of the three populations suggests that the populations of Makobe and Senga are composed of two colour-morphs of males, one with a darker underside (i.e. black) that represents the relatively unique colour-space of Makobe males and one with a lighter underside (i.e. blue) that represents the relatively unique colour-space of Python males. Several populations of P. nyererei including the population of Python are known to feature males with blue undersides and other populations including Makobe are known to feature black underside males [33,35]. Our data suggest that both colour-morphs can exist within individual populations (Senga, Makobe) and that females of all populations mate with males of both colour-morphs, but in very different proportions.
Females of the different P. nyererei populations did not only show intraspecific differences in directional sexual selection and intraspecific assortative mating, but also strong interspecific-assortative mating. Females of all three populations mated species-assortatively, choosing to mate exclusively (Python and Makobe) or almost so (Senga) with P. nyererei males rather than with males of the closely related, para-allopatric sister-species P. igneopinnis. Males of all three populations have a common colour component found on the dorsum and in the dorsal fin (i.e. a colour range from red to orange to magenta). This colour component is also present in P. igneopinnis males, but much less extensive. This main difference in colour between the two species could possibly underlie species-assortative mating of P. nyererei females. Pundamilia igneopinnis males are completely black on the whole body and bright orange is confined to the edge and posterior part of the dorsal fin. Red coloration on the dorsum in P. igneopinnis is confined to dorsal areas on the head.
Previous studies by Maan and co-workers demonstrated that the colours red, yellow and black are under directional sexual selection in P. nyererei populations [32,33]. Based on behavioural preference experiments, they showed that wild-caught females from Makobe and from Kissenda Islands (i.e. an island similar in light conditions, turbidity and in male coloration to Python Island) both preferred males with higher redscores [32,33]. They further showed that Makobe females preferred males with high yellowscores and showed a trend to prefer males with high blackscores [33]. Makobe males in their study also had significantly higher red- and blackscores compared with Kissenda males, but males of both populations did not differ in yellowscores [33]. In an earlier study by Maan et al. [32], where mate choice experiments were conducted, wild-caught females mated significantly more with males with higher blackscores and showed a trend to mate with redder males [32]. We used slightly different criteria to define the different colours such that the colour red in the studies by Maan and co-workers is equivalent to the sum of three colours red, orange and magenta in our study, yet the results from both studies are very similar.
This study and the behavioural mate preference tests by Maan and co-workers were done in aquaria with clear water and under broad-spectrum illumination, and resulted in similar findings. This suggests that female mate preference is not simply a consequence of signal transmission properties of the water. It suggests that the relationship between male coloration and female mating preference is not mediated by environmental variation (i.e. ambient light conditions) alone, but that male coloration and female mating preferences have evolved between populations owing to this or other variation, resulting in intraspecific preference–trait coevolution [33]. Such coevolution between mating signals and preferences within a population is theoretically expected [7,8,43] and could play a role in speciation if sexual isolation arises owing to mating trait divergence between populations resulting in population- or species-assortative mating [43]. Experimental work in Pundamilia has shown that red and blue male coloration and female behavioural preference for red versus blue male coloration are both heritable [45,46]. Earlier experimental work has shown that male colour variation among populations of P. nyererei is also heritable [21]. The drivers of such preference–trait coevolution can be stochastic (e.g. mutation and genetic drift [5,6] or deterministic (e.g. sensory drive or ecological selection [9,10]) and both deterministic and stochastic processes can interact with one another (i.e. run-away sexual selection [7]).
Sexual selection can be a powerful evolutionary force; not only is it a driver of the evolution of mating traits within a population, it can also potentially drive differentiation between populations or species and has been suggested to be an important factor in speciation [1–4]. Yet mating traits may not only be subject to sexual selection, but also ecological selection, and these two forces may either effect divergence synergistically or antagonistically [47]. Coloration in P. nyererei populations from different islands covaries positively with water transparency, which causes a dramatic change in the environmental light spectrum from turbid to clear water islands [17, 30]. These different underwater light environments have been proposed to generate variation in the strength of sexual selection [21] and possibly generate divergent selection between para-allopatric populations of P. nyererei resulting in the observed differences in colour between these conspecific populations [30,31]. Our findings suggest that population differences in within-population mating preferences for male colour components predict between-population differences in coloration for the populations of Makobe and Python, but not Senga. Further, our interpopulation and interspecific mate choice results suggest that male nuptial colour may mediate population- and species-assortative mating, and that population-assortative mating may be a simple direct extension of within-population colour preference. These results highlight the important role in speciation that has been attributed to female mate choice and male colour in African cichlids [20–23].
Supplementary Material
Acknowledgements
We thank Andreas Taverna for fish caretaking, Selina Räber for Photoshop advice and Julia Schwarzer for an automated script to extract colourscores. We further thank Martine E. Maan and all the members of the Fish Ecology and Evolution laboratory for valuable comments. The experiment was authorized by the veterinary office of the canton of Lucerne, Switzerland (licence number: LU04/07).
Data accessibility
The complete dataset is deposited on Dryad data (doi:10.5061/dryad.dc8dq).
Authors' contributions
O.M.S., M.E.R.P. and O.S. conceived the experiments, O.M.S. designed and performed the experiments, O.M.S. and R.T. processed the data, O.M.S. and J.M.A.-R. analysed the data, O.M.S. and O.S. wrote the manuscript and all authors gave final approval of the manuscript.
Competing interests
We have no competing interests.
Funding
This research was supported by a Swiss National Science Foundation grant no. (31003A-118293).
References
- 1.Coyne JA, Orr HA. 2004. Speciation. Sunderland, MA: Sinauer Associates. [Google Scholar]
- 2.West-Eberhard MJ. 1983. Sexual selection, social competition, and speciation. Quart. Rev. Biol. 58, 155–183. ( 10.1086/413215) [DOI] [Google Scholar]
- 3.Panhuis TM, Butlin R, Zuk M, Tregenza T. 2001. Sexual selection and speciation. Trends Ecol. Evol. 16, 364–371. ( 10.1016/S0169-5347(01)02160-7) [DOI] [PubMed] [Google Scholar]
- 4.Ritchie MG. 2007. Sexual selection and speciation. Annu. Rev. Ecol. Evol. Syst. 38, 79–102. ( 10.1146/annurev.ecolsys.38.091206.095733) [DOI] [Google Scholar]
- 5.Lewontin RC. 1974. The genetic basis of evolutionary change. New York, NY: Columbia University Press. [Google Scholar]
- 6.Kimura M. 1983. The neutral theory of molecular evolution. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 7.Fisher RA. 1930. The genetical theory of natural selection. Oxford, UK: Oxford University Press. [Google Scholar]
- 8.Prum RO. 2010. The Lande-Kirkpatrick mechansim is the null model of evolution by intersexual selection: implications for meaning, honesty and design in intersexual signals. Evolution 64, 3085–3100. ( 10.1111/j.1558-5646.2010.01054.x) [DOI] [PubMed] [Google Scholar]
- 9.Endler JA. 1992. Signals, signal conditions, and the direction of evolution. Am. Nat. 139, 125–153. ( 10.1086/285308) [DOI] [Google Scholar]
- 10.Kolm N, Amcoff M, Mann RP, Arnqvist G. 2012. Diversification of a food-mimicking male ornament via sensory drive. Current Biol. 22, 1440–1443. ( 10.1016/j.cub.2012.05.050) [DOI] [PubMed] [Google Scholar]
- 11.Kuijper B, Pen I, Weissing FJ. 2012. A guide to sexual selection theory. Annu. Rev. Ecol. Evol. Syst. 43, 287–311. ( 10.1146/annurev-ecolsys-110411-160245) [DOI] [Google Scholar]
- 12.Tobias JA, Aben J, Brumfield RT, Derryberry EP, Halfwerk W, Slabbekoorn H, Seddon N.. 2010. Song divergence by sensory drive in amazonian birds. Evolution 64, 2820–2839. ( 10.1111/j.1558-5646.2010.01067.x) [DOI] [PubMed] [Google Scholar]
- 13.Leal M, Fleishman LJ. 2002. Evidence for habitat partitioning based on adaptation to environmental light in a pair of sympatric lizard species. Proc. R. Soc. Lond. B 269, 351–359. ( 10.1098/rspb.2001.1904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cocroft RB, Rodriguez RL, Hunt RE. 2008. Host shifts, the evolution of communication, and speciation in the Enchenopa binotata species complex of treehoppers. In Specialization, speciation, and radiation: the evolutionary biology of herbivorous insects (ed. Tilmon KJ.), pp. 88–100. Berkeley, CA: University of California Press. [Google Scholar]
- 15.Boughman JW. 2002. How sensory drive can promote speciation. Trends Ecol. Evol. 17, 571–577. ( 10.1016/S0169-5347(02)02595-8) [DOI] [Google Scholar]
- 16.Boughman JW. 2001. Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature 411, 944–948. ( 10.1038/35082064) [DOI] [PubMed] [Google Scholar]
- 17.Seehausen O, et al. 2008. Speciation through sensory drive in cichlid fish. Nature 455, 620–626. ( 10.1038/nature07285) [DOI] [PubMed] [Google Scholar]
- 18.Seehausen O. 2006. African cichlid fish: a model system in adaptive radiation research. Proc. R. Soc. B 273, 1987–1998. ( 10.1098/rspb.2006.3539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maan ME, Sefc KM. 2013. Colour variation in cichlid fish: developmental mechanisms, selective pressures and evolutionary consequences. Semin. Cell Dev. Biol. 24, 516–528. ( 10.1016/j.semcdb.201305.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dominey W. 1984. Effects of sexual selection and life history on speciation: species flock in African cichlids and Hawaiian Drosophila. In Evolution of fish species flocks (eds Echelle AA, Kornfield I), pp. 231–250. Orono, ME: University of Maine at Orono Press. [Google Scholar]
- 21.Seehausen O, van Alphen JJM, Witte F. 1997. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277, 1808–1811. ( 10.1126/science.277.5333.1808) [DOI] [Google Scholar]
- 22.Salzburger W. 2009. The interaction of sexually and naturally selected traits in the adaptive radiations of cichlid fishes. Mol. Ecol. 18, 169–185. ( 10.1111/j.1365-294X.2008.03981.x) [DOI] [PubMed] [Google Scholar]
- 23.Wagner CE, Harmon L, Seehausen O. 2012. Ecological opportunity and sexual selection together predict adaptive radiation. Nature 487, 366–370. ( 10.1038/nature11144) [DOI] [PubMed] [Google Scholar]
- 24.Seehausen O. 2009. Progressive levels of trait divergence along a ‘speciation transect’ in the Lake Victoria cichlid fish Pundamilia. In Ecological reviews: speciation and patterns of diversity (eds Butlin R, Bridle J, Schluter D), pp. 155–176. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 25.Seehausen O. 1997. Distribution of and reproductive isolation among color morphs of a rock dwelling Lake Victoria cichlid. Ecol. Freshw. Fish. 6, 59–66. ( 10.1111/j.1600-0633.1997.tb00145.x) [DOI] [Google Scholar]
- 26.Seehausen O, van Alphen JJM. 1998. The effect of male coloration on female mate choice in closely related Lake Victoria cichlids (Haplochromis nyererei complex). Behav. Ecol. Sociobiol. 42, 1–8. ( 10.1007/s002650050405) [DOI] [Google Scholar]
- 27.Selz OM, Pierotti MER, Maan ME, Schmid C, Seehausen O. 2014. Female preference for male color is necessary and sufficient for assortative mating in 2 cichlid sister species. Behav. Ecol. 25, 612–626. ( 10.1093/beheco/aru024) [DOI] [Google Scholar]
- 28.Carleton KL, Parry JW, Bowmaker JK, Hunt DM, Seehausen O. 2005. Colour vision and speciation in Lake Victoria cichlids of the genus Pundamilia. Mol. Ecol. 14, 341–4353. ( 10.1111/j.1365-294X.2005.02735.x) [DOI] [PubMed] [Google Scholar]
- 29.Hofmann CM, O'Quin KE, Marshall NJ, Cronin TW, Seehausen O, Carelton KL. 2009. The eyes have it: regulatory and structural changes both underlie cichlid visual pigment diversity. PLoS Biol. 7, e1000266 ( 10.1371/journal.pbio.1000266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castillo Cajas RF, Selz OM, Ripmeester EAP, Seehausen O, Maan ME. 2012. Species-specific relationships between water transparency and male coloration within and between two closely related Lake Victoria cichlid species. Int. J. Evol. Biol. 2012, 1–12. ( 10.1155/2012/161306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maan ME, van Rooijen AMC, van Alphen JJM, Seehausen O. 2008. Parasite mediated sexual selection and species divergence in Lake Victoria cichlid fish. Biol. J. Linn. Soc. 94, 53–600. ( 10.1111/j.1095-8312.2008.00989.x) [DOI] [Google Scholar]
- 32.Maan ME, Seehausen O, Söderberg L, Johnson L, Ripmeester EAP, Mrosso HDJ, Taylor MI, van Dooren TJ, van Alphen JJM. 2004. Intraspecific sexual selection on a speciation trait, male coloration, in the Lake Victoria cichlid Pundamilia nyererei. Proc. R. Soc. Lond. B 271, 2445–2452. ( 10.1098/rspb.2004.2911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maan ME, Seehausen O, Van Alphen JJM. 2010. Female mating preferences and male coloration covary with water transparency in a Lake Victoria cichlid fish. Biol. J. Linn. Soc. 99, 398–406. ( 10.1111/j.1095-8312.2009.01368.x) [DOI] [Google Scholar]
- 34.Seehausen O. 1996. Lake Victoria rock cichlids: taxonomy, ecology, and distribution. Zevenhuizen, The Netherlands: Verduijn Cichlids. [Google Scholar]
- 35.Seehausen O, Lippitsch E, Bouton N, Zwennes H. 1998. Mbipi, the rock-dwelling cichlids of Lake Victoria: description of three new genera and fifteen new species (Teleostei). Ichthyol. Explor. Freshw. 9, 129–228. [Google Scholar]
- 36.Witte-Maas ELM, Witte F. 1985. Haplochromis nyererei, a new cichlid fish from Lake Victoria named in honour of Mwalimu Julius Nyerere, President of Tanzania. Leiden, The Netherlands: Brill. [Google Scholar]
- 37.Konijnendijk N, Joyce DA, Mrosso HDJ, Egas M, Seehausen O. 2011. Community genetics reveal elevated levels of sympatric gene flow among morphologically similar but not among morphologically dissimilar species of Lake Victoria cichlid fish. Int. J. Evol. Biol. 2011, 616320 ( 10.4061/2011/616320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terai Y, et al. 2006. Divergent selection on opsins drives incipient speciation in Lake Victoria cichlids. PLoS Biol. 12, e433 ( 10.1371/journal.pbio.0040433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fryer G, Iles TD. 1972. The cichlid fishes of the Great Lakes of Africa: their biology and evolution. Edinburgh, UK: Oliver and Boyd. [Google Scholar]
- 40.Selz OM, Thommen R, Maan ME, Seehausen O. 2014. Behavioural isolation may facilitate homoploid hybrid speciation. J. Evol. Biol. 27, 275–289. ( 10.1111/jeb.12287) [DOI] [PubMed] [Google Scholar]
- 41.Vandeputte M, Mauger S, Dupont-Nivet M. 2006. An evaluation of allowing for mismatches as a way to manage genotyping errors in parentage assignment by exclusion. Mol. Ecol. Notes 6, 265–267. ( 10.1111/j.1471-8286.2005.01167.x) [DOI] [Google Scholar]
- 42.Endler JA. 1990. On the measurement and classification of colour in studies of animal colour patterns. Biol. J. Linn. Soc. 41, 315–352. ( 10.1111/j.1095-8312.1990.tb00839.x) [DOI] [Google Scholar]
- 43.Lande R. 1981. Models of speciation by sexual selection on polygenic traits. Proc. Natl Acad. Sci. USA 78, 3721–3725. ( 10.1073/pnas.78.6.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pauers MJ, McKinnon JS. 2012. Sexual selection on color and behaviour within and between cichlid populations: implications for speciation. Curr. Zool. 58, 475–483. ( 10.1093/czoolo/58.3.475) [DOI] [Google Scholar]
- 45.Haesler MP, Seehausen O. 2005. Inheritance of female mating preference in a sympatric sibling species pair of Lake Victoria cichlids: implications for speciation. Proc. R. Soc. B 272, 237–245. ( 10.1098/rspb.2004.2946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magalhaes IS, Seehausen O. 2010. Genetics of male nuptial colour divergence between sympatric sister species of a Lake Victoria cichlid fish. J. Evol. Biol. 23, 914–924. ( 10.1111/j.1420-9101.2010.01960.x) [DOI] [PubMed] [Google Scholar]
- 47.Maan ME, Seehausen O. 2011. Ecology, sexual selection and speciation. Ecol. Lett. 14, 591–602. ( 10.1111/j.1461-0248.2011.01606.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete dataset is deposited on Dryad data (doi:10.5061/dryad.dc8dq).