Abstract
Vision is a sensory modality of fundamental importance for many animals, aiding in foraging, detection of predators and mate choice. Adaptation to local ambient light conditions is thought to be commonplace, and a match between spectral sensitivity and light spectrum is predicted. We use opsin gene expression to test for local adaptation and matching of spectral sensitivity in multiple independent lake populations of threespine stickleback populations derived since the last ice age from an ancestral marine form. We show that sensitivity across the visual spectrum is shifted repeatedly towards longer wavelengths in freshwater compared with the ancestral marine form. Laboratory rearing suggests that this shift is largely genetically based. Using a new metric, we found that the magnitude of shift in spectral sensitivity in each population corresponds strongly to the transition in the availability of different wavelengths of light between the marine and lake environments. We also found evidence of local adaptation by sympatric benthic and limnetic ecotypes to different light environments within lakes. Our findings indicate rapid parallel evolution of the visual system to altered light conditions. The changes have not, however, yielded a close matching of spectrum-wide sensitivity to wavelength availability, for reasons we discuss.
Keywords: visual ecology, local adaptation, evolution, opsin, gene expression, Gasterosteus aculeatus
1. Background
Sensory systems are often thought to be under strong natural selection [1] and are predicted to evolve to better correspond to signals in the local environment [2]. For example, sensitivity of the visual system to different wavelengths of light is expected to evolve to match roughly the availability of wavelengths [3,4], increasing ability to catch photons and detect contrast between objects and background [5–7]. However, few studies have tested the adaptive significance of spectral sensitivity across the whole visual spectrum. The degree of matching between spectral sensitivity of organisms and their light environment across the spectrum has not been quantified.
Aquatic organisms provide excellent opportunities to test for local adaptation and quantify matching [8]. This is because differential attenuation of wavelengths of light with water depth and by suspended particulates result in dramatic and predictable changes in local light spectra [9]. For example, the transition from marine to fresh waters is usually accompanied by a large reduction in the availability of ultraviolet (UV) wavelengths, largely because of an increase in the amount of dissolved organics [9,10].
We used threespine stickleback (Gasterosteus aculeatus), which inhabit both marine and freshwater habitats, to investigate predicted evolutionary changes in visual adaptations of populations to the different ambient light environments. Marine stickleback invaded and adapted to numerous lakes and streams at the end of the last ice age (approx. 12 000 years ago) [11]. First, we tested for parallel evolution of opsin gene expression and spectral sensitivity over the visual light spectrum among these derived freshwater populations, which would represent strong evidence of natural selection [12].
Second, using the extant marine form as a proxy for the ancestral state, we evaluated the extent to which shifts in the spectral sensitivity of freshwater populations are correlated with shifts in the ambient light environment, and whether the outcome improves the match to local ambient light spectra. Finally, we tested for parallel divergence of spectral sensitivity of multiple pairs of sympatric limnetic and benthic stickleback ecotypes (or ‘species pairs') to finer scale heterogeneity in the light environment within lakes. In each of the three species pairs analysed here, the benthic stickleback forage in the vegetated littoral regions of the lake and deeper sediments, whereas limnetics are pelagic, found in the open water and near rocky cliffs [13]. The benthic environment contains relatively more long wavelengths than the open water [14].
We focused on expression of opsin genes, which encode the light-sensitive G-protein-coupled receptors that are expressed in retinal rod and cone cells. Opsins conjugate to vitamin A derived chromophores and play an important role in colour vision by mediating the conversion of photons into electrochemical signals, which initiate a neuronal response that is perceived by the brain [15]. The clear and well-characterized link between opsin genotype (coding sequence) and spectral phenotype (wavelength of maximal absorption, λmax) make opsins particularly useful for studying sensory adaptation [16]. Opsin mediated shifts in spectral sensitivity can be achieved by changes in opsin protein-coding sequence (e.g. [17]) and by changes in levels of gene expression (e.g. [18]). We studied gene expression because analysis of whole genomes of marine and freshwater stickleback has not found consistent differences in opsin gene coding sequence between marine and freshwater populations [19]. Compared to other fishes, stickleback have relatively few (four) opsins, with a single functional opsin gene in each of the four cone opsin subfamilies: short-wavelength sensitive 1 (SWS1), short-wavelength sensitive 2 (SWS2), middle-wavelength sensitive (RH2) and long-wavelength sensitive (LWS) [20]. We measured expression levels of each of the four unique cone opsin genes in 11 stickleback populations. We also measured expression in fish from two populations raised in a common laboratory environment to test the extent to which it is genetically determined.
We used opsin gene expression levels to estimate spectrum-wide spectral sensitivity to evaluate two general expectations. First, the advantages of photon capture and contrast should result in spectral sensitivity evolving roughly to correspond with wavelength availability [3,4]. We measure this correspondence (matching) with the correlation across wavelengths between spectral sensitivity and two measures of light availability: irradiance (photons of each wavelength available at a specific water depth) and transmission (indicating the absorption of specific wavelengths by water). Large discrepancies between spectral sensitivity and light availability in specific regions of the visual spectrum might suggest specialized visual functions. Second, changes in wavelength availability from marine to fresh water should lead to similar shifts in spectral sensitivity (local adaptation). For example, as some wavelengths become scarce in the new environment and others common, relative to the ancestral environment, we expect spectral sensitivity to shift to correspond [2]. Throughout, we use the whole-light spectrum to study association, rather than studying associations between summary measures such as the median. We introduce a new metric to quantify the correlation between shift in spectral sensitivity and the transition between light environments.
Shifts in spectral sensitivity can additionally be achieved by differential use of vitamin A-derived chromophores [21,22]. Conjugation of an opsin to an A1 chromophore (11-cis retinal) leads to a shorter wavelength of maximal absorption (λmax) than conjugation to an A2 chromophore (3-dehydro 11-cis retinal) [21]. Switches in chromophore use have been shown to occur in fishes over ontogeny [23] and between habitats via phenotypic plasticity [22]. Fishes in the ocean generally use A1 chromophores, while freshwater fishes have a mixture of A1 and A2 chromophores (varying from completely A1 to completely A2) [22]. Complete use of A2 is generally found in lakes whose waters are strongly stained with tannins (e.g. [24]), and such lakes are not included in our study. To account for possible variation in chromophore use, we model the effects of changes in chromophore and describe how this affects our measures of local adaptation and spectral matching of opsin expression in stickleback.
2. Material and methods
(a). Sampling
Six gravid females were collected from each of 11 populations inhabiting different breeding environments in the Strait of Georgia region of British Columbia, Canada. Collections were made under the Species At Risk Act collection permit 236 and British Columbia Fish Collection permit NA-SU12-76311. The samples came from two marine locations, three lakes containing just a single species of stickleback and three lakes containing stickleback species pairs (see the electronic supplementary material, §1 and table S1 for site details). Fish were euthanized at the collection site and eye tissue was immediately preserved in RNAlater® (Qiagen, The Netherlands) and then kept at –20°C for up to a month before RNA was extracted.
(b). Opsin expression and spectral sensitivity
The expression of each of the stickleback's four unique cone opsin genes (SWS1, SWS2A, RH2-1 and LWS [20]) was measured using a standard reverse-transcriptase quantitative polymerase chain reaction protocol (details in the electronic supplementary material, §2). We normalized the absolute number of transcripts for each gene from each individual by dividing the expression of a given opsin by the sum of the expression of all four opsins to get relative opsin expression. We also measured gene expression of a reference gene, Beta actin, and calculated the expression of each opsin gene relative to it.
All statistical analyses in the paper were conducted in R v. 3.0.2 [25]. To test for differences in mean expression of each opsin gene between marine and freshwater populations, we used a linear mixed-effects model (using the nlme package, [26]) with water type (marine or fresh) as fixed effect and location as a random effect. For this comparison, individuals from the benthic and limnetic species in a given location were combined and treated as a single population. Results were the same when only the benthics, or only the limnetics, were used instead. In separate analyses, we tested for differences in gene expression between the sympatric benthic and limnetic species in three lakes, with lake as a random effect and ecotype as a fixed effect in the model. We treat lake populations as independent replicates that require no phylogenetic correction. This is justified by the geological origins of lakes, which are in separate drainages and were accessible via the sea for a limited period of time. Previous studies show that phylogenies of freshwater stickleback populations in British Columbia based on putatively neutral markers are well approximated by a star phylogeny (e.g. [27]).
We bred three families of one marine population (Oyster Lagoon) and three families of one benthic population (Priest Lake) by in vitro fertilization and reared them under laboratory conditions in stand-alone 100 l tanks with fluorescent lights. A gravid adult female from each family was euthanized and her opsin expression was quantified as described earlier. We used linear models to test differences between laboratory-reared marine and freshwater fish and between laboratory- and wild-reared fish from the same populations.
Upon finding differences in mean opsin expression between marine and freshwater stickleback, and between sympatric benthic and limnetic stickleback, we estimated how they translated into differences in spectral sensitivity. We calculated a spectral sensitivity curve Si (350–700 nm) for each individual i based on its relative expression of the four opsin genes, and using the absorbance templates from Govardovskii et al. [28] and estimates by Flamarique et al. [24] of the wavelength of maximum absorbance (λmax) of each opsin gene (details in §3 of the electronic supplementary material). This model assumes that opsin expression contributes additively to spectral sensitivity; at this point in time, it is a necessary simplification as we still lack empirically informed models that describe and generalize any potential inhibitory interactions among opsins during signal integration and interpretation.
Chromophore (A1 and A2) ratios in the surveyed freshwater populations are not known. Based on empirical observations [24], we assumed that marine stickleback used 100% A1 in the ocean. We estimated spectral sensitivity of stickleback in fresh water using three different chromophore ratios representing the extremes: 100% A1; 50% A1 and 50% A2; and 100% A2. We assumed that benthic and limnetic stickleback have the same A1 : A2 ratio.
(c). Association between spectral sensitivity and ambient light
We measured the spectral conditions of each location, with the exception of Cranby Lake and Little Quarry Lake. We used two measures to quantify the ambient light environment: irradiance and transmission. Irradiance measures the abundance of photons at each wavelength in the environment at a given point in time. Irradiance measurements of side-welling light (Is) were taken at 10, 20, 50, 100 and 200 cm depth at 10 or more sites within each sampling location using a cosine corrector attached to a spectrophotometer (Ocean Optics, USA). In subsequent analyses, we used the irradiance at 50 cm. A limitation of using irradiance to quantify available light is that it varies with depth and with the weather and the angle of the sun. Transmission is the relative rate of loss of photons of a given wavelength per unit distance travelled through water. Transmission is a property of the body of water and may be less variable than irradiance, at least on short time scales. Transmission was measured as the light extinction coefficient with depth (Ks) (method for calculation outlined in the electronic supplementary material, §5).
To test for local adaptation, we developed a statistic to quantify the association between the shift in spectral sensitivity and the transition in light environment, from marine to fresh water, across all wavelengths for each lake population. First, we chose a marine population (Oyster Lagoon) to represent the ancestral phenotype and breeding environment. Next, we constructed transmission (Ks) and irradiance (Is) curves by calculating at each wavelength (λ) the median from all samples within a location. At each wavelength, we then subtracted the median value of the reference marine location from the median value in each freshwater location. This yielded change in transmission (ΔKs) and change in irradiance (ΔIs) values at every wavelength (λ) at each freshwater location. A positive value of ΔIs at a given wavelength indicates that there are more photons of that wavelength (λ) present at the freshwater location relative to the marine environment. A positive value of ΔKs at a given wavelength (λ) indicates greater light transmission (fewer photons lost as light travels through water) at the freshwater location than at the reference marine location.
Change in spectral sensitivity ΔS was calculated similarly, as follows. We calculated the median sensitivity at each wavelength (λ) of the sample of individuals from the reference marine population. Change in sensitivity was calculated for each freshwater individual as the difference between its sensitivity curve and the median marine curve. Finally, for each freshwater individual, we calculated the correlation coefficient (r) of the change in sensitivity (ΔS) against the change in light environment (ΔKs or ΔIs), with each wavelength yielding a data point for each freshwater individual. A positive r indicates that regions of the spectrum with increased irradiance (or transmission) are correlated with increased spectral sensitivity, and regions of the spectrum with a decrease in irradiance (or transmission) are correlated with decreased spectral sensitivity. We used a mixed-effects model (with population as a random effect) to test whether mean correlation coefficients (r) differed significantly from zero.
We carried out a similar analysis of local adaptation of spectral sensitivity between the sympatric species in relation to differences in their local light environments. For each lake, we used the limnetic population and the pelagic environment as the reference. Other calculations were the same as described above for the marine and freshwater comparison (see the electronic supplementary material, §§5 and 6, tables S3 and S4, and figures S1 and S2, for further details and justification of our reference populations).
To quantify the degree to which populations are matched to their native light environments, we estimated the correlation, wavelength by wavelength, between each population's mean spectral sensitivity and the transmission and irradiance measured in its local environment. The significance of the mean correlation was tested separately for marine and freshwater populations using linear models.
Because analyses of local adaptation and matching involved a suite of tests that incorporated different measures of light environment and three chromophore scenarios, we adjusted the p-values for multiple testing in each table of results using the ‘BH’ false discovery rate method [29] and the p.adjust function in R (electronic supplementary material, tables S3–S5). Raw p-values are reported in the main paper and adjusted p-values are reported in the statistics tables in the electronic supplementary material. In all cases, significant p-values remained significant after the correction for multiple testing.
3. Results
(a). Opsin expression and spectral sensitivity
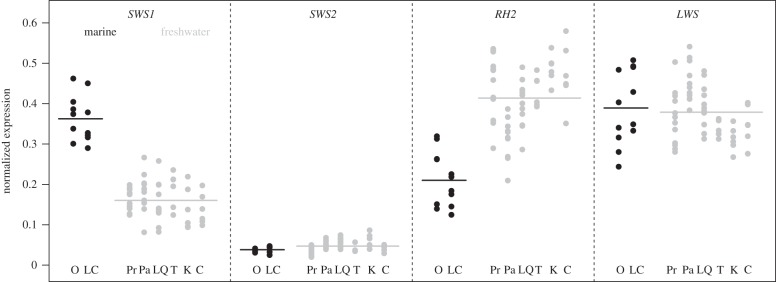
Freshwater stickleback populations had significantly lower expression of the SWS1 (UV) opsin gene than the marine populations (difference = −0.20 ± 0.02 s.e., F1,6 = 145.2, p < 0.001) and higher expression of the RH2 (green) opsin gene (difference = 0.21 ± 0.06 s.e., F1,6 = 18.1, p = 0.005). We did not detect a significant difference in the other two opsin genes, LWS (red) (difference = 0.02 ± 0.04 s.e., F1,6 = 0.2, p = 0.68) and SWS2 (blue) (difference = −0.009 ± 0.008 s.e., F1,6 = 1.2, p = 0.31) (figure 1). Differences in SWS1 and RH2 remained significant if expression was calculated relative to the reference gene Beta actin (SWS1 difference = 2.1 ± 0.3 s.e., F1,6 = 49.2, p < 0.001; RH2 difference = 2.97 ± 0.9 s.e., F1,6 = 10.7, p = 0.017). Thus, we proceeded using cone opsin proportion as our metric of gene expression when modelling spectral sensitivity, as this has been shown to be best for making inferences about overall colour vision capacities [30].
Figure 1.
Normalized cone opsin gene expression of marine and freshwater populations. Marine populations are indicated in black and freshwater populations in grey. Horizontal lines indicate the mean of all populations; circles indicate individual fish. O, Oyster Lagoon; LC, Little Campbell River; Pr, Priest Lake; Pa, Paxton Lake; LQ, Little Quarry Lake; T, Trout Lake; K, Kirk Lake; C, Cranby Lake.
These differences in overall expression translated into large differences in estimated spectral sensitivity in two portions of the spectrum (figure 2). Freshwater fish had reduced sensitivity in the 350–375 nm (UV and violet) region of the spectrum, and they had greater sensitivity in the 450–600 nm (blue and green) region relative to both marine populations.
Figure 2.
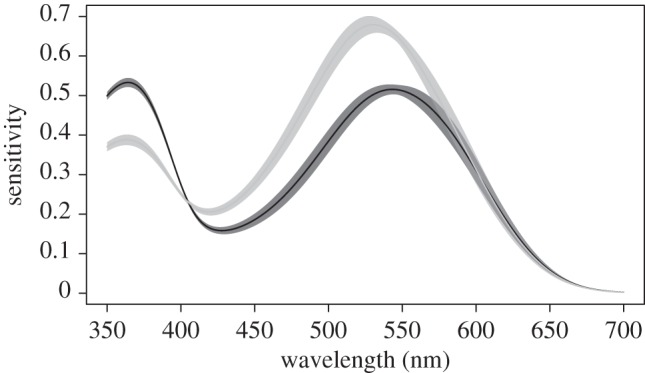
Estimated spectral sensitivity of marine and freshwater populations assuming both use only the A1 chromophore. Marine populations are indicated in black and freshwater in grey. The thin lines are the fitted values of spectral sensitivity from the mixed-effects model. The shaded regions are 1 s.d. above and below the fitted values, with standard errors also derived from the mixed-effects model.
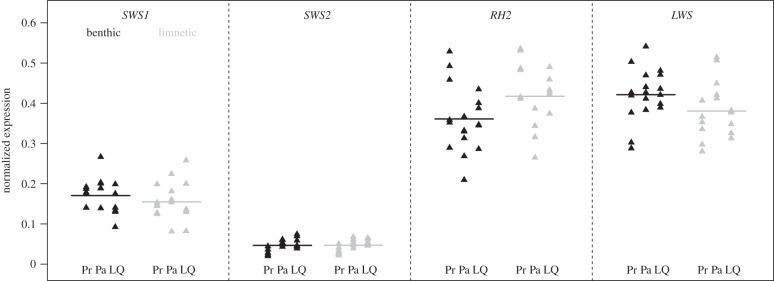
Within lakes, we found that the limnetic stickleback populations had significantly greater RH2 (green) expression than the benthics (difference = 0.05 ± 0.02 s.e., F1,31 = 7, p = 0.01), and benthics had greater LWS (red) expression (0.04 ± 0.02 s.e., F1,31 = 4.3, p = 0.05). However, the magnitudes of the differences were small (figure 3). The expression of SWS1 and SWS2 opsins did not differ significantly (p > 0.29) between the two species (figure 3). The difference in RH2 expression between the species was still significant when expression was calculated relative to Beta actin gene expression (difference = 1.3 ± 0.6 s.e., F1,31 = 4.4, p = 0.04), but the difference in LWS was not (difference = 0.3 ± 0.84 s.e., F1,31 = 0.12, p = 0.70). These differences in expression translate to reduced sensitivity in the 525–575 nm (green) region of the spectrum and increased sensitivity in the portion of the spectrum above 600 nm (red) in benthics compared with limnetics (electronic supplementary material, figure S3).
Figure 3.
Normalized cone opsin gene expression of benthic and limnetic populations. The benthic populations are in black and limnetic populations in grey. Horizontal lines indicate the mean of all populations; triangles indicate individual fish. Location names abbreviated as: Pr, Priest Lake; Pa, Paxton Lake; LQ, Little Quarry Lake.
(b). Laboratory rearing
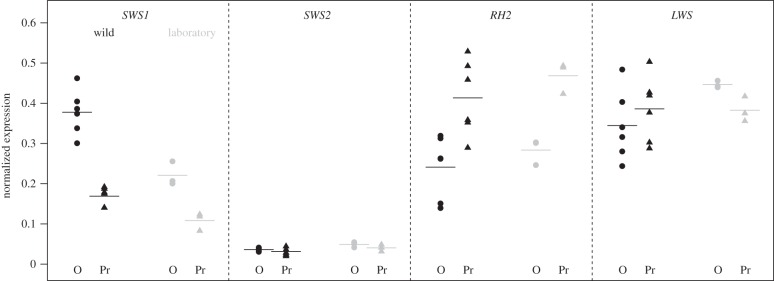
In the laboratory, Oyster Lagoon (marine) and Priest benthic fish (freshwater) had similar expression differences as in the wild (figure 4). SWS1 gene expression remained different between the marine and freshwater populations in the laboratory (difference 0.11 ± 0.02 s.e., F1,4 = 27.1, p = 0.01) as did RH2 (difference 0.18 ± 0.03 s.e., F1,4 = 40.1, p = 0.003). The difference in SWS1 was, however, greater in the wild samples, as indicated by an interaction between rearing condition (wild or laboratory) and population of origin (effect size = 0.096 ± 0.039 s.e., t1,4 = 2.486, p = 0.03). No other interactions were significant (all p > 0.17). Finally, we also detected a small difference in LWS expression between the two populations in the laboratory only (figure 4; 0.06 ± 0.02 s.e., F1,4 = 11.5, p = 0.03). Additional tests examining changes in the gene expression of laboratory-reared fish from each population compared to their wild counterparts are outlined in the electronic supplementary material, §4.
Figure 4.
Opsin expression in wild and laboratory-reared fish from a marine (O, Oyster Lagoon) and freshwater location (Pr, Priest Lake). Wild fish are indicated in black, laboratory-reared fish in grey. Horizontal lines indicate the mean of the population, and points indicate individual fish.
(c). Association between shifts in spectral sensitivity and ambient light
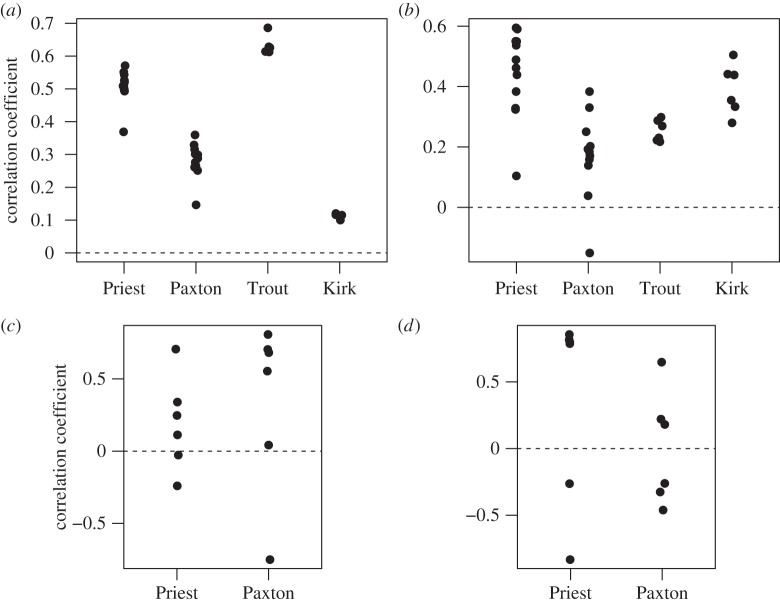
The shift in spectral sensitivity from marine to freshwater environments was positively correlated with the change in ambient light spectrum, when sensitivity was estimated assuming that both populations used only the A1 chromophore. On average, the correlation measured using transmission (mean r = 0.39 ± 0.12 s.e., t1,31 = 3.3, p = 0.002; figure 5a) was of similar magnitude when using irradiance (mean r = 0.32 ± 0.06 s.e., t1,31 = 4.95, p < 0.0001; figure 5b). These correlations arose primarily from shifts in the short- (UV-blue) and middle-wavelength (green) regions (electronic supplementary material, figure S4). Decreased transmission of UV (350–400 nm) and violet (380–450 nm) in the freshwater environment (indicated by values below the dashed line in the electronic supplementary material, figure S4) correspond with decreased sensitivity to these wavelengths in freshwater populations. Increased transmission of blue (450–495 nm) and green (495–570 nm) wavelengths in freshwater is correlated with increased sensitivity to these wavelengths. Freshwater populations varied considerably in the strength of the correlation (figure 5).
Figure 5.
(a) Correlations between shifts in spectral sensitivity of individuals from freshwater populations and differences in local light transmission relative to the reference marine population, Oyster Bay. (b) As in (a) but using irradiance to measure light environment shift. (c) Correlations between shifts in spectral sensitivity between sympatric benthic and limnetic stickleback species and differences in local light transmission. (d) As in (c) but using irradiance to compare light environments.
These results isolate the effects of shifts in spectral sensitivity caused by changes in opsin gene expression in freshwater, when controlling for chromophore. We also measured the effects of these expression changes if combined with a hypothetical increase in the use of the A2 chromophore in these freshwater populations. The correlation between shifts in spectral sensitivity and transmission weakens slightly when a 50 : 50 mix of A1 and A2 chromophores is projected (mean r = 0.22 ± 0.12 s.e., t1,31 = 1.85, p = 0.07). When 100% A2 chromophore is used, the correlation between shifts in sensitivity and transmission weakens further (mean r = 0.14 ± 0.09 s.e., t1,31 = 1.53, p = 0.14) and the correlation between shifts in sensitivity and irradiance becomes negative (mean r = −0.48 ± 0.05 s.e., t1,31 = −9.3, p ≤ 0.0001) (see the electronic supplementary material, table S3 for details, including adjusted p-values).
Within species pair lakes, there was a moderate, although not quite significant, correlation between divergence in spectral sensitivity and the difference in transmission (modelled using the A1 chromophore; figure 5c; mean r = 0.27 ± 0.13 s.e., t1,10 = 1.97, p = 0.077). This correlation was not significant for the difference in irradiance (figure 5d; mean r = 0.18 ± 0.18 s.e., t1,10 = 1.00, p = 0.339). The results were similar when other chromophore ratios were used to estimate spectral sensitivity, assuming that ratios were the same in both sympatric forms (see the electronic supplementary material, table S4 for details, including adjusted p-values).
(d). Match of spectral sensitivity to ambient light
Despite strong correlations between shifts in spectral sensitivity and changes in the distribution of available wavelengths, spectral sensitivity is not closely matched to wavelength availability in either marine or freshwater environments. The mean correlation between spectral sensitivity of freshwater fish and ambient light in lakes, while statistically significant, was small (0.07 ± 0.03 for transmission and 0.12 ± 0.02 for irradiance). This low level of matching has arisen multiple times in parallel in lake stickleback, which suggests that natural selection favours it. Substituting the chromophore did little to alter the mean correlation for transmission (although it became statistically insignificant) and slightly changed the strength for irradiance (see the electronic supplementary material, §7 and table S5 for details, including adjusted p-values). In the marine environment, the mean correlations between marine spectral sensitivity and transmission or irradiance are negative (r = −0.66 ± 0.16 s.e. and r = −0.11 ± 0.07 s.e., respectively). The main cause of the strong negative correlation in marine waters is the excessive UV sensitivity compared with UV light availability. Nevertheless, UV expression declines in fresh water, where these wavelengths are even more scarce, contributing to the observed correlation between shifts in sensitivity and the change in wavelength distribution.
4. Discussion
Our findings indicate that there has been rapid parallel evolution of opsin gene expression and spectral sensitivity across the light spectrum in freshwater stickleback populations. All surveyed freshwater populations have their spectral sensitivity shifted towards blue and green wavelengths, and away from ultraviolet and violet, relative to the marine populations. This has been accomplished entirely by shifts in opsin gene expression rather than protein sequence changes. We provide evidence that this difference has a genetic basis, as the main differences in expression were maintained in two laboratory-reared populations. Our analyses also reveal a strong association between shifts in spectral sensitivity and changes in light transmission from marine to fresh water environments, suggesting that these shifts are in an adaptive direction. On a smaller scale, we also find support for parallel adaptive divergence of gene expression and spectral sensitivity within lakes, between sympatric limnetic and benthic species. The evolution of the visual system in stickleback has been rapid, as these freshwater populations have evolved within the last 12 000 years after the last glacial maxima [11].
The degree of phenotypic parallelism in opsin expression and spectral sensitivity that we describe is unprecedented over such a short time span. Nine independently derived populations exhibit the same direction of shift in opsin expression following the colonization of freshwater. In East African cichlids, parallel evolutionary divergence of opsin expression has been detected between species within two of the three major lake cichlid radiations [31], but these radiations are much older than the freshwater stickleback populations studied here. Our findings are in line with previous work in stickleback, which has found extensive parallel evolution of morphological traits and patterns of genomic divergence among freshwater populations [19,32,33]. Some but not all of this morphological parallelism involves changes at the same underlying genes, which frequently represents adaptation from a common ancestral pool of standing genetic variation [32]. Possibly, the parallelism we observe in spectral sensitivity also represents adaptation from a common pool of standing genetic variation, which would help to explain the speed of evolution in this trait in stickleback. Further genetics work is required to test this idea.
The result from our laboratory-rearing experiment suggests a substantial genetic component to the population differences in opsin expression. This contrasts with many other systems in which differential opsin gene expression and/or spectral sensitivity is largely phenotypically plastic (e.g. [34]). For example, wild bluefin killifish (Lucania goodie) living in clear springs and tannin-stained waters exhibit large differences in their opsin gene expression [34]; however, light treatment and rearing experiments in the laboratory have shown that most of these differences are owing to environmental effects [34].
Smaller but detectable differences in opsin expression and sensitivity between limnetic and benthic stickleback inhabiting the same lake were repeated in multiple lakes, suggesting a role for natural selection in divergence of visual systems on a small, within-lake scale. Benthics had slightly higher estimated sensitivity to red wavelengths than did limnetics, in accordance with a more red-shifted local light environment. Previous work using optomotor behavioural response assays indicated that limnetic stickleback in Enos Lake have higher red wavelength sensitivity than the benthic population from the same lake, and similar red wavelength sensitivity to the benthic in Paxton Lake [14]. By contrast, we found higher expression of long-wavelength opsins in benthics compared with limnetics. Future work is required to determine how these differences in opsin expression affect foraging and mate choice in stickleback, as has been suggested in Lake Victoria cichlids [35].
Early work in the field of visual ecology focused on the hypothesis that spectral sensitivity should evolve to maximize an individual's photon catch [5–7]. Tests of this hypothesis have examined the relationship between the λmax of visual pigments (opsins) and the wavelengths most prevalent in ambient environment and have often found a strong relationship (e.g. [36,37]). However, detection of contrast and colour discrimination also probably shapes the evolution of spectral sensitivity. With multiple functions, it may be difficult to predict a priori the evolved degree of spectrum-wide matching of spectral sensitivity to the available light spectrum. We did not find a close match in freshwater populations, and indeed, the correlation was negative in marine populations. The low match in marines is driven by their high estimated sensitivity to short wavelengths such as UV, despite the relative rarity of these light wavelengths in the marine environment compared to mid-wavelengths. The low degree of matching suggests that increasing photon capture alone is unlikely to explain the evolution of spectral sensitivity. Predicting a shift in sensitivity with change in light spectrum may be more straightforward: reduced investment in capturing specific wavelengths that are increasingly rare is expected. For example in the deep sea, long-wavelength light is rare, and some deep sea fish have lost long-wave-sensitive opsins and shifted their sensitivity towards shorter wavelengths [37]. Similarly, we found that freshwater stickleback have reduced expression of short wavelengths, which are even scarcer in freshwater than in the sea. Nevertheless, freshwater fish retain relatively high sensitivity to UV light compared with background irradiance.
One possible explanation for the low match between sensitivity and ambient wavelengths is that high expression of pigments whose sensitivity is offset from the dominant wavelengths of the environment could play an important role in contrast detection under low-light conditions [36]. For example in stickleback, UV wavelengths are important for detection of zooplankton prey against the background light [38]. This idea is consistent with the observed trend towards reduced UV opsin expression in freshwater stickleback populations, since most are less zooplanktivorous than marine stickleback [39]. Experimental work in other fish species has also shown that reduced UV sensitivity coincides with reduced zooplanktivory and zooplankton foraging ability [40,41]. A second possible explanation for the low match between spectral sensitivity and ambient light is that detection of specific wavelengths might be important for mate choice and intraspecific signalling. Short (UV-blue) and long wavelengths (yellow-red) are important signals for mate choice in stickleback [42], as male nuptial coloration often involves blue and red pigmentation [43], as well as reflection in the UV [44]. Tuning of perception towards these nuptial signals and detection of contrast among them could also contribute to the mismatch of sensitivity to available light. It is also conceivable that our estimates of sensitivity, which do not account for non-additive signal integration during neuronal processing, underestimate the environmental correlation.
A2 opsin chromophore complexes do not necessarily act synergistically with changes in opsin expression to produce adaptive shifts in spectral sensitivity. In the populations surveyed, substitution of A1 chromophores with A2 chromophores weakens the relationship between shifts in spectral sensitivity and shifts in ambient light. While the empirical ratios of A1 and A2 in the wild are unknown for these freshwater populations, our analyses suggest that A2 domination would be unlikely. A2 dominated retinas result in shifts in spectral sensitivity that do not correlate to shifts in these environments, and thus are unlikely to be in an adaptive direction. This was a somewhat surprising result as A2 chromophores are commonly used by many species of fishes found in freshwater lakes or streams [22]. The potentially maladaptive shifts seen when substituting to A2 are a result of overshooting long-wavelength sensitivity relative to the prevalence of these wavelengths in the surveyed freshwater lakes. This finding is consistent with work suggesting A2-dominated retinas are common for threespine stickleback from dystrophic lakes that are strongly red-shifted relative to the marine environment, as A2 use in such an environment would probably result in shifts in an adaptive direction [24].
In this study, we provide three lines of evidence to suggest that observed shifts in spectral sensitivity are adaptive: we show that they have evolved repeatedly, are genetically based and that regions of the spectrum that differ between marine and freshwater locations are largely the same regions that exhibit differences in spectral sensitivity between populations. The methods used in this study help to understand the direction of evolution of spectral sensitivity, and its relationship with ambient light. However, our approach does not allow us to disentangle the relative contribution of selection on colour discrimination, contrast detection and photon capture to shifts in spectral sensitivity. Future experimental and theoretical work will be required to determine the importance of selection on each of these functions.
Supplementary Material
Acknowledgements
We thank Loren Rieseberg for use of his RT-qPCR machine and Narina Jabari for field assistance. We also thank Chad Brock, Molly Cummings, Mark Kirkpatrick, John Taylor and the late Gregory Di Valentin for valuable discussions.
Ethics
Collections were made under the Species At Risk Act collection permit 236 and British Columbia Fish Collection permit NA-SU12-76311. Animals were treated in accordance with University of British Columbia Animal Care protocols (Animal Care permit A11-0402)
Data accessibility
The raw data and accompanying meta data are archived in the Dryad database: http://dx.doi.org/10.5061/dryad.1mc01.
Authors' contributions
D.J.R., D.S. and T.V. conceived of and designed the study; D.J.R., G.L.O. and T.V. collected the data; D.J.R., T.V. and N.H. analysed the data; T.V. and N.H. developed the statistical method; D.J.R. wrote the manuscript with help from T.V. and D.S. All authors contributed to the editing.
Competing interests
The authors have no competing interests.
Funding
This research was funded by NSERC grants to D.S. and N.H., fellowships to D.J.R. G.L.O. and T.V., an SICB grant-in-aid of research to D.J.R. and an NSF grant no. (IOS 1145468) to T.V.
References
- 1.Endler JA. 1991. Variation in the appearance of guppy colour patterns to guppies and their predators under different visual conditions. Vis. Res. 31, 587–608. ( 10.1016/0042-6989(91)90109-I) [DOI] [PubMed] [Google Scholar]
- 2.Endler JA. 1992. Signals, signal conditions, and the direction of evolution. Am. Nat. 139, S125–S153. ( 10.1086/285308) [DOI] [Google Scholar]
- 3.Munz FW, McFarland WN. 1977. Evolutionary adaptations of fishes to the photic environment. In The visual system in vertebrates (ed. Crescitelli F.), pp. 194–274. New York, NY: Springer. [Google Scholar]
- 4.Bowmaker JK, Govardovskii VI, Shukolyukov SA, Zueva LV, Hunt DM, Sideleva VG, Smirnova OG. 1994. Visual pigments and the photic environment: the cottoid fish of Lake Baikal. Vis. Res. 34, 591–605. ( 10.1016/0042-6989(94)90015-9) [DOI] [PubMed] [Google Scholar]
- 5.Clarke GL. 1936. On the depth at which fish can see. Ecology 17, 452–456. ( 10.2307/1931845) [DOI] [Google Scholar]
- 6.Denton EJ, Warren FJ. 1957. The photosensitive pigments in the retinae of deep-sea fish. J. Mar. Biol. Assoc. UK 36, 651–662. ( 10.1017/S0025315400025911) [DOI] [Google Scholar]
- 7.Munz FW. 1958. The photosensitive retinal pigments of fishes from relatively turbid coastal waters. J. Gen. Physiol. 42, 445–459. ( 10.1085/jgp.42.2.445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lythgoe JN. 1979. Ecology of vision. Oxford, UK: Oxford University Press. [Google Scholar]
- 9.Kirk JTO. 1994. Light and photosynthesis in aquatic ecosystems. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 10.Kirk JTO. 1977. Use of a quanta meter to measure attenuation and underwater reflectance of photosynthetically active radiation in some inland and coastal south-eastern Australian waters. Aust. J. Mar. Freshwater Res. 28, 9–21. ( 10.1071/MF9770009) [DOI] [Google Scholar]
- 11.Bell MA, Foster SA. 1994. The evolutionary biology of the threespine stickleback. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.Schluter D, Nagel LM. 1995. Parallel speciation by natural selection. Am. Nat. 146, 292–301. ( 10.1086/285799) [DOI] [Google Scholar]
- 13.McPhail JD. 1992. Ecology and evolution of sympatric sticklebacks (Gasterosteus): evidence for a species-pair in Paxton Lake, Texada Island, British Columbia. Can. J. Zool. 70, 361–369. ( 10.1139/z92-054) [DOI] [Google Scholar]
- 14.Boughman JW. 2001. Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature 411, 944–948. ( 10.1038/35082064) [DOI] [PubMed] [Google Scholar]
- 15.Shichida Y, Imai H. 1998. Visual pigment: G-protein-coupled receptor for light signals. Cell Mol. Life Sci. 54, 1299–1315. ( 10.1007/s000180050256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoyama S. 1995. Amino acid replacements and wavelength absorption of visual pigments in vertebrates. Mol. Biol. Evol. 12, 53–61. ( 10.1093/oxfordjournals.molbev.a040190) [DOI] [PubMed] [Google Scholar]
- 17.Yokoyama S, Radlwimmer FB, Blow NS. 2000. Ultraviolet pigments in birds evolved from violet pigments by a single amino acid change. Proc. Natl Acad. Sci. USA 97, 7366–7371. ( 10.1073/pnas.97.13.7366) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carleton KL, Kocher TD. 2001. Cone opsin genes of African cichlid fishes: tuning spectral sensitivity by differential gene expression. Mol. Biol. Evol. 18, 1540–1550. ( 10.1093/oxfordjournals.molbev.a003940) [DOI] [PubMed] [Google Scholar]
- 19.Jones FC, et al. 2012. The genomic basis of adaptive evolution in threespine sticklebacks. Nature 484, 55–61. ( 10.1038/nature10944) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rennison DJ, Owens GL, Taylor JS. 2012. Opsin gene duplication and divergence in ray-finned fish. Mol. Phylogenet. Evol. 62, 986–1008. ( 10.1016/j.ympev.2011.11.030) [DOI] [PubMed] [Google Scholar]
- 21.Hárosi FI. 1994. An analysis of two spectral properties of vertebrate visual pigments. Vis. Res. 34, 1359–1367. ( 10.1016/0042-6989(94)90134-1) [DOI] [PubMed] [Google Scholar]
- 22.Toyama M, et al. 2008. Presence of rhodopsin and porphyropsin in the eyes of 164 fishes, representing marine, diadromous, coastal and freshwater species: a qualitative and comparative study. Photochem. Photobiol. 84, 996–1002. ( 10.1111/j.1751-1097.2008.00344.x) [DOI] [PubMed] [Google Scholar]
- 23.Flamarique IN. 2005. Temporal shifts in visual pigment absorbance in the retina of Pacific salmon. J. Comp. Phys. A 191, 37–49. ( 10.1007/s00359-004-0573-9) [DOI] [PubMed] [Google Scholar]
- 24.Flamarique IN, Cheng CL, Bergstrom C, Reimchen TE. 2013. Pronounced heritable variation and limited phenotypic plasticity in visual pigments and opsin expression of threespine stickleback photoreceptors. J. Exp. Biol. 216, 656–667. ( 10.1242/jeb.078840) [DOI] [PubMed] [Google Scholar]
- 25.R Development Core Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 26.Pinheiro J, Bates D, DebRoy S, Sarkar D, orpR Core Team 2015. nlme: linear and nonlinear mixed effects models. R package version 3.1-119. See http://CRAN.R-project.org/package=nlme.
- 27.Orti G, Bell MA, Reimchen TE, Meyer A. 1994. Global survey of mitochondrial DNA sequences in the threespine stickleback: evidence for recent migrations. Evolution 48, 608–622. ( 10.2307/2410473) [DOI] [PubMed] [Google Scholar]
- 28.Govardovskii VI, Fyhrquist N, Reuter TOM, Kuzmin DG, Donner K. 2000. In search of the visual pigment template. Vis. Neurosci. 17, 509–528. ( 10.1017/S0952523800174036) [DOI] [PubMed] [Google Scholar]
- 29.Benjamini Y, Yekutieli D. 2001. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 29, 1165–1188. ( 10.1214/aos/1013699998) [DOI] [Google Scholar]
- 30.Fuller RC, Claricoates KM. 2011. Rapid light-induced shifts in opsin expression: finding new opsins, discerning mechanisms of change, and implications for visual sensitivity. Mol. Ecol. 20, 3321–3335. ( 10.1111/j.1365-294X.2011.05180.x) [DOI] [PubMed] [Google Scholar]
- 31.O'Quin KE, Hofmann CM, Hofmann HA, Carleton KL. 2010. Parallel evolution of opsin gene expression in African cichlid fishes. Mol. Biol. Evol. 27, 2839–2854. ( 10.1093/molbev/msq171) [DOI] [PubMed] [Google Scholar]
- 32.Colosimo PF, et al. 2005. Widespread parallel evolution in sticklebacks by repeated fixation of ectodysplasin alleles. Science 307, 1928–1933. ( 10.1126/science.1107239) [DOI] [PubMed] [Google Scholar]
- 33.Hohenlohe PA, Bassham S, Etter PD, Stiffler N, Johnson EA, Cresko WA. 2010. Population genomics of parallel adaptation in threespine stickleback using sequenced RAD tags. PLoS Genet. 6, e1000862 ( 10.1371/journal.pgen.1000862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuller RC, Carleton KL, Fadool JM, Spady TC, Travis J. 2005. Genetic and environmental variation in the visual properties of bluefin killifish, Lucania goodei. J. Evol. Biol. 18, 516–523. ( 10.1111/j.1420-9101.2005.00886.x) [DOI] [PubMed] [Google Scholar]
- 35.Seehausen O, et al. 2008. Speciation through sensory drive in cichlid fish. Nature 455, 620–626. ( 10.1038/nature07285) [DOI] [PubMed] [Google Scholar]
- 36.Loew ER, Lythgoe JN. 1978. The ecology of cone pigments in teleost fishes. Vis. Res. 18, 715–722. ( 10.1016/0042-6989(78)90150-5) [DOI] [PubMed] [Google Scholar]
- 37.Hunt DM, Dulai KS, Partridge JC, Cottrill P, Bowmaker JK. 2001. The molecular basis for spectral tuning of rod visual pigments in deep-sea fish. J. Exp. Biol. 204, 3333–3344. [DOI] [PubMed] [Google Scholar]
- 38.Rick IP, Bloemker D, Bakker T. 2012. Spectral composition and visual foraging in the three-spined stickleback (Gasterosteidae: Gasterosteus aculeatus L.): elucidating the role of ultraviolet wavelengths. Biol. J. Linnean Soc. 105, 359–368. ( 10.1111/j.1095-8312.2011.01796.x) [DOI] [Google Scholar]
- 39.Hagen DW. 1967. Isolating mechanisms in threespine sticklebacks (Gasterosteus). J. Fish Board Can. 24, 1637–1692. ( 10.1139/f67-138) [DOI] [Google Scholar]
- 40.Bowmaker JK, Kunz YW. 1987. Ultraviolet receptors, tetrachromatic colour vision and retinal mosaics in the brown trout Salmo trutta: age-dependent changes. Vis. Res. 27, 2101–2108. ( 10.1016/0042-6989(87)90124-6) [DOI] [PubMed] [Google Scholar]
- 41.Flamarique IN. 2013. Opsin switch reveals function of the ultraviolet cone in fish foraging. Proc. R. Soc. B 280, 20122490 ( 10.1098/rspb.2012.2490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rick IP, Bakker TC. 2008. Colour signalling in conspicuous red sticklebacks: do ultraviolet signals surpass others? BMC Evol. Biol. 8, 189 ( 10.1186/1471-2148-8-189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowland WJ. 1994. Proximate determinants of stickleback behaviour: an evolutionary perspective. In The evolutionary biology of the threespine stickleback (eds Bell MA, Foster SA), pp. 29–344. Oxford, UK: Oxford University Press. [Google Scholar]
- 44.Rick IP, Modarressie R, Bakker TC. 2004. Male three-spined sticklebacks reflect in ultraviolet light. Behaviour 141, 1531–1541. ( 10.1163/1568539042948222) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data and accompanying meta data are archived in the Dryad database: http://dx.doi.org/10.5061/dryad.1mc01.