Abstract
Gaze following, or co-orienting with others, is a foundational skill for human social behaviour. The emergence of this capacity scaffolds critical human-specific abilities such as theory of mind and language. Non-human primates also follow others' gaze, but less is known about how the cognitive mechanisms supporting this behaviour develop over the lifespan. Here we experimentally tested gaze following in 481 semi-free-ranging rhesus macaques (Macaca mulatta) ranging from infancy to old age. We found that monkeys began to follow gaze in infancy and this response peaked in the juvenile period—suggesting that younger monkeys were especially attuned to gaze information, like humans. After sexual maturity, monkeys exhibited human-like sex differences in gaze following, with adult females showing more gaze following than males. Finally, older monkeys showed reduced propensity to follow gaze, just as older humans do. In a second study (n = 80), we confirmed that macaques exhibit similar baseline rates of looking upwards in a control condition, regardless of age. Our findings indicate that—despite important differences in human and non-human primate life-history characteristics and typical social experiences—monkeys undergo robust ontogenetic shifts in gaze following across early development, adulthood and ageing that are strikingly similar to those of humans.
Keywords: gaze following, social cognition, primates, cognitive development, life history
1. Introduction
Gaze following, or co-orienting with others, is a foundational skill in human social cognition [1]. This behaviour first emerges in human infancy around six months of age [2], and scaffolds the development of more sophisticated socio-cognitive capacities [3]. By their first birthday, infants begin to establish joint attention with others [4,5]; follow others' gaze in more complex situations, such as behind barriers [6]; and use gaze direction as a cue to others' communicative intentions [7,8]. Furthermore, infants' early gaze-following responses predict their later theory of mind and language skills in toddlerhood [9,10], and disruptions of the typical early development of social attention in autism highlight the critical importance of this skill in the acquisition of typical human social behaviour and language [11–13]. Importantly, patterns of human social attention are not static after childhood. For example, in human adults (but not infants), women exhibit greater responsivity to gaze cues than do men [14–17]. Finally, older adults show declines in both the propensity to respond to gaze cues and the flexibility of their responses during ageing [18–20]. Overall, these patterns suggest that human gaze following shows characteristic changes across the life course (including during infancy, sexual maturity and senescence), and can map onto important differences in overall socio-cognitive functioning across individuals.
Some non-human animals also follow gaze, although there is interspecific variation in the types of cues that different species use to determine the direction of another individual's gaze, as well as the degree to which these co-orienting responses may reflect reasoning about another individual's line of sight versus more reflexive processes [21,22]. However, much less is known about the developmental time course over which these skills emerge in non-human animals. Comparative studies of cognitive development in other species can address the origin of human-like social capacities, such as what sorts of experiences are necessary for these skills to emerge [23–26]. For example, the acquisition of co-orienting responses may depend on strongly canalized developmental pathways that generally emerge in primates living in social groups of a certain complexity. Under this view, human and many non-human primate species might exhibit similar developmental patterns of gaze following. By contrast, the experiences necessary to spur the emergence of gaze-following skills over ontogeny—and, therefore, the underlying socio-cognitive mechanisms supporting the response—may be distinct in humans. In this case, humans should exhibit patterns of gaze development that are not shared with other primates. Along these lines, several theories directly link the evolution of our species' cognitive abilities to our unique life-history patterns [27,28]. In particular, human cognition may have co-evolved with relevant human life-history characteristics, such as an extended juvenile period that allows for the acquisition and refinement of cognitive skills, and increased longevity that allows for the exploitation of these skills. If human cognition and human life history did evolve in tandem, then species with different life-history traits should exhibit distinct patterns of cognitive development.
Current evidence suggests that some non-human primates do in fact acquire gaze-following behaviours over a different developmental time course than humans. For example, some macaque species appear to develop gaze-following skills at a slower rate than humans [29–31], with responses to some gaze cues (such as eye direction) not emerging until adulthood [30]. Similarly, apes may not show basic co-orienting responses when others move the direction of their gaze, nor more complex forms of reasoning about others' line of sight (e.g. accounting for barriers and distractors) until they are several years old [24,32–36]. Overall, this evidence supports the proposal that gaze following in non-human primates requires more extensive experience with relevant social interactions than is necessary for humans (e.g. [29]). Moreover, there is little evidence that non-human primates exhibit human-like sex differences in social attention [21,37,38] (but see [39]), which also suggests that human gaze development may be uniquely sensitive to either social experiences or hormonal mechanisms that come into play after sexual maturity. This early divergence in patterns of gaze-following development may play a critical role in the origins of major differences in the social-cognitive skills of humans and other species [23]. These conclusions are limited, however, because previous studies on the comparative development of gaze following have often tested only a few individuals longitudinally (e.g. [32,33,40]). Moreover, to our knowledge no previous study has examined gaze following in non-human animals ranging from early infancy to old age, hindering comparisons with human gaze following which is known to change across the entire lifespan.
To address this gap, we studied the development of gaze following in rhesus macaques (Macaca mulatta) in the largest cross-sectional sample of non-human animals tested to date (n = 481). Rhesus monkeys are a highly social Old World monkey, and our subject population semi-free ranges in large, mixed-sex social groups with wide age variation [41]. This unique population therefore allowed us to test specific individuals across the lifespan because the animals live in naturalistic conditions with species-typical social experiences. Importantly, rhesus monkeys show robust similarities to humans in several social-cognitive capacities: they follow others' gaze [35,42,43]; preferentially attend to socially relevant information such as conspecific's eyes and emotional expressions [44,45]; use this contextual information when interpreting gaze cues [39,46,47]; and use information about where others are looking to infer their visual perspective and knowledge [48–50]. Yet macaques and humans also exhibit major differences in life-history characteristics: macaques show relatively faster growth and brain maturation during the juvenile period, no period of reproductive cessation (menopause), and a shorter absolute lifespan than humans [51–53]. The fact that macaques and humans show many shared capacities for social cognition while exhibiting major differences in life-history traits provides a powerful test of whether humans and non-humans exhibit shared developmental patterns for gaze following.
For practical reasons, we tested monkeys in a simple experimental gaze-following task in which they could follow a human's gaze upwards. We used a human actor to ensure that the gaze stimulus was as constant as possible across individuals and conditions. We adopted this approach because rhesus monkeys spontaneously follow the gaze of both conspecifics [42,43] and humans [35]. The actor rotated her entire head upward (with her eyes open), because previous work indicated that macaques show robust gaze-following responses to such cues [35,36], and primates generally are less sensitive to eye cues alone [21,54]. Although it remains possible that monkeys of different ages had different levels of experience with humans and thus responded differentially to the human actor, we note that as a whole this population is highly habituated to human observers from infancy. Moreover, a prior study found that watching a human actor direct their gaze did not impact monkeys' subsequent gaze following in a later encounter [29]. Overall, these factors endorse our approach of using the tightly controlled actions of a human demonstrator.
Using this simple task, we assessed several features of the monkeys' responses to the actor's change in gaze direction upwards (see the electronic supplementary material, videos S1 and S2). First, we examined whether monkeys co-oriented by looking upwards. Such co-orienting behaviours can stem from different underlying psychological processes—from reflexive response to others' orientation, to more complex inferences about others' line of sight and perceptual experience [21,22,55,56]. We, therefore, also examined two additional features of their behaviour to disentangle the cognitive mechanisms mediating their responses. First, we examined whether monkeys flexibly habituated to the experimenter's repeated head movement across trials in the absence of a target—indicating that they adjusted their response based on situational context [35] rather than merely reflexively co-orienting when another individual shifted her gaze. Second, we examined whether monkeys made multiple independent looks to identify the (absent) target of gaze. In particular, we measured whether monkeys looked up multiple times, echoing previous work examining whether primates ‘check back’ with actors to assess their true line of sight [35,57]. In this naturalistic context, however, we could only assess whether monkeys made multiple independent looks (e.g. looked up, looked away and then looked up again). Importantly, previous work [35,43] has shown that rhesus monkeys follow the specific gaze direction of an actor (i.e. they look up more in the test situation but rarely do so in a control condition when the actor does not look up). In order to assess patterns of gaze habituation across trials in naive animals, we did not include control trials in the first study, but we did include them in the second study specifically to address this issue.
2. Study 1: developmental changes in gaze following
Each monkey completed one experimental session lasting up to four trials. In each trial, an experimenter captured the monkey's attention and then looked straight up with both her head and eyes for 10 s. We then measured whether the monkey also looked up during this time period.
(a). Subjects
We tested 481 rhesus macaques (219 males and 262 females, ranging from two weeks to 28.5 years old) from the Cayo Santiago population, a group of over 1200 free-ranging, individually identifiable monkeys. We required that monkeys successfully complete at least one trial to be included; a small number of additional monkeys were excluded because they ran away without being identified, were not looking at the start of their first trial during coding, or were not in view for the full 10 s of the trial (see the electronic supplementary material for details).
(b). Procedure
Two experimenters approached a calmly sitting monkey (1–2 m away). Experimenter 1 (E1), the actor, first attracted the monkey's attention to her face (by calling ‘monkey’ and/or clapping her hands). She then looked directly up while simultaneously saying ‘now’ (see the electronic supplementary material, figure S1); this auditory cue marked the start of the trial. Experimenter 2 (E2), the cameraperson, stood next to E1 and filmed the monkey's face; she said ‘stop’ after the 10 s trial had concluded. We tested monkeys sitting in the vicinity of a tree so that the experimenter's look was consistent with the possibility of a target of attention being present. We, consequently, refrained from testing in locations when another monkey was actually present above the subject, to avoid any possible visual and auditory confounds. To assess monkeys' habituation to repeated gazing, we presented monkeys with up to four identical trials. Across the four trials, E1 tried to attract the monkey's attention for the next trial as soon as the previous one concluded, with no pause. Monkeys were free-ranging on the island during the test and thus may voluntarily leave the testing area before completing all possible trials. If this happened, E1 (who could not see the monkey's previous responses, as she had been looking up during the test) determined whether or not to end the session if the monkey ran away or moved to an inaccessible location before the entire four trials could be completed.
(c). Data coding and analysis
Two independent coders scored each trial. A primary coder examined all trials from the study, and two reliability coders each scored approximately half of the trials (see the electronic supplementary material for all coding details). Individual trial clips were randomized during coding so coders were blind to trial number. Each coder independently identified the start of the trial (e.g. when E1 said ‘now’) and examined the subsequent 10 s period frame-by-frame to judge: (i) whether the monkey ever looked upwards (using either their eyes or entire head); (ii) the total duration of looking upwards; (iii) the monkey's latency to look up; and (iv) the total number of discrete looks (e.g. looking up, looking away and then looking up again). Coders had high reliability for all measures, including agreement for whether the monkey looked up (κ = 0.92 for first reliability coder; κ = 0.92 for second), duration of time spent looking up (rp = 0.96 for first coder, rp = 0.95 for second) and total number of discrete looks (rp = 0.87 for first coder; rp = 0.92 for second).
For our analyses, we classified individuals into age cohorts based on life-history transitions in this species: infants up to 1 year (completion of weaning); juveniles up to 5 years (the onset of sexual maturity); adults up to 15 years and older monkeys over 15 years (monkeys in this population have a median lifespan of 15 years, only rarely exceeding 25 years [58]). We then used the glmer function from the LME4 software package in R to model whether monkeys followed gaze (see the electronic supplementary material). We fitted binomial models to a logit link function using maximum likelihood, including random subject intercepts to account for repeated trials within subjects. A feature of generalized linear mixed models (GLMM) is that it can account for unequal repeats across subjects [59], which is important as subjects did not always complete all four trials (as they were free-ranging during tests). We conducted post hoc Tukey comparisons of model factors using the glht function in the multcomp package, and compared the fit of different models using likelihood ratio tests [60].
(d). Results and discussion
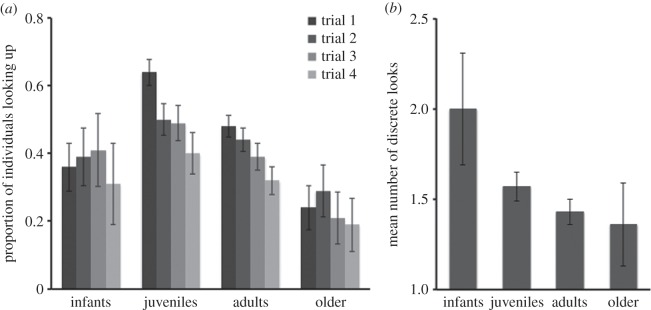
We first examined monkeys' tendency to follow gaze over the lifespan, and found that the rate at which the monkeys looked up varied across age cohorts. While 36.2 ± 7.1% of infants looked up on their first trial, 64.4 ± 3.9% of juveniles, 48.3 ± 3.2% of adults and only 24.4 ± 6.5% of older monkeys followed gaze (figure 1a; electronic supplementary material, table S1). In other words, the tendency to follow gaze emerged in infancy, peaked in juveniles, and then declined across adulthood and old age. Using GLMM, we first fitted a base model that included random subject intercepts to account for repeated measures, and trial number as a covariate to account for any within-session shifts in gaze following. To assess the importance of life-history stage on gaze following, we then added age cohort in the second model. Including cohort significantly improved fit as compared to the base model (χ23 = 30.10, p < 0.001); pairwise comparisons revealed that juveniles followed gaze more than all other groups, and younger adults further followed gaze more than older adults (Tukey tests: p < 0.01 for all significant cases). In the third model, we added sex as a predictor to assess if the monkeys showed human-like sex differences in responses. This further increased model fit compared with the second model (χ21 = 5.64, p < 0.05), revealing that females were more likely to follow gaze than males (see table 1 for parameters from the full model), as in humans [14,15].
Figure 1.
Gaze-following responses across the lifespan (study 1). Monkeys completed an experimental gaze-following task in which a human attracted their attention and then looked up. (a) Juveniles and adults exhibited flexible habituation by decreasing their responses across four repeated trials. (b) Younger monkeys made more multiple discrete looks in order to locate the (absent) target of the experimenter's gaze. Error bars indicate s.e.
Table 1.
Factors influencing propensity to follow gaze in macaques' development (study 1). (Predictors from the full (best fit) model. Trial number (1–4) was included as a covariate across models, and we added cohort and sex in successive models to test their importance. Values in bold are predictors that reached significance of p < 0.05.)
| factor | estimate | s.e. | Z | p-value |
|---|---|---|---|---|
| trial number | −0.308 | 0.062 | −4.979 | <0.001 |
| sex (female baseline) | −0.402 | 0.170 | −2.370 | <0.05 |
| cohort: juveniles versus infants | 1.017 | 0.328 | 3.097 | <0.01 |
| cohort: adults versus infants | 0.436 | 0.311 | 1.404 | 0.18 |
| cohort: older adults versus infants | −0.701 | 0.411 | −1.706 | 0.18 |
| cohort: adults versus juveniles | −0.581 | 0.192 | −3.034 | <0.01 |
| cohort: older adults versus juveniles | −1.718 | 0.338 | −5.086 | <0.001 |
| cohort: older adults versus adults | −1.137 | 0.320 | −3.557 | <0.005 |
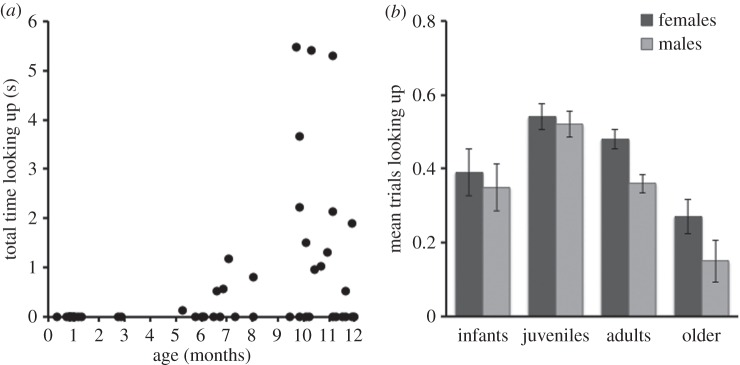
We next examined the factors that predicted gaze following within each cohort. We were particularly interested in: (i) when gaze following first emerged in younger monkeys; (ii) when sex differences in monkey gaze following emerged; and (iii) whether different cohorts habituated by showing reductions in their propensity to look up across trials, our first index of the cognitive processes supporting gaze following. To do so, we used GLMM to model the importance of sex, trial number and age (in years) as a covariate within each cohort separately, with random subject intercepts to account for repeated measures as before. We found that gaze-following responses had different predictors across age cohorts. Infants (n = 47, 25 females, 22 males) did not show habituation or sex differences in responses, but model fit was improved by including age (χ21 = 17.89, p < 0.001). The youngest monkey in our sample that followed gaze was 5.5 months old, indicating the capacity to follow gaze was present during the second half of the first year of life and the tendency to do so increased through the infant period (figure 2a). In juveniles (n = 149, 79 females, 70 males), the inclusion of only trial number improved model fit (χ21 = 15.14, p < 0.001). That is, juveniles were less likely to look up over subsequent trials, indicating that flexible habituation to repeated gazes emerges in the juvenile period. For adult macaques (n = 240, 126 females, 114 males), model fit was improved by including both trial number (χ21 = 13.76, p < 0.001), and then also sex as a predictor (χ21 = 6.92, p < 0.01). Thus, adults continued to show flexible habituation, but also exhibited a human-like sex difference in social attention following maturity, as adult females gaze followed more than males (figure 2b). Finally, none of these predictors improved model fit in older adults (n = 45, 32 females, 13 males), indicating that flexible control over gaze following declined during monkey ageing (see the electronic supplementary material for details and tables S2–S5 for models parameters from each cohort).
Figure 2.
Gaze-following emergence in infancy and sex differences at maturity (study 1). (a) The propensity to follow gaze initially emerged in the first year of life. The duration of upwards gazing by the 47 infants on their first trial, plotted by age in months. (b) In adulthood, females began to follow gaze more robustly than males. Mean gaze-following responses by sex across cohorts (averaging all completed trials). Error bars indicate s.e.
We next examined the number of discrete looks that monkeys made, our second measure of the cognitive processes supporting gaze following (figure 1b). We predicted that if monkeys exhibit enhanced sensitivity to gaze cues in infancy, then infants should make the most discrete looks in an effort to locate the (absent) target. We, therefore, analysed trial-one behaviour of monkeys who followed gaze on that trial (e.g. before they could receive any feedback that there was in fact no target). Within the subset of monkeys who followed gaze (n = 240), we found that the number of discrete looks upwards was negatively correlated with age (rp = −0.17, p < 0.01). Whereas infants made an average of 2.0 ± 0.31 looks, this declined to 1.36 ± 0.20 in older adults. This pattern could reflect age-related maturation of control processes that override reflexive gaze following, greater sensitivity of infants to a persistent gaze cue, or overall greater attention to humans by infants.
We next examined several alternative explanations for the overall pattern of age-related change in gaze following. First, we tested whether differences in habituation across cohorts might reflect age differences in the total number of trials that monkeys completed. Across all monkeys, mean trial completion was 2.9 ± 0.06, with 48.9% of monkeys completing all four trials (the modal trial completion number). We found that infants completed slightly fewer trials than adults (infant mean: 2.51 ± 0.18 trials; juveniles 2.81 ± 0.10; adults 3.02 ± 0.08; older adults 3.00 ± 0.19 trials), but that differences in trial completion cannot account for our overall pattern of results, particularly the increase in juvenile gaze following compared with adults and older adults (see the electronic supplementary material). Second, we considered whether cohorts differed in their baseline tendency to look up. One possibility is that juveniles' higher rates of gaze following actually reflect a higher rate of baseline upward looks (rather than responses to the experimenter's actions). We, therefore, examined whether there were differences in the latency or duration of the juveniles' looks that differentiate their looking relative to the other age groups. We predicted that baseline looking that did not occur in response to the experimenter's actions should: (i) be randomly distributed across the trial, rather than occurring soon after the experimenter looked up; and (ii) last a shorter duration, consistent with scanning rather than trying to locate a specific target in that location. To test this, we examined the latency and duration of looks in first trials of individuals who did look up (see the electronic supplementary material for details). However, we found no significant differences in either response latency (F3,236 = 1.44, p > 0.23, n.s.) or gaze duration (F3,236 = 1.95, p > 0.12, n.s.) across cohorts. In this way, the pattern of gazing in individuals who did respond was similar across ages. These results consequently suggest similar processes drove gaze following across age cohorts, but we returned to this issue in study 2.
3. Study 2: gaze direction control
In study 2, we directly measured whether there were baseline differences in the monkeys' tendency to look up across cohorts. Previous work has shown that rhesus monkeys follow the specific gaze direction of an actor (i.e. looking up more in a test condition than in a control condition where the actor did not look up [35,43]) and overall patterns of looking in these studies did not support the possibility that the age effects were driven by baseline rates of looking up. Nevertheless, we directly tested this possibility in study 2, in which we presented monkeys (n = 80 monkeys) with a similar procedure to that of study 1 but here the experimenter alternated the direction of her gaze (up versus down).
(a). Subjects
We retested a subset of 80 individuals (39 males and 41 females) who had previously completed the first study, a subsample of individuals ranging from 1.5 to 22 years old. We tested individuals in the order in which we encountered them, with the selection for inclusion in this study blind to subjects' performance in study 1. Monkeys were never tested on the same day as their participation in study 1 (typically, at least two weeks or more later). Monkeys had to successfully complete at least the first two trials (one up and one down trial) for the within-subjects condition comparison; additional monkeys were excluded because they did not successfully complete at least two trials (see the electronic supplementary material).
(b). Procedure and coding
Study 2 used the same procedure as in study 1, but across the four trials E1 alternated whether she looked straight up or straight down; we counterbalanced whether an individual received an up trial or a down trial first. We chose to contrast the up trials with down trials, rather than use a ‘no look’ control (where the experimenter attracts the subject's attention and then gazes directly at the subject, rather than looking up) that has been more typically used in previous studies with apes [35,57], because this type of extended direct gaze is generally perceived as a threat in this population of monkeys. We instead used a ‘downwards look’ in order to provide a fair contrast with the up trials, in that the experimenter captured the monkey's attention and then looked in a specific direction, without introducing additional confounds such as increased arousal due to directed gaze.
As in study 1, trials were clipped and randomized so that the two coders were blind to condition (see electronic supplementary material for all coding details). We coded whether the monkeys looked up across trials, so we could use identical coding procedures as in the first study. That is, this control further served as a measure of baseline rates of looking up in a situation where the experimenter had not looked up, but her actions were otherwise identical to the test in terms of attracting the monkey's attention and then diverting her gaze.
(c). Results and discussion
Overall, monkeys looked up across 40.4 ± 4.1% of all completed up trials, but did so on only 14.1 ± 2.9% of all down trials (see figure 3 for performance by age cohort). We used GLMMs to examine what factors predicted whether individuals would look up. We first fitted a base model that included random subject intercepts, condition order (random assignment to experience either an up or down trial first) and trial number as a covariate to account for within-session habituation. In the second model, we added age as a covariate to examine if propensity to follow gaze declined (as infants were not included here, we predicted gaze would have a linear effect on gaze following in this age range). We found that gaze following indeed declined with age, as this predictor improved model fit compared with the base model (χ21 = 16.72, p < 0.001). Finally, we tested the efficacy of our experimental manipulation by adding trial condition (up or down) as a predictor. This further improved model fit (χ21 = 34.98, p < 0.001), indicating that the monkeys were more likely to look up after the experimenter looked up, compared with the control condition where she looked in a different direction (see table 2 for full model parameters).
Figure 3.
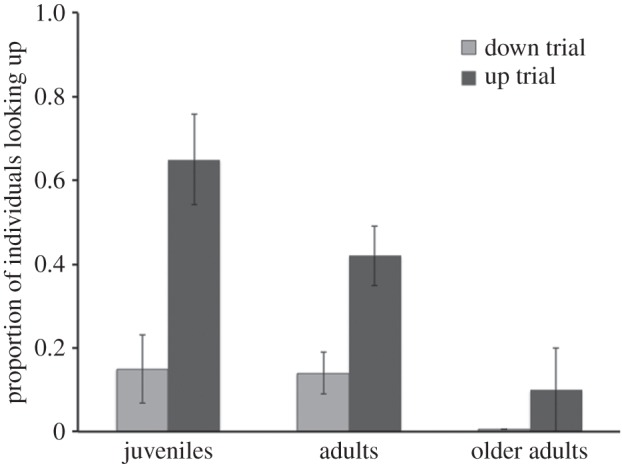
Responses to different gaze directions (study 2). The control study assessed how monkeys responded to the experimenter looking up versus down (as a measure of baseline upward looking). Proportion individuals looking up on each monkey's first up and down trial (order counterbalanced). While gaze-following responses declined with age in the up trials, monkeys of all ages exhibit similar low rates of baseline looks on down trials. Error bars indicate s.e.
Table 2.
Factors influencing propensity to follow gaze in direction control study (study 2). (Predictors from the full (best fit) model. Trial number (1–4) as a covariate and the factor condition order (direction of experimenter's look on the first trial) were included as predictors across models, and we added age and trial condition in successive models to test their importance. Values in bold are predictors that reached significance of p < 0.05.)
| factor | estimate | s.e. | Z | p-value |
|---|---|---|---|---|
| trial number | 0.038 | 0.156 | 0.247 | 0.80 |
| cond. order (down first baseline) | −0.900 | 0.459 | −1.959 | <0.051 |
| age | −0.224 | 0.061 | −3.665 | <0.001 |
| trial cond. (down trial baseline) | 2.037 | 0.401 | 5.085 | <0.001 |
We next examined how responses on up and down trials separately related to age (see the electronic supplementary material, tables S6 and S7 for details). We found that subject age was a significant predictor of responses on up trials, as including age increased model fit (χ21 = 14.74, p < 0.001). Specifically, gaze-following responses declined with age, replicating the basic results from study 1. By contrast, including age did not increase model fit for down trials (χ21 = 1.58, p > 0.20, n.s.), indicating similar rates of baseline looks across this age range. Study 2, therefore, replicated the main results from study 1, showing that rates of gaze following declined between the juvenile period and old age. In addition, we confirmed that baseline rates of looking upwards in this situation—when the experimenter's actions were otherwise identical in terms of capturing the monkey's attention, but then she looked in a different direction—remained at similar low rates across all ages. Consequently, our main developmental results from study 1 probably reflect differences in gaze-following responses, not baseline reactivity.
4. General discussion
Our findings indicate that gaze following in rhesus macaques shares several of the important developmental changes observed in humans. First, in study 1, we found a dramatic increase in monkeys' propensity to follow gaze between six months and 1 year of age, showing that rhesus macaque gaze following is present in infancy, as it is in typically developing humans. Human infants and toddlers are especially attuned to other's gaze [1], which has been interpreted to mean that young children use these sorts of gaze cues as a window into other peoples' mental states and communicative intentions [3,7]. Although we cannot directly address whether monkeys interpreted gaze cues in the same way, we found that younger monkeys were also particularly sensitive to others' gaze, as the frequency of gaze following peaked in juvenile monkeys. Second, our paradigm also allowed us to test for human-like patterns of gaze following in later life. Adult women are more responsive to gaze cues than are adult men [14,15], and we found a similar pattern in adult rhesus monkeys. This finding has not been consistently reported for non-human primates [21,37,38] (but see [39]), though there is some evidence for increased sociality in female infant monkeys [61]. Gender differences in human cognition may arise from diverse sources, including socialization, cultural norms of behaviour and biological variation in underlying neurobiological systems. Comparative data from species that lack human-like culture can help disentangle these diverse influences on cognition. Monkeys generally lack socially learned norms of behaviour, yet nonetheless showed sex differences in gaze following. Given that these differences emerged in adulthood, one possibility is that social attention in monkeys is influenced by the hormonal mechanisms driving sexual maturation. Finally, we observed that older monkeys are less likely to follow gaze, which is the first empirical demonstration that monkeys and humans show similar changes in social attention during healthy ageing [19,20]. While some previous work has shown that adult chimpanzees exhibit declines in gaze following during ageing [62], this effect has not been reported previously for macaques. Remarkably, older monkeys followed gaze at frequencies similar to those displayed by infants. Importantly, the results from study 2 suggest that age-related changes in gaze following did not reflect baseline differences in the tendency to look upwards more generally.
We also discovered that the developmental changes we observed in gaze following were associated with changes in other processes that support flexible behaviour. We found age-related changes in habituation to repeated gaze cues across trials, as well as the likelihood of making multiple discrete looks in the direction of the (absent) target. Although some infants followed gaze, flexible patterns of habituation to uninformative gaze cues were not observed until the juvenile period. In addition, we found that juveniles showed both the highest overall propensity to follow gaze, as well as the most flexibility in dampening their responses across trials when there was no actual target of the experimenter's gaze. While adults continued to show this pattern of habituation, older adults did not. These findings suggest diminished control over reflexive gaze following in older adults. Finally, younger monkeys that did follow gaze made the most discrete looks in the direction of the absent target. Together, these findings demonstrate that ontogenetic changes in human social attention parallel those in rhesus macaques, despite differences in socialization and culture between the two species.
Our large-scale design goes beyond all previous comparative developmental studies of primate gaze following by probing this behaviour across the entire lifespan. While some previous work also found high rates of gaze following in juvenile rhesus macaques compared with adults [35], our work further illuminates changes in gaze following during maturity and ageing. Furthermore, our measures of habituation and repeated looking allowed us to also assess some aspects of the cognitive processes underlying gaze following. Our results directly contrast with other work in closely related species. For example, evidence indicates that adult pigtail macaques (Macaca nemestrina) show more robust gaze-following responses than juveniles to human demonstrators [30]. One possibility is that differences observed between rhesus and pigtail macaques reflect minor variation in procedure across studies. However, it is also possible that these developmental patterns reflect true species differences. Pigtail macaques are characterized by higher levels of social tolerance and conciliation than rhesus macaques, who are comparatively more competitive and despotic [63]. This difference in social organization may be reflected in interspecific patterns of social-cognitive development. Future work would profit from testing more primate species to determine how differences in social structure affect the development of gaze processing across species.
Taken together, our findings indicate that macaque gaze following over the lifespan follows a remarkably human-like trajectory, even though their life-history characteristics are quite different from humans in terms of patterns of early growth, as well as reproductive senescence and ageing [51–53]. Rhesus macaques' differences in patterns of maturation, but similarities in cognitive development, bear on recent proposals arguing that the evolution of human life history and the evolution of human cognition are intertwined. In particular, these proposals link our species' extended life-history patterns to the pace at which human cognitive capacities develop: our relatively long juvenile period may allow the enhancement and elaboration of sophisticated cognitive skills, and our long lifespan then allows for the exploitation of the cognitive skills we have acquired [27,28]. Yet our results suggest that humans and rhesus monkeys can show quite similar patterns of psychological development—at least for this foundational socio-cognitive skill.
Why might this be the case? One possibility is that humans and other primates do not necessarily differ in the pace of development of some critical skills, but rather in the developmental patterns that link the emergence of different skills across ontogeny [23,24]. In humans, several more complex capacities emerge after and build upon earlier-emerging gaze-following skills—including joint attention, theory of mind and language [1,3,9]. Although gaze following is present in both infant macaques and human infants, macaques (and other primates) do not subsequently develop language or human-like mentalizing skills, such as the ability to reason about false beliefs [50]. Moreover, some socio-cognitive capacities that do emerge in primate development—such as understanding others' goals—are not clearly related to gaze following in non-humans [24], even though gaze following is linked to later-emerging social skills in humans. This suggests that the developmental sequence between early emerging socio-cognitive abilities and later-emerging socio-cognitive capacities in humans may not be shared with other species. Thus, although humans and other primates may show a similar developmental pace for the emergence of gaze following, this may have no bearing of the pattern of development of other socio-cognitive capacities in non-human primates.
Supplementary Material
Acknowledgements
We thank Lindsey Jones and Samantha Monier for assistance with data collection, and Linda Chang and Janelle Gagnon for data coding. The authors are grateful to Angelina Ruiz Lambides, Nahiri Rivera Barreto and Giselle Caraballo Cruz for their research support.
Ethics
All behavioural tests were approved by the Cayo Santiago IACUC and the Yale IACUC, and adhered to guidelines for animal research at this site.
Data accessibility
Data are available on Dryad http://dx.doi.org/10.5061/dryad.16n63.
Authors' contributions
A.G.R., A.M.A, M.L.P. and L.R.S. designed the study; A.G.R. and A.M.A. collected the data; A.G.R analysed the data; and A.G.R., A.M.A., M.L.P. and L.R.S. wrote the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by the NIMH (R01MH096875) and an NCRR grant CM-5-P40RR003640-13 award to the Caribbean Primate Research Center, the University of Puerto Rico-Medical Sciences Campus. L.R.S. was supported by a James S. McDonnell Foundation Award (no. 220020242) and Yale University.
References
- 1.Flom R, Lee K, Muir D. 2007. Gaze-following: its development and significance. Mahwah, NJ: Erlbaum. [Google Scholar]
- 2.D'Entremont B, Hains SMJ, Muir DW. 1997. A demonstration of gaze following in 3 to 6 month-olds. Infant Behav. Dev. 20, 569–572. ( 10.1016/S0163-6383(97)90048-5) [DOI] [Google Scholar]
- 3.Wellman HM. 2011. Developing a theory of mind. In The Blackwell handbook of cognitive development (ed. Goswami U.), pp. 167–187. New York, NY: Wiley-Blackwell. [Google Scholar]
- 4.Butterworth G, Cochran E. 1980. Towards a mechanism of joint visual attention in human infancy. Int. J. Behav. Dev. 3, 253–272. ( 10.1177/016502548000300303) [DOI] [Google Scholar]
- 5.Carpenter M, Nagell K, Tomasello M. 1998. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monogr. Soc. Res. Child Dev. 63, 1–143. ( 10.2307/1166214) [DOI] [PubMed] [Google Scholar]
- 6.Moll H, Tomasello M. 2004. 12- and 18-month-old infants follow gaze to spaces behind barriers. Dev. Sci. 7, F1–F9. ( 10.1111/j.1467-7687.2004.00315.x) [DOI] [PubMed] [Google Scholar]
- 7.Csibra G. 2010. Recognizing communicative intentions in infancy. Mind Lang. 25, 141–168. ( 10.1111/j.1468-0017.2009.01384.x) [DOI] [Google Scholar]
- 8.Senju A, Csibra G. 2008. Gaze following in human infants depends on communicative signals. Curr. Biol. 18, 668–671. ( 10.1016/j.cub.2008.03.059) [DOI] [PubMed] [Google Scholar]
- 9.Brooks R, Meltzoff AN. 2005. The development of gaze following and its relation to language. Dev. Sci. 8, 535–543. ( 10.1111/j.1467-7687.2005.00445.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks R, Meltzoff AN. 2008. Infant gaze following and pointing predict accelerated vocabulary growth through two years of age: a longitudinal, growth curve modeling study. J. Child Lang. 35, 207–220. ( 10.1017/S030500090700829X) [DOI] [PubMed] [Google Scholar]
- 11.Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. 2004. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev. Psychol. 40, 271–283. ( 10.1037/0012-1649.40.2.271) [DOI] [PubMed] [Google Scholar]
- 12.Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. 2009. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature 459, 257–263. ( 10.1038/nature07868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volkmar F, Chawarska K, Klin A. 2005. Autism in infancy and early childhood. Annu. Rev. Psychol. 56, 315–336. ( 10.1146/annurev.psych.56.091103.070159) [DOI] [PubMed] [Google Scholar]
- 14.Deaner RO, Shepherd SV, Platt ML. 2007. Familiarity accentuates gaze cueing in women but not men. Biol. Lett. 2, 64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayliss AP, di Pellegrino G, Tipper SP. 2005. Sex differences in eye gaze and symbolic cueing of attention. Q. J. Exp. Psychol. A 58, 631–650. ( 10.1080/02724980443000124) [DOI] [PubMed] [Google Scholar]
- 16.Mundy P, Block J, Delgado C, Pomares Y, van Hecke AV, Parlade MV. 2007. Individual differences and the development of joint attention in infancy. Child Dev. 78, 938–954. ( 10.1111/j.1467-8624.2007.01042.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alwall N, Johansson D, Hansen S. 2010. The gender difference in gaze-cueing: associations with empathizing and systemizing. Pers. Individual Differ. 49, 729–732. ( 10.1016/j.paid.2010.06.016) [DOI] [Google Scholar]
- 18.Kuhn G, Pagano A, Maani S, Bunce D. 2015. Age-related decline in the reflexive component of overt gaze following. Q. J. Exp. Psychol. 68, 1073–1081. ( 10.1080/17470218.2014.975257) [DOI] [PubMed] [Google Scholar]
- 19.Slessor G, Phillips LH, Bull R. 2008. Age-related declines in basic social perception: evidence from tasks assessing eye-gaze processing. Psychol. Aging 23, 812–822. ( 10.1037/a0014348) [DOI] [PubMed] [Google Scholar]
- 20.Slessor G, Venturini C, Bonny EJ, Insch PM, Rokaszewicz A, Finnerty AN. 2016. Specificity of age-related differences in eye-gaze following: evidence from social and nonsocial stimuli. J. Gerontol. B 71, 11–22. ( 10.1093/geronb/gbu088) [DOI] [PubMed] [Google Scholar]
- 21.Rosati AG, Hare B. 2009. Looking past the model species: diversity in gaze-following skills across primates. Curr. Opin. Neurobiol. 19, 45–51. ( 10.1016/j.conb.2009.03.002) [DOI] [PubMed] [Google Scholar]
- 22.Shepherd SV. 2010. Following gaze: gaze-following behavior as a window into social cognition. Front. Integr. Neurosci. 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosati AG, Wobber V, Hughes K, Santos LR. 2014. Comparative developmental psychology: how is human cognitive development unique? Evol. Psychol. 12, 448–473. ( 10.1177/147470491401200211) [DOI] [PubMed] [Google Scholar]
- 24.Wobber V, Herrmann E, Hare B, Wrangham R, Tomasello M. 2014. Differences in the early cognitive development of children and great apes. Dev. Psychobiol. 56, 574–573. ( 10.1002/dev.21125) [DOI] [PubMed] [Google Scholar]
- 25.Matsuzawa T. 2007. Comparative cognitive development. Dev. Sci. 10, 97–103. ( 10.1111/j.1467-7687.2007.00570.x) [DOI] [PubMed] [Google Scholar]
- 26.Gomez JC. 2005. Species comparative studies and cognitive development. Trends Cogn. Sci. 9, 118–125. ( 10.1016/j.tics.2005.01.004) [DOI] [PubMed] [Google Scholar]
- 27.Kaplan H, Hill K, Lancaster J, Hurtado AM. 2000. A theory of human life history evolution: diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185. ( 10.1002/1520-6505(2000)9:4%3C156::AID-EVAN5%3E3.0.CO;2-7) [DOI] [Google Scholar]
- 28.Bjorklund DF, Green B. 1992. The adaptive nature of cognitive immaturity. Am. Psychol. 47, 46–54. ( 10.1037/0003-066X.47.1.46) [DOI] [Google Scholar]
- 29.Ferrari PF, Coude G, Gallese V, Fogassi L. 2008. Having access to others’ mind through gaze: the role of ontogenetic and learning processes in gaze-following behavior of macaques. Soc. Neurosci. 3, 239–249. ( 10.1080/17470910701429065) [DOI] [PubMed] [Google Scholar]
- 30.Ferrari PF, Kohler E, Fogassi L, Gallese V. 2000. The ability to follow eye gaze and its emergence during development in macaque monkeys. Proc. Natl Acad. Sci. USA 97, 13 997–14 002. ( 10.1073/pnas.250241197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teufel C, Gutmann A, Pirow R, Fischer J. 2010. Facial expressions modulate the ontogenetic trajectory of gaze-following among monkeys. Dev. Sci. 13, 913–922. ( 10.1111/j.1467-7687.2010.00956.x) [DOI] [PubMed] [Google Scholar]
- 32.Okamoto-Barth S, Tomonaga M, Tanaka M, Matsuzawa T. 2008. Development of using experimenter-given cues in infant chimpanzees: longitudinal changes in behavior and cognitive development. Dev. Sci. 11, 98–108. ( 10.1111/j.1467-7687.2007.00658.x) [DOI] [PubMed] [Google Scholar]
- 33.Tomasello M, Carpenter M. 2005. The emergence of social cognition in three young chimpanzees. Monogr. Soc. Res. Child Dev. 70, vii. ( 10.1111/j.1540-5834.2005.00336.x) [DOI] [PubMed] [Google Scholar]
- 34.Okamoto S, Tomonaga M, Ishii K, Kawai N, Tanaka M, Matsuzawa T. 2002. An infant chimpanzee (Pan troglodytes) follows human gaze. Anim. Cogn. 5, 107–114. ( 10.1007/s10071-002-0133-z) [DOI] [PubMed] [Google Scholar]
- 35.Tomasello M, Hare B, Fogleman T. 2001. The ontogeny of gaze following in chimpanzee, Pan troglodytes, and rhesus macaques, Macaca mulatta. Anim. Behav. 61, 335–343. ( 10.1006/anbe.2000.1598) [DOI] [Google Scholar]
- 36.Tomonaga M, Tanaka M, Matsuzawa T, Myowa-Yamakoshi M, Kosugi D. 2004. Development of social cognition in infant chimpanzees (Pan troglodytes): face recognition, smiling, gaze, and the lack of triadic interactions. Jpn Psychol. Res. 46, 227–235. ( 10.1111/j.1468-5584.2004.00254.x) [DOI] [Google Scholar]
- 37.Herrmann E, Call J, Hernadez-Lloreda MV, Hare B, Tomasello M. 2007. Humans have evolved specialized skills of social cognition: the cultural intelligence hypothesis. Science 317, 1360–1366. ( 10.1126/science.1146282) [DOI] [PubMed] [Google Scholar]
- 38.Hopkins W, Misiura M, Reamer L, Schaeffer J, Mareno MC, Schapiro S. 2014. Poor receptive joint attention skills are associated with atypical gray matter asymmetry in the posterior superior temporal gyrus of chimpanzees (Pan troglodytes). Front. Psychol. 5, 7 ( 10.3389/fpsyg.2014.00007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paukner A, Anderson JR, Fogassi L, Ferrari PF. 2007. Do facial gestures, visibility or speed of movement influence gaze following responses in pigtail macaques? Primates 48, 241–244. ( 10.1007/s10329-006-0024-z) [DOI] [PubMed] [Google Scholar]
- 40.Matsuzawa T, Tomonaga M, Tanaka M. 2006. Cognitive development in chimpanzees. Tokyo, Japan: Springer. [Google Scholar]
- 41.Rawlins RC, Kessler MJ. 1987. The Cayo Santiago macaques: history, behavior and biology. Albany, NY: State University of New York Press. [Google Scholar]
- 42.Emery NJ, Lorincz EN, Perrett DI, Oram MW, Baker CI. 1997. Gaze following and joint attention in rhesus monkeys (Macaca mulatta). J. Comp. Psychol. 111, 286–293. ( 10.1037/0735-7036.111.3.286) [DOI] [PubMed] [Google Scholar]
- 43.Tomasello M, Call J, Hare B. 1998. Five primate species follow the visual gaze of conspecifics. Anim. Behav. 55, 1063–1069. ( 10.1006/anbe.1997.0636) [DOI] [PubMed] [Google Scholar]
- 44.Shepherd SV, Deaner RO, Platt ML. 2006. Social status gates social attention in monkeys. Curr. Biol. 16, R119–R120. ( 10.1016/j.cub.2006.02.013) [DOI] [PubMed] [Google Scholar]
- 45.Hoffman KL, Gothard KM, Schmid MC, Logothetis NK. 2007. Facial-expression and gaze-selective responses in the monkey amygdala. Curr. Biol. 17, 766–772. ( 10.1016/j.cub.2007.03.040) [DOI] [PubMed] [Google Scholar]
- 46.Goossens BMA, Dekleva M, Reader SM, Sterck EHM, Bolhuis JJ. 2008. Gaze following in monkeys is modulated by observed facial expressions. Anim. Behav. 75, 1673–1681. ( 10.1016/j.anbehav.2007.10.020) [DOI] [Google Scholar]
- 47.Mosher CP, Zimmerman PE, Gothard KM. 2011. Videos of conspecifics elicit interactive looking patterns and facial expressions in monkeys. Behav. Neurosci. 125, 639–652. ( 10.1037/a0024264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rochat MJ, Serra E, Fadiga L, Gallese V. 2008. The evolution of social cognition: goal familiarity shapes monkeys’ action understanding. Curr. Biol. 18, 227–232. ( 10.1016/j.cub.2007.12.021) [DOI] [PubMed] [Google Scholar]
- 49.Flombaum JI, Santos LR. 2005. Rhesus monkeys attribute perceptions to others. Curr. Biol. 15, 447–452. ( 10.1016/j.cub.2004.12.076) [DOI] [PubMed] [Google Scholar]
- 50.Martin A, Santos LR. 2016. What cognitive representations support primate theory of mind? Trends Cogn. Sci. 20, 375–382. ( 10.1016/j.tics.2016.03.005) [DOI] [PubMed] [Google Scholar]
- 51.Leigh SR. 2004. Brain growth, life history, and cognition in primate and human evolution. Am. J. Primatol. 62, 139–164. ( 10.1002/ajp.20012) [DOI] [PubMed] [Google Scholar]
- 52.Bogin B, Smith BH. 1996. Evolution of the human life cycle. Am. J. Hum. Biol. 8, 703–716. ( 10.1002/(SICI)1520-6300(1996)8:6%3C703::AID-AJHB2%3E3.0.CO;2-U) [DOI] [PubMed] [Google Scholar]
- 53.Alberts SC, et al. 2013. Reproductive aging patterns in primates reveal that humans are distinct. Proc. Natl Acad. Sci. USA 110, 13 440–13 445. ( 10.1073/pnas.1311857110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tomasello M, Hare B, Lehmann H, Call J. 2007. Reliance on head versus eyes in the gaze following of great apes and human infants: the cooperative eye hypothesis. J. Hum. Evol. 52, 314–320. ( 10.1016/j.jhevol.2006.10.001) [DOI] [PubMed] [Google Scholar]
- 55.Okamoto-Barth S, Call J, Tomasello M. 2007. Great apes’ understanding of other individuals’ line of sight. Psychol. Sci. 18, 462–468. ( 10.1111/j.1467-9280.2007.01922.x) [DOI] [PubMed] [Google Scholar]
- 56.Emery NJ. 2000. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci. Biobehav. Rev. 24, 581–604. ( 10.1016/S0149-7634(00)00025-7) [DOI] [PubMed] [Google Scholar]
- 57.Bräuer J, Call J, Tomasello M. 2005. All great ape species follow gaze to distant locations and around barriers. J. Comp. Psychol. 119, 145–154. ( 10.1037/0735-7036.119.2.145) [DOI] [PubMed] [Google Scholar]
- 58.Hoffman CL, Higham JP, Mas-Rivera A, Ayala JE, Mastripieri D. 2010. Terminal investment and senescence in rhesus macaques (Macaca mulatta) on Cayo Santiago. Behav. Ecol. 21, 972–978. ( 10.1093/beheco/arq098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baayen RH. 2008. Analyzing linguistic data. A practical introduction to statistics. Cambridge, MA: Cambridge University Press. [Google Scholar]
- 60.Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. ( 10.1016/j.tree.2008.10.008) [DOI] [PubMed] [Google Scholar]
- 61.Simpson EA, Nicolini Y, Shetler M, Suomi SJ, Ferrari PF, Pauker A. 2016. Experience-independent sex differences in newborn macaques: females are more social than males. Sci. Rep. 6, 19669 ( 10.1038/srep19669) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lacreuse A, Russell JL, Hopkins W, Herndon JG. 2014. Cognitive and motor aging in female chimpanzees. Neurobiol. Aging 35, 623–632. ( 10.1016/j.neurobiolaging.2013.08.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thierry B. 2007. Unity in diversity: lessons from macaque societies. Evol. Anthropol. 16, 224–238. ( 10.1002/evan.20147) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on Dryad http://dx.doi.org/10.5061/dryad.16n63.