Abstract
Habitat degradation is a global problem and one of the main causes of biodiversity loss. Though widespread, the mechanisms that underlie faunal changes are poorly understood. In tropical marine systems, corals play a crucial role in forming habitat, but coral cover on many reefs is declining sharply. Coral degradation affects the olfactory cues that provide reliable information on the presence and intensity of threat. Here, we show for the first time that the ability of a habitat generalist to learn predators using an efficient and widespread method of predator learning is compromised in degraded coral habitats. Results indicate that chemical alarm cues are no longer indicative of a local threat for the habitat generalist (the damselfish, Pomacentrus amboinensis), and these cues can no longer be used to learn the identity of novel predators in degraded habitats. By contrast, a rubble specialist and congeneric (Pomacentrus coelestis) responded to olfactory threat cues regardless of background environment and could learn the identity of a novel predator using chemical alarm cues. Understanding how some species can cope with or acclimate to the detrimental impacts of habitat degradation on risk assessment abilities will be crucial to defining the scope of resilience in threatened communities.
Keywords: chemical alarm cue, coral reef fishes, habitat degradation, predator–prey, risk assessment, threat learning
1. Introduction
The degradation of habitats is the largest cause of population extinction and community change worldwide [1,2]. This degradation is caused by global, regional and local issues. As habitats are fragmented, degraded and lost, the services they supply to the organisms that contribute to those habitats are disrupted [3]. These services include food, shelter and crucial biological interactions such as symbioses [4]. Changes to habitats may directly or indirectly affect an organism's fitness or survival and in doing so influence the viability of a local population [5,6]. In the ocean, many of the mortal effects of habitat degradation involve modifications to the vulnerability of prey to predators [7–9]. As the characteristics of habitats are changed, the balance of senses prey use to assess risk may also have to change, modifying the way that prey interact with known predators [10,11]. The changing nature of a habitat also means that predators that do not normally use the intact habitat expand their range to include the degraded habitat and so prey must face novel predators within their modified habitat [12].
Tropical coral reefs are one of the most species-diverse ecosystems on the planet, but are also most vulnerable to climate-driven change, such as ocean acidification, ocean warming and the increased frequency of storms [13]. Even the Great Barrier Reef, arguably the best managed of the world's coral reefs, has shown a major decline in live coral cover over the last 20 years [14,15]. As reefs degrade to a more algal-dominated landscape, monitoring studies indicate that there are dramatic changes in the composition of fish species. On a timescale measured by years, these changes are driven by recruitment (e.g. [16]) and a change in shelter as the nursery function of live coral diminishes. Decreased topographic complexity reduces the number of potential microhabitats and shelter sites for new settlers [7]. The changes in fish abundance and species composition that occur when coral habitats degrade are rapid [17], but little is known of the mechanisms that lead to this rapid shift in fish assemblages.
One mechanism by which reef degradation can potentially impact fish assemblages is by altering the way fish learn the identity of novel predators, such that they undertake an inappropriate antipredator behaviour when faced with a threat [18]. Almost all marine fishes have a larval phase that divorces them from their natal habitat. Larvae can bring with them some information about relevant threats through parental inheritance [19], but this will prove of limited use when the identity of predators themselves is unpredictable. Fish typically can learn threats in a number of ways, the most dangerous of which is a direct encounter with a predator, where a predator makes an unsuccessful strike, or the prey survives capture to subsequently escape (e.g. [20]). A safer and near-ubiquitous method of learning threats involves chemical associative learning, where a chemical cue released from a damaged conspecific (or ecologically relevant individual; known as a chemical alarm cue) is paired with chemical or visual cues of a novel predator, subsequently labelling the predator as a threat [21]. Here, no direct encounter with a predator is required by the individual(s) learning the predator. The frequency of pairing [22] or social reinforcement [23,24] in part determines the level of threat. If this important mechanism for learning olfactory and visual threats is hindered in any way, then the ability of fishes to react in an appropriate way when faced with a threat is severely impacted (e.g. [25]). The consequences will be most dramatic for predator-naive fish that enter their juvenile habitat at the end of their pelagic larval phase where predation may remove over 50% of recruits within the first 2 days [26].
Two recent studies found that fish that were either on or within a habitat that was extensively degraded did not respond to chemical alarm cues with an antipredator response [9,27]. However, even though they do not appear to respond appropriately to alarm cues, it is unknown whether they fail to learn the identity of novel predators using the normal method of associative learning. Other studies have found that while fish may not behaviourally respond to chemical alarm cues under sub-threshold levels, they are still able to use these cues to learn the identity of novel predators [28,29]. Moreover, because coral death and habitat degradation is spatially patchy [30], the patchiness is likely to affect the presence and concentrations of active chemicals released from the degraded substratum and this may influence the efficacy of alarm cues realized by prey. Fish on a patch of live coral among a background of degraded habitat may still be able to use alarm cues to learn threats, through a buffering of chemicals within the boundary layer around the live coral. Currently, it is unknown whether degraded habitats affect learning or the role that spatial patchiness of habitats may play in the manifestation of this effect.
This study examined the ability of a habitat generalist to learn predators in degraded coral habitats using chemical associative learning. Newly settled juveniles of the common ambon damselfish, Pomacentrus amboinensis, were placed in the field either in a background habitat that was healthy or degraded, on coral habitat patches that were composed of either live or dead coral. Through chemical associative learning, fish were taught the identity of a novel predator (dottyback, Pseudochromis fuscus) in the field. Fish antipredator responses were later tested to determine whether they had managed to learn the identity of the predator in the four habitat contexts (2 patch types × 2 background habitats; figure 1). We predicted that the lack of an antipredator response [9,27] would likely mean that the habitat generalist would not be able to learn the identity of novel predators. We also predicted that living on a healthy live habitat patch within a degraded habitat would not assist in their ability to learn threats due to the chemical interference of the background habitat (through alarm cue masking or cue modification). For comparison, we also examined the ability of a congeneric rubble specialist, Pomacentrus coelestis, to use chemical alarm cues as indicators of risk in degraded coral habitats, and also whether they could use these alarm cues to learn a novel odour as a threat. Our findings suggest that habitat degradation greatly impacts the learning of threats for some species, but others have found ways around this serious problem.
Figure 1.
Examples of live or dead coral patch treatments placed within healthy or degraded background habitats: (a) dead coral within degraded habitat (note cue tube to the right of the patch), (b) dead coral within healthy habitat, (c) live coral within degraded habitat and (d) live coral within healthy habitat.
2. Material and methods
(a). Study species and location
The ambon damselfish, P. amboinensis, is a common fish within coral reef fish communities of the Indo-Pacific (especially on the Great Barrier Reef), and adults are found in highest densities in shallow areas with a mixture of sand, rubble and live hard coral [31,32]. Juveniles settle from the larval phase after 15–23 days (at about 10–12 mm SL, standard length) to a broad range of habitats including live coral (70% of settlers), dead coral (20%) and rubble (10%) [32]. Fish used in the present studies were all caught in light traps as they entered the vicinity of the reef at night to settle. At dawn, captured individuals were brought back to the Lizard Island research station where they were placed in 16 l flow-through seawater holding tanks (densities of approx. 10–20 fish/tank). Fish were kept for 1–2 days and fed ad libitum twice daily with newly hatched Artemia sp. nauplii prior to release onto experimental patch reefs. Fish captured in light traps had no previous association with the fish community associated with the hard reef and were naive to bottom-dwelling predators that feed on juvenile and adult life stages [18].
Previous research on P. amboinensis has found that the newly settled fish have an innate antipredatory response to damage-released chemical cues from the skin of conspecifics both in the field and laboratory [27]. This antipredatory response involves reduced foraging and activity, and increased shelter use, and is similar to the reaction shown in many other damselfishes (e.g. [18,33]).
The neon damselfish, P. coelestis, exclusively inhabits rubble areas that have high flow conditions in lagoons or seaward reefs. It is a planktivore, and juveniles settle directly into the adult habitat at about approximately 14 mm SL. A recent study showed that P. coelestis juveniles are able to learn in the laboratory to associate odours with a threat through associative learning by the simultaneous exposure to a novel odour and a damage-released conspecific chemical alarm cue (G. Lienart, M. Ferrari, M. McCormick 2014, unpublished data). Fish react to the learnt threat with a typical antipredatory response involving a decrease in feeding and activity. Pomacentrus coelestis were captured, transported and maintained in exactly the same way as P. amboinensis.
In the current experiment with P. amboinensis, we used the dottyback Pseudochromis fuscus as the predator, which is a voracious predator on newly settled damselfishes [34]. Dottybacks were collected using hand nets and a dilute solution of clove oil anaesthetic and brought back to the research station, where they were placed individually in 1 l mesh containers and fed dead juvenile fishes.
Live healthy and dead-algae-covered hard coral (Pocillopora damicornis) were used as the small habitat patches that fishes were released onto during the experiment. The dead coral still had the same skeletal structure as live coral, so the topographic complexity was similar among patches and treatments.
(b). Experimental design
Experimental trials were conducted on the reef within small sand patches surrounded by one of two background habitats: hard coral reef (composed of a typical diversity of live and dead coral habitats) or degraded rubble habitat composed of dead hard coral that had been degraded by invertebrates, algae and bioeroders (figure 1). Ten small patch reefs (30 × 15 × 20 cm) of either live healthy Pocillopora damicornis (a bushy hard coral), or dead algal and invertebrate-covered P. damicornis were assembled with treatments alternated on the small sand patches within either of the two background habitats (figure 1). To avoid any contamination between patch reefs, there was a minimum of 3 m between patches and divers moved in an up-current direction during the experiment. A single juvenile P. amboinensis was placed onto each patch reef and allowed to acclimate for a minimum of 30 min before initial conditioning. Conditioning involved exposing the fish to 60 ml of a 50 : 50 mixture of chemical alarm cues and predator odour. This cocktail was slowly delivered by a 60 ml syringe via a 3 m plastic tube that was attached up-current at the edge of the patch reef using metal skewers (figure 1a). The tube outflow was positioned so that injected cues were carried over the focal fish by the prevailing current. The delivery tube was then slowly flushed with a further 60 ml of seawater collected from the background habitat relevant to the trial. Conditioned P. amboinensis were then left for approximately 4 h after which their behavioural response to one of three different chemical cues was quantified: (i) skin extracts from damaged conspecifics (i.e. chemical alarm cues), (ii) predator odour and (iii) seawater (blank control). The behaviour of focal fish was quantified for 3 min before (pre-stimulus period) and 3 min after (post-stimulus period) the addition of a test cue (as per previously established protocols [9,27]. Cues were delivered to the focal fish through a delivery tube positioned up-current of the patch reef as previously described (60 ml cues + 60 ml seawater flush). Behaviour was quantified (described below) 1 min after the initiation of cue injection. In summary, this gave us a 3 cues (chemical alarm cue, predator odour, seawater) × 2 patch types (live and rubble) × 2 background habitats (degraded and healthy) design (n = 15–26 fish).
To examine whether a rubble specialist would respond to chemical alarm cues in dead habitats, we placed P. coelestis on dead coral patches with a background of degraded coral. The experimental set-up was the same as above though the design differed, with fish exposed to three treatments: (i) skin extracts from damaged conspecifics, (ii) skin extract controls from a phylogenetically and ecologically distant heterospecific fish (Apogon fragilis, Apogonidae: controlling for a response to the damaged skin of any fish) and (iii) seawater controls.
Once the ability to use chemical alarm cues in P. coelestis was determined we undertook a second experiment to determine whether they could use the chemical alarm cue to learn the odour of a novel predator. This second experiment was conducted in exactly the same way as the learning experiment for P. amboinensis, with the exception that it was only carried out in a degraded background with the fish on dead coral (i.e. worse-case scenario).
To prepare the alarm cue underwater, one recently settled juvenile P. amboinensis (or P. coelestis, depending on the experiment) caught with a hand-net was placed into a 75 × 125 mm click-sealed bag filled with approximately 250 ml of seawater. This seawater came from the habitat that was the relevant experimental background for the replicate. Fish were euthanized by a quick blow to the brain case and the epidermis of the fish was lightly scratched using a scalpel blade that had been placed in the bag. For the initial conditioning, a syringe was used to extract 30 ml of the alarm cue and then 30 ml predator odour from a similar sized clip-seal bag that contained a live dottyback (approx. 75 ml standard length in 1 l of seawater) who had been in the bag for around 30 min. The pairing of the alarm cue with the predator odour (i.e. chemical associative learning) should subsequently label the predator odour as a threat [21,25]. Alarm cues were used within 10 min of production.
The behaviour of fish was assessed by a SCUBA diver positioned at least 1.5 m away from the patch reef. Four aspects of activity and behaviour were estimated for each 3 min sampling period: bite rate (successful and unsuccessful strikes), total distance moved (cm), maximum distance ventured from shelter (cm) and boldness. Distance measures were estimated in reference to the known length of the patch reef. Boldness was assessed using a continuous scale between 0 and 3 where: 0 is hiding in hole and seldom emerging; 1 is retreating to hole when scared and taking more than 5 s to re-emerge, weakly or tentatively striking at food; 2 is shying to shelter of patch when scared but quickly emerging, purposeful strikes at food; and 3 is not hiding when scared, exploring around the coral patch and striking aggressively at food [8]. At the end of the 3 min observation period, the fish was approached with a pencil and the fish's reaction and latency to emerge from shelter was taken into account in the assessment of boldness. This boldness measure has been shown to be repeatable [35,36] and initially high boldness has been associated with higher survival in other studies using newly settled damselfishes [31,37]. Three minute behavioural assessments have previously been found to be sufficiently long to obtain a representative estimate of an individual's behaviour [32,35].
(c). Statistical analyses
To determine whether there was an effect of background habitat or coral treatment on pre-stimulus behaviours of P. amboinensis a two-factor MANOVA was undertaken on bite rate (bites/3 min), total distance moved (cm over 3 min), maximum distance ventured away from shelter (cm) and boldness.
To determine the effect of background habitat, coral treatment and cue on behaviour, data were converted into the changes in magnitude of behavioural variables (post-stimulus–pre-stimulus). This minimized variance due to inherent differences in behaviour among individuals. The proportional change in behaviour was analysed for three variables (bite rate, total distance moved and boldness). Change in maximum distance ventured from shelter, as a measure of the willingness to take risk, was expressed in centimetres, because it did not show the same high levels of variability among individuals. A three-factor MANOVA was used to test for their equality between background habitats (healthy or degraded), coral treatments (live or dead coral patch) and cues (seawater, chemical alarm cue or predator odour), together with the interactions among the three factors. The nature of significant differences found by MANOVA was then explored with three-factor ANOVAs, followed by Tukey's HSD means comparisons. Replication ranged from 15 to 26, so type III sums of squares were employed.
To determine whether P. coelestis responded to chemical alarm cues within a degraded habitat, a one-factor MANOVA tested for the equality of behavioural change among the three treatments (seawater, conspecific chemical alarm cue, damage cues from a heterospecific). Likewise, to determine whether P. coelestis could use chemical alarm cues to learn a novel predator odour in degraded habitat, a one-factor MANOVA was also used to test for differences in behavioural change among the three experimental treatments (seawater, chemical alarm cue; predator odour seawater). The behavioural variables for both experiments included in the analysis were bite rate, total distance moved, maximum distance ventured and boldness (n = 15 and 10–13 for the respective experiments). ANOVAs on the individual variables were used to determine the nature of the significant difference found by MANOVA and significant differences were further evaluated with Tukey's HSD means comparisons. Examinations of the distribution of residuals (residual analysis) were used to examine normality and homogeneity of variance for all datasets, and all variables were found not to require transformation.
3. Results
In general, the behaviour and space use of P. amboinensis did not differ between coral treatments and background habitats, with a MANOVA showing no significant effects or interactions detected prior to the introduction of olfactory cues (p > 0.05, electronic supplementary material, table S1).
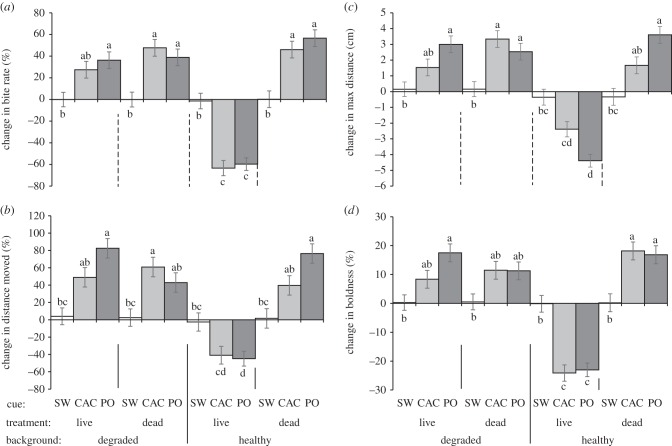
However, P. amboinensis juveniles responded differently when exposed to olfactory cues, with their response determined by a three-way interaction between background habitat, coral treatment and cue type (MANOVA interaction: F4,382 = 8.767, p < 0.0001; figure 2). ANOVA showed that there were significant three-way interactions among factors for all four behavioural variables (p < 0.0001, electronic supplementary material, table S2; figure 2). Overall, there was almost no change in behaviour of P. amboinensis in response to the seawater control (figure 2a–d). There was a significant response to the chemical alarm cue, with the fish's response dependent upon the type of background habitat and coral treatment. Fish that were on live coral within a healthy coral background responded to chemical alarm cues with a typical antipredatory response; a reduction in bite rate (approx. 70%, figure 1a), total distance moved (40%, figure 2b), maximum distance ventured (2.5 cm, figure 2c) and boldness (25%, figure 2d). By contrast, those that had any association with dead habitat, whether resident on a dead coral patch or on a patch of live coral within a degraded habitat, did not respond to chemical alarm cues with a reduction in activity (figure 2). Rather these fish became significantly more active, showing increases in all variables, particularly when resident on a dead coral patch.
Figure 2.
Change in behavioural response of the ambon damselfish Pomacentrus amboinensis to three olfactory cues (seawater, SW; chemical alarm cues, CAC or predator odour, PO), while resident on patch reefs composed of either live or dead coral that were set among a background habitat of healthy or degraded coral (see figure 1 for habitat photographs). The previously naive fish had been taught through associative learning on their resident habitat patch that the predator odour represented a threat. Behaviours recorded were: (a) bite rate, (b) total distance moved, (c) maximum distance ventured away from shelter and (d) boldness. Errors are standard errors and letters above bars represent groupings of means from Tukey's post-hoc tests (n = (left to right) 20, 15, 15, 19, 15, 15, 17, 18, 26, 15, 15, 15).
Newly settled P. amboinensis learnt that the predator odour represented a threat only when they were exposed to the pairing of chemical alarm cues and predator odour while on a live coral patch within a matrix of healthy coral (figure 2). Once again, when fish were exposed to cues from dead habitat, either through the patch they resided on or through the degraded habitat that surrounded them, they responded to predator odour with an increase in all behavioural variables, which was not consistent with an antipredatory response. When in a background of live coral on a live coral patch fish responded to predator odour with a similar intensity of response as they did to the chemical alarm cue (figure 2).
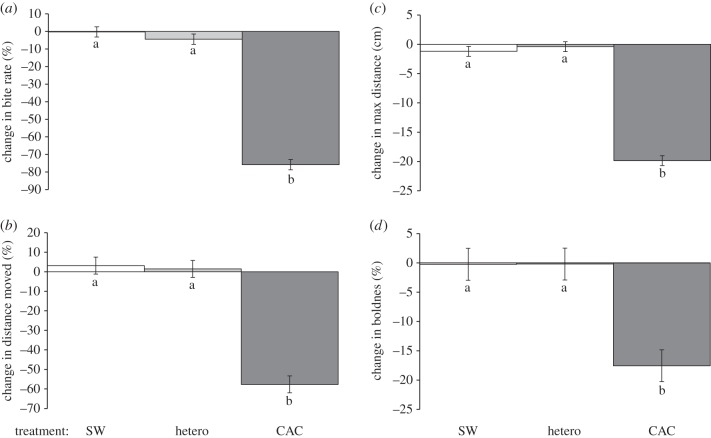
By contrast, P. coelestis reacted with an overt antipredator response to conspecific chemical alarm cues, with a decrease in bite rate, activity, boldness and distance from shelter when residing on a dead coral patch with a background of degraded coral (MANOVA, F8,80 = 9.425, p < 0.0001; figure 3). Univariate analyses indicated that there was a significant treatment effect for all four variables measured (p < 0.0001, electronic supplementary material, table S3 and figure 3). For all variables, there was no significant change in behaviour in response to seawater or heterospecific skin extracts. However, in response to chemical alarm cue fish reduced bite rate (by 75%, figure 3a), total distance moved (by 58%, figure 3b), maximum distance ventured (by 20%, figure 3c) and boldness (by 18%, figure 3d).
Figure 3.
Change in behavioural response of the neon damselfish Pomacentrus coelestis to three olfactory cues (SW; heterospecific skin cues, hetero; CAC), when resident on a dead coral patch within a background habitat of degraded coral. Behaviours recorded were: (a) bite rate, (b) total distance moved, (c) maximum distance ventured away from shelter and (d) boldness. Errors are standard errors and letters above bars represent groupings of means from Tukey's post-hoc tests (n = 15).
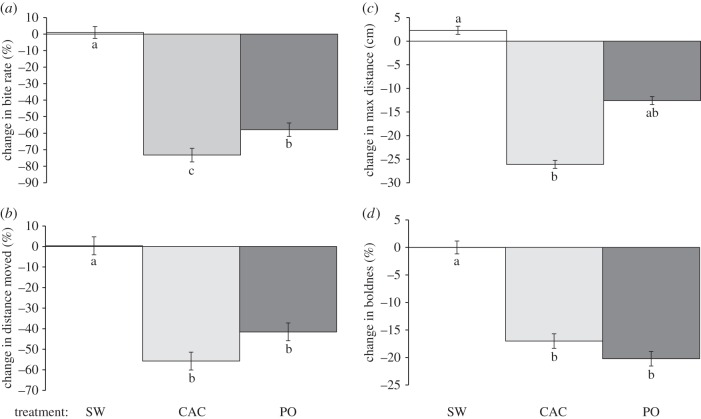
Pomacentrus coelestis was also found to learn a novel predator odour in the degraded coral habitat, with a significant difference in the behaviour of fish when exposed to seawater, chemical alarm cues and predator odour after they have previously been conditioned to learn the predator odour (MANOVA, F8,56 = 14.969, p < 0.0001; figure 4). ANOVA indicated that generally the alarm cue and predator odour elicited a similar response in the fish, with a reduction in bite rate, total distance moved, maximum distance ventured and boldness (electronic supplementary material, table S4 and figure 4). These differences were significantly reduced from the seawater controls, with the exception of maximum distance ventured, where the predator odour treatment did not differ from either the seawater control or chemical alarm cue treatments (figure 4c).
Figure 4.
Change in behavioural response of the neon damselfish Pomacentrus coelestis to three olfactory cues (SW, CAC and PO), when resident on a dead coral patch within a background habitat of degraded coral and pre-conditioned to recognize PO as a threat. Behaviours recorded were: (a) bite rate, (b) total distance moved, (c) maximum distance ventured away from shelter and (d) boldness. Errors are standard errors and letters above bars represent groupings of means from Tukey's post-hoc tests (n = 10–13).
4. Discussion
Learning plays a key role in predator avoidance, but our results suggest that habitat degradation will disrupt the associative learning abilities of some reef fish prey. For a generalist species, coral degradation resulted in the loss of an important olfactory method of learning about threats, while a rubble specialist still responded to risk cues in degraded coral habitats. The generalist lost the crucial ability to learn about novel predators through chemical associative learning [21]. Our previous research suggests that this may be because the chemical alarm cues are modified by the chemistry associated with dead coral and rubble habitats [9] (see discussion below). The loss of this mechanism of cataloguing predators is potentially devastating as it is one of the main mechanisms by which organisms can safely learn predator threats without having to experience the threat first hand [38]. Previous studies have shown that learning predators in this way greatly improves survival at highly vulnerable periods, such as the transition between life-history stages or during habitat shifts associated with moving from nursery habitats, when individuals enter new habitats that contain novel predators [18,39]. How the rubble specialist manages to circumvent the problems associated with recognizing the olfactory threat cues experienced by P. amboinensis is presently unknown, but indicates that some species have the capacity to get around this major problem.
For many organisms, particularly those with complex life cycles where newly metamorphosed juveniles enter a different environment from their parents, individuals must be able to identify predators rapidly to maximize the probability of survival. Research shows that mortality levels are very high immediately following these life-history transitions [40,41]; in coral reefs, it can be absolute, but averages about 50% over the first few days after settlement onto the reef [26]. This initially high mortality usually asymptotes quickly (Type III trajectory; [42]). What is unclear is the extent to which this rapid improvement in survival is because the most vulnerable individuals have been removed from the population, or whether it reflects the speed at which individuals learn to effectively evade local predators (e.g. [43]). If the process of cataloguing predators is hindered, then this loss of learning in degraded habitats may affect how rapidly survival improves after a habitat transition and the link between recruitment magnitude and cohort persistence.
Because the generalist cannot use chemical alarm cues in dead coral or rubble-dominated habitats, the expectation would be that they would be uncommon in that habitat. However, the focal species commonly inhabits degraded areas. Previous research indicates that this distribution is generated through a combination of processes. Pomacentrus amboinensis settles to a broad range of habitats [44], but is found largely at the base of the reef on rubble and live coral due to a mixture of differential mortality and competition [31,32,45]. Juveniles survive best when they are within the breeding territories of males of the same species, which are located at the base of the reef among patchy live and dead coral [31]. Here, males guard eggs in a benthic nest through an approximate 5-day embryonic phase prior to them hatching and entering the pelagic environment. A number of the egg predators that receive the most aggression from the males are also key predators on recently settled fishes, resulting in higher survival of juveniles over this highly vulnerable juvenile early life-stage at the base of the reef [31]. Interspecific interactions with a common sympatric congeneric, the lemon damselfish P. moluccensis, also reinforces this distribution [32]. But how do our generalists manage to survive in degraded habitats when they do not respond appropriately to the most reliable indicators of threat, a chemical alarm cue or learn via chemical associative learning?
Coral reef fishes live in a diverse community where resources are finely partitioned among similar species. This allows ample opportunity for prey to identify potential predators using public information that does not require the use of chemical alarm cues. Social learning is a means by which bystanders can learn by associating the reaction of nearby fish to the transient occurrence of a stimulus (e.g. a predator swimming past or odour signature of a predator [24,46]). Social learning can occur not only within a species, but across species that live in the same environment [23,24,47]. In this way, information that has been learnt through direct means by an individual (either direct risk, or through associative learning) can be passed to the rest of the local guild members who do not need to experience the direct risk first hand. Species that can still use chemical alarm cues in degraded habitat can also generalize the risk-related information that comes from closely related species [48]. Social learning also allows this generalized information to be passed to species that cannot use chemical alarm cues. Thus, species such as P. amboinensis that cannot learn through associative learning may be able to learn by observing the reaction of closely related species whose capacity to use chemical alarm cues is unaffected by habitat degradation, such as P. coelestis.
It is presently unclear why damage-released chemicals from the generalist are no longer effective in degraded habitats. Lönnstedt et al. [27] demonstrated using a tank experiment that a small amount of seawater that had been in contact with dead algal-covered coral was sufficient to effectively deactivate the species chemical alarm cues. Because the current study was conducted wholly in the field, it is presently unclear whether it is the processes of learning that is disrupted, or the process involved in the recognition of learned cues. Why the chemical alarm cues from the rubble specialist, P. coelestis, are not affected is unknown. Currently, the structure of damage-released alarm pheromones is only known for freshwater ostariophysan fishes [49], where several taxa have been found to respond to hypoxanthine-3-N-oxide (H3NO). However, the chemical structure of alarm cues in marine fishes is yet to be established. It may be that despite being closely related species, the structure of the chemical alarm cue molecule may differ sufficiently to be differentially affected by the chemistry of the environment. However, given the extent to which chemical alarm cues appear to be used to provide information about threat among closely related damselfish species [50], this hypothesis appears unlikely. The underlying mechanism explaining how some species are able to respond to olfactory threat cues in degraded habitats has yet to be revealed, and we encourage further work into finding the chemical structure of alarm pheromones for marine fishes.
To be able to understand how communities will respond to the global problem of rapid habitat degradation, we must examine how degradation may modify the interconnections among species in communities [51]. The interaction between a predator and its prey is one of the strongest links between species in interaction networks and this interaction has at its core how prey learn the identity of predators and the effectiveness of escape strategies. This study highlights that the outcome and dynamic of a predator–prey interaction are not just determined by the two parties, but also the state of the environment in which they live. Chemical cues are commonly used to learn and reinforce the identity of predators in aquatic prey. Our results show that predator learning via olfactory threat cues was impaired in degraded coral reef habitats for P. amboinensis, a generalist species. However, a closely related species, P. coelestis which is only found in degraded coral habitats was still able to use olfactory threat cues to assess risk in situ, and to learn new threats. These findings suggest that the process by which prey learn the identity of novel predators is specific to how chemical information is processed by the sensory systems of different taxa. Our study provides a strong indication that some species are able to behaviourally offset the potentially devastating impacts of habitat degradation on predator learning abilities. Determining the mechanisms that make some species susceptible to the modification of chemical threat cues in degraded habitats, the ways by which they compensate for the loss of this important source of information, and their potential to adapt over time will greatly enhance our understanding of how communities will respond to habitat degradation.
Supplementary Material
Acknowledgements
We thank Jamie McWilliam, Bridie Allan and Maya Santangelo for their help as dive buddies.
Ethics
This research corresponds to Australian laws and regulations under the animal ethics guidelines of James Cook University (permit numbers: A 2005).
Data accessibility
Data are provided on the Tropical Data Hub http://dx.doi.org/10.4225/28/55A45055A0C12.
Authors' contributions
M.I.M. and O.M.L. designed the experiment. M.I.M. carried out the data collection and analysis and wrote the initial draft of the paper. O.M.L. substantially contributed to manuscript revisions.
Competing interests
We declare we have no competing interests.
Funding
This project was supported through the ARC Centre of Excellence for Coral Reefs research grant no. (EI140100117).
References
- 1.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 2.McCauley DJ, Pinsky ML, Palumbi SR, Estes JA, Joyce FH, Warner RR. 2015. Marine defaunation: animal loss in the global ocean. Science 347, 1255641 ( 10.1126/science.1255641) [DOI] [PubMed] [Google Scholar]
- 3.Edwards DP, Tobias JA, Sheil D, Meijaard E, Laurance WF. 2014. Maintaining ecosystem function and services in logged tropical forests. Trends Ecol. Evol. 29, 511–520. ( 10.1016/j.tree.2014.07.003) [DOI] [PubMed] [Google Scholar]
- 4.Valiente-Banuet A, et al. 2014. Beyond species loss: the extinction of ecological interactions in a changing world. Funct. Ecol. 29, 299–307. ( 10.1111/1365-2435.12356) [DOI] [Google Scholar]
- 5.Aubry LM, Rockwell RF, Cooch EG, Brook RW, Mulder CPH, Koons DN. 2013. Climate change, phenology, and habitat degradation: drivers of gosling body condition and juvenile survival in lesser snow geese. Glob. Change Biol. 19, 149–160. ( 10.1111/gcb.12013) [DOI] [PubMed] [Google Scholar]
- 6.Carey C. 2014. Climate change, extinction risks, and reproduction of terrestrial vertebrates. In Reproductive sciences in animal conservation (eds Holt WV, Brown JL, Comizzoli P), pp. 35–54. New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
- 7.Pratchett MS, et al. 2008. Effects of climate-induced coral bleaching on coral-reef fishes—ecological and economic consequences. Oceanogr. Mar. Biol. 46, 251–296. ( 10.1201/9781420065756.ch6) [DOI] [Google Scholar]
- 8.McCormick MI. 2009. Behaviourally mediated phenotypic selection in a disturbed coral reef environment. PLoS ONE 4, e7096 ( 10.1371/journal.pone.0007096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lönnstedt OM, McCormick MI, Chivers DP, Ferrari MCO. 2014. Habitat degradation is threatening reef replenishment by making fish fearless. J. Anim. Ecol. 83, 1178–1185. ( 10.1111/1365-2656.12209) [DOI] [PubMed] [Google Scholar]
- 10.Dixson DL, Munday PL, Jones GP. 2010. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 13, 68–75. ( 10.1111/j.1461-0248.2009.01400.x) [DOI] [PubMed] [Google Scholar]
- 11.McCormick MI, Lönnstedt OM. 2013. Degrading habitats and the effect of topographic complexity on risk assessment. Ecol. Evol. 3, 4221–4229. ( 10.1002/ece3.793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feary DA, Pratchett MS, Emslie M, Fowler AM, Figueira WF, Luiz OJ, Nakamura Y, Booth DJ. 2014. Latitudinal shifts in coral reef fishes: why some species do and others do not shift. Fish Fish. 15, 593–615. ( 10.1111/faf.12036) [DOI] [Google Scholar]
- 13.Hughes TP, Bellwood DR, Connolly SR, Cornell HV, Karlson RH. 2014. Double jeopardy and global extinction risk in corals and reef fishes. Curr. Biol. 24, 2946–2951. ( 10.1016/j.cub.2014.10.037) [DOI] [PubMed] [Google Scholar]
- 14.De'ath G, Fabricius KE, Sweatman H, Puotinen M. 2012. The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl Acad. Sci. USA 109, 17 995–17 999. ( 10.1073/pnas.1208909109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GBRMPA. 2014. Great Barrier Reef Outlook Report 2014. Townsville, Australia: Great Barrier Reef Marine Park Authority. [Google Scholar]
- 16.Jones GP, McCormick MI, Srinivasan M, Eagle JV. 2004. Coral decline threatens fish biodiversity in marine reserves. Proc. Natl Acad. Sci. USA 101, 8251–8253. ( 10.1073/pnas.0401277101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham NA, Jennings S, MacNeil MA, Mouillot D, Wilson SK. 2015. Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518, 94–97. ( 10.1038/nature14140) [DOI] [PubMed] [Google Scholar]
- 18.Lönnstedt OM, McCormick MI, Meekan MG, Ferrari MCO, Chivers DP. 2012. Learn and live: the role of predator experience in influencing prey behaviour and survival. Proc. R. Soc. B 279, 2091–2098. ( 10.1098/rspb.2011.2516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giesing ER, Suski CD, Warner RE, Bell AM. 2010. Female sticklebacks transfer information via eggs: effects of maternal experience with predators on offspring. Proc. R. Soc. B 278, 1753–1759. ( 10.1098/rspb.2010.1819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lönnstedt OM, McCormick MI. 2015. Damsel in distress: captured damselfish prey emit chemical cues that attract secondary predators and improve escape chances. Proc. R. Soc. B 282, 20152038 ( 10.1098/rspb.2015.2038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrari MCO, Wisenden BD, Chivers DP. 2010. Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. Can. J. Zool. 88, 698–724. ( 10.1139/Z10-029) [DOI] [Google Scholar]
- 22.Mitchell MD, McCormick MI, Ferrari MCO, Chivers DP. 2011. Friend or foe? The role of latent inhibition in predator and non-predator labelling by coral reef fishes. Anim. Cogn. 14, 707–714. ( 10.1007/s10071-011-0405-6) [DOI] [PubMed] [Google Scholar]
- 23.Crane AL, Ferrari MCO. 2013. Social learning of predation risk: a review and prospectus. In Social learning theory: phylogenetic considerations across animal, plant, and microbial taxa (ed. Clark KB.), pp. 53–82. New York, NY: Nova Science Publisher. [Google Scholar]
- 24.Manassa RP, McCormick MI, Chivers DP. 2013. Socially acquired predator recognition in complex ecosystems. Behav. Ecol. Sociobiol. 67, 1033–1040. ( 10.1007/s00265-013-1528-3) [DOI] [Google Scholar]
- 25.Lönnstedt OM, McCormick MI. 2013. Ultimate predators: lionfish have evolved to circumvent prey risk assessment abilities. PLoS ONE 8, e75781 ( 10.1371/journal.pone.0075781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almany GR, Webster MS. 2006. The predation gauntlet: early post-settlement mortality in coral reef fishes. Coral Reefs 25, 19–22. ( 10.1007/s00338-005-0044-y) [DOI] [Google Scholar]
- 27.Lönnstedt OM, McCormick MI, Chivers DP. 2013. Degraded coral disrupts innate antipredator responses of fish. Ecol. Evol. 3, 38–47. ( 10.1002/ece3.388) [DOI] [Google Scholar]
- 28.Brown GE, Adrian JC, Patton T, Chivers DP. 2001. Fathead minnows learn to recognize predator odour when exposed to concentrations of artificial alarm pheromone below their behavioural-response threshold. Can. J. Zool. 79, 2239–2245. ( 10.1139/z01-194) [DOI] [Google Scholar]
- 29.Mirza RS, Chivers DP. 2003. Response of juvenile rainbow trout to varying concentrations of chemical alarm cue: response thresholds and survival during encounters with predators. Can. J. Zool. 81, 88–95. ( 10.1139/z02-216) [DOI] [Google Scholar]
- 30.Marshall PA, Baird AH. 2000. Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs 19, 155–163. ( 10.1007/s003380000086) [DOI] [Google Scholar]
- 31.McCormick MI, Meekan MG. 2007. Social facilitation of selective mortality. Ecology 88, 1562–1570. ( 10.1890/06-0830) [DOI] [PubMed] [Google Scholar]
- 32.McCormick MI, Weaver C. 2012. It pays to be pushy: intracohort interference competition between two reef fishes. PLoS ONE 7, e42590 ( 10.1371/journal.pone.0042590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrari MCO, Dixson DL, Munday PL, McCormick MI, Meekan MG, Sih A, Chivers DP. 2011. Intrageneric variation in tolerance of coral reef fishes to ocean acidification: implications for climate change projections on marine communities. Glob. Change Biol. 17, 2980–2986. ( 10.1111/j.1365-2486.2011.02439.x) [DOI] [Google Scholar]
- 34.Feeney WE, Lönnstedt OM, Bosiger YJ, Martin J, Jones GP, Rowe RJ, McCormick MI. 2012. High rate of prey consumption in a small predatory fish on coral reefs. Coral Reefs 31, 909–918. ( 10.1007/s00338-012-0894-z) [DOI] [Google Scholar]
- 35.White JR, Meekan MG, McCormick MI. 2015. Individual consistency in the behaviors of newly-settled reef fish. PeerJ 3, e961 ( 10.7717/peerj.961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White JR, Meekan MG, McCormick MI, Ferrari MCO. 2013. A comparison of measures of boldness and their relationships to survival in young fish. PLoS ONE 8, 268900 ( 10.1371/journal.pone.0068900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuiman LA, Meekan MG, McCormick MI. 2010. Maladaptive behavior reinforces a recruitment bottleneck in newly settled reef fishes. Oecologia 164, 99–108. ( 10.1007/s00442-010-1712-3) [DOI] [PubMed] [Google Scholar]
- 38.Chivers DP, Smith RJF. 1998. Chemical alarm signalling in aquatic predator–prey systems: a review and prospectus. Ecoscience 5, 338–352. [Google Scholar]
- 39.Chivers DP, Mirza RS, Johnston JG. 2002. Learned recognition of heterospecific alarm cues enhances survival during encounters with predators. Behaviour 139, 929–938. ( 10.1163/156853902320387909) [DOI] [Google Scholar]
- 40.Gosselin LA, Qian P. 1997. Juvenile mortality in benthic marine invertebrates. Mar. Ecol. Prog. Ser. 146, 265–282. ( 10.3354/meps146265) [DOI] [Google Scholar]
- 41.Byström P, Persson L, Wahlström E. 1998. Competing predators and prey: juvenile bottlenecks in whole-lake experiments. Ecology 79, 2153–2167. ( 10.2307/176718) [DOI] [Google Scholar]
- 42.McCormick MI, Hoey AS. 2004. Larval growth history determines juvenile growth and survival in a tropical marine fish. Oikos 106, 225–242. ( 10.1111/j.0030-1299.2004.13131.x) [DOI] [Google Scholar]
- 43.Mirza RS, Chivers DP. 2000. Predator-recognition training enhances survival of brook trout: evidence from laboratory and field-enclosure studies. Can. J. Zool. 78, 2198–2208. ( 10.1139/z00-164) [DOI] [Google Scholar]
- 44.McCormick MI, Moore JAY, Munday PL. 2010. Influence of habitat degradation on fish replenishment. Coral Reefs 29, 537–546. ( 10.1007/s00338-010-0620-7) [DOI] [Google Scholar]
- 45.McCormick MI. 2012. Lethal effects of habitat degradation on fishes through changing competitive advantage. Proc. R. Soc. B 279, 3899–3904. ( 10.1098/rspb.2012.0854) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown C, Laland K. 2011. Social learning in fishes. In Fish cognition and behavior (eds Brown C, Laland KN, Krause J), pp. 240–257. London, UK: Blackwell Publishing Ltd. [Google Scholar]
- 47.Griffin A. 2004. Social learning about predators: a review and prospectus. Anim. Learn. Behav. 32, 131–140. ( 10.3758/BF03196014) [DOI] [PubMed] [Google Scholar]
- 48.Mitchell MD, Cowman PF, McCormick MI. 2012. Are chemical alarm cues conserved within the coral reef fish family Pomacentridae? PLoS ONE 7, e47428 ( 10.1371/journal.pone.0047428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown GE, Adrian JC, Smyth E, Leet H, Brennan S. 2000. Ostariophysan alarm pheromones: laboratory and field tests of the functional significance of nitrogen oxides. J. Chem. Ecol. 26, 139–154. ( 10.1023/A:1005445629144) [DOI] [Google Scholar]
- 50.Mitchell MD, McCormick MI, Ferrari MCO, Chivers DP. 2013. Generalization of learned predator recognition in coral reef ecosystems: how cautious are damselfish? Funct. Ecol. 27, 299–304. ( 10.1111/1365-2435.12043) [DOI] [Google Scholar]
- 51.Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. 2010. A framework for community interactions under climate change. Trends Ecol. Evol. 25, 325–331. ( 10.1016/j.tree.2010.03.002) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are provided on the Tropical Data Hub http://dx.doi.org/10.4225/28/55A45055A0C12.