Abstract
Understanding the evolutionary responses of organisms to thermal regimes is of prime importance to better predict their ability to cope with ongoing climate change. Although this question has attracted interest in free-living organisms, whether or not infectious diseases have evolved heterogeneous responses to climate is still an open question. Here, we ran a common garden experiment using the fish ectoparasite Tracheliastes polycolpus, (i) to test whether parasites living in thermally heterogeneous rivers respond differently to an experimental thermal gradient and (ii) to determine the evolutionary processes (natural selection or genetic drift) underlying these responses. We demonstrated that the reaction norms involving the survival rate of the parasite larvae (i.e. the infective stage) across a temperature gradient significantly varied among six parasite populations. Using a Qst/Fst approach and phenotype–environment associations, we further showed that the evolution of survival rate partly depended upon temperature regimes experienced in situ, and was mostly underlined by diversifying selection, but also—to some extent—by stabilizing selection and genetic drift. This evolutionary response led to population divergences in thermal tolerance across the landscape, which has implications for predicting the effects of future climate change.
Keywords: pre-adaptation, ectoparasites, generalism, thermal reaction norms, common garden experiment, Qst/Fst
1. Introduction
Species are facing the new challenge of intense and rapid climate change [1,2]. They can respond to these changes by shifting their geographical distribution to track their favourable habitats [2–7]. They can also cope with climate change by adapting in situ to ongoing changes, either through phenotypic plasticity or microevolution [1–3]. Alternatively, some populations may have been selected for higher thermal tolerance, which would provide them—in a context of climate change—with an advantage over those with more narrow tolerance ranges. These populations should better survive future climatic change, and could also expand their ranges by replacing local populations with narrow thermal tolerance [4–6].
This hypothesis implicitly suggests that populations respond differently to current climatic conditions, so that this variation in responses can then be advantageous to face future climate change [7,8]. Local adaptation, genetic drift and founder effects are processes that can all generate population variation (both on mean values and reaction norms) in important traits such as thermal tolerance [8]. For instance, populations currently living in environments with high temperature fluctuations may evolve thermal generalism, whereas populations living in stable environments should evolve thermal specialization towards the local optima [9]. We can expect generalist populations to better cope with future climate change [9]. Thus, rather than considering a species as a uniform entity, this hypothesis considers a species as a heterogeneous entity that may include population subsets that would better fit future climate change [7].
Infectious diseases are particularly sensitive to climate change [10]. Warming temperatures are expected to affect virulence [11] and dynamics of transmission [12,13], as well as physiological development and survival rate of pathogens. At the same time, hosts may undergo an increased susceptibility to infection due to thermal stresses [10,14]. Because both host and pathogen traits can be affected by temperature, predicting the outcome of future climate change on the spatial distribution of infectious diseases is still a great challenge [10,15]. The evolutionary potential of pathogens is predicted to be high, and actually higher than that of hosts [16]. As such, many studies have highlighted the adaptation of pathogen genotypes to their local host genotypes (parasite-genotype × host-genotype interactions), demonstrating a strong heterogeneity within pathogen meta-populations in virulence-related traits [17,18]. Surprisingly, the possibility for parasite-genotype × environment interactions has attracted less attention (but see [9,10,19–22]), which can however potentially lead to heterogeneous responses of parasite populations to climatic variation [23].
The main goal of this study was to test experimentally whether or not parasite populations living in thermally heterogeneous landscapes respond differently to an experimental thermal gradient. To test this, we used the fish ectoparasite Tracheliastes polycolpus as a model. Tracheliastes polycolpus is a species of copepod whose females infect many freshwater fish [24] and partially or completely destroy fins [25], which leads to reduced fitness in hosts [26]. We conducted a common garden experiment to test the null hypothesis that larvae (i.e. the infective stage of the parasite) collected from adult parasites originating from rivers with different thermal regimes share similar reaction norms (in term of survival time) across an experimental gradient of water temperature ranging from 16°C to 22°C. This parasite lives in highly heterogeneous climatic environments [27] and effective population sizes are relatively small [28], suggesting that both local adaptation and genetic drift may generate population divergence for traits related to thermal tolerance. For these reasons, we expect to reject the null hypothesis that larvae from different rivers will respond homogeneously to a thermal gradient. We then used a Qst/Fst approach [29,30], combined with an approach linking thermal reaction norms observed in the experiment to thermal variation measured in the field to gain further insights into the evolutionary mechanisms (genetic drift or natural selection) that may generate contrasted responses to thermal regimes in wild populations.
2. Material and methods
(a). Biological model
Tracheliastes polycolpus is a parasitic copepod infecting several freshwater fish species of the Cyprinid family, the rostrum dace Leuciscus burdigalensis being the main host [24]. Only adult females are parasitic: they feed on the epithelial cells and mucus of fins, leading to decreased host fitness [26]. Each female is fecundated by a single dwarf male (i.e. gonochoric and monogamous species; O.R., S.B. & G.L. 2013, unpublished data) and produces two egg sacs each containing up to 165 eggs [29]. Female parasites lay free copepodid larvae in the water column. This larval stage is the infective stage of the parasite, during which it actively infects (within 3–5 days) other host individuals.
(b). Sampling sites
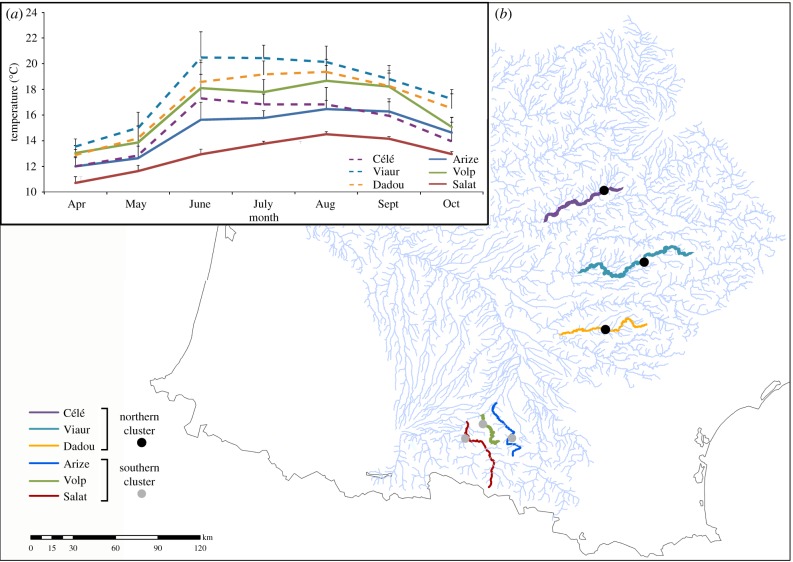
Six rivers were selected in the Garonne River Basin in southern France (Arize, Célé, Dadou, Salat, Viaur and Volp rivers; figure 1b). Rivers were selected based on their thermal regimes and on the level of genetic differentiation of their parasite populations. Specifically, using neutral genetic markers, we previously demonstrated that two genetic clusters of T. polycolpus coexist in this river basin [28] (see also the electronic supplementary material, appendix S1), and we selected three rivers within each genetic cluster that varied in their thermal regime (figure 1a). A first genetic cluster (northern cluster) grouped the Célé, Viaur and Dadou rivers, whereas the second cluster (southern cluster) grouped the Arize, Volp and Salat rivers (figure 1b).
Figure 1.
Populations of the parasite Tracheliastes polycolpus sampled in southwestern France. (a) Representation of mean monthly water temperature (±s.d.) for each river sampled, from April 2014 to October 2014. (b) Map of the six sampled rivers and their affiliation to the two genetic clusters (northern and southern clusters). Black circles indicate the sampling sites of T. polycolpus from the northern cluster; grey circles indicate the sampling sites of the southern cluster.
The water temperature (to the nearest 0.1°C) of each river was recorded every 30 min from April 2014 to October 2014, which represents the active period of parasite. To do so, we deployed two automatic data recorders (Hobo) along a 200 m stretch of each river, along which parasites were sampled (see figure 1b for the site locations). The thermal regime of each river was characterized from these raw data by calculating the mean monthly water temperature (°C), the mean annual water temperature (°C) and the annual coefficient of variation (CV). The thermal regime recorded for the six rivers strongly varied, with the Salat reaching a maximum mean monthly temperature of approximately 14.5°C in contrast to the approximately 20.5°C measured for the warmest river (Viaur; table 1 and figure 1a). Rivers also strongly differed in their CV, with the largest CV observed for the Volp (7.39°C) and the lowest in the Salat river (3.27°C; table 1).
Table 1.
Description of the environmental variables characterizing the six sampled sites (distance from the source and mean temperature from April to September ± coefficient of variation). The number of hosts (Leuciscus burdigalensis) infected with adults females carrying mature eggs and collected at each sampling site, the total number of parasites (Tracheliastes polycolpus) brought to the laboratory and total number of hatched larvae used in the experiment are also mentioned.
| river | distance from the source (km) | mean temperature ± CV | number of hosts | number of parasites with successful hatching | number of larvae |
|---|---|---|---|---|---|
| Célé | 35 | 15.27 ± 7.72 | 5 | 5 | 56 |
| Viaur | 129 | 18.07 ± 6.46 | 8 | 9 | 121 |
| Dadou | 70 | 17.04 ± 6.01 | 3 | 4 | 80 |
| Arize | 40 | 14.79 ± 5.7 | 8 | 11 | 115 |
| Volp | 31 | 16.60 ± 7.39 | 6 | 10 | 100 |
| Salat | 69 | 12.94 ± 3.27 | 11 | 15 | 131 |
(c). Parasite sampling
Parasites were collected from daces during summer 2014. Daces were sampled by electric-fishing at a single site in each river (figure 1) along an approximate 200 m river stretch. We sampled as many daces as possible so as to ensure the sampling of several parasites with mature egg clutches per site, and to avoid that all parasites came from a single dace (table 1). All adult parasites carrying mature eggs (i.e. eggs with four visible black dots [31]) were gently removed from their hosts, dropped into a 50 ml vial filled with river water and brought alive to the laboratory.
At the laboratory, we separated the two egg sacs from each female parasite using a sterile scalpel, and we placed each pair of egg sacs in a single well from a 24-well plate. Wells were filled with 2 ml of aerated and de-chlorinated tap water maintained at a temperature close to the one measured in natura during sampling (±1°C). Water in the wells was changed every day until hatching.
(d). Experimental design
We recorded the hatching of the eggs five times a day every 4 h (from 07.00 to 23.00). Once hatched, each larva was individually placed in a 24-well plate cell and plates were distributed in one of three thermal treatments: 16°C, 19°C or 22°C. We ensured that at least three larvae from each adult parasite were available for each of the three thermal treatments. This ensured that the progeny from each clutch (i.e. from a female fecundated by a single male) was split into all treatments, and that the interaction parasite × genotype per thermal treatment was replicated at least three times.
Thermal treatments were chosen as follows: the intermediate thermal treatment (19°C) was based on a preliminary analysis (in French rivers) indicating that prevalence in wild populations was maximal at a temperature around 19°C [32]; the two additional treatments (16°C and 22°C) were included to mimic a large thermal gradient and to cover the range of temperatures observed at our sampling sites (see the electronic supplementary material, table S1). To maintain a constant temperature and prevent external contaminations, the 24-well plates containing the parasite larvae were placed in water baths, differing in water temperatures, in a climatic chamber. Water baths were set to 16°C (±0.1°C), 19°C (±0.2°C) and 22°C (±0.3°C) using waterproof heating systems (Tetra). Water temperature was monitored throughout using automatic data recorders (Hobo) placed in each water bath.
(e). Experimental survey
A total of 603 larvae hatched from 54 parasites from 41 hosts were tested in the common garden experiment (electronic supplementary material, table S2). Larvae were individually monitored five times a day at 4 h intervals (from 07.00 to 23.00) under a binocular microscope, to assess whether they were alive or dead (a larva was considered dead when it was observed to be totally immobile for 1 min). To limit observation biases, the observer was different at each successive observation and larvae were removed from the plate only after three consecutive recordings as dead. We changed 1 ml of water in each well every day of the experiment. We quantified the survival time for each larva as the time (in minutes) from hatching to the first of the three observations for which it was observed as dead.
(f). Molecular analysis
To quantify neutral genetic differentiation (Fst) between parasite populations, we genotyped a total of 187 individuals from the six sampling sites (approx. 30 individuals per site; see the electronic supplementary material, table S3). Sixteen microsatellite loci specifically designed for T. polycolpus were amplified and genotyped [24,28].
(g). Statistical analysis
(i). Comparisons of survival time and reaction norms
We first tested for significant differences in survival time (among populations and climatic treatments) and in reaction norms (i.e. response to the thermal gradient) among populations using linear mixed-effects models (LMMs) with the survival time (scaled to the mean) as the dependent variable, thermal treatments and the river identity as fixed categorical factors, as well as the two-term interaction resulting from these two factors. Thermal treatment was treated as a categorical (rather than continuous) variable to more efficiently account for potential nonlinear response curves and to facilitate the interpretation of two-way interactions. Adult parasite identity was nested within host identity as random terms. A significant interaction term indicates different reactions norms among populations. A Gaussian error-term distribution was assumed, as there was no strong sign of non-normality of the data. This model was run for all six rivers combined, and for rivers from each of the two genetic clusters separately.
To assess the effect of the genetic background of parasites in shaping reaction norms, we ran an LMM with a similar structure, except that river identity was replaced by the identity of their genetic cluster (northern or southern clusters).
In a series of two models, we then directly tested whether or not the thermal regimes of the rivers significantly affected reaction norms. Thermal regimes of the rivers were characterized by their mean annual water temperatures and their annual CV. We set two LMMs in which one of these two latter characteristics was a fixed continuous factor, and in which thermal treatments (categorical) and the resulting two-term interaction were included (the dependent variable and the random structure were as above). In this type of model, a significant interaction would indicate that reaction norms vary according to in situ thermal conditions (for instance, the slope in survival between 16°C and 19°C varies according to in situ thermal characteristics).
(h). Quantitative trait analyses
To investigate whether differences in survival time and reaction norms between populations was driven by natural selection or genetic drift, we used a classical Qst/Fst comparison [30]. Global Fst was estimated for each locus independently. Global Qst was estimated as 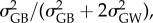 with
with 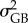 and
and 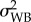 representing the between- and within-population components of the additive variance (VA) for each trait, respectively [29]. These components were extracted from random-linear models for six quantitative traits: the survival time measured for each temperature treatment (three quantitative traits; Q16, Q19 and Q22) and the reaction norms (i.e. slope values) between survival measured for each thermal treatment (three quantitative traits; Qs(16–19), Qs(19–22) and Qs(16–22)). For Q16, Q19 and Q22, variance components were extracted from a random-intercepts linear model (with Gaussian error-term distribution) with the survival time (scaled to the mean) as the dependent variable, adult parasite identity (i.e. assuming that each female mated with a single male, hence leading to a full-sib design), host identity (to account for variance in survival that was due to the condition of the host, [31]) and population identity as random terms. From this model,
representing the between- and within-population components of the additive variance (VA) for each trait, respectively [29]. These components were extracted from random-linear models for six quantitative traits: the survival time measured for each temperature treatment (three quantitative traits; Q16, Q19 and Q22) and the reaction norms (i.e. slope values) between survival measured for each thermal treatment (three quantitative traits; Qs(16–19), Qs(19–22) and Qs(16–22)). For Q16, Q19 and Q22, variance components were extracted from a random-intercepts linear model (with Gaussian error-term distribution) with the survival time (scaled to the mean) as the dependent variable, adult parasite identity (i.e. assuming that each female mated with a single male, hence leading to a full-sib design), host identity (to account for variance in survival that was due to the condition of the host, [31]) and population identity as random terms. From this model, 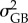 was estimated as the variance related to the population identity term, whereas
was estimated as the variance related to the population identity term, whereas 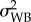 was twice the variance associated with the adult parasite identity term [29,33]. For Qs(16–19), Qs(19–22) and Qs(16–22), variance components were extracted from a random slopes and intercepts linear model (with Gaussian error-term distribution) with the survival time (scaled to the mean) as the dependent variable, and in which the slopes between treatments were allowed to vary among adult parasites (to estimate
was twice the variance associated with the adult parasite identity term [29,33]. For Qs(16–19), Qs(19–22) and Qs(16–22), variance components were extracted from a random slopes and intercepts linear model (with Gaussian error-term distribution) with the survival time (scaled to the mean) as the dependent variable, and in which the slopes between treatments were allowed to vary among adult parasites (to estimate 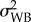 related to slopes) and populations (to estimate
related to slopes) and populations (to estimate 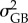 related to slopes). We also included the host identity as an intercept-only random term. Global Fst and Qst were estimated for all rivers combined, all rivers from the northern cluster only, and all rivers from the southern cluster only.
related to slopes). We also included the host identity as an intercept-only random term. Global Fst and Qst were estimated for all rivers combined, all rivers from the northern cluster only, and all rivers from the southern cluster only.
Qst and Fst values were compared statistically by estimating the overlap between their respective 95% CIs (assuming that overlapping 95% CIs indicate non-significant differences between Qst and Fst, [34]). The 95% CIs were obtained using a resampling procedure for global Qst [35] and were estimated from the distribution of Fst values measured for each loci for global Fst [33].
All statistical analyses were performed using R language and the following packages: ‘admbglmm’ [36], ‘lme4’ [37] and ‘hierfstat’ [38].
3. Results
(a). Comparisons of survival time and reaction norms
Larvae survival time significantly differed among temperature treatments, but not among rivers (table 2a). There was no significant interaction between treatments and rivers, indicating that reaction norms were statistically similar among rivers (table 2a and figure 2a). Overall, mean survival time was the highest at 16°C (mean = 5275.45 ± 2069.40 min), and decreased at 19°C and 22°C (4495.65 ± 1694.08 min and 3600.07 ± 1421.67 min, respectively; figure 2a). On the contrary, the model including the genetic cluster affiliation of each river revealed a significant interaction term between the cluster identity and temperature treatments (table 2b), indicating that the thermal reaction norms significantly differed between the two genetic clusters. Specifically, in the southern genetic cluster the survival time at 16°C was significantly higher than at 19°C (Tukey post-hoc test, p < 0.01), whereas in the northern genetic cluster the survival time was similar between these two temperature treatments (Tukey post-hoc test, p = 0.833; electronic supplementary material, table S4; figure 2b). The slope between these two temperature treatments was therefore slower in the northern than in the southern genetic cluster. There were no significant interactions between treatments and rivers when each cluster was considered separately (table 2c,d).
Table 2.
Results of linear mixed models used to determine the effects of experimental treatment on larval survival of the parasite Tracheliastes polycolpus. Bold p-values indicate significant at significance level of 0.05.
| degree of freedom | f-value | p-value | |
|---|---|---|---|
| (a) the effect of river identity (all populations combined) | |||
| temperature treatments | 2, 560 | 24.812 | <0.001 |
| river identity | 5, 560 | 0.916 | 0.441 |
| treatments × rivers | 10, 560 | 1.261 | 0.249 |
| (b) the effect of genetic cluster | |||
| temperature treatments | 2, 572 | 64.816 | <0.001 |
| genetic clusters | 1, 572 | 0.076 | 0.783 |
| treatments × clusters | 2, 572 | 3.484 | 0.031 |
| (c) the effect of river identity (populations form the southern cluster) | |||
| temperature treatments | 2, 319 | 25.351 | <0.001 |
| river identity | 2, 319 | 1.733 | 0.178 |
| treatments × rivers | 4, 319 | 0.329 | 0.858 |
| (d) the effect of river identity (populations from the northern cluster) | |||
| temperature treatments | 2, 238 | 6.642 | 0.002 |
| river identity | 2, 238 | 0.539 | 0.583 |
| treatments × rivers | 4, 238 | 0.958 | 0.431 |
| (e) the effect of mean annual water temperature | |||
| temperature treatments | 2, 572 | 85.539 | <0.001 |
| mean annual water temperature | 1, 572 | 1.797 | 0.441 |
| treatments × mean annual water temperature | 2, 572 | 3.967 | 0.019 |
| (f) the effect of coefficient of variation | |||
| temperature treatments | 2, 572 | 85.677 | <0.001 |
| coefficient of variation | 1, 572 | 2.324 | 0.128 |
| treatments × coefficient of variation | 2, 572 | 1.135 | 0.322 |
Figure 2.
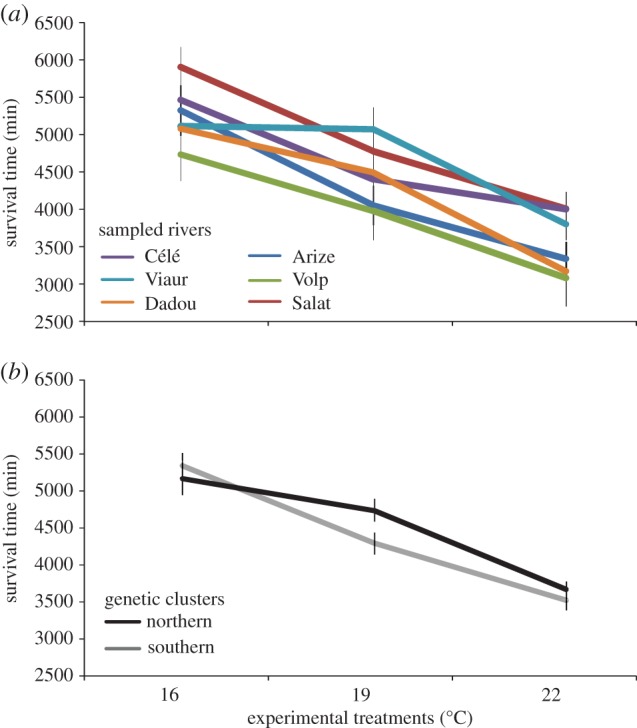
Survival reaction norms of Tracheliastes polycolpus larvae exposed to three different experimental temperatures (16°C, 19°C, 22°C). (a) Representation of the mean survival time (±s.e.) of individuals from the six rivers sampled. (b) Representation of the survival reaction norms of individuals from the two genetic clusters: northern cluster (Célé, Viaur, Dadou) and southern cluster (Arize, Volp, Salat).
Finally, we found that the specific thermal regime—and more specifically the mean annual water temperature—of each river significantly affected the reaction norms. Indeed, we found a significant interaction between temperature treatments and the mean annual water temperature of each river (table 2e), whereas there was no significant interaction between the CV measured for each river and the temperature treatment (table 2f). This indicated that the slope of reaction norms between each pair of experimental treatments (i.e. the difference in mean survival time between each pair of treatments) significantly varies according to the mean annual water temperature of each river. Most specifically, we found that the difference in survival time between the 16°C and 19°C treatments was highly correlated to the mean annual water temperature (r² = 0.75); difference in survival time between these two treatments was higher in rivers with a low mean annual water temperature, whereas difference in survival time was close to zero for the river with the highest water temperature (figure 3). Relationships were weakest (and had different slopes) for other reaction norms between treatment pairs (figure 3).
Figure 3.
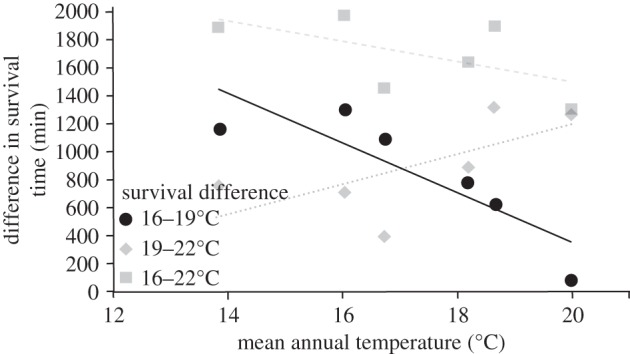
Representation of difference in survival time of Tracheliastes polycolpus between pairs of experimental treatments for each river according to the mean annual temperature in each river sampled. Black circles represent the differences between 19°C and 16°C treatments for each river, the background grey diamonds represent the differences between 22°C and 19°C treatments, and grey squares the differences between 22°C and 16°C treatments. Solid black lines indicate the regression slope for 19°C and 16°C differences, and dashed grey lines indicate the regression slope for 22°C and 19°C differences, and 22°C and 16°C treatments.
(b). Quantitative trait analyses
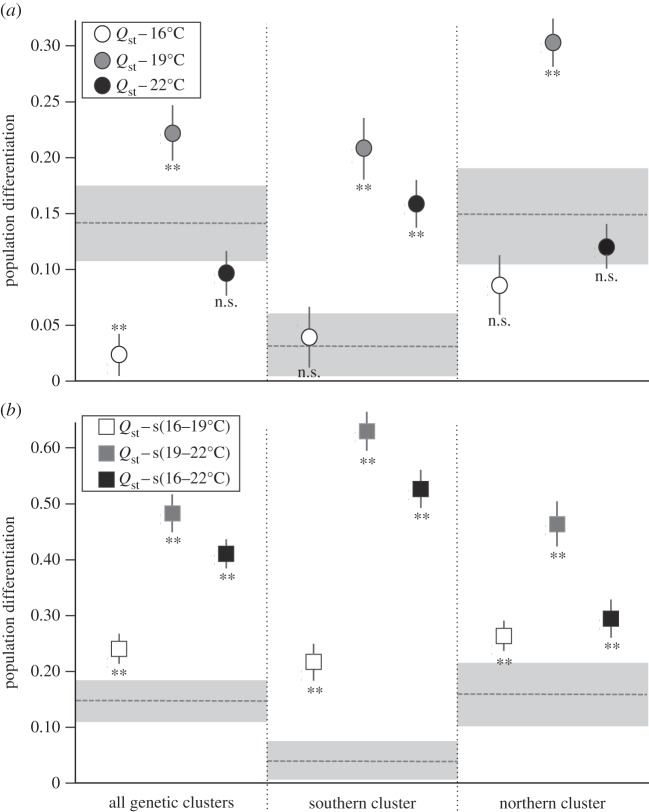
Global Fst was 0.145 (±0.065) when all rivers were considered, 0.153 (±0.078) for rivers of the northern cluster and as low as 0.032 (±0.028) for rivers from the southern cluster (figure 4a,b). Pairwise Fst among the six rivers ranged from 0.02 (Arize versus Salat rivers) to 0.18 (Viaur versus Volp rivers) with an average of 0.11 (electronic supplementary material, table S5).
Figure 4.
Comparison of quantitative (Qst, dots) and neutral (Fst, horizontal dashed lines) genetic differentiations among T. polycolpus populations. (a) Comparisons for quantitative traits (survival time) measured at each experimental temperature (survival times at 16°C, white dots; survival times at 19°C, grey dots; survival times at 22°C, black dots), and (b) comparisons for quantitative traits (differences in survival time) measured between each experimental temperature (difference in survival times between 16°C and 19°C, white dots; difference in survival times between 19°C and 22°C, grey dots; difference in survival times between 16°C and 22°C, black dots). In both (a,b), comparisons are performed for all six populations from the two genetic clusters (all genetic clusters), the three populations from the southern cluster, or the three populations from the northern cluster. The 95% CIs are the vertical lines for the Qst and the grey areas for the Fst. When 95% CIs of Qst and Fst do not overlap, this indicates significant differences between Qst and Fst, which are denoted as double asterisks. n.s. indicates not significant when the 95% CIs overlap.
Regarding Qst/Fst comparisons, differentiations at quantitative traits were overall larger for traits involving slopes than for traits synthetizing mean survival (see the y-axes on figure 4a versus 4b), irrespectively of the comparison involved (all rivers combined or rivers from the respective genetic clusters). Moreover, we found significant signals of diversifying natural selection (Qst > Fst, with non-overlapping 95% CI) for several traits (and for all comparisons) including the survival time measured at 19°C (Q19; figure 4a) and all slope values (Qs(16–19), Qs(19–22) and Qs(16–22); figure 4b). Interestingly, we found a clear pattern of stabilizing selection (Qst < Fst, with non-overlapping 95% CI) for the survival time measured at 16°C (Q16), although this conclusion holds true only when the comparisons involved all rivers. In other cases, differentiation at Q16 was still low, but similar to neutral differentiation (Qst ≈ Fst, with overlapping 95% CI; figure 4a). For Q22, we found patterns of diversifying selection among rivers from the southern cluster, but not for other comparisons for which Qst did not vary from the neutral expectation (figure 4a,b).
4. Discussion
Most previous studies on host–parasite interactions focused on coevolution between each protagonist, providing evidence for the adaptation of parasites to their local host genotypes [17,18]. However, less attention was paid to the possibility for adaptation of parasites to local abiotic factors, although it has been shown that important traits can be constrained by the environment [39–41]. Herein, we provide experimental evidence that local adaptation to in situ climatic conditions can led to the evolution of a major fitness trait in the ectoparasite Tracheliastes polycolpus (i.e. the survival rate of the free-living infective stage in the surrounding environment). Survival rate is a critical trait as it determines the transmission propensity of the parasite [42]. Interestingly, we showed that local adaptation led to the occurrence of diverging responses of survival rate to current climatic conditions in wild populations, but also that stabilizing selection and/or genetic drift played a role in some situations. This important finding raises the possibility that some populations will be better suited to cope with ongoing climatic changes [8,43].
We found that raising water temperature significantly reduced larvae survival in all parasite populations investigated, with the highest survival rate observed at 16°C. Although we failed to detect significant differences in reaction norms among rivers when considering only their identity, a series of additional tests demonstrated that parasites were locally adapted to in situ climatic conditions. For instance, reaction norms significantly varied between the two main genetic clusters to which parasite populations belong. Populations from the southern genetic cluster tended to experience an increase in mortality with raising temperatures, whereas for populations from the northern genetic cluster, the difference in survival between the cold (16°C) and intermediate (19°C) treatments was less striking. These differences in survival time may appear subtle at first glance, but nonetheless correspond to an approximate 75% divergence between the two clusters regarding the difference in survival rate between 16°C and 19°C (i.e. the absolute difference in survival time between 16°C and 19°C was 1050 min for the southern cluster and only 382 min for the northern cluster; figure 2b). The time of exposure of infective stages is an extremely important parameter for predicting infection rate in many parasites [44–46]. However, to what extent these differences are biologically relevant in T. polycolpus remains an unexplored question. We can expect them to be of major importance as the environment in which infective stages of T. polycolpus are living are extremely vast compared with their body size and the abundance of the main host; all strategies helping at finding this needle (the host) in this haystack (the environment) should be highly selected for. These differences in thermal tolerance between the two clusters may be due to genetic drift (as clusters were identified according to neutral markers [28]) or adaptation to local climatic conditions (e.g. the mean annual temperature was 14.8 ± 1.9°C and 16.8 ± 2.7°C for the southern and northern clusters, respectively). Based on this analysis, the two processes cannot be ruled out given that genetic and environmental dissimilarities are strongly confounded. However, we further identified strong ‘phenotype–environment associations’ as differences in thermal regimes between rivers (i.e. mean annual water temperature) significantly explained differences in thermal tolerance between populations. There was notably a strong relationship between thermal regimes measured in the field and differences in survival rate measured experimentally between the 16°C and 19°C treatments; populations living in colder waters evolved restricted thermal tolerance (thermal specialization towards an optimum, as survival rate was much higher at 16°C), whereas populations living in hotter waters (and also experiencing larger annual temperature range; figure 1a) evolved a wider range of thermal tolerance (thermal generalization, as survival rates were similar at both 16°C and 19°C). If a slight increase in temperature (16–19°C) is detrimental, extreme variation (16–22°C) dramatically decreased T. polycolpus larvae survival. This result supports the idea that, although high temperatures should increase growth rate (e.g. [14]), a trade-off with survival rate might limit these benefits [47]. Interestingly, we failed to find differences between populations when considering the upper experimental temperature (approx. 22°C) in comparisons. This result corroborates recent findings demonstrating that variation in thermal environments impacts plasticity in lower thermal limits but not in upper critical thermal limits, which may ultimately bind acclimation to warming climate [48].
These ‘phenotype–environment associations’ strongly suggest gene × environment interactions, and hence local adaptation of parasite populations to thermal regimes. This conclusion was reinforced by strong signals of divergent selection in the Qst/Fst comparison for several of the traits we investigated. Indeed, genetic differentiation measured at several quantitative traits related to survival rates and reaction norms was significantly higher than expected under the neutral expectation of an effect of genetic drift. This is to our knowledge one of the few studies using a Qst/Fst approach to identify the footprint of natural selection due to non-biotic factors in parasites. These phenotypic divergences probably reflect variation in the strength and direction of selective pressures among rivers [49]; populations evolved different adaptive responses leading to local adaptation of thermal tolerance [50]. Most particularly, natural selection participated to the divergence of populations for the survival measured at 19°C, which may indicate a particular adaptation to rivers with relatively high water temperature regimes. Interestingly, we found that—in contrast—survival rate measured at 16°C was more likely to be under stabilizing selection for T. polycolpus. This is actually not surprising, as we have demonstrated that survival rate was higher at this temperature for most populations, which means that this temperature probably constitutes an optimum. It is hence likely that selection makes populations converge towards this optimum. By contrast, some populations (i.e. those living in relatively hot waters) may have diverged towards a second optimum close to 19°C. This could explain why we found that the reaction norm between survival at 16°C and 19°C was strongly related to the local thermal regime of rivers.
Although these adaptive responses probably arise through selection on additive genetic variance, our experimental design does not permit us to rule out the possible influence of non-additive genetic effects and/or non-genetic (yet transmitted) effects [51]. Most notably, environmental effects experienced early during egg development and/or maternal effects during the ontogeny can determine the future thermal performance of larvae [52,53]. Trans-generational plasticity and maternal effects have been observed across a wide range of organisms, and we can suspect that they may be of great importance for parasite larvae as they ensure transmission rate among hosts. However, studies on trans-generational plasticity and maternal effects on parasites remain scarce (but see [54]), whereas parasite-induced maternal (and paternal) effects in hosts have been largely demonstrated [55,56]. Disentangling (additive) genetic versus non-genetic effects using specific experimental designs with multiple generations will be an important next step to better predict the responses of parasites to changes in thermal conditions.
We observed that the identity of the host on which parasites were sampled determined (in some cases) a non-negligible part of the variance observed in parasite survival time (i.e. up to 25%, not shown). We already demonstrated that fitness traits (e.g. egg number, parasite body size) in T. polycolpus were partly determined by the host identity and body condition [31], which calls for further integrative studies considering all environmental components into the evolution of life-history traits in parasites. Moreover, the effects of thermal change on parasites are concomitant with parallel effects on their hosts, which can change coevolutionary dynamics. For example, hosts can experience enhanced resistance (e.g. [16]), can change their avoidance behaviour (e.g. [14]) or can acclimate faster than parasites [45]. Thus, different components of the host–parasite interaction (e.g. transmission, virulence, resistance and tolerance) can differentially be altered by the abiotic environment [57], which supports the view that abiotic factors can modulate the trajectories of host–parasite coevolution, resulting in complex three-way ‘genotype-by-genotype-by-environment’ interactions [19,57,58].
To conclude, our findings demonstrate that parasite species can be composed of sub-populations differing in their tolerance to thermal regimes, which suggests that the ghost of evolution past can generate populations with different susceptibility to future climate change [59]. For instance, given the global temperature rising in rivers, and the increasing and instability of climate regimes in rivers these environments [60], we can expect that T. polycolpus populations with higher thermal range tolerance (i.e. those from the northern cluster) will better fit the novel climatic conditions. However, this basic prediction would hold true only if the thermal generalism is not associated with costs, for instance, related to host generalism. Hence, given the complexity of gene-by-gene-by-environment interactions, it seems obvious that the impact of climate change will strongly alter the rate and direction of coevolutionary dynamics at both the species and population levels [8,41,61,62]. Projections of disease dynamics under climate change should explicitly consider these complex interactions in spatially and temporally heterogeneous landscapes given the diverse possible outcomes of environmentally mediated antagonistic interactions [15,57].
Supplementary Material
Acknowledgements
We warmly thank Leopold Ghinter and Emeric Mahé for their valuable help with laboratory experiments. Comments raised by two reviewers greatly improved the manuscript. The genetic data were generated at the molecular genetic technical facilities of the Genopole Midi-Pyrénées (Toulouse, France). This work was undertaken at SEEM, which forms part of the ‘Laboratoire d'Excellence’ (LABEX) entitled TULIP (ANR-10-LABX-41).
Data accessibility
Experimental raw data used to perform analyses are available at Dryad (doi:10.5061/dryad.2th2m).
Authors' contributions
E.M.-G., G.L. and S.B. designed the experiment and E.M.-G. coordinated the study; E.M.-G., O.R., N.C., G.L. and S.B. conducted parasite sampling; E.M.-G., O.R., N.C. and G.L. carried out the experimental laboratory work; E.M.-G. ran the statistical analyses; E.M.-G., S.B., O.R. and G.L. wrote the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This work was funded by the Agence Nationale de la Recherche (project INCLIMPAR, grant no. ANR-11-JSV7-0010) and by BiodivERsA (project PROBIS). E.M.-G. was supported by a PhD grant from the French Ministry for Education and Sciences.
References
- 1.Gienapp P, Teplitsky C, Alho JS, Mills JA, Merilä J. 2008. Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178. ( 10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann AA, Sgrò CM. 2011. Climate change and evolutionary adaptation. Nature 470, 479–485. ( 10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 3.Merilä J, Hendry AP. 2014. Climate change, adaptation, and phenotypic plasticity: the problem and the evidence. Evol. Appl. 7, 1–14. ( 10.1111/eva.12137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Firn J, et al. 2011. Abundance of introduced species at home predicts abundance away in herbaceous communities. Ecol. Lett. 14, 274–281. ( 10.1111/j.1461-0248.2010.01584.x) [DOI] [PubMed] [Google Scholar]
- 5.Parker JD, et al. 2013. Do invasive species perform better in their new ranges? Ecology 94, 985–994. ( 10.1890/12-1810.1) [DOI] [PubMed] [Google Scholar]
- 6.Carroll SP, Jørgensen PS, Kinnison MT, Bergstrom CT, Denison RF, Gluckman P, Smith TB, Strauss SY, Tabashnik BE. 2014. Applying evolutionary biology to address global challenges. Science 346, 1245993 ( 10.1126/science.1245993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sultan SE, Spencer HG. 2002. Metapopulation structure favors plasticity over local adaptation. Am. Nat. 160, 271–283. ( 10.1086/341015) [DOI] [PubMed] [Google Scholar]
- 8.Valladares F, et al. 2014. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 17, 1351–1364. ( 10.1111/ele.12348) [DOI] [PubMed] [Google Scholar]
- 9.Ketola T, Mikonranta L, Zhang J, Saarinen K, Örmälä A-M, Friman V-P, Mappes J, Laakso J. 2013. Fluctuating temperature leads to evolution of thermal generalism and preadaptation to novel environments. Evolution 67, 2936–2944. ( 10.1111/evo.12148) [DOI] [PubMed] [Google Scholar]
- 10.Altizer S, Ostfeld RS, Johnson PTJ, Kutz S, Harvell CD. 2013. Climate change and infectious diseases: from evidence to a predictive framework. Science 341, 514–519. ( 10.1126/science.1239401) [DOI] [PubMed] [Google Scholar]
- 11.Landis SH, Sundin J, Rosenqvist G, Roth O. 2012. Behavioral adjustments of a pipefish to bacterial Vibrio challenge. Behav. Ecol. Sociobiol. 66, 1399–1405. ( 10.1007/s00265-012-1395-3) [DOI] [Google Scholar]
- 12.Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD. 2002. Climate warming and disease risks for terrestrial and marine Biota. Science 296, 2158–2162. ( 10.1126/science.1063699) [DOI] [PubMed] [Google Scholar]
- 13.Brooks DR, Hoberg EP. 2007. How will global climate change affect parasite–host assemblages? Trends Parasitol. 23, 571–574. ( 10.1016/j.pt.2007.08.016) [DOI] [PubMed] [Google Scholar]
- 14.Macnab V, Barber I. 2012. Some (worms) like it hot: fish parasites grow faster in warmer water, and alter host thermal preferences. Glob. Change Biol. 18, 1540–1548. ( 10.1111/j.1365-2486.2011.02595.x) [DOI] [Google Scholar]
- 15.Lafferty KD. 2009. Calling for an ecological approach to studying climate change and infectious diseases. Ecology 90, 932–933. ( 10.1890/08-1767.1) [DOI] [PubMed] [Google Scholar]
- 16.Raffel TR, Romansic JM, Halstead NT, McMahon TA, Venesky MD, Rohr JR. 2013. Disease and thermal acclimation in a more variable and unpredictable climate. Nat. Clim. Change 3, 146–151. ( 10.1038/nclimate1659) [DOI] [Google Scholar]
- 17.Greischar MA, Koskella B. 2007. A synthesis of experimental work on parasite local adaptation. Ecol. Lett. 10, 418–434. ( 10.1111/j.1461-0248.2007.01028.x) [DOI] [PubMed] [Google Scholar]
- 18.Hoeksema JD, Forde SE. 2008. A meta-analysis of factors affecting local adaptation between interacting species. Am. Nat. 171, 275–290. ( 10.1086/527496) [DOI] [PubMed] [Google Scholar]
- 19.Laine A-L. 2008. Temperature-mediated patterns of local adaptation in a natural plant–pathogen metapopulation. Ecol. Lett. 11, 327–337. ( 10.1111/j.1461-0248.2007.01146.x) [DOI] [PubMed] [Google Scholar]
- 20.Vale PF, Stjernman M, Little TJ. 2008. Temperature-dependent costs of parasitism and maintenance of polymorphism under genotype-by-environment interactions. J. Evol. Biol. 21, 1418–1427. ( 10.1111/j.1420-9101.2008.01555.x) [DOI] [PubMed] [Google Scholar]
- 21.Berkhout BW, Lloyd MM, Poulin R, Studer A. 2014. Variation among genotypes in responses to increasing temperature in a marine parasite: evolutionary potential in the face of global warming? Int. J. Parasitol. 44, 1019–1027. ( 10.1016/j.ijpara.2014.07.002) [DOI] [PubMed] [Google Scholar]
- 22.Thomas MB, Blanford S. 2003. Thermal biology in insect–parasite interactions. Trends Ecol. Evol. 18, 344–350. ( 10.1016/S0169-5347(03)00069-7) [DOI] [Google Scholar]
- 23.Rohr JR, Dobson AP, Johnson PTJ, Kilpatrick AM, Paull SH, Raffel TR, Ruiz-Moreno D, Thomas MB. 2011. Frontiers in climate change-disease research. Trends Ecol. Evol. 26, 270–277. ( 10.1016/j.tree.2011.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lootvoet A, Blanchet S, Gevrey M, Buisson L, Tudesque L, Loot G. 2013. Patterns and processes of alternative host use in a generalist parasite: insights from a natural host–parasite interaction. Funct. Ecol. 27, 1403–1414. ( 10.1111/1365-2435.12140) [DOI] [Google Scholar]
- 25.Loot G, Poulet N, Reyjol Y, Blanchet S, Lek S. 2004. The effects of the ectoparasite Tracheliastes polycolpus (Copepoda: Lernaeopodidae) on the fins of rostrum dace (Leuciscus leuciscus burdigalensis). Parasitol. Res. 94, 16–23. ( 10.1007/s00436-004-1166-9) [DOI] [PubMed] [Google Scholar]
- 26.Blanchet S, Méjean L, Bourque J-F, Lek S, Thomas F, Marcogliese DJ, Dodson JJ, Loot G. 2009. Why do parasitized hosts look different? Resolving the ‘chicken–egg’ dilemma. Oecologia 160, 37–47. ( 10.1007/s00442-008-1272-y) [DOI] [PubMed] [Google Scholar]
- 27.Cardon M, Loot G, Grenouillet G, Blanchet S. 2011. Host characteristics and environmental factors differentially drive the burden and pathogenicity of an ectoparasite: a multilevel causal analysis. J. Anim. Ecol. 60, 657–667. ( 10.1111/j.1365-2656.2011.01804.x) [DOI] [PubMed] [Google Scholar]
- 28.Rey O, Fourtune L, Paz-Vinas I, Loot G, Veyssière C, Roche B, Blanchet S. 2015. Elucidating the spatio-temporal dynamics of an emerging wildlife pathogen using approximate Bayesian computation. Mol. Ecol. 24, 5348–5363. ( 10.1111/mec.13401) [DOI] [PubMed] [Google Scholar]
- 29.Merilä J, Crnokrak P. 2001. Comparison of genetic differentiation at marker loci and quantitative traits. J. Evol. Biol. 14, 892–903. ( 10.1046/j.1420-9101.2001.00348.x) [DOI] [Google Scholar]
- 30.Leinonen T, McCairns RJS, O'Hara RB, Merilä J. 2013. QST–FST comparisons: evolutionary and ecological insights from genomic heterogeneity. Nat. Rev. Genet. 14, 179–190. ( 10.1038/nrg3395) [DOI] [PubMed] [Google Scholar]
- 31.Loot G, Poulet N, Brosse S, Tudesque L, Thomas F, Blanchet S. 2011. Determinants of life-history traits in a fish ectoparasite: a hierarchical analysis. Parasitology 138, 848–857. ( 10.1017/S003118201100014X) [DOI] [PubMed] [Google Scholar]
- 32.Mullet V. 2013. Effet des facteurs environnementaux sur la prévalence et la richesse allélique de Tracheliastes polycolpus. MSc thesis, University Paul Sabatier, France.
- 33.Whitlock MC, Guillaume F. 2009. Testing for spatially divergent selection: comparing QST to FST. Genetics 183, 1055–1063. ( 10.1534/genetics.108.099812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood JLA, Tezel D, Joyal D, Fraser DJ. 2015. Population size is weakly related to quantitative genetic variation and trait differentiation in a stream fish. Evolution 69, 2303–2318. ( 10.1111/evo.12733) [DOI] [PubMed] [Google Scholar]
- 35.Manly BFJ. 2006. Randomization, bootstrap and Monte Carlo methods in biology, 3rd edn Boca Raton, FL: CRC Press. [Google Scholar]
- 36.Skaug H, Fournier D, Nielsen A. 2006. glmmADMB: generalized linear mixed models using AD model builder. R package version 0.3. See http://admb-project.org/redmine/projects/issues/repository/revisions/1/entry/trunk/examples/admb-re/glmmadmb/glmmADMB/html/glmm.admb.html. [Google Scholar]
- 37.Bates D, Mächler M, Bolker BM, Walker SC. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 38.Goudet J. 2005. hierfstat, a package for r to compute and test hierarchical F-statistics. Mol. Ecol. Notes 5, 184–186. ( 10.1111/j.1471-8286.2004.00828.x) [DOI] [Google Scholar]
- 39.McCoy KD, Boulinier T, Schjorring S, Michalakis Y. 2002. Local adaptation of the ectoparasite Ixodes uriae to its seabird host. Evol. Ecol. Res. 4, 441–456. [Google Scholar]
- 40.Mostowy R, Engelstädter J. 2011. The impact of environmental change on host–parasite coevolutionary dynamics. Proc. R. Soc. B 278, 2283–2292. ( 10.1098/rspb.2010.2359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrison E, Laine A-L, Hietala M, Brockhurst MA. 2013. Rapidly fluctuating environments constrain coevolutionary arms races by impeding selective sweeps. Proc. R. Soc. B 280, 20130937 ( 10.1098/rspb.2013.0937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hudson P, Rizzoli A, Grenfell B, Heesterbeek H, Dobson A. 2002. The ecology of wildlife diseases. Oxford, UK: Oxford University Press. [Google Scholar]
- 43.Salamin N, Wüest RO, Lavergne S, Thuiller W, Pearman PB. 2010. Assessing rapid evolution in a changing environment. Trends Ecol. Evol. 25, 692–698. ( 10.1016/j.tree.2010.09.009) [DOI] [PubMed] [Google Scholar]
- 44.Hauck R, Hafez HM. 2013. Experimental infections with the protozoan parasite Histomonas meleagridis: a review. Parasitol. Res. 112, 19–34. ( 10.1007/s00436-012-3190-5) [DOI] [PubMed] [Google Scholar]
- 45.Paull SH, Raffel TR, LaFonte BE, Johnson PTJ. 2015. How temperature shifts affect parasite production: testing the roles of thermal stress and acclimation. Funct. Ecol. 29, 941–950. ( 10.1111/1365-2435.12401) [DOI] [Google Scholar]
- 46.Ben-Ami F, Regoes RR, Ebert D. 2008. A quantitative test of the relationship between parasite dose and infection probability across different host–parasite combinations. Proc. R. Soc. B 275, 853–859. ( 10.1098/rspb.2007.1544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee W-S, Monaghan P, Metcalfe NB. 2013. Experimental demonstration of the growth rate–lifespan trade-off. Proc. R. Soc. B 280, 20122370 ( 10.1098/rspb.2012.2370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gunderson AR, Stillman JH. 2015. Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proc. R. Soc. B 282, 20150401 ( 10.1098/rspb.2015.0401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porcher E, Giraud T, Goldringer I, Lavigne C. 2004. Experimental demonstration of a causal relationship between heterogeneity of selection and genetic differentiation in quantitative traits. Evolution 58, 1434–1445. ( 10.1111/j.0014-3820.2004.tb01725.x) [DOI] [PubMed] [Google Scholar]
- 50.Kawecki TJ, Ebert D. 2004. Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241. ( 10.1111/j.1461-0248.2004.00684.x) [DOI] [Google Scholar]
- 51.Danchin É, Charmantier A, Champagne FA, Mesoudi A, Pujol B, Blanchet S. 2011. Beyond DNA: integrating inclusive inheritance into an extended theory of evolution. Nat. Rev. Genet. 12, 475–486. ( 10.1038/nrg3028) [DOI] [PubMed] [Google Scholar]
- 52.Mousseau TA, Fox CW. 1998. The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407. ( 10.1016/S0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- 53.Salinas S, Brown SC, Mangel M, Munch SB. 2013. Non-genetic inheritance and changing environments. Non-Genet. Inherit. 1, 38–50. ( 10.2478/ngi-2013-0005) [DOI] [Google Scholar]
- 54.Benesh DP. 2013. Parental effects on the larval performance of a tapeworm in its copepod first host. J. Evol. Biol. 26, 1625–1633. ( 10.1111/jeb.12165) [DOI] [PubMed] [Google Scholar]
- 55.Garnier R, Boulinier T, Gandon S. 2012. Coevolution between maternal transfer of immunity and other resistance strategies against pathogens. Evolution 66, 3067–3078. ( 10.1111/j.1558-5646.2012.01665.x) [DOI] [PubMed] [Google Scholar]
- 56.Kaufmann J, Lenz TL, Milinski M, Eizaguirre C. 2014. Experimental parasite infection reveals costs and benefits of paternal effects. Ecol. Lett. 17, 1409–1417. ( 10.1111/ele.12344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolinska J, King KC. 2009. Environment can alter selection in host–parasite interactions. Trends Parasitol. 25, 236–244. ( 10.1016/j.pt.2009.02.004) [DOI] [PubMed] [Google Scholar]
- 58.Sternberg ED, Thomas MB. 2014. Local adaptation to temperature and the implications for vector-borne diseases. Trends Parasitol. 30, 115–122. ( 10.1016/j.pt.2013.12.010) [DOI] [PubMed] [Google Scholar]
- 59.Ryan SJ, McNally A, Johnson LR, Mordecai E, Ben-Horin T, Paaijmans K, Lafferty KD. 2014. Changing physiological suitability limits of malaria transmission in Africa under climate change. See http://arxiv.org/14077612Q-Bio. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Vliet MTH, Franssen WHP, Yearsley JR, Ludwig F, Haddeland I, Lettenmaier DP, Kabat P. 2013. Global river discharge and water temperature under climate change. Glob. Environ. Change 23, 450–464. ( 10.1016/j.gloenvcha.2012.11.002) [DOI] [Google Scholar]
- 61.Thompson JN. 2005. The geographic mosaic of coevolution. Chicago, IL: University of Chicago Press. [Google Scholar]
- 62.Schade FM, Shama LNS, Wegner KM. 2014. Impact of thermal stress on evolutionary trajectories of pathogen resistance in three-spined stickelback (Gasterosteus aculeatus). BMC Evol. Biol. 14, 64 ( 10.1186/s12862-014-0164-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Experimental raw data used to perform analyses are available at Dryad (doi:10.5061/dryad.2th2m).