Abstract
Metal-hyperaccumulating plants, which are hypothesized to use metals for defence against pests and pathogens, provide a unique context in which to study plant–pathogen coevolution. Previously, we demonstrated that the high concentrations of zinc found in leaves of the hyperaccumulator Noccaea caerulescens provide protection against bacterial pathogens, with a potential trade-off between metal-based and pathogen-induced defences. We speculated that an evolutionary arms race between zinc-based defences in N. caerulescens and zinc tolerance in pathogens might have driven the development of the hyperaccumulation phenotype. Here, we investigate the possibility of local adaptation by bacteria to the zinc-rich environment of N. caerulescens leaves and show that leaves sampled from the contaminated surroundings of a former mine site harboured endophytes with greater zinc tolerance than those within plants of an artificially created hyperaccumulating population. Experimental manipulation of zinc concentrations in plants of this artificial population influenced the zinc tolerance of recovered endophytes. In laboratory experiments, only endophytic bacteria isolated from plants of the natural population were able to grow to high population densities in any N. caerulescens plants. These findings suggest that long-term coexistence with zinc-hyperaccumulating plants leads to local adaptation by endophytic bacteria to the environment within their leaves.
Keywords: hyperaccumulators, endophytes, local adaptation, zinc
1. Introduction
Metal-hyperaccumulating plants maintain exceptionally high concentrations of metals in their aerial tissues [1–4], often exceeding 1% of tissue dry biomass for elements such as zinc, cadmium, nickel and manganese, a phenotype that is thought to protect them from herbivory [5–12] and disease [13–15]. There is evidence that the use of metals in defence has led to an evolutionary trade-off between metal hyperaccumulation and some pathogen-induced defences [16–18]. At present, however, little evidence is available concerning the effect of metal hyperaccumulation and metal-based defences upon the endophytic bacteria of hyperaccumulator plants. Two studies have surveyed the endophytes associated with nickel hyperaccumulator plants on natural serpentine soils [19,20]. Both reported that these bacteria showed high nickel tolerance, supporting the notion that the plants provide a high-metal environment for bacteria. Bacterial nickel tolerance was found to vary in proportion to nickel concentrations in different tissues, while bacterial population densities decreased as nickel concentrations increased [19]. Similarly, high cadmium tolerance was found for the bacterial endophytes of a cadmium hyperaccumulator growing on mine tailings [21]. Thus, high metal tolerance is associated with bacterial growth within metal-hyperaccumulating plants.
In previous work, we demonstrated that zinc concentrations in the aerial tissues of plants of a natural Noccaea caerulescens (synonym Thlaspi caerulescens) population growing on an abandoned lead–zinc mine at Hafna, Snowdonia, UK, [22] are higher than those found to prevent the growth of pathogenic Pseudomonas syringae bacteria under laboratory conditions [15], suggesting that zinc tolerance is important for naturally occurring endophytes of these plants. Here, we investigate the possibility that the endophytic pseudomonads of metal hyperaccumulators have developed increased metal tolerance as a result of local adaptation to the metal-rich environment of the hyperaccumulator leaves, a scenario that might drive the evolution of further metal hyperaccumulation in plants that use metals as a defence against pathogenic microorganisms.
In this study, endophytic Pseudomonas associated with a natural population of the zinc hyperaccumulator N. caerulescens were characterized and compared with those associated with an artificial field population. Bacterial zinc tolerance and virulence on N. caerulescens were determined and the distribution of these traits across a phylogeny of the isolated bacteria was examined. The results provide evidence that bacteria isolated from the natural population of N. caerulescens are better able to grow and cause disease in this metal hyperaccumulator. These findings support the idea of local adaptation by endophytic bacteria communities in an established, natural population of N. caerulescens, and strengthen the concept of plant pathogens as a selective force in the evolution of metal hyperaccumulation.
2. Material and methods
(a). Field sites
Plants of N. caerulescens (J.Presl & C.Presl) F.K.Mey. (=Thlaspi caerulescens J.Presl & C.Presl) were studied at two field locations: an artificial population cultivated at Wytham, Oxfordshire, UK (51°47′ N, 1°39′ W) and a natural population growing on wasteland at Hafna mine, an abandoned lead–zinc mine in Snowdonia National Park, Conwy, UK (53°07′ N, 3°49′ W [22]). The artificial population was generated from seed collected from N. caerulescens at Prayon, Belgium (50°35′ N, 5°40′ E [23]). Plants were precultured in a glasshouse for eight weeks on soil supplemented with zinc oxide to contain either 0, 1, 2 or 5 g kg−1 Zn (w/w). They were then transferred to two closely adjacent field sites at Wytham. At each site, plants grown on different zinc treatments were split equally between three randomized blocks.
(b). Measurement of leaf zinc concentrations
Leaf material was sampled from plants of N. caerulescens at all field sites and pools of 10–30 plants per treatment created. Zinc content in pooled samples of leaf biomass was measured following oven drying and extraction in concentrated nitric acid by atomic absorption spectrophotometry as described [24].
(c). Endophyte collection, identification and in planta growth assays
Noccaea caerulescens leaves were collected at both field sites, sealed into plastic bags and transferred to the laboratory for further analysis. From the artificial population at Wytham, 10 leaves per plant per zinc treatment were sampled. From the natural population at Hafna mine, 10 leaves per plant were sampled. Incidence of disease symptoms in sampled plants was recorded. Leaves were surface sterilized by successive immersion in 10% (v/v) sodium hypochlorite solution and 100% ethanol for 5 min each. The sterilized leaves were rinsed in sterile, distilled water and dried. Each leaf was then macerated and incubated at room temperature for 5 min in 1 ml of potassium phosphate buffer at pH 6.8. One hundred microlitre aliquots of this buffer were spread onto King's B (KB) agar, which is particularly conducive to the growth of Pseudomonas species [25]. Plates were incubated at 28°C for 48 h. Colonies were picked into KB broth and incubated overnight. Cultures were supplemented with 50% (v/v) glycerol to a final concentration of 20% (v/v) glycerol and stored at –80°C. All endophyte strains were subjected to LOPAT and GATTa assays [26]. In addition, 16S, rpoD and gyrB genes were sequenced (primers are given in electronic supplementary material, table S1) and used as queries for NCBI BLAST. In planta growth and pathogenicity assays were carried out as described [15].
(d). Measurements of bacterial zinc tolerance
All bacterial strains isolated from N. caerulescens plants were grown in KB broth supplemented with a range of zinc concentrations from 0 to 20 mM, and the increase in OD600 of the medium over 24 h recorded. Bacterial growth response curves for zinc were then created and used to determine the concentration of zinc causing a 50% reduction in bacterial growth (IC50; [27]). Growth response curves were also clustered by shape, using k-means clustering in ‘R’ [28], into groups or classes representing different patterns of response to zinc. k-means clustering maps data to the nearest mean value, where ‘k’ is the number of means; here, k = 4 was selected as this gave clear, discrete clusters. Strains in group 4 were the most tolerant, able to grow at 15 mM Zn, whereas those in group 1 showed strongly reduced growth even at 5 mM Zn. Both measures of zinc tolerance were used to describe the strains.
(e). Phylogeny reconstruction
Sequences of endophyte rpoD genes were used to build a phylogenetic tree. Alignment was carried out using MUSCLE (EBI [29,30]). Reference strain sequences were chosen based on BLAST results for the endophytic bacteria and obtained from NCBI (www.ncbi.nlm.nih.gov). GenBank IDs are given in electronic supplementary material, table S2. Following the initial alignment, strains with poor alignment to the rest or with poor sequence quality were removed from the analysis. Remaining sequences were trimmed to the region which consistently aligned well across all sequences, and the alignment repeated. Resulting good-quality alignments were used to produce a single gene tree. rpoD gene sequences were obtained from 60% (144/244) of isolated strains and 60% (86/144) of these sequences were of sufficient quality and sufficiently well aligned to use in phylogeny reconstruction. Phylogeny reconstruction was performed in MEGA [31] by maximum parsimony [32], using the Close-Neighbour-Interchange algorithm [33] with search level 3 in which the initial trees were obtained with the random addition of sequences with 10 replicates. An estimate of the trees' robustness was obtained by conducting a bootstrap analysis [34] with 500 replicates.
3. Results
(a). Zinc hyperaccumulation is negatively correlated with disease incidence in a natural population of Noccaea caerulescens
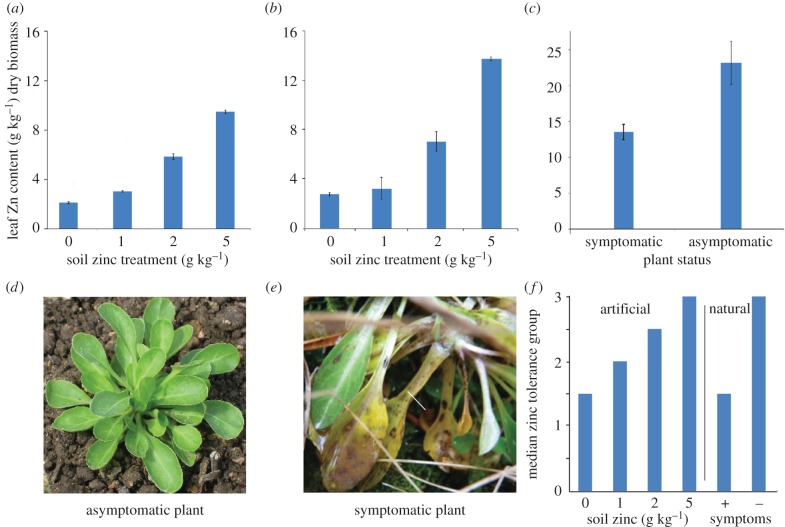
To determine whether an established population of zinc-hyperaccumulating plants would host a population of locally adapted pathogens able to cause disease in high-zinc conditions, we compared zinc concentration and disease incidence in N. caerulescens plants of the natural population at Hafna Mine, Snowdonia, to N. caerulescens plants established in an artificial population at two field sites at Wytham. N. caerulescens plants at Wytham showed significantly increased leaf zinc concentration when grown on increasingly high-zinc soil, at both sites (figure 1a,b) (ANOVAs: Site 1, p < 0.0005; Site 2, p = 0.009). Plants from the natural population displayed high concentrations of zinc in their aerial tissues, above the threshold of 1.0% of tissue dry biomass regarded as defining zinc hyperaccumulation [35]. There were two spatially separate patches of N. caerulescens at the Hafna Mine site. Interestingly, the plants in these two sub-populations contained significantly different concentrations of zinc (ANOVA, p = 0.0001) (figure 1c), with the sub-population that showed higher zinc concentrations consisting of plants that appeared healthy (figure 1d) while the other sub-population contained plants displaying disease symptoms (figure 1e). No plants of the artificial field population in Wytham displayed symptoms of disease at any time.
Figure 1.
Zinc accumulation, tolerance and disease incidence in natural and artificial Nocaea caerulescens populations. Zinc concentration in leaves of plants of the artificial population (Prayon genotype) grown at two distinct field sites at Wytham, UK, was measured at the end of the experimental period (a,b). Values shown are means ± s.e. (n = 3 samples of 10–30 pooled plants; at least three technical replicates performed per sample). Two spatially distinct sub-populations of N. caerulescens were distinguished in the natural population at Hafna Mine, Snowdonia; two leaves per plant were sampled from randomly selected plants from each patch and zinc concentrations measured (c); these two sub-populations were designated ‘asymptomatic’ and ‘symptomatic’ (d,e, respectively), as disease was observed in one only. Strains from both the artificial and natural populations were grown on KB medium supplemented with a range of zinc concentrations from 0 to 20 mM, and the increase in OD600 of the medium over 24 h recorded. Bacterial growth curves were then clustered using k-means clustering into ‘tolerance group’ representing different patterns of response to zinc (see electronic supplementary material, figure S1). Median zinc tolerance group (1–4, where 1 = least tolerant and 4 = most tolerant) of endophytic bacteria isolated from plants of the artificial population of N. caerulescens grown at Wytham, on four different zinc soil treatments is shown (f), as is median zinc tolerance group of endophytic bacteria isolated from plants of the natural population in Snowdonia, from spatially separate patches in which symptoms were (+) or were not (−) apparent (f). (Online version in colour.)
(b). Plants of the natural population of Noccaea caerulescens, and plants with higher zinc concentrations, hosted endophytes with greater zinc tolerance
To investigate whether the leaves of zinc-hyperaccumulating N. caerulescens provide a local environment that imposes a selective pressure in favour of zinc tolerance, we measured and compared the zinc tolerance of bacteria isolated from plants of the artificial and natural populations. Zinc tolerance curves showing bacterial growth versus zinc concentration were produced for each endophyte strain, which were used to determine IC50 values for zinc for each strain. The endophytes isolated from leaves from the artificial population had a greater range of zinc tolerances by this measure, but the median for the strains isolated from the natural population was significantly higher than that for the artificial population (IC50 of 9.5 versus 7.0 mM, respectively; Mann–Whitney test: p < 0.0005). Zinc tolerance curves for individual strains were clustered by shape using k-means clustering. These clusters then served to define four tolerance groups, with group 1 being the least, and group 4 the most, tolerant strains (electronic supplementary material, figure S1).
The median tolerance group of the endophytes increased as the plant zinc concentration increased, either with zinc treatment in the artificial population or between the two patches of plants in Snowdonia (figure 1f). Kruskal–Wallis tests on these data produce significant H-statistics (Wytham zinc treatments, p = 0.014; Snowdonia sub-populations, p < 0.0001). In conjunction with the results shown in figure 1a–c, this indicates that greater zinc concentrations within the plants were correlated with higher zinc tolerance among their endophytes.
(c). Artificial and natural populations of Noccaea caerulescens supported different bacterial endophytes
The majority of isolated strains from both artificial and natural populations belonged to the genus Pseudomonas, with some representatives of Xanthomonas, Erwinia, Enterobacter, Pantoea, Escherichia and Aeromonas. Pseudomonads were of particular interest, as previous work has shown that some Pseudomonas species can be pathogenic on N. caerulescens [15]. By focusing on Pseudomonas, direct comparison of the results obtained here to those in our previous study was facilitated [15]. KB agar was therefore selected as an isolation medium [25]. Since KB is biased in favour of pseudomonads, we limit further consideration to this genus. The composition of the two populations was widely different, with Wytham plants supporting mainly strains whose top BLAST hit was to P. aeruginosa, whereas Snowdonia plants supported mainly strains whose top BLAST hit was to P. syringae, P. graminis and P. fluorescens (electronic supplementary material, figure S2).
(d). Phylogenies of Pseudomonas endophyte strains from natural and artificial field populations of Noccaea caerulescens suggest multiple origins of zinc tolerance
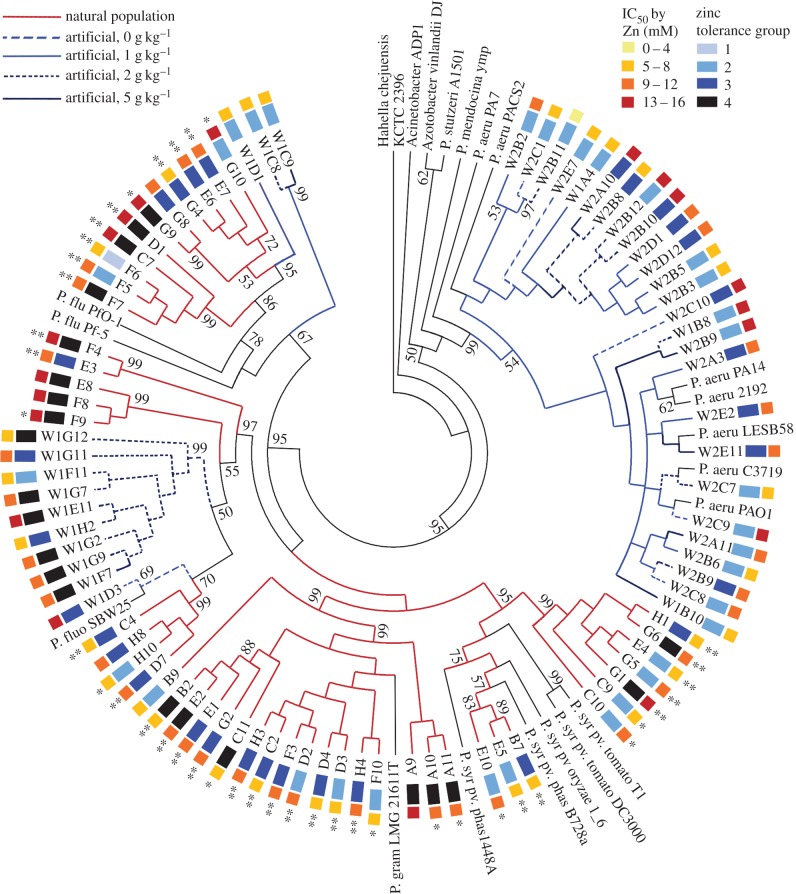
To investigate whether zinc-tolerant strains of endophytes were related, an rpoD gene tree was created for all endophytic pseudomonads for which high-quality rpoD gene sequences could be obtained. Figure 2 shows the resulting tree with both measures of zinc tolerance (IC50 and tolerance group) indicated. Highly zinc-tolerant strains are distributed across the tree and are not confined to strains isolated from either population of N. caerulescens. Also included are reference strains from the genus Pseudomonas, and non-Pseudomonas reference strains Hahella chejuensis, Azotobacter vinelandii and Acinetobacter ADP1. These show this tree to be in agreement with phylogenies produced by other researchers for Pseudomonas [36], with strains of Cluster I separated from Cluster II, and, within the clusters, the aeruginosa, syringae and fluorescens complexes differentiated. These deep branches in the tree also have high bootstrap support (99%, 99% and 97%, respectively; figure 2). The average IC50 for zinc in these three species complexes were similar (9.8 mM, 9.5 mM and 9.0 mM, respectively), and species complex was not a significant predictor of IC50 (ANOVA, p = 0.52). On the other hand, tolerance groups were not uniformly distributed between the three species complexes, with more group 4 strains in the syringae and fluorescens complexes, and more group 2 strains in the aeruginosa complex (χ2-goodness-of-fit test to uniform distribution of groups; p < 0.001). Smaller monophyletic clades with high bootstrap support can be seen within the syringae and fluorescens clusters, some of which contain strains with similar zinc tolerances, while others include a range of zinc IC50 values and tolerance groups.
Figure 2.
rpoD gene tree of Wytham and Snowdonia strains. The bootstrap consensus tree from 120 most parsimonious trees is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) is shown next to those branches where the bootstrap value exceeds 50% [27]. Branches are coloured red for endophytes of plants from the natural population in Snowdonia and blue for endophytes of plants from the artificial population at Wytham. Within the natural population, ** indicates that the plant from which the endophyte was isolated was symptomatic, whereas * indicates that the plant was from the same spatial sub-population as the symptomatic plants; no asterisk indicates that the isolate was from the asymptomatic sub-population. Within the artificial population, blue branches are further colour-coded to indicate the zinc treatment applied to the plant from which the endophyte was extracted (light blue: dashed: no added zinc, solid: 1 g zinc added per kilogram of soil; dark blue: dashed 2 g kg−1, solid: 5 g kg−1). Zinc tolerance groups based on groupings for all strains on KB are shown in black and shades of blue, with group 4 (highest) in black, through to group 1 (lowest) in pale blue. Zinc concentrations giving IC50 for strains are indicated with a heat map where red is highest (13–16 mM Zn) and yellow lowest (0–4 mM).
(e). Artificial and natural populations of Noccaea caerulescens supported different bacterial endophytes
To identify the strains isolated, we sequenced 16S, rpoD and gyrB genes and used the results to query the NCBI database using BLAST [37] (electronic supplementary material, table S3). Notably, a higher incidence of strains closely related to P. syringae was found in Snowdonia (electronic supplementary material, figure S2), which is consistent with disease incidence at this site, since at least some strains of P. syringae are pathogenic on N. caerulescens [15]. Endophytes identified as P. syringae and P. graminis also caused symptoms in N. caerulescens on reinoculation (electronic supplementary material, table S4). Further details about the identity of different bacterial strains at the two sites and about reinoculation experiments are given in the electronic supplementary material.
(f). On inoculation into Noccaea caerulescens plants grown on low zinc, symptoms were observed with Pseudomonas syringae and Pseudomonas graminis strains
For further insight into whether any of the strains isolated from the Snowdonia plants could be responsible for observed disease symptoms, all strains from these plants were inoculated into N. caerulescens plants (from Prayon, Belgium, as used for the artificial population) grown on low zinc in the laboratory. Plants were assessed at 24 h, and those showing rapid necrosis were excluded from further analysis due to possible onset of a non-host response. Symptom development in the remainder was recorded over 72 h. Of the 83 strains isolated, 14 induced symptoms (electronic supplementary material, table S4). All these had at least one top BLAST hit to Pseudomonas; in 10 cases, there was a top BLAST hit to P. syringae or P. graminis (electronic supplementary material, table S3). Considering the known role of many P. syringae strains as causative agents of disease (e.g. [15]), these species are plausible candidates to be the causal agents of the observed disease symptoms.
(g). Pathogenicity of endophytes on Noccaea caerulescens depends on both zinc tolerance and association with the natural population of Noccaea caerulescens
To determine the effect of zinc tolerance on the virulence of candidate pathogens, six endophyte strains belonging to the species P. syringae (determined using sequence data and LOPAT and GATTa assays) and the previously studied laboratory strain P. syringae pv. maculicola M4 were inoculated into N. caerulescens plants (from Prayon, Belgium) grown with 0.04, 10, 30 or 300 µM zinc. Four strains were isolated from the natural N. caerulescens population, and displayed a range of zinc tolerances; the remaining two were from the artificial N. caerulescens population and displayed high-zinc tolerance (table 1). As previously reported [15], Psm was able to multiply within plants grown on 0.04 and 10 µM Zn, but showed reduced or no growth in plants grown on 30 µM or 300 µM Zn.
Table 1.
Zinc tolerance of strains selected for in planta growth assays, showing zinc tolerance groups as defined by k-means clustering (electronic supplementary material, figure S1) and IC50 values for zinc.
| strain | zinc tolerance group | IC50 [Zn] (mM) |
|---|---|---|
| W1D7 | 2 | 10.0 |
| W1E11 | 1 | 11.3 |
| SnD3 | 3 | 7.63 |
| SnD4 | 3 | 6.75 |
| SnC10 | 3 | 11.8 |
| SnB11 | 3 | 7.06 |
| Psm | not tested | 7.25 |
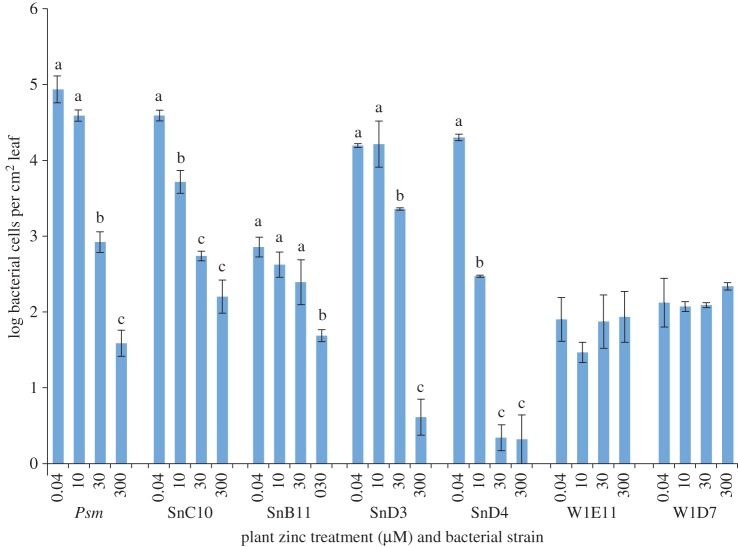
Bacterial growth in planta is shown in figure 3. The four strains isolated from the natural population of N. caerulescens were all able to grow in planta when the plants were grown on 0.04 µM Zn. The ability of these strains to multiply in plants grown on higher concentrations of zinc was correlated with their zinc tolerance, with the most tolerant, strain SnC10 (IC50 for Zn = 11.8 mM) able to multiply in all plants, although with reduced growth at higher zinc; strains SnB11 and SnD3 (IC50 = 7.1 and 7.6 mM, respectively) able to multiply only in plants grown on 30 µM Zn or lower, again with less success at higher zinc; and the least tolerant strain, SnD4 (IC50 = 6.7 mM), only able to multiply in the plants grown on 0.04 µM Zn. Psm has an IC50 of 7.25 mM, placing it between SnB11 and SnD3, whose in planta behaviour it closely replicated. By contrast, neither of the strains isolated from the artificial population of N. caerulescens was able to multiply in planta, regardless of plant zinc treatment, despite the high in vitro zinc tolerances of these two bacterial strains (IC50 = 10 and 11.3 mM).
Figure 3.
Growth of selected bacterial strains isolated from leaves of N. caerulescens plants of natural (SnC10, SnB11, SnD3, SnD4) or artificial populations (W1E11, W1D7). Plants were grown on 0.04, 10, 30 or 300 µM Zn and bacteria were inoculated into 10-week-old plants at 106 cfu ml−1 in 10 mM MgCl2. Pseudomonas syringae pv. maculicola is included for comparison. Bacterial cell counts were estimated by plating three independent samples of homogenized leaf tissue onto KB-CFC agar after 0 and 5 days. Representative day 5 data from one of two replicate experiments is shown; no differences in bacterial population were detected on day 0. Zinc was a significant predictor of bacterial numbers at 5 days post-inoculation for Psm and all strains isolated from the natural population, but not for strains isolated from the artificial population (GLMs; p ≤ 0.0005, <0.0005, 0.011, <0.0005, <0.0005, 0.638 and 0.693, for strains from left to right across the graph). Where zinc had a significant effect, Bonferroni's simultaneous comparisons were carried out. Means that were not significantly different (within each strain) are marked with the same letter; error bars represent ± s.e. (Online version in colour.)
4. Discussion
In this work, we investigated the possibility that populations of bacterial endophytes of metal-hyperaccumulating plants become locally adapted to the metal-rich environment they encounter within these plants. Previously, we have demonstrated that endophytic bacteria of the zinc hyperaccumulator N. caerulescens show higher zinc tolerance when compared with bacteria pathogenic on related non-accumulator plants [15], an idea supported by other studies of bacteria associated with metal-hyperaccumulating plants [18,19,38–40]. Considered alongside evidence for a role of hyperaccumulated metals in defence of plants such as N. caerulescens against disease [13–15,41,42], this suggests that local adaptation of pathogens to high-metal concentrations in the rhizosphere and phyllosphere of metal-accumulating plants might drive the evolution of further defensive hyperaccumulation in a form of coevolutionary arms race [15,43].
Here, we have shown that bacteria isolated from the zinc-hyperaccumulating plant N. caerulescens display a degree of zinc tolerance correlated with the zinc concentrations found in the leaves of the plants from which they were isolated. This result supports the idea that the leaf environment provided by this zinc hyperaccumulator exerts a selection pressure for zinc tolerance. While in theory it is possible that disease leads to reduced metal accumulation, any reduction in metal uptake capacity would occur as a result of disease symptoms; zinc accumulation, however, occurs throughout the life of the plant, so that any measureable differences in the zinc content of aerial tissues are likely to predate the infection. Additionally, this is demonstrated in laboratory experiments (e.g. [15]) in which plants were first cultivated, in a healthy state, on a range of Zn concentrations and then artificially inoculated with P. syringae, resulting in symptoms with severity inversely proportional to measured plant Zn content. On the other hand, there is now an extensive body of evidence indicating that cross-talk occurs between metal and biotic stress signalling at the level of ROS (e.g. [15]), hormones (e.g. [44]), transcriptomic and proteomic changes (including enzymes of the secondary metabolism, PR proteins and more, see [45] for review). This cross-talk may confer cross-protection against disease when plants are exposed to and accumulate heavy metals such as zinc. Additionally, there is mounting evidence (e.g. [6,7,15,16]) that metal hyperaccumulation does indeed protect these plants from disease, as would be expected under the hypothesis that an evolutionary arms race taking place in the zinc-rich environment of hyperaccumulators and their surroundings is responsible for driving the evolution of the metal accumulation trait.
We have also compared the zinc tolerance of bacteria isolated from a natural population (Snowdonia) of N. caerulescens plants to those isolated from an artificial population (Wytham) created for this study. The preponderance of more zinc-tolerant bacteria originating from the Snowdonia site, and of less zinc-tolerant bacteria from the Wytham site, suggests that the natural population of N. caerulescens at Snowdonia provided a selection pressure acting in favour of zinc-tolerant bacteria. The scattered distribution of zinc tolerance across the rpoD gene tree implies multiple origins of zinc tolerance, within and among groups of related bacteria, suggesting that this trait might be acquired relatively easily, as seen in a library of transposon mutants of P. syringae pv. maculicola [15]. This supports the hypothesis that, if metal accumulation evolved as a defence, rapidly evolving pathogens are likely to have driven a subsequent arms race leading to the progressive enhancement of the metal-accumulation phenotype [16].
It is possible that differences in plant genotype between the N. caerulescens plants in the natural and artificial environments may have influenced the establishment of endophytic bacteria. However, differences in endophyte zinc tolerance that covary with plant zinc content are seen at the within-genotype level. Thus, the findings shown in figure 1f, that the median zinc tolerance group into which isolated endophytes fall is positively correlated with plant zinc content in the natural population, and with the zinc treatment on which the plants were grown in the artificial population, indicate that the plant zinc content affects the zinc tolerance of its bacterial endophytes in a manner independent of plant genotype.
Interestingly, while IC50 values for zinc are not predicted by the species complexes into which a strain falls on the rpoD tree, zinc-tolerance group percentages do vary between these phylogenetic groups. Strains in the aeruginosa complex are exclusively found in tolerance groups 2 and 3, both of which show fairly linear zinc dose–response curves (electronic supplementary material, figure S1). Strains in the syringae and fluorescens complexes are, respectively, equally or more likely to fall into zinc tolerance group 4 than groups 2 and 3. Group 4 strains show little or no growth reduction until zinc concentrations are fairly high (5–10 mM zinc; electronic supplementary material, figure S1). The ability to withstand up to 10 mM zinc without growth reduction might give these bacteria an advantage when growing endophytically in N. caerulescens plants exposed to high zinc.
Notably, there are only three monophyletic groups with high bootstrap support (99%) containing exclusively highly zinc-tolerant strains (IC50 > 9 mM Zn and tolerance group 3 or 4) isolated from plants of the natural population. These are very small groups (G8 & 9, F4 & E3, E8, F8 & F9), suggesting that the trait was recently acquired. Elsewhere on the tree, such strains tend to be found in clades with high bootstrap support that contain a greater variety of zinc tolerance phenotypes (for example, the highly tolerant strains G1 and G9, which are found alongside a range of less tolerant strains), again implying recent, repeated acquisitions of tolerance. Zinc-tolerant strains isolated from the artificial N. caerulescens population, by contrast, dominate, and are exclusively found within one clade (BS 99%) within the fluorescens complex.
This might indicate that the established, high-zinc environment of the natural population tends to select for the zinc tolerance trait wherever it arises, while the zinc-tolerant bacteria from the artificial site represent an earlier evolution of tolerance unrelated to the sudden, artificial appearance of Noccaea plants. The scattered distribution of zinc-tolerant bacteria across the tree could also reflect horizontal gene transfer among bacteria in Snowdonia, in addition to mutation towards greater zinc tolerance.
While pathogens isolated from the natural population were able to infect N. caerulescens under laboratory conditions, the reverse was not true of even highly zinc-tolerant bacteria from the artificial population. We have previously shown that the type 3 secretion system (T3SS), used to deliver bacterial disease effectors to the host, is essential for the growth of P. syringae pv. maculicola (Psm) in planta in N. caerulescens [15]. This indicates that, despite the importance of zinc as a defence [15] and the loss of certain other defences by the metal hyperaccumulators [16,17], the plants retain defences against which the T3SS is necessary, probably including some elements of PAMP-triggered immunity (PTI: [46,47]). It is also possible that some elements of effector-triggered immunity (ETI: [46,48]) are retained. Thus, to infect N. caerulescens, bacteria require both zinc tolerance and the ability to overcome pathogen-induced plant defences. As a result, N. caerulescens may only be vulnerable to pathogenic bacteria such as P. syringae, which possess the T3SS and effectors, but which have also been exposed to a zinc-rich environment and acquired zinc tolerance. We hypothesize that at the former mine site in Snowdonia, the natural population of N. caerulescens has selected for zinc-tolerant, N. caerulescens-specific pathogens. These are able to infect N. caerulescens, while endophytic bacteria from the artificial population, with no long-term exposure to N. caerulescens, proved non-pathogenic.
In conclusion, this study provides evidence for local adaptation of endophytic bacteria to the zinc-rich environment of leaves of N. caerulescens plants growing on a zinc-rich substrate. Pathogenicity, however, requires more than simply zinc tolerance, and was only found to be prevalent in the natural population. It will be of interest to determine which elements of PTI and ETI are retained by N. caerulescens, and whether these occur in all N. caerulescens, or vary with the degree of zinc availability, tolerance and hyperaccumulation in different populations. Investigation of endophytes and pathogens of other populations, and their ability to infect plants of other populations, would contribute to a more complete picture of the emergence and ongoing evolution of hyperaccumulation and associated endophytes.
Supplementary Material
Acknowledgements
The authors would like to thank Mr Nigel Fisher, Conservator of Wytham Woods, and Mr Phil Smith, for their invaluable assistance in setting up experiments at Wytham, and for the use of the field station facilities.
Data accessibility
rpoD, gyrB and 16S sequences used in BLAST searches and to reconstruct the endophyte phylogeny in figure 2 are deposited in GenBank; accession numbers KX181734 - KX181845. rpoD sequence alignment and tree are deposited in TreeBase; http://purl.org/phylo/treebase/phylows/study/TB2:S18035. All other data are deposited in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.qb74m.
Author contributions
H.N.F. developed and performed experiments, analysed data and wrote the manuscript; H.M. performed experiments; A.M. produced tolerance clusters; J.A.C.S. and G.M.P. supervised the work and wrote the manuscript.
Competing interests
The authors declare no competing interests.
Funding
H.N.F. was funded by a PhD studentship from the Natural Environment Research Council (NERC).
References
- 1.Jaffré T, Brooks RR, Lee J, Reeves RD. 1976. Sebertia acuminata: a hyperaccumulator of nickel from New Caledonia. Science 193, 579–580. ( 10.1126/science.193.4253.579) [DOI] [PubMed] [Google Scholar]
- 2.Brooks RR, Lee J, Reeves RD, Jaffré T. 1977. Detection of nickeliferous rocks by analysis of herbarium species of indicator plants. J. Geochem. Explor. 7, 49–57. ( 10.1016/0375-6742(77)90074-7) [DOI] [Google Scholar]
- 3.Pollard AJ. 2000. Metal hyperaccumulation: a model system for coevolutionary studies. New Phytol. 146, 179–181. ( 10.1046/j.1469-8137.2000.00651.x) [DOI] [PubMed] [Google Scholar]
- 4.Assunção AGL, Schat H, Aarts MGM. 2003. Thlaspi caerulescens, an attractive model species to study heavy metal hyperaccumulation in plants. New Phytol. 159, 351–360. ( 10.1046/j.1469-8137.2003.00820.x) [DOI] [PubMed] [Google Scholar]
- 5.Boyd RS, Martens SN. 1992. The raison d'être for metal hyperaccumulation by plants. In The vegetation of ultramafic (serpentine) soils (eds Baker AJM, Proctor J, Reeves RD), pp. 279–289. Andover, MA: Intercept Limited. [Google Scholar]
- 6.Boyd RS. 2007. The defense hypothesis of elemental hyperaccumulation: status, challenges and new directions. Plant Soil 293, 153–176. ( 10.1007/s11104-007-9240-6) [DOI] [Google Scholar]
- 7.Boyd RS. 2012. Plant defense using toxic inorganic ions: conceptual models of the defensive enhancement and joint effects hypotheses. Plant Sci. 195, 88–95. ( 10.1016/j.plantsci.2012.06.012) [DOI] [PubMed] [Google Scholar]
- 8.Vesk PA, Reichman SM. 2009. Hyperaccumulators and herbivores—a Bayesian meta-analysis of feeding choice trials. J. Chem. Ecol. 35, 289–296. ( 10.1007/s10886-009-9607-7) [DOI] [PubMed] [Google Scholar]
- 9.Behmer ST, Lloyd CM, Raubenheimer D, Stewart-Clark J, Knight J, Leighton RS, Harper FA, Smith JAC. 2005. Metal hyperaccumulation in plants: mechanisms of defence against insect herbivores. Funct. Ecol. 19, 55–66. ( 10.1111/j.0269-8463.2005.00943.x) [DOI] [Google Scholar]
- 10.Cheruiyot DJ, Boyd RS, Moar WJ. 2013. Exploring lower limits of plant elemental defense by cobalt, copper, nickel, and zinc. J. Chem. Ecol. 39, 666–674. ( 10.1007/s10886-013-0279-y) [DOI] [PubMed] [Google Scholar]
- 11.Kazemi-Dinan A, Barwinski A, Stein RJ, Krämer U, Müller C. 2015. Metal hyperaccumulation mediates defence against herbivores in the field and improved growth. Entomol. Exp. Appl. 157, 3–10. ( 10.1111/eea.12333) [DOI] [Google Scholar]
- 12.Kazemi-Dinan A, Thomaschky S, Stein RJ, Krämer U, Müller C. 2014. Zinc and cadmium hyperaccumulation act as deterrents towards specialist herbivores and impede the performance of a generalist herbivore. New Phytol. 202, 628–639. ( 10.1111/nph.12663) [DOI] [PubMed] [Google Scholar]
- 13.Boyd RS, Shaw J, Martens SN. 1994. Nickel hyperaccumulation defends Streptanthus polygaloides (Brassicaceae) against pathogens. Am. J. Bot. 81, 294–300. ( 10.2307/2445455) [DOI] [Google Scholar]
- 14.Ghaderian Y, Lyon A, Baker AJM. 2000. Seedling mortality of metal hyperaccumulator plants resulting from damping off by Pythium spp. New Phytol. 146, 219–224. ( 10.1046/j.1469-8137.2000.00645.x) [DOI] [PubMed] [Google Scholar]
- 15.Fones HN, Davis CAR, Rico A, Fang F, Smith JAC, Preston GM. 2010. Metal hyperaccumalation armors plants against disease. PLoS Pathog. 6, e1001093 ( 10.1371/journal.ppat.1001093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fones HN, Eyles CJ, Bennett MH, Smith JAC, Preston GM. 2013. Uncoupling of reactive oxygen species accumulation and defence signalling in the metal hyperaccumulator plant Noccaea caerulescens. New Phytol. 199, 916–924. ( 10.1111/nph.12354) [DOI] [PubMed] [Google Scholar]
- 17.Llugany M, Martin SR, Barceló J, Poschenrieder C. 2013. Endogenous jasmonic and salicylic acids levels in the Cd-hyperaccumulator Noccaea (Thlaspi) praecox exposed to fungal infection and/or mechanical stress. Plant Cell Rep. 32, 1243–1249. ( 10.1007/s00299-013-1427-0) [DOI] [PubMed] [Google Scholar]
- 18.Kazemi-Dinan A, Sauer J, Stein RJ, Krämer U, Müller C. 2015. Is there a trade-off between glucosinolate-based organic and inorganic defences in a metal hyperaccumulator in the field? Oecologia 178, 369–378. ( 10.1007/s00442-014-3218-x) [DOI] [PubMed] [Google Scholar]
- 19.Barzanti R, Ozinol F, Bazziculupo M, Gabbrielli R, Galardi F, Gonnelli C, Mengoni A. 2007. Isolation and characterization of endophytic bacteria from the nickel hyperaccumulator plant Alyssum bertolonii. Microbiol. Ecol. 53, 306–316. ( 10.1007/s00248-006-9164-3) [DOI] [PubMed] [Google Scholar]
- 20.Idris R, Trifonova R, Puschenreiter M, Wenzel WW, Sessitsch A. 2004. Bacterial communities associated with flowering plants of the Ni hyperaccumulator Thlaspi goesingense. Appl. Environ. Microbiol. 70, 2667–2677. ( 10.1128/AEM.70.5.2667-2677.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo S, et al. 2011. Analysis and characterization of cultivable heavy metal-resistant bacterial endophytes isolated from Cd-hyperaccumulator Solanum nigrum L. and their potential use for phytoremediation. Chemosphere 85, 1130–1138. ( 10.1016/j.chemosphere.2011.07.053) [DOI] [PubMed] [Google Scholar]
- 22.Bennett J, Vernon RW. 1990. Mines of the Gwydyr Forest: part 2. The Hafna Mine, Llanrwst and some early ventures in Gwydyr Nant. Cuddington, UK: Gwydyr Mines Publications. [Google Scholar]
- 23.Dechamps C, Noret N, Mozek R, Escarré J, Lefèbvre C, Gruber W, Meerts P. 2008. Cost of adaptation to a metalliferous environment for Thlaspi caerulescens: a field reciprocal transplantation approach. New Phytol. 177, 167–177. [DOI] [PubMed] [Google Scholar]
- 24.Roosens N, Verbruggen N, Meerts P, Ximénez-Embún P, Smith JAC. 2003. Natural variation in cadmium tolerance and its relationship to metal hyperaccumulation for seven populations of Thlaspi caerulescens from western Europe. Plant Cell Environ. 26, 1657–1672. ( 10.1046/j.1365-3040.2003.01084.x) [DOI] [Google Scholar]
- 25.King EO, Ward MK, Raney DE. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 44, 301–307. [PubMed] [Google Scholar]
- 26.Schaad N, Jones J, Chun W. 2001. A laboratory guide for identification of plant pathogenic bacteria, 3rd edn St Paul, MN: American Pathological Society Press. [Google Scholar]
- 27.Soothill JS, Ward R, Girling AJ. 1992. The IC50: an exactly defined measure of antibiotic sensitivity. J. Antimicrob. Chemother. 29, 137–139. ( 10.1093/jac/29.2.137) [DOI] [PubMed] [Google Scholar]
- 28.R Development Core Team. 2008. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 29.Edgar RC. 2004. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinf. 5, 113 ( 10.1186/1471-2105-5-113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acid Res. 35, 1792–1797. ( 10.1093/nar/gkh340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24, 1596–1599. ( 10.1093/molbev/msm092) [DOI] [PubMed] [Google Scholar]
- 32.Eck RV, Dayhoff MO. 1996. Atlas of protein sequence and structure. Silver Sprigs, MD: National Biomedical Research Foundation. [Google Scholar]
- 33.Nei M, Kumar S. 2000. Molecular evolution and phylogenetics. New York, NY: Oxford University Press. [Google Scholar]
- 34.Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791. ( 10.2307/2408678) [DOI] [PubMed] [Google Scholar]
- 35.Baker AJM, Brooks RR. 1989. Terrestrial higher plants which hyperaccumulate metallic elements—a review of their distribution, ecology and phytochemistry. Biorecovery 1, 81–126. [Google Scholar]
- 36.Yamamoto S, Kasai H, Arnold DL, Jackson RW, Vivian A, Harayama S. 2000. Phylogeny of the genus Pseudomonas: intrageneric structure reconstructed from the nucleotide sequences of gyrB and rpoD genes. Microbiology 146, 2385–2394. ( 10.1099/00221287-146-10-2385) [DOI] [PubMed] [Google Scholar]
- 37.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215, 403–410. ( 10.1016/S0022-2836(05)80360-2) [DOI] [PubMed] [Google Scholar]
- 38.Idris R, Kuffner M, Bodrossy L, Puschenreiter M, Monchy S, Wenzel WW, Sessitsch A. 2006. Characterization of Ni-tolerant methylobacteria associated with the hyperaccumulating plant Thlaspi goesingense and description of Methylobacterium goesingense sp. nov. Systemat. Appl. Microbiol. 29, 634–644. ( 10.1016/j.syapm.2006.01.011) [DOI] [PubMed] [Google Scholar]
- 39.Xiao X, Luo S, Zeng G, Wei W, Wan Y, Chen L, Yang L, Chen J, Xi Q. 2010. Biosorption of cadmium by endophytic fungus (EF) Microsphaeropsis sp. LSE10 isolated from cadmium hyperaccumulator Solanum nigrum L. Bioresour. Technol. 101, 1668–1674. ( 10.1016/j.biortech.2009.09.083) [DOI] [PubMed] [Google Scholar]
- 40.Chen L, Luo S, Chen J, Wan Y, Li X, Liu C, Liu F. 2014. A comparative analysis of endophytic bacterial communities associated with hyperaccumulators growing in mine soils. Environ. Sci. Pollut. Res. 21, 7538–7547. ( 10.1007/s11356-014-2670-9) [DOI] [PubMed] [Google Scholar]
- 41.Hanson B, Garifullina GF, Lindblom SD, Wangeline A, Ackley A, Kramer K, Norton AP, Lawrence CB, Pilon-Smits EAH. 2003. Selenium accumulation protects Brassica juncea from invertebrate herbivory and fungal infection. New Phytol. 159, 461–469. ( 10.1046/j.1469-8137.2003.00786.x) [DOI] [PubMed] [Google Scholar]
- 42.Hörger A, Fones HN, Preston GM. 2013. The current status of the defence hypothesis in relation to pathogens. Front. Plant Sci. 4, 395–406. ( 10.3389/fpls.2013.00395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fones HN, Preston GM. 2013. Trade-offs between metal hyperaccumulation and induced disease resistance in metal hyperaccumulator plants. Plant Pathol. 62, 63–71. ( 10.1111/ppa.12171) [DOI] [Google Scholar]
- 44.Freeman JL, Garcia D, Kim D, Hopf A, Salt DE. 2005. Constitutively elevated salicylic acid signals glutathione-mediated nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Physiol. 137, 1082–1091. ( 10.1104/pp.104.055293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahsan N, Renaut J, Komatsu S. 2009. Recent developments in the application of proteomics to the analysis of plant responses to heavy metals. Proteomics 9, 2602–2621. ( 10.1002/pmic.200800935) [DOI] [PubMed] [Google Scholar]
- 46.Jones JD, Dangl JL. 2006. The plant immune system. Nature 444, 323–329. ( 10.1038/nature05286) [DOI] [PubMed] [Google Scholar]
- 47.Ausubel FM. 2005. Are innate immune signaling pathways in plants and animals conserved? Nature Immunol. 6, 973–979. ( 10.1038/ni1253) [DOI] [PubMed] [Google Scholar]
- 48.Navarro L, Zipfel C, Rowland O, Keller I, Robatzek S, Boller T, Jones JD. 2004. The transcriptional innate immune response to flg22. Interplay and overlap with Avr gene-dependent defense responses and bacterial pathogenesis. Plant Physiol. 135, 1113–1128. ( 10.1104/pp.103.036749) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
rpoD, gyrB and 16S sequences used in BLAST searches and to reconstruct the endophyte phylogeny in figure 2 are deposited in GenBank; accession numbers KX181734 - KX181845. rpoD sequence alignment and tree are deposited in TreeBase; http://purl.org/phylo/treebase/phylows/study/TB2:S18035. All other data are deposited in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.qb74m.