Abstract
Objectives
The potential effect of hormonal contraception on HIV-1 acquisition and transmission represents an important public health issue. Several observational studies have suggested an association between the use of hormonal contraception, in particular injectable depot medroxyprogesterone acetate (DMPA), and an increased risk of HIV-1 acquisition and transmission. We and others have previously demonstrated that DMPA acts as a potent inhibitor of innate and adaptive immune mechanisms. The study presented here addresses the immunomodulatory properties of several common progestins with a potential to replace DMPA.
Study design
To identify safe alternatives to DMPA, we tested the effect of commonly used progestins on the function of human primary T cells and plasmacytoid dendritic cells (pDCs) obtained from the blood of healthy premenopausal women.
Results
Medroxyprogesterone acetate (MPA) inhibited the activation of T cells and pDCs in response to T cell receptor and Toll-like receptor (TLR)-mediated activation at physiological concentrations. Etonogestrel (ETG) exerted a partial suppressive activity at high concentrations. In sharp contrast, norethisterone (NET) and levonorgestrel (LNG) did not exhibit detectable immunosuppressive activity.
Conclusion
Evidence indicating the immunosuppressive properties of DMPA strongly suggests that DMPA should be discontinued and replaced with other forms of long-term contraception. Since NET and LNG do not exert immunosuppressive properties at physiological concentrations, these progestins should be considered as alternative contraceptives for women at high risk of HIV-1 infection.
Keywords: hormonal contraception, HIV-1, AIDS, progestins, DMPA, NET, LNG, ETG
Introduction
Contraception represents a critical component of preventive health care. It provides women with a control over their reproductive health, reduces the number of unintended pregnancies, decreases maternal and infant mortality and morbidity, reduces recourse to abortion, lowers the risk of mother-to child transmission of HIV-1, and provides additional benefits including reduction of poverty and improved access to education. The use of injectable hormonal contraceptives is highly popular as it provides multiple advantages over other forms of contraception including high effectiveness and a long-term effect [1, 2]. Depot medroxyprogesterone acetate (DMPA; Depo-Provera), a progestin-only contraceptive typically administered in the form of a 3-monthly intramuscular injection, is one of the most commonly used contraceptives in sub-Saharan Africa and other areas with high HIV-1 prevalence [1, 2]. It is estimated that 20–50 million women worldwide use DMPA and the number is steadily increasing [1, 3, 4]. In some countries, DMPA is the method of choice for over 50% of women using modern methods of contraception [1, 2]. Unfortunately, multiple observational studies suggest an association between the use of hormonal contraception and increased risk of HIV-1 acquisition and transmission [5–12]. In most studies, the adjusted hazard ratio of HIV-1 acquisition associated with the use of DMPA is higher than that linked to the use of oral contraceptives or other forms of contraception [7–13]. Many studies do not distinguish between the two most common forms of injectable contraception, DMPA and norethisterone enanthate (NET-EN), and little attention has been placed on differential pharmacological and biological effects of these two contraceptives. Recent re-analysis of data by Heffron et al. showed that, within the injectable users, women outside of South Africa (consistent with DMPA usage) had higher HIV-1 risk (adjusted HR = 3.9) than women living in South Africa where NET-EN is used more widely [13]. None of the three studies that specifically assessed the effect of NET-EN found a significant association with HIV-1 acquisition [3, 14, 15]; however, more data is urgently needed. Non-human primate studies demonstrate that DMPA enhances the risk of SIV acquisition via vaginal exposure and suggest that DMPA increases viral levels in the acute phase of infection and reduces the protective effect of prior immunization [16–19]. Recently, we have demonstrated that MPA suppresses the production of key T cell-derived regulators of cellular and humoral immunity involved in the induction of immune response to invading pathogens including IFNγ, IL-2, IL-4, IL-6, IL-12, MIP-1α, and TNFα [20]. Importantly, MPA inhibits the function of pDCs and reduces the production of IFNα in response to Toll-like receptor (TLR) -7, -8 and -9 ligands. Furthermore, MPA prevents the downregulation of HIV-1 coreceptors CXCR4 and CCR5 on activated T cells and causes increased replication of HIV-1 in activated peripheral blood mononuclear cells (PBMCs) [20]. Immunosuppressive properties of DMPA have been consistently demonstrated in various models [4, 5, 16–18, 20–29].
The effect of hormonal contraception on HIV-1 acquisition and transmission represents a critical global public health issue. Recent WHO meeting on programmatic and research priorities for contraception for women at risk of HIV identified the research addressing the association between various methods of hormonal contraception and HIV acquisition and transmission as a top priority, with an emphasis on injectables and other long-term methods [30]. Accumulated studies indicating the immunosuppressive properties of DMPA [4, 5, 16–18, 20–29], and the epidemiological evidence demonstrating an association between DMPA use and increased risk of HIV-1 and other infections [5–13, 31–35] strongly suggest that the use of DMPA should be discontinued, especially in areas with high HIV-1 prevalence. However, withdrawal of DMPA from family planning programs without offering equally effective forms of contraception is not warranted as it could result in a sharp increase in unwanted births, unsafe abortions, and maternal and infant mortality. In some regions up to nine additional maternal deaths could occur for every case of HIV-1 averted [36–38]. Replacement of DMPA with condoms would result in a significant increase of unintended pregnancies due to high failure rates [37]. Thus, it is critical to identify contraceptive regimen that could effectively replace DMPA without exerting undesired side effects.
Most family planning programs strongly favor long-term methods of contraception due to higher efficacy, reliability, and ease of use. Norethisterone (NET)-based injectables are commonly used in resource-limited countries [1, 3, 9, 31, 39–41]. Levonogestrel (LNG) or etonogestrel (ETG)-releasing devices or implants are highly effective and reversible methods of long-term contraception [42–44]. ETG, LNG, and NET are considered for use in HIV-1-endemic areas; however, their safety in regard to the effect on immune system and HIV-1 transmission has not been validated [1, 3, 4, 9, 39, 40, 45]. Identification of contraceptives that do not suppress the protective properties of the immune system is critical for the selection of safe hormonal contraception in areas with high HIV-1 prevalence [4, 36, 37, 40]. In order to find safe alternatives to DMPA, we analyzed the effect of commonly used progestins on the adaptive and innate immune system. Importantly, the presented data indicate that, at physiological levels, NET and LNG do not suppress cytokine production by activated T cells or pDCs.
Materials and Methods
Study participants and sample collection
All procedures involving the use of human subjects were approved by the Institutional Review Board of the University of Alabama at Birmingham. Informed consent was obtained from all participants. All volunteers were recruited at UAB, Birmingham, Alabama. The volunteers were healthy pre-menopausal women; blood was collected at 10 to 22 days post last menstruation. 100 ml of acid citrate dextrose (ACD)-treated blood was collected by personnel trained in phlebotomy.
Materials
All cell culture reagents were obtained from Mediatech Inc. (Manassas, VA), unless indicated otherwise. Etonogestrel was obtained from Bosche Scientific (New Brunswick, NJ); all other synthetic hormones were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies for flow cytometry were purchased from eBioscience, (San Diego, CA), unless listed otherwise. Progesterone (P4; pregn-4-ene-3,20-dione), medroxyprogesterone acetate (MPA; 17α-hydroxy-6α-methylprogesterone acetate), norethisterone (NET; 19-nor-17α-ethynyltestosterone), levonorgestrel (LNG; L-norgestrel), and etonogestrel (ETG; 3-keto-desogestrel) were dissolved in DMSO (Mediatech Inc., cell culture grade) at a concentration of 10 mM and stored in aliquots at −80°C prior use. Aldrithiol-2-inactivated HIV-1 virus (AT2-HIV, MN strain, lot P3944) and microvesicle-only control (lot P4176) were obtained from the AIDS and Cancer Virus Program, National Cancer Institute, Frederick, MD.
Cytokine production in vitro
PBMCs were cultured in phenol red-free RPMI medium containing 10% heat-inactivated charcoal dextran-scrubbed fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 2 mM GlutaMAX (Invitrogen, Carlsbad CA), 100 IU/ml penicillin, and 100 µg/ml streptomycin. Isolated PBMCs were incubated in the presence of the solvent (DMSO) alone or indicated concentrations of progestin and activated for an additional 24 hrs with T cell activation/expansion MACSi microbeads coated with antibodies against CD2, CD3, and CD28 antigens (Miltenyi Biotec, Auborn, CA) at 1:2 bead to cell ratio in the presence or absence of progestins. The concentration of IFNγ in the media was determined by ELISA (eBiosciences); the concentrations of 26 cytokines and chemokines were determined using the 26-plex MILLIPLEX Human Cytokine/Chemokine Panel kit (Millipore, Billerica, MA) as described [20].
pDC activation and intracellular cytokine staining assay
PBMCs (1.5 × 106 cells/ml) were pre-incubated for 6 hrs in the presence or absence of hormones and subsequently stimulated with either 0.2 µg/ml TLR-7/8 ligand R848 (Invivogen, San Diego, CA), 2 µM TLR-9 ligand CpG ODN2216 (Hycult Biotech, Uden, The Netherlands), or 900 ng/ml p24CA equivalent AT2-inactivated HIV-1. 1 µl of GolgiPlug (BD biosciences, San Diego, CA) was added to the cell culture immediately following the addition of R848, or 6 hrs post- addition of CpG or AT2 HIV, respectively. 20 hrs post the initiation of stimulation, cells were collected and stained for pDC markers (CD123-PE-Cy7 (eBioscience) and CD303-APC (BCDA-2; Miltenyi)). Cells were permeabilized using the Cytofix/Cytoperm kit (BD) and stained intracellularly with IFNα-PE (BD) and TNFα-FITC mAbs. Samples were analyzed on LSR-II flow cytometer (BD) and data analysis was performed using the FACSDiva (BD) software [20].
Statistical Analysis
Data were analyzed using Student’s t-test. A standard level of statistical significance α = 0.05 was used; all reported p-values are two-sided. GraphPad Prism 5 (GraphPad Software Inc., LaJolla, CA) statistical and graphing software package was used. Heat maps were created with Matrix2png (http://www.bioinformatics.ubc.ca/matrix2png/).
Results
MPA but not NET or LNG inhibits cytokine production by activated T cells
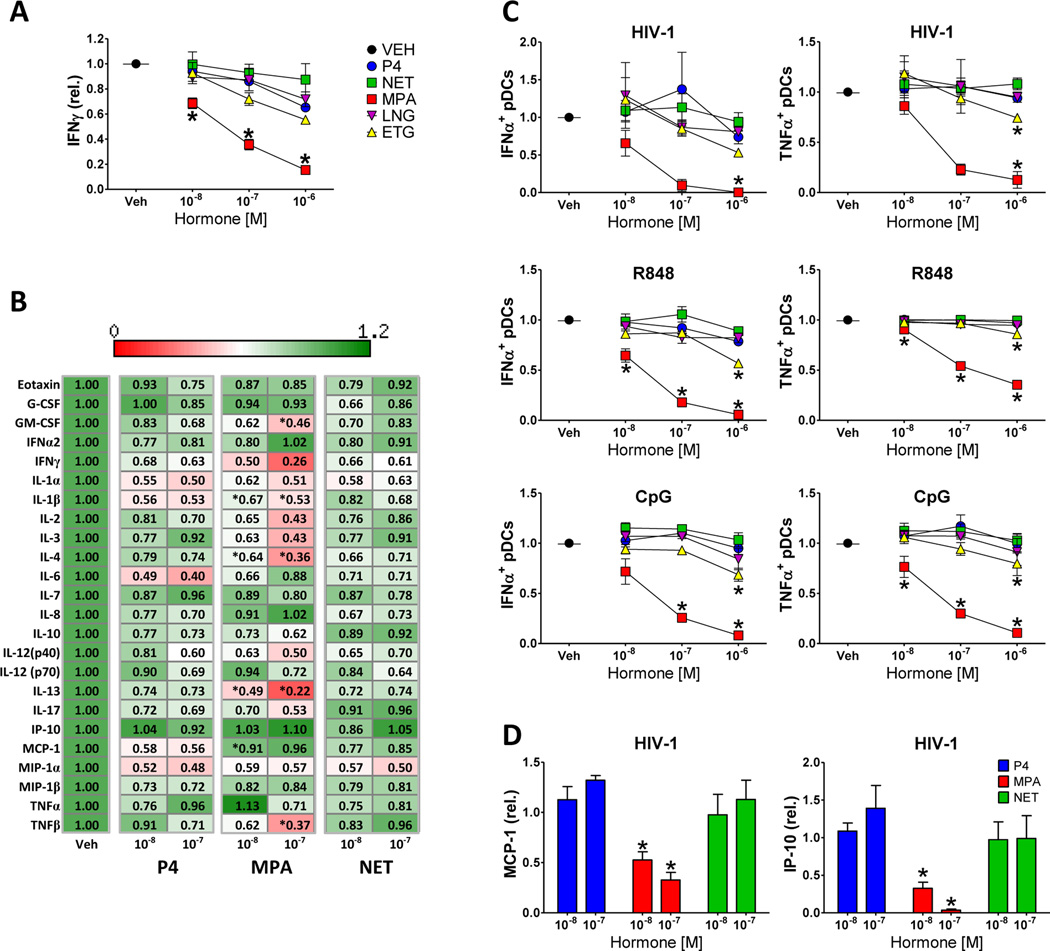
To compare the potential immunomodulatory effects of progestins, PBMCs obtained from healthy female volunteers were incubated with T cell-activating MACSi microbeads coated with antibodies against CD2, CD3, and CD28 antigens in the absence or presence of increasing concentrations of progesterone (P4), MPA, NET, LNG, or ETG. As demonstrated in Figure 1A, MPA exerted a significant inhibitory effect on the production of IFNγ by CD2/CD3/CD28-stimulated PBMCs at a concentration of 10−8 M and higher. At 10−6 M, ETG displayed a significant inhibition of T cell function compared to untreated control but not compared to cells treated with P4. In contrast, NET and LNG did not exert a significant suppressive effect on IFNγ production compared to P4 at any concentration tested. In contrast to a previous study where the immunosuppressive effect of MPA was observed at a minimal concentration of 10−7 M [20], here we report a significant suppressive effect at concentrations as low as 10−8 M. Due to the low solubility of LNG in ethanol, the stock solutions of progestins in the current study were prepared in DMSO. This is in contrast to the previous study where all progestin stock solutions were prepared in ethanol [20]. To test the putative effect of the solvent, the suppressive properties of MPA dissolved in DMSO or ethanol were directly compared. As shown in Supplemental Figure 1, MPA dissolved in DMSO consistently exerted its suppressive effect on T cells at a lower concentration than MPA dissolved in ethanol. The mechanism underlying the effect of the solvent on progestin-mediated T cell suppression in vitro is unclear.
Figure. 1. MPA but not NET or LNG suppresses cytokine production by activated mononuclear cells and pDCs.
A) PBMCs obtained from healthy female volunteers were incubated for 24 hrs in the presence of vehicle (Veh; DMSO) or indicated concentrations of progestins and activated for an additional 24 hrs with microbeads coated with antibodies against CD2, CD3, and CD28 antigens. Graph represents the concentration of IFNγ in the culture media. Data are normalized values relative to vehicle control; mean ± standard error of the mean (SEM) of three donors is shown. B) Heat map representation of the effect of P4, MPA, and NET on cytokine production by mononuclear cells stimulated with microbeads coated with antibodies against CD2/CD3/CD28. Data are presented as normalized values relative to vehicle; means of three independent experiments are presented. * denotes statistically significant difference (p < 0.05) compared to P4-treated samples at respective concentrations. C) Graphic representation of the effect of P4, MPA, NET, LNG, and ETG at the indicated concentrations on intracellular production of IFNα and TNFα by pDCs stimulated with AT2-inactivated HIV-1, R848, or CpG. Normalized data from three independent donors corrected for background are presented. D) Effect of P4, MPA, and NET on the accumulation of IP-10 (CXCL10) and MCP-1 in cell culture supernatants of HIV-1-stimulated PBMCs. Data from three independent experiments are normalized to vehicle control ± SEM. P4, progesterone; MPA, medroxyprogesterone acetate; NET, norethisterone; LNG, levonorgestrel; ETG, etonogestrel. * denotes statistically significant difference (p < 0.05) of progestin-treated samples compared to the P4-treated samples.
Importantly, the inhibitory effect of MPA on cytokine production was not limited to IFNγ. As shown in the heat map representation in Figure 1B, the presence of 10−8 M or 10−7 M MPA caused a significant reduction in the production of IL-1β, IL-4, IL-13, GM-CSF, and TNFβ by PBMCs incubated with TCR-activating microbeads. In contrast, the effect of NET on cytokine production by activated PBMCs was comparable or lower than that of P4.
MPA but not NET or LNG inhibits cytokine production by activated pDCs
pDCs represent a key immune population functioning as a sentinel recognizing early viral and bacterial infections via TLR-7/8 and TLR-9. Suppression of pDC function may tip the balance between viral replication and immune control at an early stage of infection towards the benefit of the virus. We have recently shown that the activation of pDCs is modulated by MPA [20]. To address the effect of other commonly used progestins, PBMCs were pre-incubated with P4, MPA, NET, LNG or ETG for 6 hours prior to stimulation with AT-2-inactivated HIV-1 virus, TLR-7/8-specific ligand R848, or TLR-9-specific ligand CpG. Following 20 hours of stimulation, the intracellular production of IFNα and TNFα in pDCs defined as CD123+CD303+ cells was determined [20]. As demonstrated in Figure 1C, MPA inhibited HIV-1, R848, and CpG-induced IFNα and TNFα production by stimulated pDCs at concentrations as low as 10−8 M and10−7 M. ETG exerted a significant suppressive effect on pDCs at 10−6 M. In contrast, NET and LNG did not display a significant suppressive effect on cytokine production by activated pDCs.
In addition to the analysis of intracellular cytokine production, cytokine and chemokine levels in cell culture supernatants of HIV-1-stimulated PBMCs were analyzed. As shown on Figure 1D, MPA suppressed the production of IP-10 (CXCL-10) and chemotactic protein-1 (MCP-1) in response to HIV-1 at concentrations as low as 10−8 M.
Discussion
The effect of hormonal contraception on HIV-1 acquisition and transmission represents a critical global public health issue and a topic of extensive scientific and public discussion [5, 31, 36–39]. This study confirms that MPA suppresses innate and adaptive immune mechanisms at a physiological concentration [5, 20, 22, 26, 28, 29]. MPA inhibited the production of IFNγ, IL-1β, IL-4, IL-13, GM-CSF, and TNFβ by TCR-activated T cells and reduced the production of IFNα and TNFα by pDCs in response to stimulation with HIV-1 and TLR-7/8 and 9 ligands. The active suppression by MPA of pDCs and T cell effector mechanisms may favor the proliferation of a founder viral population over immune control at an early stage of infection and therefore may contribute to the observed increase in HIV-1 acquisition in DMPA users.
Following administration of 150 mg DMPA by intramuscular injection, the serum concentration of MPA reaches up to 6.5 × 10−8 M (25 ng/ml) within days after injection and decreases to about 0.3 to 2 × 10−8 M (1 to 9 ng/ml) in the following weeks [46–50]. In this study, the immunosuppressive effect of MPA in vitro was observed at 1 × 10−8 M. ETG exhibited partial immunosuppressive activity at 10−6 M, a concentration that is likely above the estimated physiological levels in contraceptive users [45, 46]. In sharp contrast, the effect of NET and LNG on pDC and T cell function was not significantly different from that of unmodified progesterone.
Progestins are designed to act primarily via the progesterone receptor (PR); however, they exert various side effects due to their diverse affinities to other members of the steroid receptor family [45, 46]. In addition to binding to PR, MPA binds with a high affinity to the glucocorticoid receptor (GR; Kd of 4 – 11 nM) that is expressed at high levels in multiple immune cell types [46, 51]. Indeed, the affinity of MPA for GR is higher than that of its natural ligand cortisol [52]. GR is a well-established regulator of immune system. The MPA-GR complex suppresses the transcription of GR-regulated genes by direct interaction with nuclear factor κB (NFκB) and activator protein-1 (AP-1), by modification of the basal transcriptional machinery, chromatin remodeling, and repression of the induction of NFκB inhibitor IκB [23, 24, 51, 53–60]. Interestingly, HIV-1-infected patients display glucocorticoid hypersensitivity associated with reduced T cell function possibly due to specific binding of Vpr to the GR co-activating motif [57, 61–63]. Thus, the sensitivity of immune cells to the effect of GR-binding progestins may be significantly enhanced in the context of HIV-1 infection. In a sharp contrast to MPA, NET and LNG have low affinity to GR and do not appear to mediate transrepression of genes involved in immune regulation [4, 45, 46, 63–65]. ETG binds GR with intermediate affinity [66, 67]. Since the GR regulates transcription of a variety of genes involved in immunity and inflammation [68, 69], the differential affinity progestins to GR (MPA >> ETG > LNG ≈ NET) is consistent with the hypothesis that progestins exert their effect on immune mechanisms primarily via the GR [4, 20, 45, 46, 51, 57, 63]. Of particular interest for public health policies are the relative effects of the two most commonly used injectable contraceptives, DMPA and NET-EN. NET-EN, typically administered as a 200 mg 2-monthly injectable, is manufactured and distributed at costs similar to DMPA [70]. Both injectable contraceptives have been shown to be highly effective and safe [65]. However, accumulating evidence suggests that DMPA but not NET-EN exerts immunosuppressive properties and increases HIV-1 infectivity [3–5, 14, 63].
In summary, we show that MPA suppresses innate and adaptive immune responses at physiological concentrations whereas NET and LNG do not exert immunosuppressive properties. The results of this study, previous studies indicating immunosuppressive properties of DMPA [5, 16–18, 21–27], and multiple epidemiological studies suggesting an association between DMPA use and increased risk of HIV-1 and other infections [5–9, 11, 12, 32–35, 40] constitute strong arguments against the continuous use of high-dose injectable DMPA in women at risk of HIV-1 infection. Importantly, it must be emphasized that, in the settings where no other form of highly effective contraception is available, the overall benefit of the use of DMPA outweighs the potential associated risks as the availability of effective methods of contraception is critical for reducing maternal morbidity and mortality. Modeling studies suggest that even if DMPA increases the risk of HIV-1 acquisition, depending on the region, withdrawal of DMPA would have a negative overall effect on public health [36–38]. Thus, DMPA must not be simply withdrawn; it must be gradually replaced with alternative forms of contraception with lower probability of a detrimental effect on HIV-1 acquisition. NET-EN or LNG-based contraceptive agents and devices represent promising candidates for safe alternatives to DMPA. Importantly, women using any form of hormonal contraception should be strongly advised to use condoms, male or female, as recommended by recent WHO guidelines [30]. Large-scale randomized controlled study addressing the safety of individual progestins in respect to immune competence and HIV-1 acquisition is urgently needed to inform policy responses in regions with varied risk of HIV-1 infection and access to medical and contraceptive services.
Supplementary Material
Implications.
The presented data suggest that, at physiological levels, the progestins NET and LNG do not suppress cytokine production by immune cells and should be considered as alternatives to DMPA; however, more in vivo testing is needed to confirm this data.
Acknowledgments
This work was supported by National Institutes of Health grants AI083027, AI103401, and AI027767.
This work was supported by National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under award number PO1 AI083027. UAB Center for AIDS Research (CFAR; funded by NIH grant P30 AI027767) Flow Cytometry Core was instrumental with flow cytometry and cytokine analysis assays. Biomedical research space used for this study was constructed with funds supported in part by NIH grant RR-20136. AT-2-inactivated HIV-1 was generously provided by Dr. J Lifson, AIDS and Cancer Virus Program, SAIC Frederick, Inc., National Cancer Institute, Frederick, supported with federal funds from NIH under contract HHSN261200800001E. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure Summary: The authors declare no competing interests.
Authorship contributions: R.P.H.H., K.G.M., and Z.H. designed and performed the research and analyzed data. R.P.H.H. and Z.H. wrote the paper.
References
- 1.United Nations, Department of Economic and Social Affairs, Population Division. World Contraceptive Use 2012. [Accessed: Jan 28, 2014];UN Report 2012. Available from: http://www.un.org/esa/population/publications/WCU2012/MainFrame.html. [Google Scholar]
- 2.Expanding access to injectable contraception. FHI 360. [Accessed: August 11, 2012]; Available from: http://www.fhi360.org/projects/progress-country-based-leadership-expanding-access-injectable-contraceptives. [Google Scholar]
- 3.Morrison CS, Skoler-Karpoff S, Kwok C, Chen PL, van de Wijgert J, Gehret-Plagianos M, et al. Hormonal contraception and the risk of HIV acquisition among women in South Africa. AIDS. 2012;26:497–504. doi: 10.1097/QAD.0b013e32834fa13d. [DOI] [PubMed] [Google Scholar]
- 4.Hapgood JP. Immunosuppressive biological mechanisms support reassessment of use of the injectable contraceptive medroxyprogesterone acetate. Endocrinology. 2013;154:985–988. doi: 10.1210/en.2013-1066. [DOI] [PubMed] [Google Scholar]
- 5.Hel Z, Stringer E, Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocrine reviews. 2010;31:79–97. doi: 10.1210/er.2009-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plummer FA, Simonsen JN, Cameron DW, Ndinya-Achola JO, Kreiss JK, Gakinya MN, et al. Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1991;163:233–239. doi: 10.1093/infdis/163.2.233. [DOI] [PubMed] [Google Scholar]
- 7.Ungchusak K, Rehle T, Thammapornpilap P, Spiegelman D, Brinkmann U, Siraprapasiri T. Determinants of HIV infection among female commercial sex workers in northeastern Thailand: results from a longitudinal study. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:500–507. doi: 10.1097/00042560-199608150-00010. [DOI] [PubMed] [Google Scholar]
- 8.Wand H, Ramjee G. The effects of injectable hormonal contraceptives on HIV seroconversion and on sexually transmitted infections. AIDS. 2012;26:375–380. doi: 10.1097/QAD.0b013e32834f990f. [DOI] [PubMed] [Google Scholar]
- 9.Morrison CS, Chen PL, Kwok C, Richardson BA, Chipato T, Mugerwa R, et al. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS. 2010;24:1778–1781. doi: 10.1097/QAD.0b013e32833a2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. The Lancet infectious diseases. 2012;12:19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeten JM, Benki S, Chohan V, Lavreys L, McClelland RS, Mandaliya K, et al. Hormonal contraceptive use, herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women. AIDS. 2007;21:1771–1777. doi: 10.1097/QAD.0b013e328270388a. [DOI] [PubMed] [Google Scholar]
- 12.Kumwenda NI, Kumwenda J, Kafulafula G, Makanani B, Taulo F, Nkhoma C, et al. HIV-1 incidence among women of reproductive age in Malawi. International journal of STD & AIDS. 2008;19:339–341. doi: 10.1258/ijsa.2007.007165. [DOI] [PubMed] [Google Scholar]
- 13.Heffron R, Rees H, Mugo N, Baeten JM. Use of hormonal contraceptives and risk of HIV-1 transmission - Authors' reply. The Lancet infectious diseases. 2012;12:510–511. doi: 10.1016/S1473-3099(12)70115-9. [DOI] [PubMed] [Google Scholar]
- 14.Myer L, Denny L, Wright TC, Kuhn L. Prospective study of hormonal contraception and women's risk of HIV infection in South Africa. Int J Epidemiol. 2007;36:166–174. doi: 10.1093/ije/dyl251. [DOI] [PubMed] [Google Scholar]
- 15.Kleinschmidt I, Rees H, Delany S, Smith D, Dinat N, Nkala B, et al. Injectable progestin contraceptive use and risk of HIV infection in a South African family planning cohort. Contraception. 2007;75:461–467. doi: 10.1016/j.contraception.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Abel K, Rourke T, Lu D, Bost K, McChesney MB, Miller CJ. Abrogation of attenuated lentivirus-induced protection in rhesus macaques by administration of depo-provera before intravaginal challenge with simian immunodeficiency virus mac239. J Infect Dis. 2004;190:1697–1705. doi: 10.1086/424600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genesca M, Li J, Fritts L, Chohan P, Bost K, Rourke T, et al. Depo-Provera abrogates attenuated lentivirus-induced protection in male rhesus macaques challenged intravenously with pathogenic SIVmac239. J Med Primatol. 2007;36:266–275. doi: 10.1111/j.1600-0684.2007.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trunova N, Tsai L, Tung S, Schneider E, Harouse J, Gettie A, et al. Progestin-based contraceptive suppresses cellular immune responses in SHIV-infected rhesus macaques. Virology. 2006;352:169–177. doi: 10.1016/j.virol.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 20.Huijbregts RP, Helton ES, Michel KG, Sabbaj S, Richter HE, Goepfert PA, et al. Hormonal contraception and HIV-1 infection: medroxyprogesterone acetate suppresses innate and adaptive immune mechanisms. Endocrinology. 2013;154:1282–1295. doi: 10.1210/en.2012-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol. 2003;77:4558–4565. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleynhans L, Du Plessis N, Black GF, Loxton AG, Kidd M, van Helden PD, et al. Medroxyprogesterone acetate alters Mycobacterium bovis BCG-induced cytokine production in peripheral blood mononuclear cells of contraceptive users. PLoS One. 2011;6:e24639. doi: 10.1371/journal.pone.0024639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bamberger CM, Else T, Bamberger AM, Beil FU, Schulte HM. Dissociative glucocorticoid activity of medroxyprogesterone acetate in normal human lymphocytes. J Clin Endocrinol Metab. 1999;84:4055–4061. doi: 10.1210/jcem.84.11.6091. [DOI] [PubMed] [Google Scholar]
- 24.Koubovec D, Vanden Berghe W, Vermeulen L, Haegeman G, Hapgood JP. Medroxyprogesterone acetate downregulates cytokine gene expression in mouse fibroblast cells. Molecular and cellular endocrinology. 2004;221:75–85. doi: 10.1016/j.mce.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Hughes GC, Thomas S, Li C, Kaja MK, Clark EA. Cutting edge: progesterone regulates IFN-alpha production by plasmacytoid dendritic cells. J Immunol. 2008;180:2029–2033. doi: 10.4049/jimmunol.180.4.2029. [DOI] [PubMed] [Google Scholar]
- 26.Cherpes TL, Busch JL, Sheridan BS, Harvey SA, Hendricks RL. Medroxyprogesterone acetate inhibits CD8+ T cell viral-specific effector function and induces herpes simplex virus type 1 reactivation. J Immunol. 2008;181:969–975. doi: 10.4049/jimmunol.181.2.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillgrass AE, Ashkar AA, Rosenthal KL, Kaushic C. Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J Virol. 2003;77:9845–9851. doi: 10.1128/JVI.77.18.9845-9851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleynhans L, Du Plessis N, Allie N, Jacobs M, Kidd M, van Helden PD, et al. The contraceptive depot medroxyprogesterone acetate impairs mycobacterial control and inhibits cytokine secretion in mice infected with Mycobacterium tuberculosis. Infect Immun. 2013;81:1234–1244. doi: 10.1128/IAI.01189-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vicetti Miguel RD, Hendricks RL, Aguirre AJ, Melan MA, Harvey SA, Terry-Allison T, et al. Dendritic cell activation and memory cell development are impaired among mice administered medroxyprogesterone acetate prior to mucosal herpes simplex virus type 1 infection. J Immunol. 2012;189:3449–3461. doi: 10.4049/jimmunol.1103054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hormonal contraception and HIV. World Health Organization; 2012. [Accessed: August 11, 2012]. Technical statement. Available from: http://www.who.int/reproductivehealth/publications/family_planning/rhr_12_8/en/index.html. [PubMed] [Google Scholar]
- 31.Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. The Lancet infectious diseases. 2013;13:797–808. doi: 10.1016/S1473-3099(13)70155-5. [DOI] [PubMed] [Google Scholar]
- 32.Morrison CS, Bright P, Wong EL, Kwok C, Yacobson I, Gaydos CA, et al. Hormonal contraceptive use, cervical ectopy, and the acquisition of cervical infections. Sex Transm Dis. 2004;31:561–567. doi: 10.1097/01.olq.0000137904.56037.70. [DOI] [PubMed] [Google Scholar]
- 33.Baeten JM, Nyange PM, Richardson BA, Lavreys L, Chohan B, Martin HL, Jr, et al. Hormonal contraception and risk of sexually transmitted disease acquisition: results from a prospective study. Am J Obstet Gynecol. 2001;185:380–385. doi: 10.1067/mob.2001.115862. [DOI] [PubMed] [Google Scholar]
- 34.Lavreys L, Chohan V, Overbaugh J, Hassan W, McClelland RS, Kreiss J, et al. Hormonal contraception and risk of cervical infections among HIV-1-seropositive Kenyan women. AIDS. 2004;18:2179–2184. doi: 10.1097/00002030-200411050-00010. [DOI] [PubMed] [Google Scholar]
- 35.Hancock EB, Manhart LE, Nelson SJ, Kerani R, Wroblewski JK, Totten PA. Comprehensive assessment of sociodemographic and behavioral risk factors for Mycoplasma genitalium infection in women. Sexually transmitted diseases. 2010;37:777–783. doi: 10.1097/OLQ.0b013e3181e8087e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain AK. Hormonal contraception and HIV acquisition risk: implications for individual users and public policies. Contraception. 2012;86:645–652. doi: 10.1016/j.contraception.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez M, Reeves M, Caughey A. Evaluating the competing risks of HIV acquisition and maternal mortality in Africa: a decision analysis. BJOG : an international journal of obstetrics and gynaecology. 2012;119:1067–1073. doi: 10.1111/j.1471-0528.2012.03402.x. [DOI] [PubMed] [Google Scholar]
- 38.Butler AR, Smith JA, Polis CB, Gregson S, Stanton D, Hallett TB. Modelling the global competing risks of a potential interaction between injectable hormonal contraception and HIV risk. AIDS. 2013;27:105–113. doi: 10.1097/QAD.0b013e32835a5a52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison CS, Nanda K. Hormonal contraception and HIV: an unanswered question. The Lancet infectious diseases. 2012;12:2–3. doi: 10.1016/S1473-3099(11)70254-7. [DOI] [PubMed] [Google Scholar]
- 40.Heffron R, Chao A, Mwinga A, Sinyangwe S, Sinyama A, Ginwalla R, et al. High prevalent and incident HIV-1 and herpes simplex virus 2 infection among male migrant and non-migrant sugar farm workers in Zambia. Sexually transmitted infections. 2011;87:283–288. doi: 10.1136/sti.2010.045617. [DOI] [PubMed] [Google Scholar]
- 41.Morroni C, Myer L, Moss M, Hoffman M. Preferences between injectable contraceptive methods among South African women. Contraception. 2006;73:598–601. doi: 10.1016/j.contraception.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Winner B, Peipert JF, Zhao Q, Buckel C, Madden T, Allsworth JE, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366:1998–2007. doi: 10.1056/NEJMoa1110855. [DOI] [PubMed] [Google Scholar]
- 43.Luukkainen T. The levonorgestrel intrauterine system: therapeutic aspects. Steroids. 2000;65:699–702. doi: 10.1016/s0039-128x(00)00177-x. [DOI] [PubMed] [Google Scholar]
- 44.Irvine GA, Campbell-Brown MB, Lumsden MA, Heikkila A, Walker JJ, Cameron IT. Randomised comparative trial of the levonorgestrel intrauterine system and norethisterone for treatment of idiopathic menorrhagia. British journal of obstetrics and gynaecology. 1998;105:592–598. doi: 10.1111/j.1471-0528.1998.tb10172.x. [DOI] [PubMed] [Google Scholar]
- 45.Stanczyk FZ, Hapgood JP, Winer S, Mishell DR., Jr Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocrine reviews. 2013;34:171–208. doi: 10.1210/er.2012-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Africander D, Verhoog N, Hapgood JP. Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids. 2011;76:636–652. doi: 10.1016/j.steroids.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Kirton KT, Cornette JC. Return of ovulatory cyclicity following an intramuscular injection of medroxyprogesterone acetate (Provera) Contraception. 1974;10:39–45. doi: 10.1016/0010-7824(74)90130-9. [DOI] [PubMed] [Google Scholar]
- 48.Nanda K, Amaral E, Hays M, Viscola MA, Mehta N, Bahamondes L. Pharmacokinetic interactions between depot medroxyprogesterone acetate and combination antiretroviral therapy. Fertility and sterility. 2008;90:965–971. doi: 10.1016/j.fertnstert.2007.07.1348. [DOI] [PubMed] [Google Scholar]
- 49.Ortiz A, Hirol M, Stanczyk FZ, Goebelsmann U, Mishell DR. Serum medroxyprogesterone acetate (MPA) concentrations and ovarian function following intramuscular injection of depo-MPA. The Journal of clinical endocrinology and metabolism. 1977;44:32–38. doi: 10.1210/jcem-44-1-32. [DOI] [PubMed] [Google Scholar]
- 50.Shrimanker K, Saxena BN, Fotherby K. A radioimmunoassay for serum medroxyprogesterone acetate. Journal of steroid biochemistry. 1978;9:359–363. doi: 10.1016/0022-4731(78)90631-3. [DOI] [PubMed] [Google Scholar]
- 51.Koubovec D, Ronacher K, Stubsrud E, Louw A, Hapgood JP. Synthetic progestins used in HRT have different glucocorticoid agonist properties. Mol Cell Endocrinol. 2005;242:23–32. doi: 10.1016/j.mce.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Kontula K, Paavonen T, Luukkainen T, Andersson LC. Binding of progestins to the glucocorticoid receptor. Correlation to their glucocorticoid-like effects on in vitro functions of human mononuclear leukocytes. Biochemical pharmacology. 1983;32:1511–1518. doi: 10.1016/0006-2952(83)90474-4. [DOI] [PubMed] [Google Scholar]
- 53.McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocrine reviews. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- 54.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocrine reviews. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 55.Smoak KA, Cidlowski JA. Mechanisms of glucocorticoid receptor signaling during inflammation. Mechanisms of ageing and development. 2004;125:697–706. doi: 10.1016/j.mad.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 56.Rogatsky I, Luecke HF, Leitman DC, Yamamoto KR. Alternate surfaces of transcriptional coregulator GRIP1 function in different glucocorticoid receptor activation and repression contexts. Proc Natl Acad Sci U S A. 2002;99:16701–16706. doi: 10.1073/pnas.262671599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hapgood JP, Tomasicchio M. Modulation of HIV-1 virulence via the host glucocorticoid receptor: towards further understanding the molecular mechanisms of HIV-1 pathogenesis. Archives of virology. 2010;155:1009–1019. doi: 10.1007/s00705-010-0678-0. [DOI] [PubMed] [Google Scholar]
- 58.Liberman AC, Refojo D, Druker J, Toscano M, Rein T, Holsboer F, et al. The activated glucocorticoid receptor inhibits the transcription factor T-bet by direct protein-protein interaction. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2007;21:1177–1188. doi: 10.1096/fj.06-7452com. [DOI] [PubMed] [Google Scholar]
- 59.Maneechotesuwan K, Yao X, Ito K, Jazrawi E, Usmani OS, Adcock IM, et al. Suppression of GATA-3 nuclear import and phosphorylation: a novel mechanism of corticosteroid action in allergic disease. PLoS medicine. 2009;6:e1000076. doi: 10.1371/journal.pmed.1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ronacher K, Hadley K, Avenant C, Stubsrud E, Simons SS, Jr, Louw A, et al. Ligand-selective transactivation and transrepression via the glucocorticoid receptor: role of cofactor interaction. Molecular and cellular endocrinology. 2009;299:219–231. doi: 10.1016/j.mce.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 61.Kino T, De Martino MU, Charmandari E, Mirani M, Chrousos GP. Tissue glucocorticoid resistance/hypersensitivity syndromes. The Journal of steroid biochemistry and molecular biology. 2003;85:457–467. doi: 10.1016/s0960-0760(03)00218-8. [DOI] [PubMed] [Google Scholar]
- 62.Mirani M, Elenkov I, Volpi S, Hiroi N, Chrousos GP, Kino T. HIV-1 protein Vpr suppresses IL-12 production from human monocytes by enhancing glucocorticoid action: potential implications of Vpr coactivator activity for the innate and cellular immunity deficits observed in HIV-1 infection. J Immunol. 2002;169:6361–6368. doi: 10.4049/jimmunol.169.11.6361. [DOI] [PubMed] [Google Scholar]
- 63.Tomasicchio M, Avenant C, Du Toit A, Ray RM, Hapgood JP. The Progestin-Only Contraceptive Medroxyprogesterone Acetate, but Not Norethisterone Acetate, Enhances HIV-1 Vpr-Mediated Apoptosis in Human CD4(+) T Cells through the Glucocorticoid Receptor. PLoS One. 2013;8:e62895. doi: 10.1371/journal.pone.0062895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nature reviews Immunology. 2010;10:365–376. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- 65.Draper BH, Morroni C, Hoffman M, Smit J, Beksinska M, Hapgood J, et al. Depot medroxyprogesterone versus norethisterone oenanthate for long-acting progestogenic contraception. The Cochrane database of systematic reviews. 2006:CD005214. doi: 10.1002/14651858.CD005214.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, et al. Classification and pharmacology of progestins. Maturitas. 2003;46(Suppl 1):S7–S16. doi: 10.1016/j.maturitas.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 67.Fuhrmann U, Slater EP, Fritzemeier KH. Characterization of the novel progestin gestodene by receptor binding studies and transactivation assays. Contraception. 1995;51:45–52. doi: 10.1016/0010-7824(94)00003-f. [DOI] [PubMed] [Google Scholar]
- 68.Herold MJ, McPherson KG, Reichardt HM. Glucocorticoids in T cell apoptosis and function. Cellular and molecular life sciences : CMLS. 2006;63:60–72. doi: 10.1007/s00018-005-5390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou J, Cidlowski JA. The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids. 2005;70:407–417. doi: 10.1016/j.steroids.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 70.Gray A. Depot-medroxyprogesterone versus norethisterone enantate for long-acting progestogenic contraception. [Accessed: 01/28/14]; doi: 10.1002/14651858.CD005214.pub2. Available from: http://apps.who.int/rhl/fertility/contraception/agcom/en/ [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.