Abstract
Impulse control disorders (ICDs) may arise in Parkinson’s disease (PD) in relation to the use of dopamine agonists (DA). A dysfunction of reward circuits is considered the main underlying mechanism. Neuroimaging has been largely used in this setting to understand the structure of the reward system and its abnormalities brought by exogenous stimulation in PD. Dopaminergic changes, such as increased dopamine release, reduced dopamine transporter activity and other changes, have been shown to be a consistent feature of ICDs in PD. Beyond the striatum, alterations of prefrontal cortical function may also impact an individuals’ propensity for impulsivity. Neuroimaging is advancing our knowledge of the mechanisms involved in the development of these behavioral addictions. An increased understanding of these disorders may lead to the discovery of new therapeutic targets, or the identification of risk factors for the development of these disorders.
Keywords: Parkinson’s disease, Impulse control disorders, Molecular imaging
1. Introduction
Impulse control disorders (ICDs) in Parkinson’s disease (PD) offer an unique window into the mechanisms of motivation, reward and addiction in the general population. All these behavioral traits are closely linked to the dopaminergic system. In PD patients, the progressive failure of the dopaminergic network and related motor symptoms (i.e. bradykinesia, rigidity and resting tremor) are treated with replacement therapies such as levodopa or dopamine agonists (DA). Approximately 14% of PD patients treated with DA may develop ICDs [1], however this incidence can be much higher (over 20%) according other reports [2]. Thus, the study of these disorders provides an opportunity for evaluating the impact of dopaminergic degeneration and therapeutic replacement on critical aspects of human behavior.
ICDs are considered behavioral addictions because of their similarities to substance abuse. Both disorders show common features like the experience of withdrawal symptoms and/or the development of tolerance [3]. The most common presentations in PD are pathological gambling, compulsive sexual behavior, compulsive buying and binge eating. Studies assessing possible triggers have found relations with traits such as higher novelty-seeking and impulsivity, but also anxiety and depression [4]. The main predisposing factor, however, has been consistently shown to be the exposure to dopaminergic therapies. It has been observed that drug-naive PD patients show similar proportions of ICDs to healthy subjects [5].
As a result of these observations, an underlying hyper-dopaminergic state has been proposed as the cause of ICDs in Parkinson’s disease. It is well known that the disease preferentially affects the dorsal (motor) striatum compared to ventral structures [6]. Therefore, dopaminergic treatment of the relatively intact ventral striatum could result in an uncontrolled activation of the reward system. On this regard, it is interesting to note some similarities with the levodopa-induced dyskinesias (LIDs). In a comprehensive review, Voon et al. [7] assessed the overlapping and common biological mechanisms of motor and behavioral complications in PD. They proposed that LIDs and behavioral disorders (i.e. ICDs, punding, and dopamine dysregulation syndrome) may be part of a continuum mediated by similar pathophysiological mechanisms acting through different basal ganglia loops. In fact, levodopa treatment may result sometimes in a dopamine dysregulation syndrome, in which patients show an increased appetite for dopaminergic medications and subsequently increase levodopa intake far beyond their prescribed dosage. However, these patients generally do not develop the classic traits of ICDs [8] observed with DA, but concurrent levodopa therapy may increase the incidence of these behavioral complications [2]. While ICDs are generally associated with PD, it is also important to remember that these may occur in other clinical conditions, like restless leg syndrome [9] and hormone replacing therapies in pituitary adenomas [10]. In these instances, DA doses required to develop ICDs were uniformly lower than in PD. Thus, it is hypothesised that the continuous stimulation of DA in a relatively intact reward system may lead to profound consequences in the response to pleasurable stimuli [11].
Research in this field has to account for the complexity of a poorly understood neurobiological system. Molecular imaging is helping to delineate its structure as well as the dynamic interactions between different components involving neurotransmitters, transporters and receptors. ICDs are a dynamic phenomenon where the effects of the neurodegenerative process in PD along with compensatory changes associated with dopamine replacement therapy and genetic vulnerabilities all have to be considered. The unraveling of all these interactions has to be accounted for in this evolving landscape.
In this review, we will describe the neural systems that are being currently targeted and the neurobiological substrate responsible for these findings, trying to outline the future directions in molecular imaging in ICDs.
2. Molecular imaging in ICD
The structural networks underlying impulse control disorders are linked to the so called reward system. Its main components (nucleus accumbens, amygdala, hippocampus, orbitofrontal and anterior cingulate cortices) are all part of the mesocorticolimbic pathway. This system is mainly regulated by dopamine and, in healthy subjects, articulates the salience of external stimuli; a thorough review on its functioning is provided by Probst et al. [12]. In brief, standard pleasurable stimuli (e.g. food, etc.) provoke a tonic dopamine response in the outer shell of the nucleus accumbens, but repeated stimuli shift this response to its core, inducing habituation. In the brainstem, dopamine autoreceptors located in substantia nigra provide an important feedback in order to regulate synaptic dopamine concentrations. As for the top-down control, the orbitofrontal and anterior cingulate cortices are responsible for weighing the importance of the reward and linking it to an appropriate response. Finally, the prefrontal cortex inhibits reward-directed response exerting a balancing effect on the system. With all these elements working correctly, a person is able to successfully adapt their behavior in a dynamic environment. In individuals suffering from addiction, however, substantial changes can occur to these structures. Tonic dopaminergic signals from the midbrain assign increased salience to addictive stimuli and, after repeated exposure, the process is shifted to dorsal striatal and sensorimotor structures, turning the stimulus–pleasure association into a stimulus-action directed towards the addictive input [13]. Finally, top-down control from the cortex seems to be impaired as well, with less activation of the prefrontal cortex.
Molecular imaging in DA-treated PD patients has largely focused on dopamine and its receptors, the autoregulatory mechanisms and the inhibitory inputs from cortical structures. Table 1 summarizes the main observations from different studies and the proposed physiopathological mechanisms. [11C] raclopride is a D2/D3 antagonist radiotracer that selectively binds to these dopaminergic receptors and competes with endogenous dopamine. This radiotracer has provided a wealth of information regarding corticostriatal control of dopamine release and tested the hyper-dopaminergic response in subjects exposed to appetitive stimuli.
Table 1.
Proposed mechanism of dopaminergic pathway malfunction and their effects.
| Systems affected by dopaminergic disfunction. | Proposed behavioral results. | References |
|---|---|---|
| Increased ventral striatal response to rewards/reward cues. | Sensitization of ventral striatal circuits to rewards resulting in increased reward seeking. | Steeves et al. [15], O’Sullivan et al. [16], Wu et al. [17] |
| Low ventral striatal D2/D3 receptor availability | Increased reward seeking/propensity for impulsivity due to blunted transmission of reward signals | Steeves et al. [15], Boileau et al. [19,20], Payer et al. [21] |
| Low DAT expression in ventral striatum | Functional downregulation (compensating for lower dopamine availability) or susceptibility trait. | Cilia et al. [24], Vriend et al. [25] |
| Low midbrain dopamine autoreceptor function | Lack of homeostatic control over striatal DA release, causing increased sensitivity to rewards/propensity for impulsivity | Ray et al. [27], Cole et al. [28] |
| Altered cortical and striatal responses to feedback when on dopamine agonists | Increased learning from reward and decreased learning from punishment, fostering the repetition of maladaptive behaviors | Van Eimeren et al. [29] |
Studies in non-PD subjects with ICDs have investigated whether higher dopamine levels were a feature of ICDs. Volkow et al. [14] scanned a group of such patients using [11C] raclopride and found decreased binding compared to controls. In PD, Steeves et al. [15] used a similar approach while exposing PD-ICD patients to a gambling paradigm in which gains and losses were represented. All of the subjects had been previously treated with DA and had developed pathological gambling (PG). In these patients, there was an increased dopamine release in the ventral striatum during the act of gambling compared to non-ICD PD patients. O’Sullivan et al. [16] found a similar decrease in [11C] raclopride binding in response to stimulating cues in PD-ICD patients compared to controls. Finally, a recent study by Wu et al. [17] tried to assess differences between single and multiple ICD patients regarding striatal dopamine release, using [11C] raclopride. No differences were found, but the same pattern of increased striatal dopamine release was observed in single and multiple ICD patients compared to non-ICD PD.
There has been a growing interest in imaging the dopamine D3 receptor because of its relation with mood, motivation and reward. Hypoactivation of this receptor has been linked to anhedonia and depression [18]. Recently, a few PET studies have been using [11C]-(+)-PHNO, a radiotracer that acts as a dopamine agonist and has increased affinity for D3 over D2 receptors. Boileau et al. [19] conducted an investigation contrasting [11C] raclopride distribution with that of [11C]-(+)-PHNO in drug naive PD patients, compared to healthy controls. The study confirmed that the D3 receptor is preferentially ventral-striatum bound, consistent with its proposed limbic role. Using the same radiotracer, a study of PG in the general population provided some interesting observations [20]. In particular, D3 binding in the substantia nigra of PG subjects substantially correlated both with gambling severity and impulsivity trait, but no significant findings were observed in the striatum. Subsequently, Payer et al. [21] studied PD-ICD patients using [11C]-(+)-PHNO and showed that D3 activity was 20% lower in the ventral striatum in these patients compared to PD controls. In sum, these reports suggest that higher levels of endogenous dopamine release and possibly lower D2/D3 receptor density may be a classic feature of behavioral forms of addiction.
The dopamine transporter modulates the availability of dopamine in the synaptic cleft by controlling its clearance. In PD, the dopamine transporter (DAT) is downregulated in order to increase available dopamine in the synapse [22]. In the general population, genetic studies have pointed to a relationship between polymorphisms in the DAT gene and binge eating [23]. In ICD-PD patients, Cilia et al. [24] used a DAT-tracer (123I-FP-CIT) to scan dopamine transporter density. Tracer binding was lower for ICD-PD patients in ventral striatum, indicating a probable functional DAT downregulation. Another possible explanation is that the lower DAT expression may not be a dynamical adjustment, but rather a pre-existing trait that may determine ICD vulnerability. In support of this hypothesis, Vriend et al. [25] tried to weigh DAT expression as a pre-existing condition in ICDs. The authors retrospectively studied a group of drug-naive PD patients and the emergence of ICDs with dopamine replacement therapies. 123I-FP-CIT scans were obtained at baseline, and the DAT binding was compared with the incidence of ICDs in a 31-month follow-up. Interestingly, the group of patients who developed ICDs consistently showed lower DAT density in the ventral striatum.
As already mentioned above, dopamine exerts its influence on a wide range of brain structures well beyond the striatum. A number of relatively new high-affinity radiotracers (e.g. [18F] fallypride; [11C] FLB-457) is available to quantify extrastriatal D2/D3 dopamine receptors. Using [18F] fallypride on healthy volunteers with amphetamine challenge, Buckholtz et al. [26] found lower D2/D3 availability in the midbrain which correlated with higher impulsivity in the Barrat Impulsiveness Scale. Lower receptor density was also associated with a higher striatal release of dopamine. Subsequently, Ray et al. [27] used [11C] FLB-457 in a group of PD patients suffering from PG while performing a gambling task. Changes in [11C] FLB-457 binding potential during gambling was reduced in PD patients with PG in the midbrain, where D2/D3 receptors are dominated by autoreceptors. The degree of change in [11C] FLB-457 binding in this region correlated with impulsivity. In the cortex, [11C] FLB-457 binding was significantly greater in the anterior cingulate cortex (ACC) in PD patients with PG, and binding in this region was also correlated with impulsivity. These findings provide evidence that PD patients with PG have dysfunctional activation of DA autoreceptors in the midbrain and low DA tone in the ACC. Thus, abnormal striatal and cortical DA homeostasis may incur vulnerability for the development of PG in PD, linked with the impulsive personality trait.
A similar link between midbrain and cortical regulatory systems was proposed by Cole at al. [28]. Their study combined [11C]-(+)-PHNO scans with functional Magnetic Resonance Imaging (fMRI) in healthy subjects. Higher midbrain D3 receptors were associated with greater connectivity with basal ganglia, but lower connectivity with salience and executive networks in the anterior insula and ACC. Though not related to patients with ICDs or impulsivity traits, this study showed the close relationship between the two modulating systems.
3. Cortical perfusion studies in ICD
Regional cerebral blood flow (rCBF) studies complement dopaminergic investigations and allow differentiating aspects of impulsive behavior at the network and system level. In an fMRI study, Van Eimeren et al. [29] imaged drug-naive PD patients while performing a gambling paradigm off-medication, after levodopa treatment and following DA treatment. Their hypothesis was that DA agonists would prevent phasic pauses in dopamine neuron firing, therefore impairing reward processing. They found that while on dopamine agonists, the rCBF activity of the lateral orbitofrontal cortex (OFC) did not decrease when reward predictions failed in the gambling task. This diminishes negative feedback and impairs punishment-related learning. Interestingly, these changes did not occur when patients received levodopa. In a subsequent study [30], ICD-PD patients instead were studied using H2(15)O PET scans to estimate blood flow changes in cortical regions. The subjects were imaged during a card selection game with probabilistic feedback. The results showed that dopamine agonists exerted differential effects in gamblers and non-gamblers. In particular, PD with PG showed DA-related reduction of activity in the left OFC, amygdala and rostral cingulate compared to non-gamblers. The diminished activation in gamblers also correlated with gambling severity. Thus, in vulnerable patients with PD, DAs produced an abnormal neuronal pattern that resembled those found in non-parkinsonian PG and drug addiction.
Antonelli et al. [31] tried as well to differentiate the motor component (impulsive actions) from the cognitive component (impulsive choices) of gambling. They imaged PD patients without ICDs while exposing them to motor (Go/no Go) and cognitive (delay discounting) tasks. The results showed that DA increase activity in the medial prefrontal and cingulate cortices while promoting impulsive choices, but motor-related impulsivity was differentially mapped to other regions (the lateral prefrontal cortex).
4. Conclusions and future directions
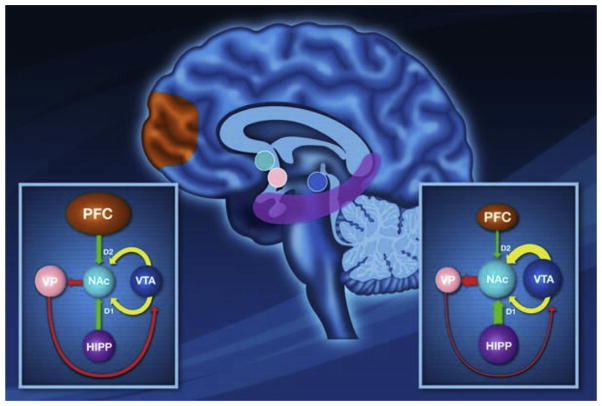
Molecular imaging has provided a great amount of information about ICDs in PD. Its findings have contributed to establish the molecular framework of the disorder, with features sometimes similar to substance abuse. Fig. 1 from Napier et al. [32] provides an overview of the neural interactions between ventral striatum and other key structures during the reward processing. This model suggests that when a task is rewarding, there is an increase in dopamine input, facilitating the hippocampal drive (D1 dependent process) to maintain focus on the current rewarded task while preventing the prefrontal cortex (D2 dependent process) from deviating from this task (left diagram). Thus, if a behavior fails to produce a reward, this would allow the prefrontal cortex to shift focus to a different response. However, according to this model, persistent activation of D2 receptors (as consequences of DA therapy in PD) may reduce the influence from the prefrontal cortex reducing flexibility and not allowing shifting behaviors towards goal-oriented task (right diagram). The continuous hippocampal drive will cause the individual to perseverate on the impulsive task. Thereby, the system is locked in this poorly controlled behavioral state.
Yet, there is still a considerable amount of unanswered questions regarding changes outside the dopaminergic system, and the interaction among different neural networks. Thus, future directions should probably include imaging of other critical neurotransmitters that may have a certain impact in ICDs. Serotonergic abnormalities have been examined in dyskinesias, and may also have a role in controlling certain behavioral complications in PD [33]. As well, another important line of research is the opioid system and its relation to reward behaviour. Schreckenberger et al. [34], using the opioidergic radioligand 18F-fluoroethyl-diprenorphine, found a correlation between scores of reward dependence and binding potential in the ventral striatum and nucleus accumbens.
Other concepts of modern neuroimaging like graph-theory analysis may also help to identify important behavioral hubs in brain networks. Thus, in conclusion, a broad spectrum of neural systems remain to be studied and an integrated approach that includes also other neuroimaging techniques is probably the most promising venture in the upcoming years.
Fig. 1.
Image adapted from Napier et al. (2015) [32]. A proposed model of dopaminergic interaction from neural regions involved in reward processing in normal condition (left diagram) and during ICDs (right diagram). See text in the main manuscript.
Acknowledgments
This work was supported by Canadian Institutes of Health Research (MOP 136778). A.P.S is supported by the Canada Research Chair program.
Footnotes
Conflict of interests
We declare no conflict of interests with the material of this manuscript.
References
- 1.Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67:589–595. doi: 10.1001/archneurol.2010.65. http://dx.doi.org/10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 2.Hassan a, Bower JH, Kumar N, Matsumoto JY, Fealey RD, Josephs Ka, et al. Dopamine agonist-triggered pathological behaviors: surveillance in the PD clinic reveals high frequencies. Park Relat Disord. 2011;17:260–264. doi: 10.1016/j.parkreldis.2011.01.009. http://dx.doi.org/10.1016/j.parkreldis.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Leeman RF, Potenza MN. Similarities and differences between pathological gambling and substance use disorders: a focus on impulsivity and compulsivity. Psychopharmacol Berl. 2012;219:469–490. doi: 10.1007/s00213-011-2550-7. http://dx.doi.org/10.1007/s00213-011-2550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voon V, Sohr M, Lang AE, Potenza MN, Siderowf AD, Whetteckey J, et al. Impulse control disorders in Parkinson disease: a multicenter case-control study. Ann Neurol. 2011;69:986–996. doi: 10.1002/ana.22356. http://dx.doi.org/10.1002/ana.22356. [DOI] [PubMed] [Google Scholar]
- 5.Weintraub D. Dopamine and impulse control disorders in Parkinson’s disease. Ann Neurol. 2008;64:93–100. doi: 10.1002/ana.21454. http://dx.doi.org/10.1002/ana.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javoy-Agid F, Taquet H, Ploska a, Cherif-Zahar C, Ruberg M. Distribution of catecholamines in the ventral mesencephalon of human brain, with special reference to Parkinson’s disease. J Neurochem. 1981;36:2101–2105. doi: 10.1111/j.1471-4159.1981.tb10843.x. http://dx.doi.org/10.1111/j.1471-4159.1981.tb10843.x. [DOI] [PubMed] [Google Scholar]
- 7.Voon V, Fernagut PO, Wickens J, Baunez C, Rodriguez M, Pavon N, et al. Chronic dopaminergic stimulation in Parkinson’s disease: from dyskinesias to impulse control disorders. Lancet Neurol. 2009;8:1140–1149. doi: 10.1016/S1474-4422(09)70287-X. http://dx.doi.org/10.1016/S1474-4422(09)70287-X. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher DA, O’Sullivan SS, Evans AH, Lees AJ, Schrag A. Pathological gambling in Parkinson’s disease: risk factors and differences from dopamine dysregulation. An analysis of published case series. Mov Disord. 2007;22:1757–1763. doi: 10.1002/mds.21611. http://dx.doi.org/10.1002/mds.21611. [DOI] [PubMed] [Google Scholar]
- 9.Tippmann-Peikert M, Park JG, Boeve BF, Shepard JW, Silber MH. Pathologic gambling in patients with restless legs syndrome treated with dopaminergic agonists. Neurology. 2007;68:301–303. doi: 10.1212/01.wnl.0000252368.25106.b6. http://dx.doi.org/10.1212/01.wnl.0000252368.25106.b6. [DOI] [PubMed] [Google Scholar]
- 10.Martinkova J, Trejbalova L, Sasikova M, Benetin J, Valkovic P. Impulse control disorders associated with dopaminergic medication in patients with pituitary adenomas. Clin Neuropharmacol. 2011;34:179–181. doi: 10.1097/WNF.0b013e3182281b2f. http://dx.doi.org/10.1097/WNF.0b013e3182281b2f. [DOI] [PubMed] [Google Scholar]
- 11.Cilia R, Van Eimeren T. Impulse control disorders in Parkinson’s disease: seeking a roadmap toward a better understanding. Brain Struct Funct. 2011;216:289–299. doi: 10.1007/s00429-011-0314-0. http://dx.doi.org/10.1007/s00429-011-0314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Probst CC, van Eimeren T. The functional anatomy of impulse control disorders. Curr Neurol Neurosci Rep. 2013;13:386. doi: 10.1007/s11910-013-0386-8. http://dx.doi.org/10.1007/s11910-013-0386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray NJ, Strafella AP. Imaging impulse control disorders in Parkinson’s disease and their relationship to addiction. 2013;120:659–664. doi: 10.1007/s00702-012-0933-5. http://dx.doi.org/10.1007/s00702-012-0933-5. [DOI] [PubMed] [Google Scholar]
- 14.Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. http://dx.doi.org/10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steeves TDL, Miyasaki J, Zurowski M, Lang AE, Pellecchia G, Van Eimeren T, et al. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain. 2009;132:1376–1385. doi: 10.1093/brain/awp054. http://dx.doi.org/10.1093/brain/awp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Sullivan SS, Wu K, Politis M, Lawrence AD, Evans AH, Bose SK, et al. Cue-induced striatal dopamine release in Parkinson’s disease-associated impulsive-compulsive behaviours. Brain. 2011;134:969–978. doi: 10.1093/brain/awr003. http://dx.doi.org/10.1093/brain/awr003. [DOI] [PubMed] [Google Scholar]
- 17.Wu K, Politis M, O’Sullivan SS, Lawrence AD, Warsi S, Bose S, et al. Single versus multiple impulse control disorders in Parkinson’s disease: an 11C-raclopride positron emission tomography study of reward cue-evoked striatal dopamine release. J Neurol. 2015:1504–1514. doi: 10.1007/s00415-015-7722-7. http://dx.doi.org/10.1007/s00415-015-7722-7. [DOI] [PubMed]
- 18.Lemke MR, Brecht HM, Koester J, Kraus PH, Reichmann H. Anhedonia, depression, and motor functioning in Parkinson’s disease during treatment with pramipexole. 2005;17 doi: 10.1176/jnp.17.2.214. http://dx.doi.org/10.1176/appi.neuropsych.17.2.214. [DOI] [PubMed] [Google Scholar]
- 19.Boileau I, Guttman M, Rusjan P, Adams JR, Houle S, Tong J, et al. Decreased binding of the D3 dopamine receptor-preferring ligand [11C]-(+)-PHNO in drug-nave Parkinson’s disease. Brain. 2009;132:1366–1375. doi: 10.1093/brain/awn337. http://dx.doi.org/10.1093/brain/awn337. [DOI] [PubMed] [Google Scholar]
- 20.Boileau I, Payer D, Chugani B, Lobo D, Behzadi A, Rusjan PM, et al. The D2/3 dopamine receptor in pathological gambling: a positron emission tomography study with [11C]-(+)-propyl-hexahydro-naphtho-oxazin and [11C]raclopride. Addiction. 2013;108:953–963. doi: 10.1111/add.12066. http://dx.doi.org/10.1111/add.12066. [DOI] [PubMed] [Google Scholar]
- 21.Payer DE, Guttman M, Kish SJ, Tong J, Strafella A, Zack M, et al. [11C]-(+)-PHNO PET imaging of dopamine D2/3 receptors in Parkinson’s disease with impulse control disorders. Mov Disord. 2015;30:160–166. doi: 10.1002/mds.26135. http://dx.doi.org/10.1002/mds.26135. [DOI] [PubMed] [Google Scholar]
- 22.Stoessl AJ, Lehericy S, Strafella AP. Imaging insights into basal ganglia function, Parkinson’s disease, and dystonia. Lancet. 2014 doi: 10.1016/S0140-6736(14)60041-6. http://dx.doi.org/10.1016/S0140-6736(14)60041-6. [DOI] [PMC free article] [PubMed]
- 23.Shinohara M, Mizushima H, Hirano M, Shioe K, Nakazawa M, Hiejima Y, et al. Eating disorders with binge-eating behaviour are associated with the s allele of the 3′-UTR VNTR polymorphism of the dopamine transporter gene. J Psychiatry Neurosci. 2004;29:134–137. [PMC free article] [PubMed] [Google Scholar]
- 24.Cilia R, Ko JH, Cho SS, van Eimeren T, Marotta G, Pellecchia G, et al. Reduced dopamine transporter density in the ventral striatum of patients with Parkinson’s disease and pathological gambling. Neurobiol Dis. 2010;39:98–104. doi: 10.1016/j.nbd.2010.03.013. http://dx.doi.org/10.1016/j.nbd.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Vriend C, Nordbeck AH, Booij J, van der Werf YD, Pattij T, Voorn P, et al. Reduced dopamine transporter binding predates impulse control disorders in Parkinson’s disease. Mov Disord. 2014:1–8. doi: 10.1002/mds.25886. http://dx.doi.org/10.1002/mds.25886. [DOI] [PubMed]
- 26.Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. http://dx.doi.org/10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ray NJ, Miyasaki JM, Zurowski M, Ko JH, Cho SS, Pellecchia G, et al. Extrastriatal dopaminergic abnormalities of DA homeostasis in Parkinson’s patients with medication-induced pathological gambling: a [11C] FLB-457 and PET study. Neurobiol Dis. 2012;48:519–525. doi: 10.1016/j.nbd.2012.06.021. http://dx.doi.org/10.1016/j.nbd.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole DM, Beckmann CF, Searle GE, Plisson C, Tziortzi AC, Nichols TE, et al. Orbitofrontal connectivity with resting-state networks is associated with midbrain dopamine D3 receptor availability. Cereb Cortex. 2012;22:2784–2793. doi: 10.1093/cercor/bhr354. http://dx.doi.org/10.1093/cercor/bhr354. [DOI] [PubMed] [Google Scholar]
- 29.Van Eimeren T, Ballanger B, Pellecchia G, Miyasaki JM, Lang AE, Strafella AP. Dopamine agonists diminish value sensitivity of the orbito-frontal cortex: a trigger for pathological gambling in Parkinson’s disease? Neuropsychopharmacology. 2009;34:2758–2766. doi: 10.1038/sj.npp.npp2009124. http://dx.doi.org/10.1038/npp.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Eimeren T, Pellecchia G, Cilia R, Ballanger B, Steeves TDL, Houle S, et al. Drug-induced deactivation of inhibitory networks predicts pathological gambling in PD. Neurology. 2010;75:1711–1716. doi: 10.1212/WNL.0b013e3181fc27fa. http://dx.doi.org/10.1212/WNL.0b013e3181fc27fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antonelli F, Ko JH, Miyasaki J, Lang AE, Houle S, Valzania F, et al. Dopamine-agonists and impulsivity in Parkinson’s disease: impulsive choices vs. impulsive actions. Hum Brain Mapp. 2014;35:2499–2506. doi: 10.1002/hbm.22344. http://dx.doi.org/10.1002/hbm.22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Napier TC, Corvol JC, Grace AA, Roitman JD, Rowe J, Voon V, et al. Linking neuroscience with modern concepts of impulse control disorders in Parkinson’s disease. Mov Disord. 2015;30:141–149. doi: 10.1002/mds.26068. http://dx.doi.org/10.1002/mds.26068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beaudoin-Gobert M, Epinat J, Metereau E, Duperrier S, Neumane S, Ballanger B, et al. Behavioural impact of a double dopaminergic and serotonergic lesion in the non-human primate. Brain. 2015:1–16. doi: 10.1093/brain/awv183. http://dx.doi.org/10.1093/brain/awv183. [DOI] [PubMed]
- 34.Schreckenberger M, Klega A, Gründer G, Buchholz H-G, Scheurich A, Schirrmacher R, et al. Opioid receptor PET reveals the psychobiologic correlates of reward processing. J Nucl Med. 2008;49:1257–1261. doi: 10.2967/jnumed.108.050849. http://dx.doi.org/10.2967/jnumed.108.050849. [DOI] [PubMed] [Google Scholar]