Abstract
Objective
Patients with Parkinson disease (PD) and mild cognitive impairment (MCI) are vulnerable to dementia and frequently experience memory deficits. This could be the result of dopamine dysfunction in corticostriatal networks (salience, central executive networks, and striatum) and/or the medial temporal lobe. Our aim was to investigate whether dopamine dysfunction in these regions contributes to memory impairment in PD.
Methods
We used positron emission tomography imaging to compare D2 receptor availability in the cortex and striatal (limbic and associative) dopamine neuron integrity in 4 groups: memory-impaired PD (amnestic MCI; n=9), PD with nonamnestic MCI (n=10), PD without MCI (n=11), and healthy controls (n=14). Subjects were administered a full neuropsychological test battery for cognitive performance.
Results
Memory-impaired patients demonstrated more significant reductions in D2 receptor binding in the salience network (insular cortex and anterior cingulate cortex [ACC] and the right parahippocampal gyrus [PHG]) compared to healthy controls and patients with no MCI. They also presented reductions in the right insula and right ACC compared to nonamnestic MCI patients. D2 levels were correlated with memory performance in the right PHG and left insula of amnestic patients and with executive performance in the bilateral insula and left ACC of all MCI patients. Associative striatal dopamine denervation was significant in all PD patients.
Interpretation
Dopaminergic differences in the salience network and the medial temporal lobe contribute to memory impairment in PD. Furthermore, these findings indicate the vulnerability of the salience network in PD and its potential role in memory and executive dysfunction.
Throughout daily life, new memories are formed and past memories are constantly drawn upon to guide behavior. Parkinson disease (PD) patients often experience memory deficits characterized by the inability to recall information, and memory impairment is a significant predictor of future dementia.1,2 Dopamine (DA) transmission is known to be crucial to normal memory function. DA signaling in the striatum facilitates learning, 3 and mesocorticolimbic DA modulates memory consolidation and retrieval in both the medial temporal lobe and cortex.4,5 Recently, we demonstrated that striatal DA depletion in a region involved in cognitive processing (associative striatum) as well as D2 receptor reductions in the bilateral insula contribute to executive dysfunction in PD.6 However, the contribution of dopaminergic dysfunction to memory impairment remains unclear. The insula along with the anterior cingulate cortex (ACC) is part of a salience network, known to causally influence activation and enable switching between the central executive network7 and the default mode network, 8,9 which cooperate to facilitate memory.10,11 It has been suggested that memory deficits in PD may be the result of dysfunctional executive networks that participate in memory retrieval and organization.12–15 Thus, due to their roles in executive processing, dopaminergic dysfunction in the salience network, the central executive network, or the striatum, which is highly interconnected with these cortical regions,16,17 could have a profound impact on memory. Alternatively, there is evidence that points to memory deficits in PD as the result of DA dysfunction in the medial temporal lobe,18,19 suggesting that memory impairment may be the result of abnormal encoding or storage of memory. The aim of this study was to assess whether dopaminergic differences in (1) the central executive network, salience network, or striatum or (2) the medial temporal lobe contribute to memory deficits in PD patients with mild cognitive impairment (MCI). We used positron emission tomography (PET) imaging to examine cortical D2 receptor availability in the dorsolateral prefrontal cortex (DLPFC) and posterior parietal cortex (PPC; central executive network); the insula and ACC (salience network); and the medial temporal lobe, as well as DA neuron integrity in the striatum in a sample of PD patients with significant memory impairment, as assessed by a full neuropsychological test battery. Furthermore, we aimed to determine whether dopaminergic changes could explain variance in memory performance, and whether changes related to memory and executive dysfunction coincide in the brain.
Subjects and Methods
Participants and Experimental Design
There were 44 subjects in total; 30 PD patients meeting the UK Parkinson Disease Society Brain Bank Criteria and 14 aged-matched healthy controls participated in the study (Table 1). Participants had no evidence of other neurological or psychiatric conditions. Patients meeting the criteria for dementia were excluded.20 All participants were matched for age and years of education and patients were matched for disease severity as measured by the Unified Parkinson Disease Rating Scale III (UPDRS-III) and for L-dopa equivalent daily dose (LEDD). The LEDD is based on theoretical equivalence to L-dopa using the following formula: L-dopa dose+L-dopa dose × ⅓ if on entacapone+bromocriptine (mg) × 10+cabergoline or pramipexole (mg) × 67+ropinirole (mg) × 20+pergolide (mg) × 100+apomorphine (mg) × 8.21 All participants received a neuropsychological assessment on medication to assess cognitive status and healthy controls showed no evidence of cognitive impairment (Table 2).
TABLE 1.
Demographic and Clinical Variables
| Variable | HC, n =14 | PD No MCI, n =11 | PD naMCI, n =10 | PD aMCI, n =9 |
|---|---|---|---|---|
| Age, yr | 67.5 (5.35) | 68.8 (3.71) | 70.2 (8.27) | 68.3 (7.40) |
| Education, yr | 17.1 (2.74) | 15.7 (2.87) | 16.7 (1.12) | 16.6 (1.94) |
| M/F | 3/11 | 3/8 | 7/3 | 7/2 |
| MoCA | 27.6 (2.21) | 26.5 (1.81) | 24.0 (2.12)a | 21.9 (3.40)b |
| BDI | 4.0 (3.96) | 5.91 (1.87) | 4.89 (3.89) | 5.78 (6.0) |
| UPDRS-III | — | 23.3 (12.03) | 20.8 (12.86) | 36.1 (16.0) |
| Disease duration, yr | — | 6.32 (4.20) | 6.11 (3.10) | 6.11 (2.85) |
| LEDD total, mg/day | — | 725 (570.85) | 611.17 (437.85) | 577.98 (245.0) |
Values are listed as mean (standard deviation).
The nonamnestic PD-MCI group had significantly lower MoCA scores compared to patients with no MCI (p = 0.004) and healthy controls (p = 0.0003).
The amnestic PD-MCI group had significantly lower MoCA scores compared to PD patients with no MCI (p = 0.001) and healthy controls (p < 0.0001). There were no significant differences in age, education, UPDRS-III, LEDD, or BDI between groups.
aMCI =amnestic MCI; BDI =Beck Depression Inventory; F =female; HC =healthy controls; LEDD =L-dopa equivalent daily dose (calculated according to Evans et al21); M =male; MCI =mild cognitive impairment; MoCA =Montreal Cognitive Assessment; naMCI =nonamnestic MCI; PD =Parkinson disease; UPDRS-III =Unified Parkinson Disease Rating Scale III.
TABLE 2.
Neuropsychological Data
| Measure | HC, n =14 | PD No MCI, n =11 | PD naMCI, n =10 | PD aMCI, n =9 |
|---|---|---|---|---|
| Digit Span forward | 0.53 (1.04) | 0.56 (0.50) | 0.04 (0.88) | −0.26 (0.54) |
| CVLT delayed free recall | 0.25 (0.92) | 0.54 (0.72) | −0.45 (1.01) | −1.17 (0.94)a,b |
| Letter-Number Sequencing | 0.15 (0.68) | 0.30 (0.64) | 0.27 (0.93) | −0.41 (0.72) |
| Visual Verbal total shifts | −0.4 (0.49) | −1.38 (2.03) | −3.34 (1.77)a,b | −2.74 (1.43)a,b |
| JLO total correct | 0.14 (0.84) | −0.07 (1.23) | −1.57 (1.78)a | −2.03 (1.13)a,b |
| Color-Word: color naming, completion time | 0.4 (0.91) | 0.12 (0.54) | −0.27 (0.66) | −0.98 (1.36) |
| Color-Word: inhibition, completion time | 0.33 (1.02) | 0.30 (0.71) | 0.01 (0.59) | −0.67 (0.78) |
| Category fluency | 0.85 (0.89) | 0.95 (0.96) | −0.73 (1.01)a | −0.46 (1.19) a,b |
| RCFT copy score | — | −0.15 (0.47) | −1.20 (1.36) | −1.28 (1.37) |
| RCFT delayed recall | — | 0.90 (1.49) | 0.45 (1.10) | −2.07 (0.34)b,c |
| Trail-Making Test, difference score | — | 0.08 (1.00) | −0.67 (1.32) | −2.48 (2.27)b |
| Boston Naming Test | — | 0.76 (0.50) | 0.45 (0.69) | 0.48 (1.31) |
| Memory composite z score | 0.23 (0.92) | 0.68 (0.83) | 0.002 (0.71) | −1.60 (0.42)a,c |
| Executive composite z score | 0.26 (0.62) | 0.044 (0.35) | −1.16 (0.54)a,b | −1.64 (1.07)a,b |
Values are listed as group-based mean z scores (standard deviation).
Significantly lower than HC.
significantly lower than PD no MCI.
significantly lower than PD naMCI, p < 0.05.
aMCI =amnestic MCI; CVLT =California Verbal Learning Test; HC =healthy controls; JLO =Judgment of Line Orientation; MCI =mild cognitive impairment; naMCI =nonamnestic MCI; PD =Parkinson disease; RCFT =Rey Complex Figure Test.
Participants were also screened for depression using the Beck Depression Inventory.22 Patients withdrew from antiparkinsonian medication for 12 hours prior to the PET scans. Participants were scanned with [11C]FLB 457, a D2 receptor ligand, which is used to measure D2 binding in cortical regions, and provides an accurate assessment of dopaminergic function in the human cortex. Participants were also scanned with [11C]dihydrotetrabenazine (DTBZ), a radioligand binding to vesicular monoamine transporter (VMAT2), which is a biomarker for DA neuron integrity in the striatum. VMAT2 is the present gold standard marker for DA neuron integrity and is well established as a method for measuring DA neuron degeneration in the striatum in PD.23–26 Subjects also underwent a high-resolution structural magnetic resonance imaging (MRI) scan for registration with the PET images. Both PET scans and MRI scans were performed on separate days to avoid excessive fatigue. Neuropsychological testing was performed within 6 months of PET scans to ensure no significant change in cognitive status. Some data from a smaller sample of these subjects have been reported previously.6 This study was approved by the research ethics committees for the Centre for Addiction and Mental Health and the University Health Network of the University of Toronto. All subjects provided written informed consent to participate.
Neuropsychological Assessment
Patients were diagnosed as PD with MCI according the recent Movement Disorders Society–commissioned task force criteria. 27 Patients were classified as PD-MCI according to the level II criteria requiring at least 1.5 standard deviations below the normative mean on at least 2 core variables of tests in a full neuropsychological test battery. The level II criteria were used, as opposed to level I, to provide more detailed neuropsychological test data for further subtyping of PD patients with MCI into amnestic (memory impaired) and nonamnestic types. All subjects were administered the full neuropsychological test battery, which requires the inclusion of at least 2 tests in each cognitive domain (memory, executive function, attention/working memory, language, visuospatial function). Core variables from each test were selected for use in the diagnosis of PD-MCI. The Disability Assessment for Dementia was used to ensure that no patients had any functional impairment due to cognition. 28 The neuropsychological test variables included the Wechsler Memory Scale III Digit Span (attention) and Letter-Number Sequencing total score (attention/working memory),29 California Verbal Learning Test II (CVLT-II) long delay free recall score (memory),30 Visual Verbal Test total number of shifts (executive),31 Judgment of Line Orientation total score (visuospatial),32 Delis–Kaplan Executive Function System (D-KEFS) Color-Word Interference, time to complete condition 1: color naming (attention), D-KEFS Color-Word Interference, time to complete condition 3: inhibition (executive), D-KEFS Verbal Fluency, total score for category fluency (language/executive), 33 Rey Complex Figure Test (RCFT) copy score (visuospatial), RCFT long delay recall score (visuospatial memory),34 Trail-Making Test (condition B-A) difference score (executive), and 30-Item Boston Naming Test, total score (language).35 All raw scores were converted into z-scores. To split PD patients into nonamnestic and amnestic subtypes, we created composite z-scores for memory performance by taking the mean of the core variables for memory, CVLT delayed recall and RCFT delayed recall, which have previously been used to create composite scores for episodic memory performance.36 A median split was used to divide PD patients with MCI into those with high and low memory performance to ensure that patients classified as amnestic MCI had the lowest overall memory performance. 37 All neuropsychological data including memory scores for the 4 groups are displayed in Table 2. For assessing relationships between PET imaging measures and our cognitive domains of interest, composite z-scores were calculated. For executive function the mean of the z-scores from the Visual Verbal Test, inhibition segment of the Color-Word Interference task, verbal (semantic) fluency, and difference score from the Trail-Making Test were used. The average of the z-scores from the CVLT long delay free recall and RCFT delayed recall were used as a measure of declarative memory function. The healthy control sample reported in our previous imaging study6 was assessed using a more limited neuropsychological test battery that did not include the RCFT, Trail-Making Test, or 30-Item Boston Naming Test.
PET Imaging
PET scanning was performed using a 3-dimensional (3D) high-resolution research tomograph (HRRT) brain tomograph (Siemens, Knoxville, TN), which measures radioactivity in 207 brain sections with a thickness of 1.22 mm each. The detectors of the HRRT are a lutetium oxyorthosilicate/lutetium–yttrium oxyorthosilicate phoswich detector, with each crystal element measuring 2 × 2 × 10 mm3. A 10-minute transmission scan, measured using a single photon point source, 137Cs (t1/2=30.2 years, Eγ=662keV) was acquired immediately before the acquisition of the emission scan to correct for attenuation. Custom-made thermoplastic facemasks together with a head-fixation system (Tru-Scan Imaging, Annapolis, MD) were used to minimize subject head movement in the scanner. Upon completion of the acquisition, the emission list mode data were rebinned into a series of 3D sinograms. For each 3D sinogram, the data were normalized with attenuation and scatter correction before applying Fourier rebinning to convert the 3D sinograms into 2D sinograms.38 The 2D sinograms were then reconstructed into image space using a 2D filtered back projection algorithm, with a ramp filter at Nyquist cutoff frequency. Each subject underwent a [11C]DTBZ and [11C]FLB 457 PET scan on separate days. For each scan, the radioactive tracer was injected as a bolus into an intravenous line placed in an antecubital vein. Emission data were acquired over 90 minutes for [11C]FLB 457 and 60 minutes for [11C]DTBZ while subjects were at rest. Subjects underwent an MRI scan on a separate day to obtain high-resolution T1-weighted and proton density–weighted structural MRIs. The MR scans were performed on a GE Discovery MR750 3.0T scanner (Milwaukee, WI, USA) equipped with an 8-channel head coil.
[11C]FLB 457 Analysis
Methods are the same as those employed in our previous imaging study.6 High-resolution T1-weighted MRIs (GE Discovery MR750 3.0T, 0.9 mm slice thickness) of each subject’s brain were transformed into standardized stereotaxic space using nonlinear automated feature matching to the Montreal Neurological Institute (MNI) template.39 PET frames were smoothed, realigned, summed, and registered to the subject MRI.40 The coregistered MRI and PET were transformed into standardized stereotaxic space using transformation parameters of the individual MRI scans. PET images were smoothed with an isotropic Gaussian kernel of 6 mm full width at half maximum (FWHM). Voxelwise [11C]FLB 457 nondisplaceable binding potential (BPND) was estimated using the simplified reference tissue model (SRTM) with the cerebellum as a reference region41,42 to generate statistical parametric images of BPND change.43
STATISTICAL ANALYSIS
Statistical maps were constructed demonstrating voxel-by-voxel BPND changes in MATLAB v7.4 (Mathworks, Natick, MA) using Statistical Parametric Mapping software (SPM8; Wellcome Department of Imaging Neuroscience, London, UK). [11C]FLB 457 BPND was compared across groups using an analysis of covariance (ANCOVA) in SPM8 for significant effects of group controlling for covariates including age, disease severity (UPDRS-III), and LEDD, followed by voxelwise post hoc independent sample t tests to examine between-group differences (SPM8). T-map images were thresholded at p<0.05 with an extent threshold of k=10. All coordinates of significant clusters are reported in MNI space. Correction within our a priori regions of interest used small volume correction for the specified brain region. Regions of changes in BPND at the extrastriatal level were considered significant at the voxel level threshold of p<0.05 after familywise error (FWE) correction for multiple comparisons. Voxelwise multiple regression analysis with composite z-scores for memory and executive function from the neuropsychological test battery was performed to determine whether there was a relationship between cognitive functions in these domains and the amount of D2 receptor binding. UPDRS-III and LEDD were used as covariates in the analysis to control for disease severity and medication effects on BPND as an outcome measure.
[11C]DTBZ Analysis
Methods were the same as those employed in previous studies. 6,44 Region of interest (ROI) analysis was performed for [11C]DTBZ to measure BPND. Analysis is similar to that described in Rusjan et al,45 using ROMI software (based on Talairach and Kabani atlases).46,47 Our striatal ROIs included the associative striatum (anterior putamen and caudate nucleus), sensorimotor, and limbic striatal subdivisions. These were delineated according to previously specified criteria.45,48 A standard brain template (International Consortium for Brain Mapping/Montreal Neurological Institute 152 MRI) containing predefined subcortical ROIs was nonlinearly transformed using SPM8 to the individual MRI (GE Discovery MR750 3.0T, proton density–weighted images, 2mm slice thickness). Individual ROIs were aligned and resliced to register with the PET images using a normalized mutual information algorithm. The occipital cortex was used as a reference region due to the lack of significant VMAT2 in this region.49 [11C]DTBZ BPND was obtained in each ROI using the SRTM41,42 with the occipital cortex time–activity curve as an input function50,51 using Receptor Parametric Mapping software. To address possible confounding effects of atrophy in the PD patient groups, partial volume effect correction was implemented using the Rousset algorithm.52
STATISTICAL ANALYSIS
A multivariate ANCOVA was used to compare [11C]DTBZ across groups of subjects in each striatal ROI while controlling for age, motor disease severity (UPDRS-III), and LEDD. Post hoc independent sample t tests were used to test for differences between groups using SPSS (v13.0; IBM, Armonk, NY) software. Results were considered significant at the threshold of p<0.05, corrected for multiple comparisons (Bonferroni).
Cortical Thickness Analysis
Cortical thickness analysis was used to investigate whether there was significant cortical thinning in our cortical regions of interest for the [11C]FLB 457 analysis using FreeSurfer software (v4.0.5, freely available at: http://surfer.nmr.mgh.harvard.edu).53,54 This software is a widely used, automated program for the analysis of brain structure.55 In brief, removal of non-brain tissue is followed by the segmentation of gray and white matter, and then alignment of each image volume to a standardized space (ie, MNI space). After intensity normalization, gray and white tissue segmentation was used as the starting point for a deformable surface algorithm that was applied to extract the pial and gray/white surfaces to estimate cortical thickness.53 Cortical thickness was calculated as the closest distance from the gray/white boundary to the gray matter/cerebrospinal fluid boundary at each vertex on the tessellated surface.55
STATISTICAL ANALYSIS
Differences between groups were assessed using a vertex-by-vertex general linear model. The model included cortical thickness as a dependent factor, group as an independent factor, and also included age, LEDD, and UPDRS-III as nuisances variables (https://surfer.nmr.mgh.harvard.edu/fswiki/FsgdFormat). Resulting maps were smoothed using a Gaussian kernel with an FWHM of 15mm. Monte Carlo Z simulations with 10,000 iterations were applied to cortical thickness maps for clusterwise correction for multiple comparisons. Results were thresholded at a probability value of 0.05 (z=1.3) corrected for multiple comparisons.
Results
D2 Receptors in Cortical Brain Networks and Memory
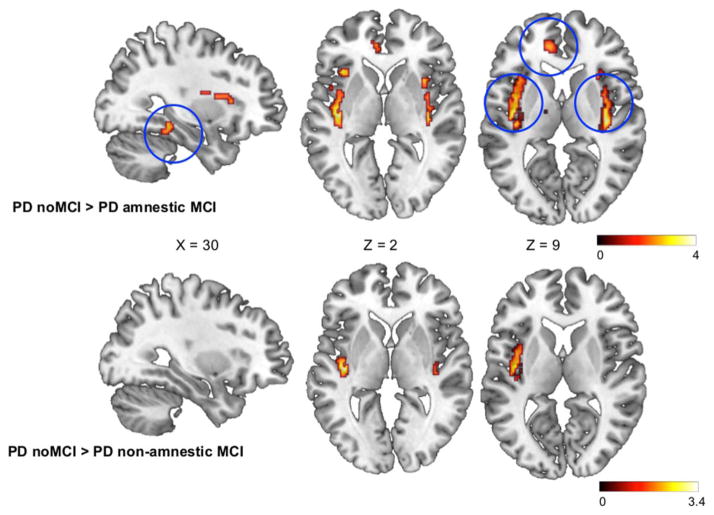
First, we aimed to investigate whether there were significant group differences in DA D2 receptors in the DLPFC, PPC, insula, ACC, or medial temporal lobe (the regions defined in our a priori hypothesis). We found a significant difference in D2 receptor availability between the 4 groups in the bilateral ACC, insula, and right parahippocampal gyrus (PHG; F2,30=8.43, p=0.01). Voxelwise t tests demonstrated a reduction in D2 receptor availability in the PD patients with amnestic MCI in the right PHG, bilateral insula, and ACC compared to healthy controls (p<0.05, Supplementary Table 1). Patients with nonamnestic MCI also demonstrated reductions in D2 availability compared to healthy controls in the right PHG and bilateral ACC, although less severe compared to the amnestic group (p<0.05, Supplementary Table 2). When comparing the MCI groups to PD patients with no MCI, the memory-impaired group had significant reductions in D2 in the right PHG and bilateral insula. They also showed a trend toward reduced binding in the left ACC; however, this did not reach significance with FWE correction (Fig 1, Supplementary Table 3). The nonamnestic MCI group only had a significant reduction in the left insula (p=0.03) and a trend toward reduction in the right insula (p=0.05) compared to patients without MCI (see Fig 1, Supplementary Table 4). Lastly, when contrasting the 2 MCI groups, PD patients with memory impairment demonstrated significantly reduced D2 in the right ACC (p=0.04) and right insula (p=0.028), as well as a trend toward reduced binding in the left ACC (p=0.07, Supplementary Table 5). Mean binding potential values extracted from each of our a priori regions of interest are displayed in Table 3. Importantly, memory was the only cognitive domain in which the amnestic MCI group displayed significantly worse performance than the nonamnestic MCI group. However, because our regions of interest included executive networks, we added executive composite z-score as a nuisance variable in the comparison between PD groups to ensure that changes seen in the amnestic MCI group were not confounded by worsening executive function. The changes in D2 receptor levels remained significant after controlling for executive composite z-score, suggesting that the group differences are independently associated with memory impairment in these regions. Notably, there was no significant difference in D2 binding between healthy controls and patients with no MCI, signifying that D2 reductions were unique to patients with cognitive impairment.
FIGURE 1.
Statistical parametric maps of regions of reduced D2 receptor availability in Parkinson disease (PD) with amnestic mild cognitive impairment (MCI; top) and nonamnestic MCI (bottom) compared to PD patients with no MCI (cognitively normal). Blue circles (top) indicate regions of the salience network (bilateral insula and left anterior cingulate cortex), as well as the right parahippocampal gyrus. Color scales represent T value.
TABLE 3.
Mean [11C]FLB 457 BPND Extracted from Cortical ROIs
| Region | HC, n =14 | PD No MCI, n =11 | PD naMCI, n =10 | PD aMCI, n =9 |
|---|---|---|---|---|
| Left DLPFC | 0.35 (0.09) | 0.32 (0.05) | 0.09 (0.09) | 0.12 (0.07) |
| Right DLPFC | 0.30 (0.10) | 0.24 (0.17) | 0.01 (0.08) | 0.02 (0.08) |
| Left parietal | 0.48 (0.12) | 0.39 (0.16) | 0.16 (0.09) | 0.17 (0.07) |
| Right parietal | 0.38 (0.10) | 0.27 (0.15) | 0.01 (0.09) | 0.03 (0.09) |
| Left MTL | 0.94 (0.11) | 0.78 (0.07) | 0.65 (0.09) | 0.47 (0.07) |
| Right MTLa | 1.13 (0.11) | 0.93 (0.12) | 0.80 (0.10) | 0.57 (0.20) |
| Left insulaa | 1.00 (0.20) | 0.81 (0.36) | 0.62 (0.06) | 0.44 (0.08) |
| Right insulaa | 1.04 (0.21) | 0.84 (0.21) | 0.52 (0.08) | 0.39 (0.07) |
| Left ACCa | 0.79 (0.15) | 0.74 (0.20) | 0.67 (0.10) | 0.40 (0.09) |
| Right ACCa | 0.80 (0.12) | 0.86 (0.31) | 0.56 (0.08) | 0.45 (0.09) |
Values are listed as group-based mean (standard error).
Indicates ROIs where significant changes were detected in the voxel-based group comparisons.
ACC =anterior cingulate cortex; aMCI =amnestic MCI; BPND = nondisplaceable binding potential; DLPFC =dorsolateral pre-frontal cortex; HC =healthy controls; MCI =mild cognitive impairment; MTL =medial temporal lobe; naMCI =nonamnestic MCI; PD =Parkinson disease; ROI =region of interest.
Striatal DA Depletion and Memory
Additionally, we aimed to determine whether striatal DA nerve terminal integrity, as measured with [11C]DTBZ, was related to memory impairment in PD patients in the associative and limbic striatum, the subdivisions involved in the modulation of memory. We found significant differences in [11C]DTBZ binding between groups in the associative striatum that remained significant after controlling for age, motor disease severity, and daily dopaminergic medication intake (F2,30=7.582, p < 0.0001). Post hoc independent sample t tests demonstrated that compared to healthy controls, significantly reduced binding was found in the PD without MCI group (p=0.005) as well as PD patients with nonamnestic MCI (p<0.0001) and PD patients with amnestic MCI (p<0.0001), indicative of severe DA neuron degeneration in all PD patient groups (Fig 2). When comparing patients with PD, the patients with amnestic MCI had significantly reduced DA neuron integrity compared to PD patients with no MCI (p=0.016, see Fig 2). We demonstrated significant group differences in [11C]DTBZ binding in the sensorimotor striatum (F2,33=14.033, p<0.0001), with significant DA denervation in all PD patient groups compared to healthy controls (p<0.0001), confirming characteristic DA depletion. No significant differences between groups were found in the limbic striatum.
FIGURE 2.

Group differences in [11C]dihydrotetrabenazine (DTBZ) binding in the associative striatum, demonstrating a downward trend in binding from healthy to Parkinson disease (PD) with amnestic mild cognitive impairment (MCI). Bars represent group means. Error bars represent standard error of the mean. *p=0.005, **p < 0.0001, ***p=0.016. BPND=nondisplaceable binding potential. [Color figure can be viewed in the online issue, which is available at www.annalsofneurology.org.]
Relationship between DA, Memory, and Executive Function
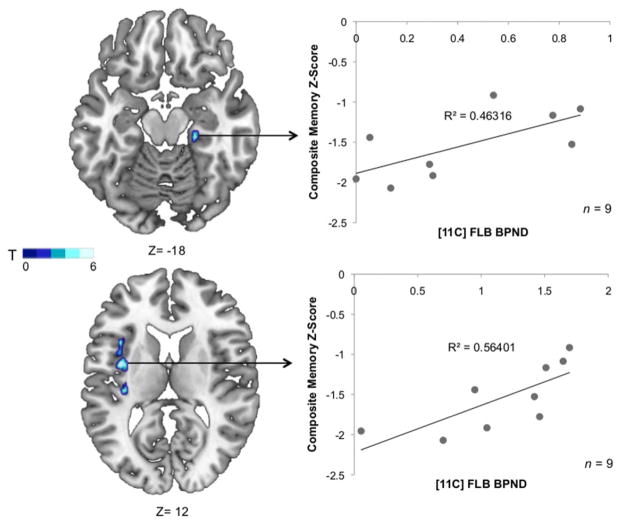
Finally, we investigated whether memory performance was related to dopaminergic changes in the brain regions demonstrating significant changes in the amnestic PD group. A voxelwise linear regression analysis demonstrated a significant positive relationship between D2 receptor availability and memory composite z-scores in PD patients with memory impairment in the right PHG (R2=0.46, T=4.38; X=22, Y=−26, Z=−18; k=16, p<0.05; Fig 3) and left insula (R2=0.56, T=4.26; X=−38, Y=−22, Z=2; k=56, p<0.05; see Fig 3), demonstrating that higher D2 availability is predictive of memory performance in PD patients with amnestic MCI in these regions. This was significant after the inclusion of executive composite z-score as a nuisance variable in the analysis, suggesting that D2 receptor binding is independently associated with memory in these regions. There were no significant relationships between memory and D2 availability in any other subject group. Furthermore, we aimed to assess whether changes in D2 in these regions were related to executive dysfunction, which could potentially overlap with the neural substrates of memory dysfunction. There was no relationship between executive dysfunction and D2 binding levels in these regions in the amnestic MCI group; however, when all PD patients with MCI (amnestic and nonamnestic, n=19) were included, there was a significant positive relationship between D2 receptor binding and executive performance in the bilateral insula and left ACC (Fig 4). No relationship was found between [11C]DTBZ binding in the associative striatum and any cognitive measure.
FIGURE 3.
Statistical map of voxels significantly associated with memory composite z-scores. Mean [11C]FLB nondisplaceable binding potential (BPND) values were extracted from a 6mm radius sphere within the parahippocampal gyrus and left insula and plotted to show the relationship between D2 receptor binding and memory performance in Parkinson disease patients with amnestic mild cognitive impairment.
FIGURE 4.
Statistical map of voxels significantly associated with executive composite z-scores. Mean [11C]FLB nondisplaceable binding potential (BPND) values were extracted from a 6mm radius sphere within the significant clusters and plotted to show the relationship between D2 receptor binding and executive performance in Parkinson disease patients with mild cognitive impairment (amnestic and nonamnestic).
We performed an additional cortical thickness analysis, to verify that the cortical D2 changes seen were not related to neuronal atrophy in PD patients. The analysis confirmed that there were no significant differences in cortical thickness between groups in any of our a priori defined regions of interest, confirming that D2 receptor measurements were not affected by significant neuronal loss.
Discussion
In this study, we established that memory impairment in PD is associated with a loss of dopaminergic function in the salience network and right PHG. PD patients with memory impairment demonstrated (1) significant D2 receptor reductions in the salience network nodes extending beyond the insula into the ACC and (2) more severe D2 reductions in the right PHG when compared to healthy controls and PD patients with no cognitive impairment. Additionally, DA D2 receptor levels in the left insula and right PHG were positively associated with memory performance. We found a strong correlation between D2 receptor levels and executive performance bilaterally in the insula and in the left anterior cingulate, regions constituting the salience network, when including all patients with MCI. Furthermore, significant DA neuron degeneration was found in PD patients with both memory and nonmemory cognitive deficits in the associative striatum.
Reduced D2 receptor availability in the anterior cingulate in addition to the insula in patients with memory impairment suggests that more severe and diffuse salience network changes contribute to memory dysfunction. There has been a growing interest in studying large-scale brain networks in neurodegenerative disease due to their selective vulnerability.56 The salience network is crucial for switching between and causally modulating the activity of the reciprocally active executive and default mode networks,7,8,57,58 and is consistently engaged during memory retrieval.59 The medial temporal lobe including the hippocampus and PHG is considered a subsystem of the default mode network.60 Thus, abnormal interaction between the salience network and medial temporal lobe due to DA dysfunction could impact the relay of information required for accurate memory retrieval. It has recently been shown that default mode network function relies on salience network integrity after traumatic brain injury, emphasizing the influence this network might have on memory.9
Reduced D2 receptor levels in the right PHG of PD patients with memory impairment are consistent with its known role in providing contextual information associated with memory,61 including semantic and visuospatial memory,62–64 which were severely impaired in our PD amnestic MCI patient group. The PHG is the major convergence point of incoming cortical information to the hippocampal formation,65 and DA signaling in this region is involved in incorporating cortical input for memory encoding and retrieval. Furthermore, the salience network and PHG are structurally and functionally connected,16,17 which may be essential for providing information pertaining to past experiences. The salience network is known to process the degree of personal relevance of stimuli to direct behavior in uncertain situations, which involves integrating visceral and cognitive/emotional information with contextual information from past experience.66 Thus, a disruption in contextual input from the PHG to this network could impact effective utilization of memory.
Our finding of a significant correlation between executive function and D2 receptor availability in nodes of the salience network in all MCI patients corroborates our previous finding of a relationship between D2 in the right anterior insula and executive function in PD patients with MCI.6 In this study, we found a correlation of both executive function (all MCI patients) and memory (only in patients with memory impairment) with D2 receptors in regions of the salience network, suggesting that its function may be multipurpose. Memory is not process pure, and relies on executive mechanisms for encoding, coordination, and retrieval.12,67 Thus, a disruption of dopaminergic modulation in the salience network could impact switching between the central executive and default mode network required for mental flexibility and the coordination of memory,7 resulting in distinct associations with poor executive and memory performance (Fig 5). From a network perspective this is plausible, and well supported by the recent findings in the literature indicating that the salience network has a binary role in modulating both the default mode and central executive networks.7,8
FIGURE 5.

Conceptual diagram demonstrating networks with reduced D2 receptor (R) binding in Parkinson disease (PD) with mild cognitive impairment and the potential impact on network interactions affecting memory and executive function. Reduced D2 in the salience network could impair engagement of the central executive network (CEN), and switching between the default mode network (DMN) and CEN. Reduced D2 in the medial temporal lobe (MTL) could disrupt the use of contextual information related to memory that is accessed by the salience network.
The absence of significant atrophy and anatomical pattern of D2 receptor loss suggests that Lewy body deposition may be the pathological mechanism affecting D2 receptor expression. The pattern of deposition according to Braak’s staging hypothesis begins in the brainstem, and then spreads to limbic regions including the medial temporal lobe, followed by the insula, and temporal and anterior cingulate cortices.68 Although cortical Lewy bodies do not directly result in neuronal death, this pathology could have an impact on D2 receptor expression in different individuals with PD. Furthermore, D2 receptor loss has been associated with disease severity in PD.69 Although we controlled for motor disease severity in the analyses, there may be an inevitable link between regional pathological progression and cognitive decline.
There were no significant changes in the hypothesized DLPFC or PPC of the executive network. Although it appears that D2 binding may be slightly lower than controls in both MCI groups, it cannot differentiate patient groups. This could be due to D2 receptor levels in these regions being less vulnerable to pathology affecting D2 receptor expression, such as Lewy body deposition. The medial temporal lobe and salience network may be also be affected earlier by a loss of D2, resulting in dysfunction of these regions in cognitive processes. As previously mentioned, the salience network plays a crucial role in engaging the central executive network, and therefore abnormalities in this network may disrupt executive mechanisms in PD, despite the lack of significant change in D2 receptor binding within the central executive network.
It is worth noting that a pathological loss of other receptor types, such as the D1 receptor, might occur in parallel to a loss of D2 receptors. Because D1 and D2 receptors act dynamically to perpetuate and reset cortical activity during cognitive tasks,70 a reduction in these receptors would have profound effects on performance in PD. Additionally, the loss of D2 receptors could have interactions with other affected neurotransmitter systems and PD-related pathology. For example, although the evidence regarding a link between amyloid and cognition in PD has been inconclusive, it has been associated with future cognitive decline, although not correlating directly with cognitive measures.71 The cholinergic system is also affected in PD and related to cognitive impairment. The cholinergic system has significant interactions with the dopaminergic system, and reduced cholinergic signaling has been associated with reducing dopaminergic function. 72 Therefore, the combined effect of a loss of function in these neurotransmitter systems could contribute to cognitive deficits seen in PD.
Our findings provide novel insight into the mechanism of memory impairment in PD. PD patients frequently experience difficulty recalling past information or events.73 The salience network and the parahippocampal gyrus are consistently engaged during memory retrieval, and thus our findings are consistent with the functional role of these regions. Additionally, the salience network being affected may impair switching between networks (central executive network and default mode network), resulting in patients becoming stuck, that is, unable to retrieve information pertaining to past experiences to be used for executive tasks (see Fig 5). Based on our findings, it seems likely that there are not completely distinct pathways for amnestic and nonamnestic MCI, but rather a more profound spread of D2 reductions in the medial temporal lobe and within the salience network affecting retrieval of information from long-term memory.
In conclusion, these results suggest that memory deficits in PD are associated with abnormal dopaminergic function in large-scale cortical networks involved in accessing memory, as well as disrupted DA signaling in the medial temporal lobe. Furthermore, aberrant relay between the PHG and salience network involved in providing contextual information to guide memory use may impact both memory and executive performance. Our findings demonstrate the vulnerability of the salience network and the PHG to dopaminergic changes in PD in the absence of atrophic changes, and specifically highlight the potential impact on memory function. Future investigations should focus on the salience network as a key vulnerable network, as well as the role of the PHG in memory decline in PD.
Supplementary Material
Acknowledgments
This study was supported by Canadian Institutes of Health Research (MOP 110962) and the National Parkinson Foundation. A.P.S. is supported by the Canada Research Chair program. L.C. is supported by a scholarship from Parkinson Society Canada.
Footnotes
Authorship
L.C. designed the study, conducted experiments, analyzed data, and wrote the manuscript; S.D.-C. aided in data collection, analysis, and manuscript preparation; Y.K. aided in data collection and manuscript preparation; B.S. aided in data analysis and manuscript preparation; I.B. aided in data analysis and manuscript preparation; R.C. aided in manuscript preparation; A.E.L. aided in manuscript preparation; S.H. aided in data analysis and manuscript preparation; P.R. aided in data analysis and manuscript preparation; A.P.S. designed the study, aided in data analysis, and wrote the manuscript.
Potential Conflicts of Interest
Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Levy G, Jacobs DM, Tang M-X, et al. Memory and executive function impairment predict dementia in Parkinson disease. Mov Disord. 2002;17:1221–1226. doi: 10.1002/mds.10280. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen KF, Larsen JP, Tysnes O-B, Alves G. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol. 2013;70:580–586. doi: 10.1001/jamaneurol.2013.2110. [DOI] [PubMed] [Google Scholar]
- 3.Shohamy D, Adcock RA. Dopamine and adaptive memory. Trends Cogn Sci. 2010;14:464–472. doi: 10.1016/j.tics.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Apitz T, Bunzeck N. Dopamine controls the neural dynamics of memory signals and retrieval accuracy. Neuropsychopharmacology. 2013;38:2409–2417. doi: 10.1038/npp.2013.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Christopher L, Marras C, Duff-Canning S, et al. Combined insular and striatal dopamine dysfunction are associated with executive deficits in Parkinson disease with mild cognitive impairment. Brain. 2014;137(pt 2):565–575. doi: 10.1093/brain/awt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiong W, Wilson SM, D’Esposito M, et al. The salience network causally influences default mode network activity during moral reasoning. Brain. 2013;136(pt 6):1929–1941. doi: 10.1093/brain/awt066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnelle V, Ham TE, Leech R, et al. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A. 2012;109:4690–4695. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci. 2006;26:916–925. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huijbers W, Pennartz CM, Cabeza R, Daselaar SM. The hippocampus is coupled with the default network during memory retrieval but not during memory encoding. PLoS One. 2011;6:e17463. doi: 10.1371/journal.pone.0017463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- 13.Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Anderson MC, Green C. Suppressing unwanted memories by executive control. Nature. 2001;410:366–369. doi: 10.1038/35066572. [DOI] [PubMed] [Google Scholar]
- 15.Kolbjørn B, Guido A, Dag A, et al. Verbal memory in drug-naive, newly diagnosed Parkinson disease. The retrieval deficit hypothesis revisited. Neuropsychology. 2011;25:114–124. doi: 10.1037/a0020857. [DOI] [PubMed] [Google Scholar]
- 16.Chikama M, McFarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997;17:9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 18.Costa C, Sgobio C, Siliquini S, et al. Mechanisms underlying the impairment of hippocampal long-term potentiation and memory in experimental Parkinson disease. Brain. 2012;135(pt 6):1884–1899. doi: 10.1093/brain/aws101. [DOI] [PubMed] [Google Scholar]
- 19.Calabresi P, Castrioto A, Di Filippo M, Picconi B. New experimental and clinical links between the hippocampus and the dopaminergic system in Parkinson disease. Lancet Neurol. 2013;12:811–821. doi: 10.1016/S1474-4422(13)70118-2. [DOI] [PubMed] [Google Scholar]
- 20.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson disease. Mov Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 21.Evans AH, Katzenschlager R, Paviour D, et al. Punding in Parkinson disease: its relation to the dopamine dysregulation syndrome. Mov Disord. 2004;19:397–405. doi: 10.1002/mds.20045. [DOI] [PubMed] [Google Scholar]
- 22.Beck A, Ward C, Mendelson M, et al. Inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 23.Frey KA, Koeppe RA, Kilbourn MR, et al. Presynaptic monoaminergic vesicles in Parkinson disease and normal aging. Ann Neurol. 1996;40:873–884. doi: 10.1002/ana.410400609. [DOI] [PubMed] [Google Scholar]
- 24.Bohnen NI, Albin RL, Koeppe RA, et al. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab. 2006;26:1198–1212. doi: 10.1038/sj.jcbfm.9600276. [DOI] [PubMed] [Google Scholar]
- 25.Martin WRW, Wieler M, Stoessl AJ, Schulzer M. Dihydrotetrabenazine positron emission tomography imaging in early, untreated Parkinson disease. Ann Neurol. 2008;63:388–394. doi: 10.1002/ana.21320. [DOI] [PubMed] [Google Scholar]
- 26.Stoessl AJ. Neuroimaging in Parkinson disease. Neurotherapeutics. 2011;8:72–81. doi: 10.1007/s13311-010-0007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reeves S, Mehta M, Howard R, et al. The dopaminergic basis of cognitive and motor performance in Alzheimer’s disease. Neurobiol Dis. 2010;37:477–482. doi: 10.1016/j.nbd.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Beyer MK, Janvin CC, Larsen JP, Aarsland D. A magnetic resonance imaging study of patients with Parkinson disease with mild cognitive impairment and dementia using voxel-based morphometry. 2007;78:254–259. doi: 10.1136/jnnp.2006.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cools R, Altamirano L, D’Esposito M. Reversal learning in Parkinson disease depends on medication status and outcome valence. Neuropsychologia. 2006;44:1663–1673. doi: 10.1016/j.neuropsychologia.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 31.Wicklund AH, Johnson N, Weintraub S. Preservation of reasoning in primary progressive aphasia: further differentiation from Alzheimer’s disease and the behavioral presentation of frontotemporal dementia. J Clin Exp Neuropsychol. 2004;26:347–355. doi: 10.1080/13803390490510077. [DOI] [PubMed] [Google Scholar]
- 32.Herwig U, Kaffenberger T, Jäncke L, Brühl AB. Self-related awareness and emotion regulation. Neuroimage. 2010;50:734–741. doi: 10.1016/j.neuroimage.2009.12.089. [DOI] [PubMed] [Google Scholar]
- 33.Hodges JR, Salmon DP, Butters N. Semantic memory impairment in Alzheimer’s disease: failure of access or degraded knowledge? Neuropsychologia. 1992;30:301–314. doi: 10.1016/0028-3932(92)90104-t. [DOI] [PubMed] [Google Scholar]
- 34.Meyers JE, Meyers KR. Rey complex figure test and recognition trial—professional manual. Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- 35.Williams BW, Wendy MACK, Henderson VW, Mack W. Boston naming test in Alzheimer’s disease. Neuropsychologia. 1989;27:1073–1079. doi: 10.1016/0028-3932(89)90186-3. [DOI] [PubMed] [Google Scholar]
- 36.Pike KE, Savage G, Villemagne VL, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130(pt 11):2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 37.Miller SL, Celone K, Depeau K, et al. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2007;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Defrise M, Kinahan PE, Townsend DW, et al. Exact and approximate rebinning algorithms for 3-D PET data. IEEE Trans Med Imaging. 1997;16:145–158. doi: 10.1109/42.563660. [DOI] [PubMed] [Google Scholar]
- 39.Collins D, Neelin P, Peters T, Evans A. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 40.Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Gunn R, Lammertsma A, Hume S, Cunningham V. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- 42.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4(3 pt 1):153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 43.Aston JA, Gunn RN, Worsley KJ, et al. A statistical method for the analysis of positron emission tomography neuroreceptor ligand data. Neuroimage. 2000;12:245–256. doi: 10.1006/nimg.2000.0620. [DOI] [PubMed] [Google Scholar]
- 44.Boileau I, Rusjan P, Houle S, et al. Increased vesicular monoamine transporter binding during early abstinence in human methamphetamine users: is VMAT2 a stable dopamine neuron biomarker? J Neurosci. 2008;28:9850–9856. doi: 10.1523/JNEUROSCI.3008-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rusjan P, Mamo D, Ginovart N, et al. An automated method for the extraction of regional data from PET images. Psychiatry Res. 2006;147:79–89. doi: 10.1016/j.pscychresns.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York, NY: Thieme Medical Publishers; 1988. [Google Scholar]
- 47.Kabani NJ, Collins DL, Evans AC. A 3d neuroanatomical atlas. Paper presented at: 4th International Conference on Functional Mapping of the Human Brain; June 7–12, 1998; Montreal, Quebec, Canada. [Google Scholar]
- 48.Mawlawi O, Martinez D, Slifstein M, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Scherman D, Raisman R, Ploska A, Agid Y. [3H]dihydrotetrabenazine, a new in vitro monoaminergic probe for human brain. J Neurochem. 1988;50:1131–1136. doi: 10.1111/j.1471-4159.1988.tb10583.x. [DOI] [PubMed] [Google Scholar]
- 50.Koeppe RA, Frey KA, Vander Borght TM, et al. Kinetic evaluation of [11C]dihydrotetrabenazine by dynamic PET: measurement of vesicular monoamine transporter. J Cereb Blood Flow Metab. 1996;16:1288–1299. doi: 10.1097/00004647-199611000-00025. [DOI] [PubMed] [Google Scholar]
- 51.Chan GL, Holden JE, Stoessl AJ, et al. Reproducibility studies with 11C-DTBZ, a monoamine vesicular transporter inhibitor in healthy human subjects. J Nucl Med. 1999;40:283–289. [PubMed] [Google Scholar]
- 52.Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med. 1998;39:904– 911. [PubMed] [Google Scholar]
- 53.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 54.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II. Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 55.Fischl B, Dale A. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;9:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seeley WW, Crawford RK, Zhou J, et al. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dhanjal NS, Wise RJS. Frontoparietal cognitive control of verbal memory recall in Alzheimer’s disease. Ann Neurol. 2014;76:241–251. doi: 10.1002/ana.24199. [DOI] [PubMed] [Google Scholar]
- 60.Andrews-Hanna JR, Reidler JS, Sepulcre J, et al. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Wagner AD. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 63.Bohbot VD, Kalina M, Stepankova K, et al. Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia. 1998;36:1217–1238. doi: 10.1016/s0028-3932(97)00161-9. [DOI] [PubMed] [Google Scholar]
- 64.Owen AM, Milner B, Petrides M, Evans AC. A specific role for the right parahippocampal gyrus in the retrieval of object-location: a positron emission tomography study. J Cogn Neurosci. 1996;8:588–602. doi: 10.1162/jocn.1996.8.6.588. [DOI] [PubMed] [Google Scholar]
- 65.Ward AM, Schultz AP, Huijbers W, et al. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp. 2014;35:1061–1073. doi: 10.1002/hbm.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 67.Levy BJ, Anderson MC. Inhibitory processes and the control of memory retrieval. Trends Cogn Sci. 2002;6:299–305. doi: 10.1016/s1364-6613(02)01923-x. [DOI] [PubMed] [Google Scholar]
- 68.Braak H, Bohl JR, Müller CM, et al. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson disease reconsidered. Mov Disord. 2006;21:2042–2051. doi: 10.1002/mds.21065. [DOI] [PubMed] [Google Scholar]
- 69.Kaasinen V, Aalto S, Nagren K, et al. Extrastriatal dopamine D(2) receptors in Parkinson disease: a longitudinal study. J Neural Transm. 2003;110:591–601. doi: 10.1007/s00702-003-0816-x. [DOI] [PubMed] [Google Scholar]
- 70.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 71.Gomperts SN, Locascio JJ, Rentz D, et al. Amyloid is linked to cognitive decline in patients with Parkinson disease without dementia. Neurology. 2013;80:85–91. doi: 10.1212/WNL.0b013e31827b1a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsukada H, Harada N, Nishiyama S, et al. Cholinergic neuronal modulation alters dopamine D2 receptor availability in vivo by regulating receptor affinity induced by facilitated synaptic dopamine turnover: positron emission tomography studies with microdialysis in the conscious monkey brain. J Neurosci. 2000;20:7067–7073. doi: 10.1523/JNEUROSCI.20-18-07067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taylor A, Saint-Cyr J, Lang A. Memory and learning in early Parkinson disease: evidence for a “frontal lobe syndrome”. Brain Cogn. 1990;13:211–232. doi: 10.1016/0278-2626(90)90051-o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.