Abstract
An organocatalytic and highly regio-, diastereo-, and enantioselective intermolecular haloetherification and haloesterification reaction of allyl amides is reported. A variety of alkene substituents and substitution patterns are compatible with this chemistry. Notably, electronically unbiased alkene substrates exhibit exquisite regio- and diastereoselectivity for the title transformation. We also demonstrate that the same catalytic system can be used in both chlorination and bromination reactions of allyl amides with a variety of nucleophiles with little or no modification.
Keywords: chloroetherification, enantioselectivity, halogenation, organocatalysis, regioselectivity
The field of catalytic asymmetric alkene halogenation has witnessed an explosive growth in recent years, with tremendous advances being made both in terms of new reaction discovery as well as mechanistic understanding. Two of the major issues that have thwarted the development of asymmetric alkene halogenations are the rapid stereochemical degradation of chiral halonium ions by olefin-to-olefin halenium transfer,[1] and isomerization of halonium ions to the open β-halocarbenium ions.[2] Not surprisingly, most early examples have reported on the intramolecular capture of halonium ions via tethered nucleophiles;[3] the proximity-driven rate enhancement of the cyclization step presumably outcompetes any stereorandomizing events. Enantioselectivities of more than 95:5 are routinely obtained with a variety of halenium precursors and nucleophiles.
More recently, the development of enantioselective intermolecular alkene halofunctionalization reactions has come into focus.[4] A number of excellent reports have shown a great deal of progress in this area.[4a, 5] Intermolecular aminohalogenation,[5b–e] haloesterifications,[5g,h,k] halohydrin[5l, 6] synthesis, and dihalogenation[5i,m] have all been reported.
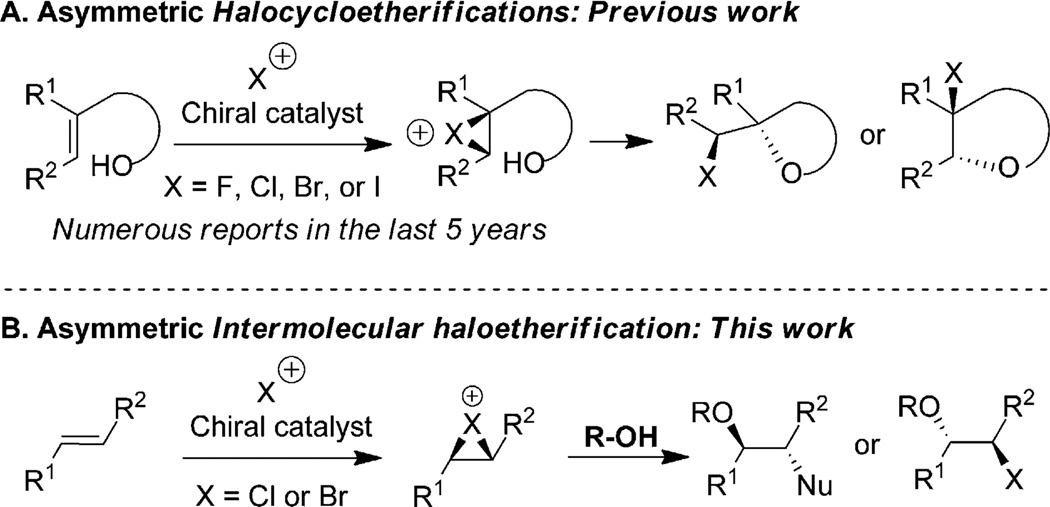
Despite this progress, numerous shortcomings are apparent. First, alcohols are yet to be demonstrated as viable nucleophiles in this chemistry despite the success seen in halocycloetherification reactions (Figure 1A and B).[7] Second, substrates with alkyl substituents on the alkene are known to afford poor or moderate levels of enantioselectivity at best.[8] Third, substrate scope studies have been limited to electronically biased alkenes and hence possible regioselectivity issues have remained unaddressed.[9] Finally, none of the catalytic systems were demonstrated to be promiscuous enough to allow for the use of different halenium sources and nucleophiles with the same substrates.
Figure 1.
Enantioselective intra- and intermolecular haloetherification of alkenes. It should be noted that the bridged halonium is used as only a demonstration of a putative intermediate, although in many instances the open carbocation would predominate. The nature of the intermediate is highly dependent on the nature of the olefin and the halogen.
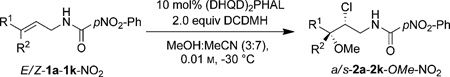
We sought to develop an enantioselective intermolecular haloetherification reaction with the intention of both demonstrating the feasibility of this unprecedented transformation as well as to address some of the limitations detailed above. We report herein the enantio-, diastereo-, and regioselective intermolecular haloetherification, haloesterification and halohydrin synthesis of a variety of alkenes, including those with no dominant bias for regioselectivity (that is, alkenes with alkyl substituents).
In our prior work, we had demonstrated that the non-nucleophilic CF3CH2OH was crucial for obtaining high yields and enantioselectivities for intramolecular cyclization of allyl amides,[3i] although traces of intermolecular incorporation of CF3CH2OH were still observed in a few cases. Crucially, these chloroether by-products were formed with exquisite diastereo- and regioselectivity (see the Supporting Information, Scheme S1 for an example). As such, this result represented a good starting point for developing a practical and general intermolecular chlorofunctionalization reaction of alkenes.
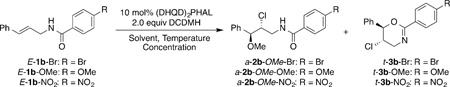
We chose the intermolecular reaction of E-1b-Br[10] with a chlorenium source and MeOH as the test bed to optimize the process. The p-bromobenzamide group was retained in these orienting studies based on our prior results.[3i] (DHQD)2PHAL (hydroquinidine 1,4-phthalazinediyl diether; 10 mol%) was employed as the catalyst along with 2.0 equiv of 1,3-dichloro-5,5-dimethylhydantoin (DCDMH) as the chlorenium source.[11] At ambient temperature both the desired chloroether product a-2b-OMe-Br and the cyclized product t-3b-Br were observed (93% combined yield) in a 1.8:1 ratio. In line with our prior studies, the cyclized product t-3b-Br had excellent enantioselectivity (96:4 e.r.), whereas the desired product a-2b-OMe-Br exhibited a lower 67:33 e.r. Lower temperatures and lower concentration led to slightly improved enantioselectivity for a-2b-OMe-Br while not significantly improving the d.r. or the 2b:3b ratio (Table 1, entries 2 and 3). Further experimentation revealed that employing MeOH as a co-solvent in MeCN led to a significant improvement in the enantioselectivity of a-2b-OMe-Br (Table 1, entry 4).
Table 1.
Orienting studies for the enantioselective intermolecular chloroetherification of E-1b-(Br/OMe/NO2).
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Substrate | Solvent | Conc. [M] | Temp. [°C] | Yield [%][a] | ratio 2b:3b[b] |
d.r. (a-2b:s-2b)[b] |
e.r. (2b)[c] |
e.r. (3b)[c] |
| 1 | E-1b-Br | MeOH | 0.03 | 24 | 93 | 1.8:1 | 6.8:1 | 67:33 | 96:4 |
| 2 | E-1b-Br | MeOH | 0.03 | −30 | 94 | 1.8:1 | 6.8:1 | 73:27 | 98:2 |
| 3 | E-1b-Br | MeOH | 0.01 | −30 | 76 | 1.8:1 | 5.7:1 | 71:29 | 98:2 |
| 4[d] | E-1b-Br | MeOH/MeCN | 0.01 | −30 | 76 | 1.3:1 | 5.8:1 | 85:15 | 99:1 |
| 5[d] | E-1b-OMe | MeOH/MeCN | 0.01 | −30 | 86 | 1.3:1 | 5.0:1 | 83:17 | 97:3 |
| 6[d] | E-1b-NO2 | MeOH/MeCN | 0.01 | −30 | 85 | 1.1:1 | 3.4:1 | 92:8 | 96:4 |
Combined yield of a-2b, s-2b, and 3b as determined by NMR analysis with MTBE as added external standard.
Determined by NMR spectroscopy.
Determined by chiral-phase HPLC.
MeOH/MeCN ratio was 3:7.
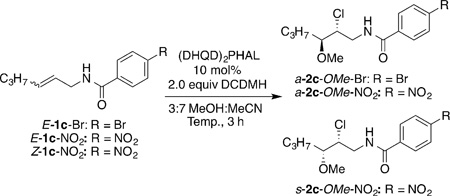
Other studies focused on varying the expendable amide moiety. While the 4-methoxybenzamide gave practically identical results (Table 1, entry 5), the electron-deficient 4-NO2-benzamide gave a significant improvement in the enantioselectivity of a-2b-OMe-NO2 (92:8 e.r.; Table 1, entry 6). As evident from these preliminary results, although useful levels of enantioinduction was seen for the intermolecular chloroetherification of E-1b-NO2, the d.r. (3.4:1) as well the ratio of 2b:3b (ca. 1:1) were not ideal. Gratifyingly, on replacing the aryl substituent on the alkene with an aliphatic group, the desired intermolecular products were obtained with near complete diastereo- and regioselectivity. For example, the reaction of E-1b-Br, bearing n-C3H7 as the alkene substituent, led to the production of a-2c-OMe-Br with more than 99:1 d.r. and r.r. (Table 2, entry 1). More importantly, a-2c-OMe-Br was formed in 92% yield and 81:19 e.r., and the formation of the cyclized product was suppressed to a mere 5%. Employing the less nucleophilic 4-NO2-benzamide in afforded exclusively a-2c-OMe-NO2 (Table 2, entry 2) in 86% yield, 87:13 e.r., more than 20:1 r.r., and more than 99:1 d.r.[12] cis-Allylic amides were even better substrates for this chemistry as compared to the trans-allylic amide counterparts. Substrate Z-1c-NO2 gave the corresponding product s-2c-OMe-NO2 in 87% yield and 99.5:0.5 e.r. (Table 2, entry 3). Reactions that were run at ambient temperatures or with lower catalyst loadings showed no loss in the diastereo- and regioselectivity and only a small decrease in the enantioselectivity (97:3 e.r.; Table 2, entries 4 and 5). Nonetheless, the yields were lower (75–79%) owing to the formation of side products arising from the competing addition of MeCN across the alkene.[13]
Table 2.
Reaction optimization for aliphatic substrates.[a]
 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | Temp [°C] | Product | Yield [%][b] | e.r.[d] |
| 1 | E-1c-Br | −30 | a-2c-OMe-Br | 92[c] | 81:19 |
| 2 | E-1c-NO2 | −30 | a-2c-OMe-NO2 | 86 | 87:13 |
| 3 | Z-1c-NO2 | −30 | s-2c-OMe-NO2 | 87 | 99.5:0.5 |
| 4 | Z-1c-NO2 | 24 | s-2c-OMe-NO2 | 75 | 97:3 |
| 5[e] | Z-1c-NO2 | −30 | s-2c-OMe-NO2 | 79 | 97:3 |
The r.r. (defined as ratio of 2:5, see Table 4) was more than 20:1 and d.r. was more than 99:1 in all instances.
Determined by NMR using MTBE as added external standard.
5% of cyclized product was also seen by NMR.
Determined by chiral-phase HPLC.
2 mol% catalyst was used.
In an effort to map out the generality of this transformation, a number of trans-disubstituted allyl amides were initially exposed to the optimized reaction conditions. Compounds E-1b-NO2 and E-1d-NO2 (Table 3, entries 1 and 2) with aryl substituents on the alkene gave moderate yields of isolated product (due to competing chlorocyclization) and fair diastereoselectivity for the corresponding products a-2b-OMe-NO2 and a-2d-OMe-NO2 (56% and 64% yields, respectively; ca. 3.3:1 d.r., mass balance in both cases was the cyclized product; Table 3, entries 1 and 2). Nonetheless, the chloroether products were formed with good enantioselectivity. Trans substrates with alkyl substituents on the olefin (Table 3, entries 3–5) predictably gave products with exquisite levels of diastereo- and regioselectivity. Additionally, high yield and e.r. was observed for a-2c-OMe-NO2 (R1 = n-C3H7). The e.r. dropped significantly on introduction of the bulky cyclohexyl group (see a-2a-OMe-NO2, 75:25 e.r.). The benzyloxy substituted compound gave only moderate yields and r.r. (62%, 7:1 r.r.), although the enantioselectivity was good (see a-2e-OMe-NO2, 89:11 e.r.).
Table 3.
Substrate scope of intermolecular chloroetherification.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Starting material | R1 | R2 | Product | Yield [%][a] | d.r. | r.r.[b] | e.r.[c] |
| 1 | E-1b-NO2 | Ph | H | a-2b-OMe-NO2[e] | 56 (49)[d] | 3.4:1 | >99:1 | 92:8 |
| 2 | E-1d-NO2 | pF-Ph | H | a-2d-OMe NO2[e] | 64 (58)[d] | 3.3:1 | >99:1 | 90:10 |
| 3 | E-1c-NO2 | n-C3H7 | H | a-2c-OMe NO2 | 86 (79) | >99:1 | >20:1 | 87:13 |
| 4 | E-1a-NO2 | CyHex | H | a-2a-OMe NO2 | 70 | >99:1 | >20:1 | 75:25 |
| 5 | E-1e-NO2 | BnOCH2 | H | a-2e-OMe-NO2 | 62 (57) | >99:1 | 7:1 | 89:11 |
| 6 | Z-1b-NO2 | H | Ph | s-2b-OMe-NO2 | 93 (82)[d] | 3.3:1 | >99:1 | 99.5:0.5 (90:10)[f] |
| 7 | Z-1f-NO2 | H | pMe-Ph | s-2f-OMe-NO2 | 87[d] | 1.3:1 | >99:1 | 98:2 (97:3)[f] |
| 8 | Z-1g-NO2 | H | pMeO-Ph | s-2g-OMe-NO2 | 80[d] | 1:1 | >99:1 | 99:1 (92:8)[f] |
| 9 | Z-1h-NO2 | H | C2H5 | s-2h-OMe-NO2 | 83 (77) | >99:1 | >20:1 | 98:2 |
| 10 | Z-1c-NO2 | H | C3H7 | s-2c-OMe-NO2 | 87 (79)g | >99:1 | >20:1 | 99.5:0.5 (99.5:0.5)[g] |
| 11 | Z-1i-NO2 | H | C5H11 | s-2i-OMe-NO2 | 83 (74) | >99:1 | >20:1 | 95:5 |
| 12 | Z-1e-NO2 | H | BnOCH2 | s-2e-OMe-NO2 | 60 (53) | >99:1 | 7:1 | 99.5:0.5 |
| 13 | Z-1j-NO2 | H | TBDPSOC2H4 | s-2j-OMe-NO2 | 79 | >99:1 | >20:1 | 99.5:0.5 |
| 14 | 1k-NO2 | Me | Me | 2k-OMe-NO2 | 59 (53) | na | na | 99:1 |
| 15[h] | E-1b-NO2 | Ph | H | ent-a-2b-OMe-NO2[e] | 42[d] | 6.2:1 | >99:1 | 75:25 |
| 16[h] | Z-1f-NO2 | H | pMe-Ph | ent-s-2f-OMe-NO2 | 82 | 1.2:1 | >99:1 | 99.5:0.5 (96:4)[f] |
| 17[h] | Z-1c-NO2 | H | C3H7 | ent-s-2c-OMe-NO2 | 69 | >99:1 | >20:1 | 95:5 |
Yield determined by NMR with MTBE as standard. Numbers in parentheses reflect yields of isolated product on a 0.1 mmol scale.
r.r. (regioselectivity) is defined as ratio of 2:5, see Table 4.
Enantioselectivity determined by chiral-phase HPLC.
Combined yield of diastereomers, Li2CO3 (15 equiv) was used as additive.
Mass balance was the cyclized product;
The e.r. values are for the minor diastereomer.
The result in parenthesis is from a 1 g reaction scale, illustrating the scalability of the method. The corresponding Ritter product[13] (6%) was also isolated.
The reactions in entries 15–17 were performed with the quasi enantiomeric (DHQ)2PHAL catalyst, yielding the enantiomeric product in each case.
Aryl-substituted Z-alkenes were exceptional substrates, leading to the intermolecular product exclusively, in good yields (73% to 80%), excellent regioselectivity (>99:1 r.r.), and high enantioselectivity (≥ 98:2 e.r.; Table 3, entries 6–8). The diastereoselectivities for these entries, however, were poor (ranging from 1:1 to 3.3:1), which was presumably due to the increased carbocation character at the benzylic position. Z-alkyl-substituted olefins afforded the desired products in near complete regio-, diastereo-, and enantioselectivity (Table 3, entries 9–13). Trisubstituted alkene 1k-NO2 provides the desired product in moderate yield and excellent enantioselectivity (59%, 99:1 e.r.). The quasi-enantiomeric catalyst, (DHQ)2PHAL, was also evaluated with four different substrates, yielding the enantiomeric products in comparable yields and selectivities (Table 3, entries 15–17, and last entry in Scheme 1). The exception was the results with the least successful category of substrates (trans-substituted aryls), which does not mirror the (DHQD)2PHAL catalyzed the reaction well (Table 3, entries 1 and 15), yielding products with lower than expected enantioselectivity.
Scheme 2.
Nucleophile scope of intermolecular chlorofunctionalization.[a,b] [a] The r.r. was more than 20:1 and d.r. was more than 99:1 in all instances. [b] Yield determined by NMR with MTBE as standard. Numbers in parentheses reflect yields of isolated products on a 0.1 mmol scale. Enantioselectivity determined by chiral-phase HPLC. [c] Reaction was run under nitrogen in the presence of molecular sieves. [d] The ratio of MeCN:H2O was 9:1, reaction temperature was −10 °C. [e] The results reflect a 1 g scale reaction. The corresponding Ritter product (10%) was also isolated. [f] The prime symbol refers to Br instead of Cl in the product. [g] Results with the quasi enantiomeric (DHQ)2PHAL catalyst (ent-2k’-OMe-NO2 was the product).
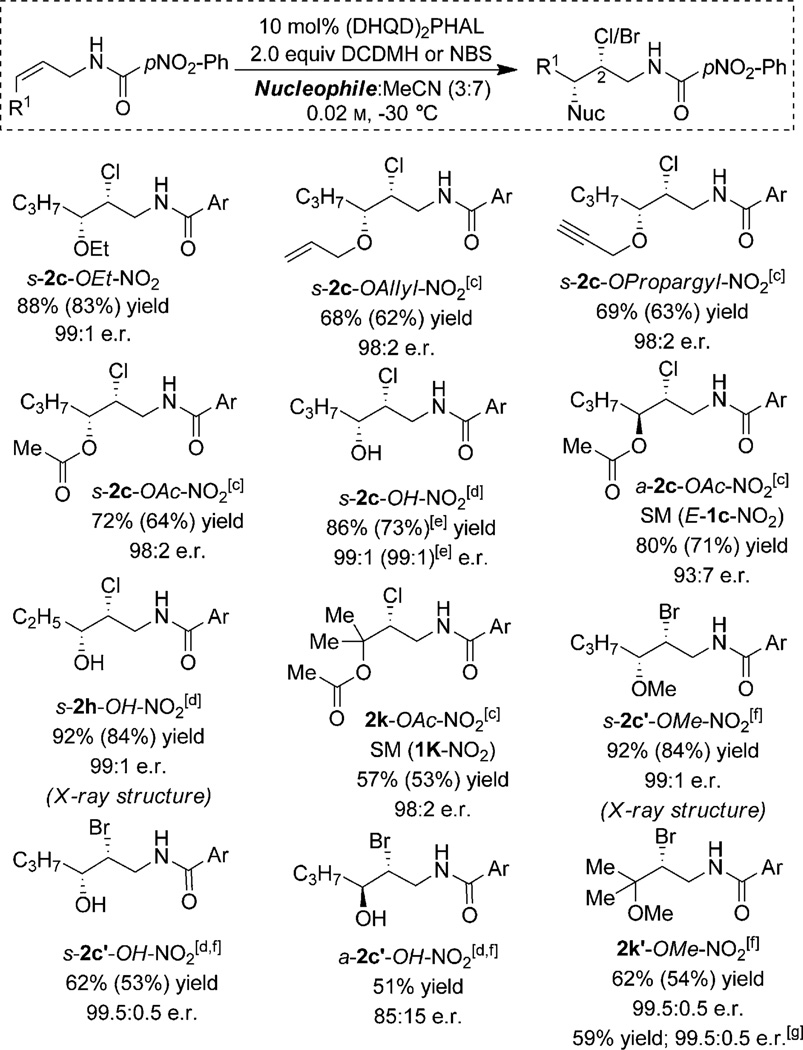
Finally, we sought to explore the scope of this reaction with regards to the nucleophilic and electrophilic components (Scheme 1). We were delighted to discover that a variety of alcohols, carboxylic acids, and water may be employed as viable nucleophiles in this chemistry with little or no modification of the optimized reaction conditions. Replacing MeOH with other alcohols such as ethanol, allyl alcohol, and propargyl alcohol as the co-solvents cleanly afford the desired products in more than 20:1 d.r. and more than 98:2 e.r. (see s-2c-OEt-NO2, s-2c-OAllyl-NO2 and s-2c-OPropargyl-NO2 in Scheme 1). These results demonstrate the feasibility of introducing diverse functional handles into the products using this chemistry, along with the highly stereoselective C–Cl and C–O bond installations during the course of the reaction.
Acetic acid can be employed also as the nucleophilic cosolvent to furnish the corresponding chloroacetates with excellent enantioselectivity with Z-, E-, as well as trisubstituted alkene substrates (≥ 93:7 e.r., see s-2c-OAc-NO2, a-2c-OAc-NO2, and 2k-OAc-NO2 in Scheme 1). Employing water as the nucleophile leads directly to the corresponding chlorohydrins in excellent yields and e.r. values (see s-2c-OH-NO2 and s-2h-OH-NO2 in Scheme 1). Finally, employing NBS in lieu of DCDMH leads to the corresponding bromoether and bromohydrin products in good yields and e.r. (see s-2c′-OMe-NO2, s-2c′-OH-NO2, a-2c′-OH-NO2, and 2k′-OMe-NO2 in Scheme 1). The quasi-enantiomeric (DHQ)2PHAL catalyst gave practically identical results favoring the opposite enantiomeric product (Scheme 1, ent-2k′-OMe-NO2). It warrants emphasis that a large excess of the nucleophile (>100 equiv) is currently required to prevent predominant formation of cyclized products. Our lab is currently in the process of addressing this limitation to enable the use of highly functionalized nucleophiles in this chemistry.
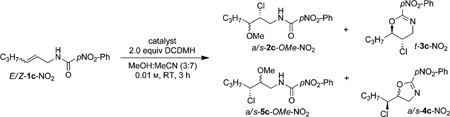
A comparison of the non-catalyzed and catalyzed chloroetherification of E-1c-NO2 and Z-1c-NO2 is illuminating (Table 4). Meticulous characterization of the products of the non-catalyzed reaction revealed a poor regioselectivity (3:1 and 4:1, respectively, for E-1c-NO2 and Z-1c-NO2) and only a marginal preference for the intermolecular capture of the putative chloriranium ion over the intramolecular capture by the amide (ca. 3:2 for E-1c-NO2 and ca. 3:1 ratio for Z-1c-NO2 substrates). Under catalyzed reaction conditions, however, the regioselectivity was superb for both E-1c-NO2 and Z-1c-NO2 (>20:1 r.r.). Additionally, the intermolecular chloroetherification product was predominant in both instances (by a ratio of 10:1 and more than 99:1 respectively, for E-1c-NO2 and Z-1c-NO2). These results underscore the exquisite levels of catalyst control in these reactions (see the Supporting Information for HPLC traces of the crude reaction mixtures). It is noteworthy that the diastereoselectivity is >99:1, even for non-catalyzed reactions. Two other facts are highly significant to report. First, the face selectivity in chlorenium delivery for the intramolecular process is the same for both E and Z substrates (see the Supporting Information, Figure S3 for chemical transformations).[12] Second, surprisingly, the olefin face selectivity of chlorenium transfer for the intramolecular process is opposite that of the intermolecular reaction (Supporting Information, Figure S4). These results lead us to conclude that two distinct mechanisms are in play that leads to either the cyclized dihydrooxazine products or the desired intermolecular addition of the nucleophile and halenium ion across the alkene in the same reaction.
Table 4.
Demonstration of catalyst control in the intermolecular chloroetherification of allyl amides.[a]
 | |||||||
|---|---|---|---|---|---|---|---|
| Substrate | Catalyst | ratio 2c:5c:3c:4c |
r.r. (2c:5c)[a] |
ratio (2c+5c):(3c+4c) |
d.r. (s-2c:a-2c) |
d.r. (s-5c:a-5c) |
d.r. (s-4c:a-4c) |
| E-1c-NO2 | none | 43:15:26:16 | 3:1 | 3:2 | <1:99 | <1:99 | <1:99 |
| E-1c-NO2 | 10% (DHQD)2PHAL | 87:4:7:2 | 21:1 | 10:1 | <1:99 | <1:99 | <1:99 |
| Z-1c-NO2 | none | 57:16:0:27 | 4:1 | 3:1 | >99:1 | >99:1 | >99:1 |
| Z-1c-NO2 | 10% (DHQD)2PHAL | 96:4:0:0 | 24:1 | >99:1 | >99:1 | >99:1 | >99:1 |
Regioselectivity (r.r.) and ratio of uncyclized to cyclized products was determined by HPLC.
A complete mechanistic picture that accounts for this nucleophile-dependent stereodivergence is yet to emerge. Nonetheless, we have recently demonstrated the importance of the nucleophile in modulating the ease of chlorenium capture by the alkene functionality by means of an anchimeric assistance.[3ac] This aspect among others is currently being looked into for explaining the observed stereoselectivity.
Supplementary Material
Acknowledgments
We are grateful to the NSF (CHE-1362812) and NIH (GM110525) for funding.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201502341.
Contributor Information
Bardia Soltanzadeh, Department of Chemistry, Michigan State University, E. Lansing, MI 48824 (USA).
Arvind Jaganathan, Engineering and Process Sciences, Core R&D, The Dow Chemical Company, Midland, MI 48674 (USA).
Richard J. Staples, Department of Chemistry, Michigan State University, E. Lansing, MI 48824 (USA)
Babak Borhan, Email: babak@chemistry.msu.edu, Department of Chemistry, Michigan State University, E. Lansing, MI 48824 (USA).
References
- 1.a) Denmark SE, Burk MT, Hoover AJ. J. Am. Chem. Soc. 2010;132:1232. doi: 10.1021/ja909965h. [DOI] [PubMed] [Google Scholar]; b) Neverov AA, Brown RS. J. Org. Chem. 1996;61:962. [Google Scholar]
- 2.a) Olah GA, Bollinger JM. J. Am. Chem. Soc. 1967;89:2993. doi: 10.1021/ja00988a034. [DOI] [PubMed] [Google Scholar]; b) Yousefi R, Ashtekar KD, Whitehead DC, Jackson JE, Borhan B. J. Am. Chem. Soc. 2013;135:14524. doi: 10.1021/ja4072145. [DOI] [PubMed] [Google Scholar]
- 3.a) Chen J, Zhou L, Yeung Y-Y. Org. Biomol. Chem. 2012;10:3808. doi: 10.1039/c2ob25327e. [DOI] [PubMed] [Google Scholar]; b) Chen Z-M, Zhang Q-W, Chen Z-H, Li H, Tu Y-Q, Zhang F-M, Tian J-M. J. Am. Chem. Soc. 2011;133:8818. doi: 10.1021/ja201794v. [DOI] [PubMed] [Google Scholar]; c) Denmark SE, Burk MT. Org. Lett. 2011;13:256. doi: 10.1021/ol203033k. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Dobish MC, Johnston JN. J. Am. Chem. Soc. 2012;134:6068. doi: 10.1021/ja301858r. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Fang C, Paull DH, Hethcox JC, Shugrue CR, Martin SF. Org. Lett. 2012;14:6290. doi: 10.1021/ol3030555. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Garzan A, Jaganathan A, Salehi Marzijarani N, Yousefi R, Whitehead DC, Jackson JE, Borhan B. Chem. Eur. J. 2013;19:9015. doi: 10.1002/chem.201300189. [DOI] [PubMed] [Google Scholar]; g) Haas J, Bissmire S, Wirth T. Chem. Eur. J. 2005;11:5777. doi: 10.1002/chem.200500507. [DOI] [PubMed] [Google Scholar]; h) Huang D, Wang H, Xue F, Guan H, Li L, Peng X, Shi Y. Org. Lett. 2011;13:6350. doi: 10.1021/ol202527g. [DOI] [PubMed] [Google Scholar]; i) Jaganathan A, Garzan A, Whitehead DC, Staples RJ, Borhan B. Angew. Chem. Int. Ed. 2011;50:2593. doi: 10.1002/anie.201006910. Angew. Chem.2011, 123, 2641. [DOI] [PubMed] [Google Scholar]; j) Jaganathan A, Staples RJ, Borhan B. J. Am. Chem. Soc. 2013;135:14806. doi: 10.1021/ja407241d. [DOI] [PubMed] [Google Scholar]; k) Kang SH, Lee SB, Park CM. J. Am. Chem. Soc. 2003;125:15748. doi: 10.1021/ja0369921. [DOI] [PubMed] [Google Scholar]; l) Kwon HY, Park CM, Lee SB, Youn JH, Kang SH. Chem. Eur. J. 2008;14:1023. doi: 10.1002/chem.200701199. [DOI] [PubMed] [Google Scholar]; m) Li H, Zhang F-M, Tu Y-Q, Zhang Q-W, Chen Z-M, Chen Z-H, Li J. Chem. Sci. 2011;2:1839. [Google Scholar]; n) Lozano O, Blessley G, Martinez del Campo T, Thompson AL, Giuffredi GT, Bettati M, Walker M, Borman R, Gouverneur V. Angew. Chem. Int. Ed. 2011;50:8105. doi: 10.1002/anie.201103151. Angew. Chem.2011, 123, 8255. [DOI] [PubMed] [Google Scholar]; o) Murai K, Matsushita T, Nakamura A, Fukushima S, Shimura M, Fujioka H. Angew. Chem. Int. Ed. 2010;49:9174. doi: 10.1002/anie.201005409. Angew. Chem.2014, 126, 7094. [DOI] [PubMed] [Google Scholar]; p) Murai K, Nakamura A, Matsushita T, Shimura M, Fujioka H. Chem. Eur. J. 2012;18:8448. doi: 10.1002/chem.201200647. [DOI] [PubMed] [Google Scholar]; q) Nakatsuji H, Sawamura Y, Sakakura A, Ishihara K. Angew. Chem. Int. Ed. 2014;53:6974. doi: 10.1002/anie.201400946. Angew. Chem.2014, 126, 7094. [DOI] [PubMed] [Google Scholar]; r) Paull DH, Fang C, Donald JR, Pansick AD, Martin SF. J. Am. Chem. Soc. 2012;134:11128. doi: 10.1021/ja305117m. [DOI] [PMC free article] [PubMed] [Google Scholar]; s) Phipps RJ, Hiramatsu K, Toste FD. J. Am. Chem. Soc. 2012;134:8376. doi: 10.1021/ja303959p. [DOI] [PubMed] [Google Scholar]; t) Tan CK, Le C, Yeung Y-Y. Chem. Commun. 2012;48:5793. doi: 10.1039/c2cc31148h. [DOI] [PubMed] [Google Scholar]; u) Veitch GE, Jacobsen EN. Angew. Chem. Int. Ed. 2010;49:7332. doi: 10.1002/anie.201003681. Angew. Chem.2010, 122, 7490. [DOI] [PMC free article] [PubMed] [Google Scholar]; v) Wang Y-M, Wu J, Hoong C, Rauniyar V, Toste FD. J. Am. Chem. Soc. 2012;134:12928. doi: 10.1021/ja305795x. [DOI] [PubMed] [Google Scholar]; w) Whitehead DC, Yousefi R, Jaganathan A, Borhan B. J. Am. Chem. Soc. 2010;132:3298. doi: 10.1021/ja100502f. [DOI] [PMC free article] [PubMed] [Google Scholar]; x) Wilking M, Mück-Lichtenfeld C, Daniliuc CG, Hennecke U. J. Am. Chem. Soc. 2013;135:8133. doi: 10.1021/ja402910d. [DOI] [PubMed] [Google Scholar]; y) Zhang W, Liu N, Schienebeck CM, Decloux K, Zheng S, Werness JB, Tang W. Chem. Eur. J. 2012;18:7296. doi: 10.1002/chem.201103809. [DOI] [PubMed] [Google Scholar]; z) Zhang W, Zheng S, Liu N, Werness JB, Guzei IA, Tang W. J. Am. Chem. Soc. 2010;132:3664. doi: 10.1021/ja100173w. [DOI] [PubMed] [Google Scholar]; aa) Zhou L, Chen J, Tan CK, Yeung Y-Y. J. Am. Chem. Soc. 2011;133:9164. doi: 10.1021/ja201627h. [DOI] [PubMed] [Google Scholar]; ab) Zhou L, Tan CK, Jiang X, Chen F, Yeung Y-Y. J. Am. Chem. Soc. 2010;132:15474. doi: 10.1021/ja1048972. [DOI] [PubMed] [Google Scholar]; ac) Ashtekar KD, Marzijarani NS, Jaganathan A, Holmes D, Jackson JE, Borhan B. J. Am. Chem. Soc. 2014;136:13355. doi: 10.1021/ja506889c. [DOI] [PMC free article] [PubMed] [Google Scholar]; ad) Jaganathan A, Borhan B. Org. Lett. 2014;16:3616. doi: 10.1021/ol500861z. [DOI] [PubMed] [Google Scholar]; ae) Yousefi R, Whitehead DC, Mueller JM, Staples RJ, Borhan B. Org. Lett. 2011;13:608. doi: 10.1021/ol102850m. [DOI] [PubMed] [Google Scholar]
- 4.a) Chen J, Zhou L. Synthesis. 2014;46:586. [Google Scholar]; b) Castellanos A, Fletcher SP. Chem. Eur. J. 2011;17:5766. doi: 10.1002/chem.201100105. [DOI] [PubMed] [Google Scholar]; c) Chemler SR, Bovino MT. ACS Catal. 2013;3:1076. doi: 10.1021/cs4001249. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Hennecke U. Chem. Asian J. 2012;7:456. doi: 10.1002/asia.201100856. [DOI] [PubMed] [Google Scholar]; e) Hennecke U. Angew. Chem. Int. Ed. 2012;51:4532. doi: 10.1002/anie.201200831. Angew. Chem.2012, 124, 4608. [DOI] [PubMed] [Google Scholar]; f) Rouf A, Taneja SC. Chirality. 2014;26:63. doi: 10.1002/chir.22268. [DOI] [PubMed] [Google Scholar]; g) Tan CK, Yu WZ, Yeung YY. Chirality. 2014;26:328. doi: 10.1002/chir.22272. [DOI] [PubMed] [Google Scholar]; h) Murai K, Fujioka H. Heterocycles. 2013;87:763. [Google Scholar]; i) Snyder SA, Treitler DS, Brucks AP. Aldrichimica Acta. 2011;44:27. [Google Scholar]; j) Tan CK, Zhou L, Yeung YY. Synlett. 2011:1335. [Google Scholar]; k) Denmark SE, Kuester WE, Burk MT. Angew. Chem. Int. Ed. 2012;51:10938. doi: 10.1002/anie.201204347. Angew. Chem.2012, 124, 11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Alix A, Lalli C, Retailleau P, Masson G. J. Am. Chem. Soc. 2012;134:10389. doi: 10.1021/ja304095z. [DOI] [PubMed] [Google Scholar]; b) Cai Y, Liu X, Zhou P, Kuang Y, Lin L, Feng X. Chem. Commun. 2013;49:8054. doi: 10.1039/c3cc44421j. [DOI] [PubMed] [Google Scholar]; c) Cai YF, Liu XH, Hui YH, Jiang J, Wang WT, Chen WL, Lin LL, Feng XM. Angew. Chem. Int. Ed. 2010;49:6160. doi: 10.1002/anie.201002355. Angew. Chem.2010, 122, 6296. [DOI] [PubMed] [Google Scholar]; d) Cai YF, Liu XH, Jiang J, Chen WL, Lin LL, Feng XM. J. Am. Chem. Soc. 2011;133:5636. doi: 10.1021/ja110668c. [DOI] [PubMed] [Google Scholar]; e) Cai YF, Liu XH, Li J, Chen WL, Wang WT, Lin LL, Feng XM. Chem. Eur. J. 2011;17:14916. doi: 10.1002/chem.201102453. [DOI] [PubMed] [Google Scholar]; f) Honjo T, Phipps RJ, Rauniyar V, Toste FD. Angew. Chem. Int. Ed. 2012;51:9684. doi: 10.1002/anie.201205383. Angew. Chem.2012, 124, 9822. [DOI] [PubMed] [Google Scholar]; g) Li G-X, Fu Q-Q, Zhang X-M, Jiang J, Tang Z. Tetrahedron: Asymmetry. 2012;23:245. [Google Scholar]; h) Li L, Su C, Liu X, Tian H, Shi Y. Org. Lett. 2014;16:3728. doi: 10.1021/ol501542r. [DOI] [PubMed] [Google Scholar]; i) Nicolaou KC, Simmons NL, Ying YC, Heretsch PM, Chen JS. J. Am. Chem. Soc. 2011;133:8134. doi: 10.1021/ja202555m. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Qi J, Fan GT, Chen J, Sun MH, Dong YT, Zhou L. Chem. Commun. 2014;50:13841. doi: 10.1039/c4cc05772d. [DOI] [PubMed] [Google Scholar]; k) Zhang W, Liu N, Schienebeck CM, Zhou X, Izhar II, Guzei IA, Tang W. Chem. Sci. 2013;4:2652. [Google Scholar]; l) Zhang Y, Xing H, Xie W, Wan X, Lai Y, Ma D. Adv. Synth. Catal. 2013;355:68. [Google Scholar]; m) Hu DX, Shibuya GM, Burns NZ. J. Am. Chem. Soc. 2013;135:12960. doi: 10.1021/ja4083182. [DOI] [PubMed] [Google Scholar]; n) Hu DX, Seidl FJ, Bucher C, Burns NZ. J. Am. Chem. Soc. 2015;137:3795. doi: 10.1021/jacs.5b01384. [DOI] [PubMed] [Google Scholar]
- 6.Brucks AP, Treitler DS, Liu SA, Snyder SA. Synthesis. 2013;45:1886. [Google Scholar]
- 7.a) Hennecke U, Muller CH, Frohlich R. Org. Lett. 2011;13:860. doi: 10.1021/ol1028805. [DOI] [PubMed] [Google Scholar]; b) Ke Z, Tan CK, Chen F, Yeung YY. J. Am. Chem. Soc. 2014;136:5627. doi: 10.1021/ja5029155. [DOI] [PubMed] [Google Scholar]; c) Müller CH, Rosner C, Hennecke U. Chem. Asian J. 2014;9:2162. doi: 10.1002/asia.201402229. [DOI] [PubMed] [Google Scholar]; d) Tay DW, Leung GY, Yeung YY. Angew. Chem. Int. Ed. 2014;53:5161. doi: 10.1002/anie.201310136. Angew. Chem.2014, 126, 5261. [DOI] [PubMed] [Google Scholar]; e) Zeng X, Miao C, Wang S, Xia C, Sun W. Chem. Commun. 2013;49:2418. doi: 10.1039/c2cc38436a. [DOI] [PubMed] [Google Scholar]
- 8.For example, the best enantioselectivities seen for aliphatic substrates in vicinal dihalogenation: 78:22 e.r. (Ref. [5i]); haloesterification: 60:40 e.r. (Ref. [5k]); halohydrin synthesis: 84:16 e.r. (one example, Ref. [5l]).
- 9.Where such substrates have been studied, the reason for poor stereoselectivity and yields has not been established. For an elegant description of the role of regioselectivity in eroding enantioselectivity in vicinal dihalogenation, see Ref. [5i].
- 10.We have opted to use a systematic naming system that enables the reader to identify the relevant components of the starting materials and products easily. Since all starting materials are substituted allyl amides, they are defined as E or Z (where appropriate), followed by a number (1a, 1b,…) that is unique to the substitution on the olefin. The naming is completed by defining the phenyl substituent of the amide moiety (NO2, Br,…), which is always on the para position. The naming of the intermolecular products precede by a or s (anti or syn), followed by a number (2a, 2b,…) that corresponds to the substituent on the parent olefin. The third component (in italics) is the identity of the nucleophile (OMe, OH,…), followed by the substituent on the phenyl group of the amide. The six-membered-ring intramolecular products are identified as either cis or trans (c or t), followed by the number that corresponds to the substituent on the parent olefin (3a, 3b,…), and then the identifier of the phenyl substituent for the amide. The five-membered-ring products are named as above, with the exception of having a or s (anti or syn) that precedes the numbering (4a, 4b,…).
- 11.See the Supporting Information for detailed DCDMH loading studies and optimization of other variables.
- 12.The absolute stereochemistry of s-2h-OH-NO2 and s-2c-OMe-NO2, and the relative stereochemistry of a-2c-OH-NO2, were established by single-crystal X-ray diffraction. Since the absolute stereochemistry of a-2c-OH-NO2 could not be determined from X-ray analysis, we resorted to the chemical transformations detailed in the Supporting Information, Figure S3 for confirmation of the structure. The absolute stereochemistry of the following compounds was determined by single-crystal X-ray diffraction (their corresponding Cambridge Structural Database deposition numbers are provided following each compound name). These are: s-4c-NO2 (1039232), s-2h-OH-NO2 (994564), a-2c-OH-NO2 (1039233), s-2c′-OMe-NO2 (1039234), t-3c-NO2 (1039235), and s-2c-OMe-NO2 (994565).
- 13.Structure of the “Ritter-type” addition product is depicted in the Supporting Information (see 2c-NHAc-NO2). Solvents other than MeCN were also evaluated as co-solvents; significantly inferior results were obtained in all instances.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.