Abstract
Background: The surveillance of patients with nondysplastic Barrett’s esophagus (NDBE) has a high cost and is of limited effectiveness in preventing esophageal adenocarcinoma (EAC). Ablation for NDBE remains expensive and controversial. Biomarkers of genomic instability have shown promise in identifying patients with NDBE at high risk for progression to EAC. Here, we evaluate the cost-effectiveness of using such biomarkers to stratify patients with NDBE by risk for EAC and, subsequently, the cost-effectiveness of ablative therapy.
Methods: A Markov decision tree was used to evaluate four strategies in a hypothetical cohort of 50-year old patients with NDBE over their lifetime: strategy I, natural history without surveillance; strategy II, surveillance per current guidelines; strategy III, ablation for all patients; strategy IV, risk stratification with use of a biomarker panel to assess genomic instability (i. e., mutational load [ML]). Patients with no ML underwent minimal surveillance, patients with low ML underwent standard surveillance, and patients with high ML underwent ablation. The incremental cost-effectiveness ratio (ICER) and incremental net health benefit (INHB) were assessed.
Results: Strategy IV provided the best values for quality-adjusted life years (QALYs), ICER, and INHB in comparison with strategies II and III. Results were robust in sensitivity analysis. In a Monte Carlo analysis, the relative risk for the development of cancer in the patients managed with strategy IV was decreased. Critical determinants of strategy IV cost-effectiveness were the complete response rate, cost of ablation, and surveillance interval in patients with no ML.
Conclusion: The use of ML to stratify patients with NDBE by risk was the most cost-effective strategy for preventive EAC treatment. Targeting ablation toward patients with high ML presents an opportunity for a paradigm shift in the management of NDBE.
Introduction
Over the last decade, significant advances have been made in our understanding of the natural history of Barrett’s esophagus and the subsequent risk for progression to advanced neoplasia. In addition, the endoscopic ablative treatment of advanced neoplasia has improved markedly. However, the management of nondysplastic Barrett’s esophagus (NDBE) continues to be based primarily on conservative endoscopic surveillance 1. The assumptions that endoscopic surveillance can accurately detect progression to high grade dysplasia (HGD) and esophageal adenocarcinoma (EAC) and that early intervention makes a positive impact on survival have not yet been demonstrated in any large controlled prospective trials. Logistical, cost, and ethical issues severely limit the feasibility of such trials. However, a series of well-conducted economic analyses looking into the most cost-effective management of NDBE have consistently demonstrated that expectant periodic endoscopic surveillance is costly and ineffective 2 3. Our earlier work indicated that an aggressive strategy of endoscopic ablation in all patients with NDBE is generally not cost-effective and would be cost-effective only if targeted to a high risk subset of patients 4. Therefore, additional management strategies, particularly ones that include risk stratification, need to be developed and evaluated.
Molecular biomarkers of genomic instability, including loss of heterozygosity (LOH) mutations near genes encoding tumor suppressor proteins (TP53 and CDKN2A), are associated with neoplastic progression in patients who have NDBE 5 6 7. Panels of such biomarkers have the potential to provide a longer detection window and, subsequently, early intervention in patients with NDBE based on their risk for neoplastic progression. Until fairly recently, this molecular testing was limited to academic and research efforts in which fresh tissue was used. However, testing can now be performed on formalin-fixed, paraffin-embedded tissue slides in a central laboratory, obviating the need for fresh tissue. These advancements have led to the development of a commercially available biomarker panel for assessing genomic instability that can be used in the clinical management of patients with Barrett’s esophagus.
Mutational load (ML) is a summary measure of genomic instability across a panel of biomarkers often mutated in EAC 5 7. ML assesses the presence and extent of genomic instability by measuring the number and clonality of LOH mutations in tissue with Barrett’s esophagus – related histology. Mutations interrogated include LOH in 17 p (TP53), 9 p (CDKN2A), 1 p (CMM1, L-myc), 3 p (VHL, HoGG1), 5q (MCC, APC), 10q (PTEN, MXI1), 17q (NME1), 18q (DCC), 21q (TFF1, PSEN2), and 22q (NF2) genomic loci. ML also assesses the presence of microsatellite instability (MSI) at these loci. In cross-sectional studies, a small percentage of NDBE-derived histological tissue had ML similar to that of higher risk histological tissue (i. e., HGD or EAC), suggesting that ML may be predictive of impending, higher risk morphological changes 5 7. Findings from a longitudinal study further demonstrate that patients who have NDBE that eventually progresses to HGD or EAC have elevated ML before the onset of histological progression 8. Thus, ML assessment has the potential to improve patient management by providing a measure of genomic instability that could signal increased risk for future progression to EAC.
Herein, we update and build upon our previous analysis of the cost-effectiveness of Barrett’s ablation strategies. We compare the current American College of Gastroenterology (ACG) guideline-recommended standard of care for patients who have NDBE with one in which ablation is indicated only for patients with NDBE who have a high risk for progression based on overall levels of genomic instability 1 9. We report cost savings and incremental gains in quality-adjusted life years with the use of ML-based risk stratification and subsequent selective endoscopic ablative therapy for the preventative treatment of EAC.
Methods
In this decision analysis, we considered a hypothetical cohort of white male patients with a mean age of 50 years in whom NDBE had recently been diagnosed during esophagogastroduodenoscopy (EGD) based on the ACG definition 10.
Model
We used commercially available decision analysis software (TreeAge Pro; TreeAge Software, Williamstown, Massachusetts, USA) to develop a hybrid model of a linear decision tree terminating in a Markov model to compare different strategies for the management of NDBE (Supplementary Fig. 1) 11. Various health and disease states (e. g., healthy, NDBE, Barrett’s esophagus with low grade dysplasia (LGD), esophageal cancer, postoperative state, and finally death), each associated with a different set of costs and utilities (Supplementary Fig. 2), were used to model the natural history of patients with Barrett’s esophagus. At entry into the model, it was considered that all members of the cohort had already undergone the index EGD confirming the diagnosis of Barrett’s esophagus without surface nodularity or masses and on biopsy would have only NDBE. At the end of each 1-year cycle of the model, they would be redistributed to different states depending on the estimated transitional probabilities among the various health states. Death was considered an absorbing state. The time horizon of the model was the lifetime of the cohort. The analysis was conducted according to the recommendations of the Panel on Cost-effectiveness in Health and Medicine for conducting and reporting a reference case analysis with a societal perspective 12.
Supplementary Fig. 1.
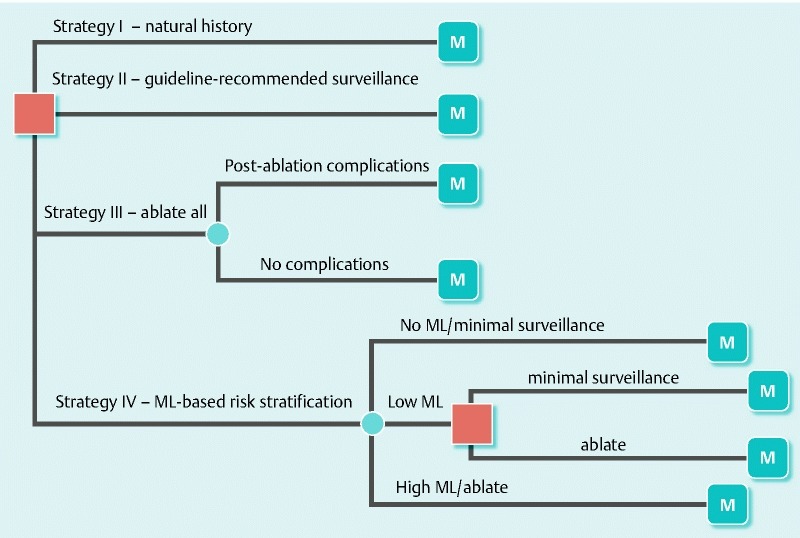
The hybrid model of the linear decision tree terminating in Markov models. In the decision tree, a square node represents the decision node at entry, the filled circles are chance nodes, and the circles with the letter M represent Markov nodes. In strategy I, after the diagnosis of nondysplastic Barrett’s esophagus (NDBE) was established, the natural history of this condition was modeled without any specific intervention. In strategy II, all patients with NDBE underwent periodic endoscopic surveillance according to the current guidelines of the American College of Gastroenterology. In strategy III, all patients with NDBE underwent endoscopic ablation. In strategy IV, all patients with NDBE underwent risk stratification for esophageal adenocarcinoma, and subsequently ablation, based on mutational load (ML); Patients with no ML underwent minimal surveillance, patients with low ML underwent standard surveillance or ablative therapy (another decision point), and patients with high ML were treated selectively with endoscopic ablation.
Supplementary Fig. 2.
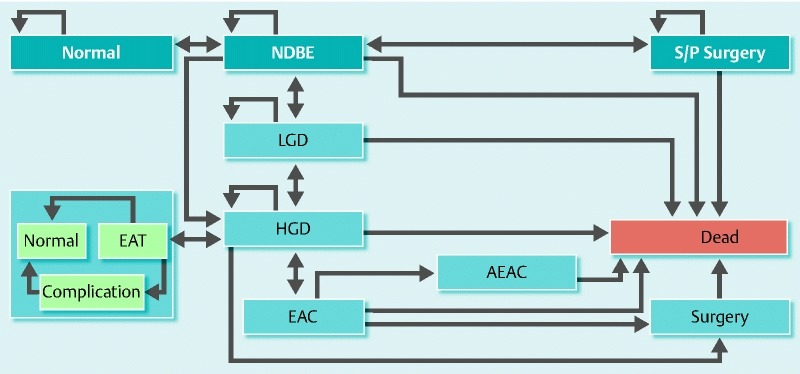
Health and disease state transitions in the Markov model. The natural history of patients with nondysplastic Barrett’s esophagus (NDBE) was modeled for various health and disease states, each associated with a different set of costs and utilities. A single arrowhead indicates transition from one state to another in the direction of the arrowhead; double arrowheads indicate that transitions in both directions are allowed in the model. Half-circle arrowheads represent states in which a patient can remain indefinitely. The light blue area represents stages associated with endoscopic ablative therapy (EAT), if allowed in the model. Normal, no Barrett’s esophagus; LGD, low grade dysplasia; HGD, high grade dysplasia; EAC, esophageal adenocarcinoma; AEAC, advanced esophageal adenocarcinoma; S/P Surgery, status post surgery.
Strategies compared
Four strategies were compared (Supplementary Methods):
Strategy I. “Natural history” of NDBE: No surveillance or interventions were used for NDBE with this strategy.
Strategy II. “Guideline-recommended surveillance” of NDBE: This strategy followed the ACG treatment guidelines for NDBE.
Strategy III. “Ablate all” patients with NDBE: Preventative endoscopic ablation therapy for NDBE was modeled primarily after a stepwise ablation procedure with the HALO Ablation System (Barrx Medical, Sunnyvale, California, USA).
Strategy IV. “ML-based risk stratification” according to levels of genomic instability: ML was assessed with BarreGen and PathFinderTG (Interpace Diagnostics, Pittsburgh, Pennsylvania, USA; formerly RedPath Integrated Pathology) in esophageal biopsy specimens from patients with NDBE, and preventative ablation was performed based on each patient’s risk for progression to EAC.
Clinical probabilities
Clinical probabilities, including transitional probabilities and performance characteristics of endoscopy and biopsy in identifying different Barrett’s esophagus – related health states, were derived from the published literature. Literature references for clinical probabilities are presented in the Supplementary Methods and Supplementary Table 1.
Supplemental Table 1. Estimates of key clinical variables in the management of nondysplastic Barrett’s esophagus.
| Input variable | Reference case estimate | Range | References |
| Annual rate of progression in an average cohort of patients with NDBE, % | |||
| HGD to cancer | 0.07 | 0.001 – 0.3 | 35 46 47 48 |
| NDBE to HGD | 0.01 | 0.0028 – 0.083 | 35 46 49 50 |
| NDBE to cancer | 0.005 | 0.001 – 0.1 | 35 51 52 53 54 55 |
| NDBE to LGD | 0.05 | 0.01 – 0.078 | 35 47 49 53 56 57 |
| LGD to HGD | 0.05 | 0.001 – 0.078 | 35 36 47 50 56 |
| LGD to cancer | 0.01 | 0.005 – 0.05 | 36 |
| Risk factor expressed as multiple of annual rate of progression in patients with | 27 28 29 30 31 | ||
| No ML | 0.1 | 0.05 – 1 | |
| Low ML | 1 | 0.5 – 5 | |
| High ML | 2.5 | 1 – 10 | |
| Prevalence of ML in patients with NDBE, % | 27 28 | ||
| Low ML | 30 | 5 – 75 | |
| High ML | 8 | 1 – 25 | |
| Annual rate of regression, % | |||
| HGD to NDBE | 0.1 | 0.01 – 0.15 | 35 47 50 |
| HGD to LGD | 0.07 | 0.05 – 0.1 | 35 46 47 48 49 50 |
| NDBE to normal | 0.0175 | 0.001 – 0.02 | 35 58 59 |
| Efficacy of surgical treatment | |||
| Probability of surgical resectability | 35 46 60 61 62 63 64 | ||
| Diagnosis by surveillance | 0.8 | 0.5 – 1.0 | |
| Diagnosis by symptoms | 0.5 | 0.1 – 0.7 | |
| Probability of curative resection | |||
| Diagnosis by surveillance | 0.7 | 0.6 – 0.9 | |
| Diagnosis by symptoms | 0.2 | 0.1 – 0.43 | |
| Efficacy of ablative therapy | |||
| Probability of complete ablation after three sittings | 0.5 | 0.1 – 1.0 | 35 38 65 66 |
| Probability of complications with ablation | 35 38 65 66 | ||
| Total | 0.1 | 0 – 0.25 | |
| Perforation | 0.05 | 0 – 0.15 | |
| Stricture | 0.05 | 0 – 0.15 | |
| Mortality, % | |||
| From esophagectomy | 0.04 | 0.02 – 0.20 | 67 68 69 70 71 |
| From endoscopy | 0.00002 | 0 – 0.00005 | 72 73 74 75 76 |
| From surgery for repair of esophageal perforation | 0.08 | 0.05 – 0.15 | 77 |
| From advanced esophageal adenocarcinoma (annual probability) | 0.6 | 0.3 – 1.0 | 34, Assumption |
| Misdiagnosis rates | |||
| Cancer called HGD | 0.175 | 0.01 – 0.0.2 | 35 78 79 80 81 |
| Cancer called LGD | 0.05 | 0.01 – 0.10 | 35 78 79 80 81 |
| HGD called cancer | 0.11 | 0.01 – 0.20 | 35 78 79 80 81 |
| HGD called LGD | 0.115 | 0.01 – 0.20 | 35 78 79 80 81 |
| LGD called cancer | 0.05 | 0.01 – 0.10 | 35 80 81 |
| LGD called HGD | 0.083 | 0.01 – 0.10 | 35 80 81 82 |
| NDBE called HGD | 0.01 | 0.0 – 0.02 | 32 81 |
| NDBE called normal | 0.01 | 0.0 – 0.0.01 | 32 81 83 |
| Normal called NDBE | 0.01 | 0.0 – 0.005 | 34 |
| Patient preferences (utilities) for health states | |||
| NDBE | 1 | 0.8 – 1.0 | 35 81 |
| LGD/HGD | 0.99 | 0.8 – 1.0 | 37 |
| After esophagectomy | 0.97 | 0.8 – 1.0 | 34 36 37 81 84 |
| Early cancer | 0.9 | 0.8 – 1.0 | 81 |
| Late cancer | 0.34 | 0 – 1 | 81 84 |
| Stricture related to ablative therapy | 0.97 | 0.85 – 1.0 | 81 |
HGD, high grade dysplasia; NDBE, nondysplastic Barrett’s esophagus; LGD, low grade dysplasia; ML, mutational load.
Cost estimates and utilities
Costs, not charges, were considered in this analysis, and a third-party payer’s perspective was taken (Supplementary Methods and Supplementary Table 2). Only direct costs were considered, and all costs were adjusted to 2013 U.S. dollars. Costs were estimated based on the national average reimbursement allowed for each coded procedure by the Centers for Medicare & Medicaid Services (CMS) during the fiscal year 2013.
Supplemental Table 2. Estimates of key costs for the management of nondysplastic Barrett’s esophagus.
| Aspect of management | Reference case cost, US$ | Range, US$ | References |
| Esophagectomy | 19,000 | 10,000 – 40,000 | 34 35 37 80 |
| EGD with ablation | 10,000 | 5000 – 25,000 | 37, Assumption |
| EGD with biopsies | 830 | 350 – 1200 | 34 35 |
| Treatment for ablation-related stricture | 2500 | 1000 – 3000 | 35 80 |
| Chemotherapy | 10,750 | ± 25 % | 32 37 |
| Radiation | 5400 | ± 25 % | 32 37 |
| Annual follow-up after surgery for early cancer (first 5 years) | 1500 | 500 – 2000 | 37 |
| Hospice | 6228 | 4600 – 7800 | 37 |
| Discount rate, % | 3 | 0 – 7 | 85 |
| ML assessment | 3100 | 2500 – 7500 | CMS |
US, United States; EGD, esophagogastroduodenoscopy; ML, mutational load; CMS, Centers for Medicare & Medicaid Services.
Sensitivity analysis
Model robustness was evaluated with sensitivity analysis based on important clinical probabilities and cost estimates. A second-order Monte Carlo simulation was performed in the hypothetical cohort of 10,000 patients with NDBE for a probabilistic sensitivity analysis. This simulation recalculates a model multiple times, incorporating uncertainties into an analysis consistent with real-life situations 13. Using tracker variables, we compared the number of patients in whom EAC developed with the different strategies.
Outcomes compared and statistical methods
The incremental cost-effectiveness ratio (ICER) and incremental net health benefit (INHB) were the primary outcomes compared among the four strategies. ICER is the difference in costs between strategies divided by the difference in outcome (life years) between strategies: for example, ICER = (Cost Strategy I – Cost Strategy II)/(Effectiveness Strategy I – Effectiveness Strategy II)]. The ICER is a measure of the added cost for each additional life year gained with a given strategy relative to another strategy.
The net health benefit (NHB) has been increasingly used in the economic evaluation of health care intervention as an alternative to the ICER. The NHB is a function of effectiveness (E), cost (C), and willingness to pay (WTP; i. e., the decision maker’s threshold ICER) according to the following formula: NHB = E – C/WTP) 14 15. The NHB is the health effect of a treatment minus the benefit that one would have obtained by investing the resources spent on a marginally effective treatment. INHB) is the difference between two NHBs. INHB is emphasized here because it is often preferred to ICER as a measure of cost-effectiveness as it is a direct interpretation of the average health gained per patient who takes the different treatment adjusted for cost and WTP. Also, the INHB, as a monotonic function of both health and cost, tends to be easier to interpret; higher values are always better. Health policy makers would favor a strategy for which the INHB takes the greatest positive value in relation to values of WTP that seem reasonable with respect to known public policy.
To evaluate the results of the Monte Carlo analysis, relative risk (RR) with 95 % confidence intervals (CIs) and number needed to treat (NNT) were calculated. NNT was defined as the number of patients that needed to be treated per a selected strategy to prevent one case of esophageal cancer.
Assumptions
This model was based on several well-accepted, published assumptions used in decision analysis models for the management of Barrett’s esophagus (Supplementary Methods).
Results
Baseline analysis
In our baseline analysis, 50-year old subjects with NDBE who received no preventative intervention and followed the natural history of NDBE progression to HGD (strategy I) had an average of 17.567 quality-adjusted life years (QALYs) at a cost of $ 12,294 per patient (Table 1). Compared with patients managed under strategy I, those managed with guideline-recommended endoscopic surveillance (strategy II) gained an additional 0.656 QALY, but at an incremental cost of $ 9068 (Table 1). Endoscopic ablation for all patients with NDBE (strategy III) had a slightly lower cost per patient than strategy II. Regardless, this cost remained incrementally higher than strategy I ($ 7033) with only a small gain in QALYs (0.616) (Table 1). Compared with strategies II and III, risk stratification with ML (strategy IV) was the preferred strategy from a cost, QALY, and ICER perspective, with the highest yield of QALYs and lowest average cost per patient. Strategy IV remained the best strategy with the lowest cost and highest number of QALYs in a modified baseline analysis with the lowest ML-based risk stratification threshold (i. e.,“low ML”) as an indicator for ablation (Table 1). In this modified analysis, any positive level of ML (i. e., both “low ML” and “high ML”) was considered an indication for ablation (patients with “no ML” were excluded).
Table 1. Results of baseline analysis comparing all management strategies for patients with nondysplastic Barrett’s esophagus.
| Strategy | Cost, $* | QALYs gained, y | ICER ($/QALY) vs. strategy I | ICER ($/QALY) vs. strategy II | ICER ($/QALY) vs. strategy III | |
| I. Natural history | 12,924 | 17.567 | – | 13,823 | 11,417 | |
| II. Guideline-recommended surveillance | 21,992 | 18.223 | 13,823 | – | 2031 | |
| III. Ablate all | 19,957 | 18.183 | 11,417 | 2031 | – | |
| IV. ML-based risk stratification | Patients with “high ML” undergo ablation. | 17,234 | 19.081 | 2847 | – 5545 | – 3032 |
| Patients with “high ML” and “low ML” undergo ablation. | 16,461 | 18.763 | 2957 | – 10,243 | – 6,028 | |
QALYs, quality-adjusted life years; ICER, incremental cost-effectiveness ratio; ML, mutational load.
Direct costs of care per patient (listed in Supplemental Table 2) from a third-party perspective accrued over the lifetime of patients as they transition from one health state to another based on the different transitional probabilities in yearly cycles, as modeled in the decision tree.
Monte Carlo analysis
In the Monte Carlo simulation, a total of 831 esophageal cancers developed in the natural course of NDBE (strategy I) over a calculated period of 174,853 person-years, for an average risk of 0.47 % per person-year. The numbers of esophageal cancers that were diagnosed under each strategy during the lifetime of this cohort were estimated at 831, 819, 596, and 402 for strategies I, II, III and IV, respectively. Thus, compared with a strategy of no preventative intervention (strategy I), the RR for the development of cancer with ML-based risk stratification (strategy IV) remained low (0.48 %, 95 %CI 0.43 – 0.54); the NNT for preventing cancer with this strategy was only 23 (95 %CI 20 – 28). Similarly, compared with guideline-recommended endoscopic surveillance (strategy II), the RR for the development of esophageal cancer with ML-based risk stratification (strategy IV) was low (0.49 %, 95 %CI 0.44 – 0.55), with an NNT of only 24 (95 %CI 21 – 28).
Supplemental Fig. 2 shows the proportions of iterations in the Monte Carlo analysis (y-axis) that are acceptable as cost-effective for each strategy against increasing WTP (x-axis). For all levels of WTP, a strategy of ML-based risk stratification (strategy IV) was the most cost-effective strategy in terms of incremental NHB (INHB), particularly when compared against the currently practiced guideline-recommended strategy of endoscopic surveillance (strategy II).
Sensitivity analysis
When a one-way sensitivity analysis was performed by varying important clinical probability and cost estimates, the model was sensitive to several clinically important model parameters. Fig.1 is a Tornado diagram of a series of one-way sensitivity analyses for NHB showing the different clinical and cost variables that impacted the NHB values of the two most competing strategies (i. e., guideline-recommended surveillance [strategy II] and ML-based risk stratification [strategy IV]). These particular variables were examined because they were supported by the most limited data. It is important to note that although the outcome values in dollar amounts changed as imputed values changed, the overall conclusion of the model with respect to NHB was unchanged; strategy IV yielded the highest NHB across a wide range of WTPs including a WTP of $50,000, which is routinely considered the threshold for an intervention to be cost-effective (Supplementary Fig. 3). Other important clinical parameters, such as the rate of complications with ablative therapy, frequency of endoscopic surveillance after complete ablation, annual probability of progression of HGD to cancer, quality of life after esophagectomy, and mortality rate after esophagectomy, did not significantly impact the conclusion of the baseline analysis (data not shown).
Fig. 1.
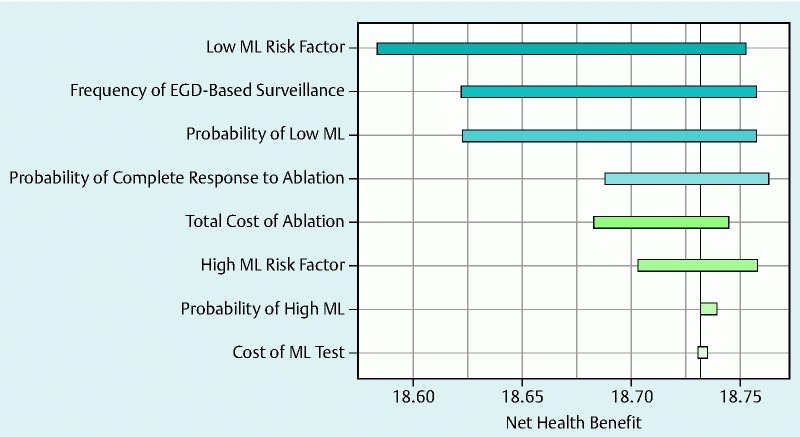
Tornado diagram for a series of one-way sensitivity analyses showing the impact of various clinical and cost variables on the net health benefit of the two most competitive strategies: a strategy based on standard surveillance (strategy II) and a strategy based on risk stratification with mutational load (strategy IV). These particular variables were examined because they were supported by the most limited available data. Although the incremental cost-effectiveness ratio changed significantly as the variables changed, the overall conclusion of the model that strategy IV yields the highest NHB at a willingness to pay of $ 50,000 was unchanged. The relative impact on the NHB is signified by the width of the horizontal bars. ML, mutational load; EGD, esophagogastroduodenoscopy.
Supplementary Fig. 3.
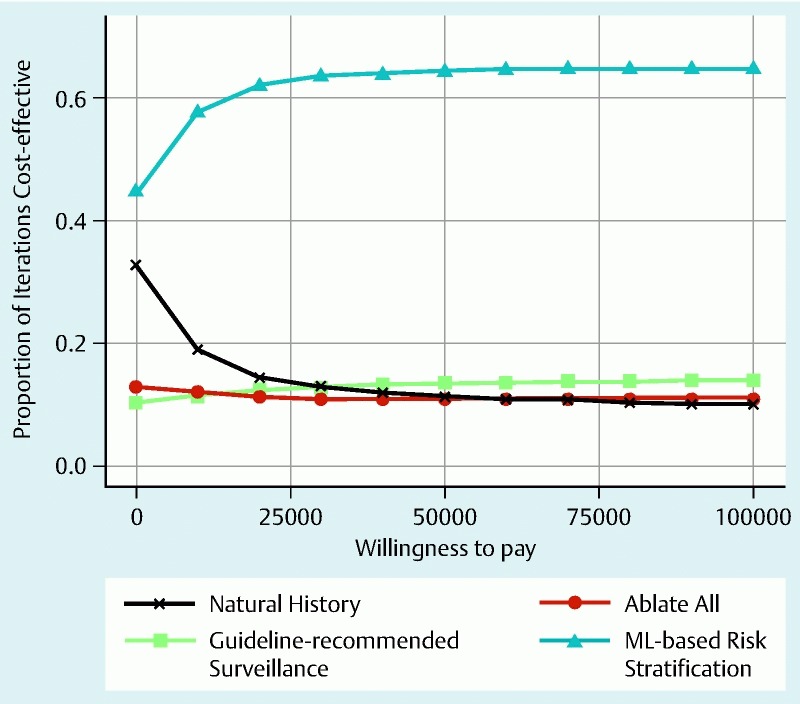
The proportions of iterations in the Monte Carlo analysis (y-axis) that are acceptable as cost-effective for each strategy against increasing willingness to pay (x-axis). A strategy of risk stratification based on mutational load (strategy IV, blue) was the most cost-effective strategy in terms of incremental net health benefit, particularly when compared with the currently practiced strategy of endoscopic surveillance (strategy II, green).
Discussion
In this cost-effectiveness analysis, we show that among competing strategies for managing patients with NDBE, risk stratification according to levels of genomic instability, with an ML-based approach, is superior to other strategies of patient management. Most importantly, this approach dominates the ACG 1 9 and other 16 guideline-recommended strategies for endoscopic surveillance. ML-based risk stratification not only yields the highest number of QALYs for patients but also costs less, resulting in comparatively lower costs per QALY gained (ICER). Moreover, it yields the highest INHB to patients, which remains robust when important cost and clinical variables are varied in sensitivity analysis. We used a modified version of a previously published decision analysis model, the internal validity of which was corroborated by the estimated lifetime risk of approximately 0.5 % per person-year for the development of EAC in the natural history arm. Such risk is supported by population-based studies of the natural history of actual patients with Barrett’s esophagus demonstrating an incidence of malignancy of approximately 0.5 % per year
Recent prospective studies have shown the safety, tolerability, and efficacy of endoscopic ablative therapy specifically in patients with NDBE 17 18 19. With well-designed studies showing high rates of complete elimination of intestinal metaplasia and eradication of pre-ablation oncogenetic abnormalities following ablation, concerns about the recurrence of metaplasia and the durability of post-ablation neo-squamous epithelium are decreasing 20 21. Recently, in another economic analysis, we studied the cost-effectiveness of a strategy based on preventative endoscopic ablative therapy in all patients with NDBE 4. Although the strategy yielded a higher number of QALYs compared with a guideline-recommended strategy of endoscopic surveillance, the cost-effectiveness of such a strategy was borderline, given the high cost of endoscopic ablation. Thus, ablation for all patients with NDBE could not be recommended for clinical practice from a health economics perspective. Of note, this economic analysis clearly called for a risk stratification strategy to identify and selectively perform ablation only in patients with NDBE who are at high risk for progression to advanced neoplasia. Consistently, the management strategy that included ML-based risk stratification of patients with NDBE, which is examined herein, was more cost-effective than ablation for all patients with NDBE.
Several biomarkers, such as DNA content abnormality (aneuploidy, tetraploidy), abnormalities at tumor suppressor gene loci (17 p and 9 p LOH), epigenetic changes (CDKN2A methylation), cell cycle markers (cyclin D1 expression), and proliferation markers, have been associated with progression from NDBE to EAC 5 6 7. In a landmark longitudinal study, Galipeau et al. 6 showed that a panel of abnormalities including 17 p LOH, DNA content irregularities (tetraploidy and aneuploidy), and 9 p LOH was the best predictor of esophageal cancer in comparison with individual biomarkers examined (RR 38.7, 95 %CI 10.8 – 138.5, P = 0.001). Patients with no baseline abnormality had a 10-year cumulative esophageal cancer incidence of 12 %, whereas patients with three abnormalities (17 p LOH, DNA aneuploidy, and 9 p LOH) had a 10-year cancer incidence of at least 79.1 % 6.
Additional studies have shown that risk for progression to EAC increases as the clonal expansion of cells with such mutations increases 22 23 24. Khara et al. recently reported the presence and extent of genomic instability by using ML in patients with NDBE and other, higher histological grades of disease 7. ML incorporated the presence and clonality of LOH mutations next to TP53 and CDKN2A, as well as eight additional genomic loci in proximity to other tumor suppressor genes. MSI mutation at these loci was also included in ML. Although some LOH and MSI damage was detected in NDBE, much more was accumulated in advanced stages of Barrett’s histology. A “high ML” level was present in up to 95 % and 96 % of patients with HGD and EAC, respectively, whereas only 8 % of patients with NDBE had a similar “high ML” level 7. In another, related longitudinal study of 69 patients with Barrett’s esophagus, Eluri et al. showed that patients with Barrett’s esophagus who had no or low levels of dysplasia but “high ML” levels were more likely to progress to HGD or EAC at a mean follow-up period of 4 years (adjusted odds ratio [OR] 166, P < 0.0001) 8.
A concern regarding the risk stratification of patients with NDBE based on biomarker panels has been the perceived high cost of biomarker testing, which led us to perform this economic analysis. Two previous decision analyses incorporated biomarker-based risk stratification strategies for the management of patients with NDBE 25 26. Both studies considered hypothetical biomarkers and were limited by significant assumptions regarding the performance characteristics and cost of these hypothetical tests. The earlier study did not consider endoscopic ablative therapy at all because it was not clinically available at the time of analysis 26. The recent study by Gordon et al. focused on the ability of biomarkers to modify NDBE surveillance intervals but did not incorporate endoscopic ablative therapy as a primary intervention 25. In our earlier work, we have shown that blanket endoscopic ablative therapy for NDBE is only borderline cost-effective 4. We have now furthered such analysis to show that limiting preventative endoscopic ablative therapy to those patients with NDBE at high risk for progression to HGD or EAC makes ablation convincingly cost-effective compared with current guideline-recommended endoscopic surveillance management.
Although our model shows promising cost-effectiveness for preventative ablation when it is limited to high risk patients with the use of biomarkers, it does have limitations. We did not account for any indirect costs and considered only stepwise radiofrequency ablation as the method of endoscopic ablation, although other ablation techniques are available. For patients with “low ML,” the endoscopic surveillance intervals were arbitrarily decided based on expert opinion, given the lack of robust long-term data on the risk for progression in these patients. However, sensitivity analyses showed that even ablation in all patients at lower risk for progression (i. e., those with “low ML”) was cost-effective and improved QALYs. The model also assumed that all specimens for ML analysis represented the worst Barrett’s-related histology present in the patient, which is a strong assumption, given the sampling variability associated with biopsy in these patients.
In summary, this economic analysis examined a strategy of using ML-based risk stratification with a commercially available panel of DNA markers to assess overall genomic instability in patients with NDBE. When endoscopic ablative therapy was selectively applied in patients at high risk for progression, the management of patients with NDBE was superior to the current guideline-recommended management strategy of conservative surveillance. Larger longitudinal studies of the use of biomarkers, such as ML, in combination with established clinical and endoscopic predictors to enhance risk stratification are urgently needed to convince clinicians and policy makers to change the current costly and ineffective practice of endoscopic surveillance.
Supplemental methods
Strategies compared
Four strategies were compared:
Strategy I. “Natural history” of nondysplastic Barrett’s esophagus (NDBE): No surveillance or interventions were used for NDBE with this strategy. Patients underwent esophagogastroduodenoscopy (EGD) only if a symptom suggestive of cancer, such as dysphagia or weight loss, developed. If cancer was discovered, patients with unresectable disease received palliative care (e. g., endoscopic therapy, chemoradiotherapy, hospice care). For those with resectable disease, the model accounted for surgery-related morbidity and mortality; it also accounted for the risk for recurrence. In addition to rates of mortality related to esophageal cancer, the U.S. life table mortality rates were incorporated into the model to account for age- and gender-specific annual mortality from all other causes.
Strategy II. “Guideline-recommended surveillance” of NDBE: This strategy followed the American College of Gastroenterology (ACG) treatment guidelines for NDBE. Patients with NDBE underwent surveillance every 3 years. If low grade dysplasia (LGD) was discovered, the frequency of surveillance was increased to annual until no dysplasia could be detected. If high grade dysplasia (HGD) was discovered, patients underwent ablation as detailed in strategy III. Patients with focal/nodular HGD underwent repeat EGD and endoscopic mucosal resection followed by ablation. Esophagectomy was considered for patients with esophageal adenocarcinoma (EAC) or persistent, diffuse, or multifocal HGD. When indicated, endoscopic therapy was performed for patients with nodular HGD or EAC. The possibility of misdiagnosis of histologic specimens, a common problem in Barrett’s esophagus, was included in the model based on published false-positive and false-negative rates. Surveillance continued to age 80.
Strategy III. “Ablate all” patients with NDBE: Preventative endoscopic ablation therapy for NDBE was modeled primarily after a stepwise ablation procedure with the HALO Ablation System (Barrx Medical, Sunnyvale, California, USA). All patients who had NDBE underwent preventative endoscopic ablation up to three times. Each time, patients incurred a risk for complications. Patients with perforations as a result of ablation were assumed to undergo esophagectomy. As noted for strategy I, the model accounted for surgery-related morbidity and mortality. Patients who had esophageal strictures as a result of ablation were assumed to undergo dilation; risks associated with dilation were included in the model. When ablation was successful, patients underwent surveillance annually for 3 years and then every 10 years in baseline analysis, with a range of 3 to every 10 years for sensitivity analysis. When ablation was incomplete, patients underwent surveillance as in strategy II and were considered to have the same risk for progression as at baseline.
Strategy IV. “Mutational load (ML) – based risk stratification” according to levels of genomic instability: ML was assessed with BarreGen and PathFinderTG (Interpace Diagnostics, Sunnyvale, California, USA; formerly RedPath Integrated Pathology) in esophageal biopsy specimens from patients with NDBE, and preventative ablation was performed based on each patient’s risk for progression to EAC. Patients with NDBE and minimal risk for progression to HGD or EAC (i. e., those with “no ML”) were followed as in strategy II, except that they underwent endoscopic surveillance every 10 years. Patients with a low risk for progression to HGD or EAC (i. e., those with “low ML”) were followed as in strategy II, although the model incorporated a second decision node to examine the possibility of managing these patients with an aggressive strategy in which they underwent endoscopic ablative therapy. Patients with a high risk for progression to HGD or EAC (i. e., those with “high ML”) underwent ablation as in strategy III. All patients with “high ML” underwent endoscopic surveillance once a year indefinitely, even if complete eradication of intestinal metaplasia was achieved.
Clinical probabilities
The MEDLINE database and abstracts from major gastroenterology meetings were searched for all relevant articles published from 1996 to 2015 by using the terms Barrett’s esophagus, esophageal cancer, gastroesophageal reflux disease, economic analysis, radiofrequency ablation, and photodynamic therapy. Also, the references of selected publications were searched manually. When specific published information was not available, expert opinion was obtained by consensus. Supplementary Table 2 lists the clinical probabilities imputed into the model.
Literature exists on the prevalence of ML in patients with NDBE, HGD, and EAC, as well as on the relative risk for progression from NDBE to EAC based on levels of genomic instability. Prevalence data for ML were primarily obtained from a recent study that assessed ML in 427 patients with NDBE; in this study, 30 % had “no ML,” 62 % had “low ML,” and 8 % had “high ML” 1. The ML-based risk for progression from NDBE to HGD was obtained from a review of the cumulative published literature citing genomic instability as a risk factor in progression of NDBE to EAC. To assess genomic instability, ML measures both loss of heterozygosity (LOH) and microsatellite instability (MSI) mutations at 9 p (CDKN2A) and 17 p (TP53), as well as at 8 additional genomic loci: 1 p (CMM1, L-myc), 3 p (VHL, HoGG1), 5q (MCC, APC), 10q (PTEN, MXI1), 17q (NME1), 18q (DCC), 21q (TFF1, PSEN2) and 22q (NF2) 27 28. In a longitudinal study, 243 patients with NDBE were evaluated at baseline for genomic instability by examining DNA content abnormalities (tetraploidy, aneuploidy) and TP53 (17 p) and CDKN2A (9 p) alterations (methylation, point mutations, and LOH). At 10 years, all abnormalities, except CDKN2A point mutation and methylation, significantly contributed to risk for progression to HGD and EAC, with relative risk by univariate analysis ranging from 2.6 (for 9 p LOH) to 10.6 (for 17 p LOH) 3. A panel of abnormalities associated with genomic instability, including 17 p LOH, DNA content irregularity, and 9 p LOH, was the best predictor of esophageal cancer, with a relative risk of 38.7 29. Additional studies have shown that risk for progression to EAC also increases with the increased clonal expansion of cells that have such cumulative DNA damage 30]. ML assesses both the presence and extent (i. e., clonality) of such mutations in Barrett’s tissue 27 28. Consistently, in a recent longitudinal study of ML, the presence of “high ML” was highly predictive of progression to HGD or EAC, with an adjusted odds ratio of 166 for progression at a mean follow-up of 4 years (P < 0.0001) 31.
Numbers extrapolated from the above studies were used in our cost analysis study to evaluate the impact of biomarkers of genomic instability assessed by ML-based risk stratification methods. In our baseline analysis for calculating annual rate of progression based on ML-associated risk, we used a conservative baseline risk ratio of 10 (range 0 – 25) for patients with low risk (i. e., those with “low ML”) and of 25 (range 5 – 50) for patients with high risk (i. e., those with “high ML”) for progression of NDBE to HGD or EAC. All risk was relative to that of patients with minimal risk for progression (i. e., those with “no ML”). A wide range of risks was examined in sensitivity analysis.
Cost estimates and utilities
Costs, not charges, were considered in this analysis, and a third-party payer’s perspective was taken (Supplementary Table 3). Only direct costs were considered, and all costs were adjusted to 2013 U.S. dollars. Costs were estimated based on the national average reimbursement allowed for each coded procedure by the Centers for Medicare & Medicaid Services (CMS) during the fiscal year 2013. Inpatient medical, surgical, and diagnostic services were assigned CPT (current procedural terminology) or DRG (diagnosis-related group) codes to identify the health care resource utilization. Outpatient data were based on ambulatory payment classification and CPT (Supplementary Table 3). For sensitivity analysis, the range of the cost estimates was obtained from published information 32 33 34 35 36 37.
For baseline analysis, the cost of ML testing was estimated at $ 3100; this figure was extrapolated from CMS reimbursement data for a similar assay related to integrated molecular pathology testing of pancreatic cystic neoplasm. The total cost of the endoscopic ablative therapy included the total cost of all procedures (performed up to 3 times) including professional fees and facility fees, which were assumed to be accrued in the first year of the model. The costs of ablation-related complications were accounted for separately.
Quality-adjusted life years (QALYs), which were the measure of effectiveness in this model, were estimated by adjusting the life expectancy of each health state by a weight or utility reflecting patient preferences for that health state. Utility values were obtained from published information (Supplementary Table 3).
Assumptions
The model and assumptions used were based on well-accepted and published decision analysis models for the management of Barrett’s esophagus 6 7 8 9 10 11. All patients with NDBE were considered potential candidates for surgical esophagectomy, if needed, and in the baseline analysis, the postoperative complication rates for esophagectomy were modeled by using data from high-volume academic centers. Any benefit in terms of reduced risk for cancer was presumed to be accrued only in the case of complete ablation of specialized columnar epithelium; patients with partial ablation had no reduction in their risk for cancer although they incurred the costs associated with endoscopic ablative therapy. Also, as previously mentioned, even patients with complete ablation continued to accrue the cost of periodic endoscopic surveillance.
We used conservative estimates in modeling the strategy based on endoscopic ablation because of the dearth of published data on Barrett’s ablation with the stepwise ablation procedure. For example, although the published article on the ablation of Barrett’s esophagus with the stepwise procedure reported a rate of complete ablation of 70 % at 1 year and of 98 % at 2.5 years, and no serious adverse event in 100 patients, in our baseline scenario we used a much more conservative estimate of 50 % as a complete response rate and did incorporate an overall complication rate of 10 % 38. These estimates of complete response in Barrett’s esophagus and safety with ablative therapy have since been confirmed in other trials of ablation in patients who have Barrett’s esophagus with and without dysplasia 39 40 41 42 43 44 45. In other assumptions, only direct costs were considered. In our experience, patients undergoing the stepwise ablation procedure for Barrett’s esophagus on an outpatient basis tolerate the procedure quite well, and no forms of short-term disutility related to uncomplicated endoscopic ablation were considered in this model. Because the patients being managed according to each strategy would be receiving acid-suppressive therapy for gastroesophageal reflux disease (GERD), the cost of such treatment was not considered in the model. For the ML-based risk stratification strategy, we assumed that biopsy specimens were of sufficient quantity and quality for ML processing.
Footnotes
Competing interests: A.D. receives research funding from Interpace Diagnostics (formerly RedPath). S.A.J., M.A.S., and K.M.C. are full-time employees of Interpace Diagnostics.
References
- 1.Spechler S J, Sharma P, Souza R F. et al. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology. 2011;140:1084–1091. doi: 10.1053/j.gastro.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 2.Inadomi J, Madanick R, Somsouk M. et al. Radiofrequency ablation is more cost-effective than endoscopic surveillance or esophagectomy among patients with Barrett’s esophagus and low-grade dysplasia. Gastroenterology. 2007;132:A53. [Google Scholar]
- 3.Inadomi J M, Sampliner R, Lagergren J. et al. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med. 2003;138:176–186. doi: 10.7326/0003-4819-138-3-200302040-00009. [DOI] [PubMed] [Google Scholar]
- 4.Das A, Wells C, Kim H J. et al. An economic analysis of endoscopic ablative therapy for management of nondysplastic Barrett's esophagus. Endoscopy. 2009;41:400–408. doi: 10.1055/s-0029-1214612. [DOI] [PubMed] [Google Scholar]
- 5.Ellsworth E, Jackson S A, Thakkar S J. et al. Correlation of the presence and extent of loss of heterozygosity mutations with histological classifications of Barrett's esophagus. BMC Gastroenterol. 2012;12:181. doi: 10.1186/1471-230X-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galipeau P C, Li X, Blount P L. et al. NSAIDs modulate CDKN2A, TP53, and DNA content risk for progression to esophageal adenocarcinoma. PLoS Med. 2007;4:e67. doi: 10.1371/journal.pmed.0040067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khara H S, Jackson S A, Nair S. et al. Assessment of mutational load in biopsy tissue provides additional information about genomic instability to histological classifications of Barrett's esophagus. J Gastrointest Cancer. 2014;45:137–145. doi: 10.1007/s12029-013-9570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eluri S, Brugge W R, Daglilar E S. et al. The presence of genetic mutations at key loci predicts progression to esophageal adenocarcinoma in Barrett's esophagus. Am J Gastroenterol. 2015;110:828–834. doi: 10.1038/ajg.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spechler S J Sharma P Souza R F et al. American Gastroenterological Association technical review on the management of Barrett's esophagus Gastroenterology 2011140e18–e52.; quiz e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampliner R E. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett's esophagus. Am J Gastroenterol. 2002;97:1888–1895. doi: 10.1111/j.1572-0241.2002.05910.x. [DOI] [PubMed] [Google Scholar]
- 11.Sonnenberg F A, Beck J R. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein M C, Siegel J E, Gold M R. et al. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 13.Doubilet P, Begg C B, Weinstein M C. et al. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5:157–177. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- 14.Heitjan D F. Fieller's method and net health benefits. Health Econ. 2000;9:327–335. doi: 10.1002/1099-1050(200006)9:4<327::aid-hec517>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 15.Stinnett A, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making. 1998;18:68–S80. doi: 10.1177/0272989X98018002S09. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald R C, di Pietro M, Ragunath K. et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut. 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 17.Fleischer D E, Overholt B F, Sharma V K. et al. Endoscopic radiofrequency ablation for Barrett's esophagus: 5-year outcomes from a prospective multicenter trial. Endoscopy. 2010;42:781–789. doi: 10.1055/s-0030-1255779. [DOI] [PubMed] [Google Scholar]
- 18.Sharma V K, Wang K K, Overholt B F. et al. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett's esophagus: 1-year follow-up of 100 patients. Gastrointest Endosc. 2007;65:185–195. doi: 10.1016/j.gie.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 19.Roorda A K, Marcus S N, Triadafilopoulos G. Early experience with radiofrequency energy ablation therapy for Barrett's esophagus with and without dysplasia. Dis Esophagus. 2007;20:516–522. doi: 10.1111/j.1442-2050.2007.00728.x. [DOI] [PubMed] [Google Scholar]
- 20.Phoa K N, van Vilsteren F G, Weusten B L. et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311:1209–1217. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- 21.Bulsiewicz W J, Kim H P, Dellon E S. et al. Safety and efficacy of endoscopic mucosal therapy with radiofrequency ablation for patients with neoplastic Barrett's esophagus. Clin Gastroenterol Hepatol. 2013;11:636–642. doi: 10.1016/j.cgh.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maley C C. Multistage carcinogenesis in Barrett's esophagus. Cancer Lett. 2007;245:22–32. doi: 10.1016/j.canlet.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Maley C C, Galipeau P C, Finley J C. et al. Genetic clonal diversity predicts progression to esophageal adenocarcinoma. Nat Genet. 2006;38:468–473. doi: 10.1038/ng1768. [DOI] [PubMed] [Google Scholar]
- 24.Maley C C, Galipeau P C, Li X. et al. The combination of genetic instability and clonal expansion predicts progression to esophageal adenocarcinoma. Cancer Res. 2004;64:7629–7633. doi: 10.1158/0008-5472.CAN-04-1738. [DOI] [PubMed] [Google Scholar]
- 25.Gordon L G, Mayne G C, Hirst N G. et al. Cost-effectiveness of endoscopic surveillance of non-dysplastic Barrett's esophagus. Gastrointest Endosc. 2014;79:242–256. doi: 10.1016/j.gie.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 26.Rubenstein J H, Vakil N, Inadomi J M. The cost-effectiveness of biomarkers for predicting the development of oesophageal adenocarcinoma. Aliment Pharmacol Ther. 2005;22:135–146. doi: 10.1111/j.1365-2036.2005.02536.x. [DOI] [PubMed] [Google Scholar]
Supplemental References
- 27.Khara H S, Jackson S A, Nair S. et al. Assessment of mutational load in biopsy tissue provides additional information about genomic instability to histological classifications of Barrett's esophagus. J Gastrointest Cancer. 2014;45:137–145. doi: 10.1007/s12029-013-9570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellsworth E, Jackson S A, Thakkar S J. et al. Correlation of the presence and extent of loss of heterozygosity mutations with histological classifications of Barrett's esophagus. BMC Gastroenterol. 2012;12:181. doi: 10.1186/1471-230X-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galipeau P C, Li X, Blount P L. et al. NSAIDs modulate CDKN2A, TP53, and DNA content risk for progression to esophageal adenocarcinoma. PLoS Med. 2007;4:e67. doi: 10.1371/journal.pmed.0040067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maley C C, Galipeau P C, Li X. et al. The combination of genetic instability and clonal expansion predicts progression to esophageal adenocarcinoma. Cancer Res. 2004;64:7629–7633. doi: 10.1158/0008-5472.CAN-04-1738. [DOI] [PubMed] [Google Scholar]
- 31.Eluri S, Brugge W R, Daglilar E S. et al. The presence of genetic mutations at key loci predicts progression to esophageal adenocarcinoma in Barrett's esophagus. Am J Gastroenterol. 2015;110:828–834. doi: 10.1038/ajg.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerson L B, Groeneveld P W, Triadafilopoulos G. Cost-effectiveness model of endoscopic screening and surveillance in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2004;2:868–879. doi: 10.1016/s1542-3565(04)00394-5. [DOI] [PubMed] [Google Scholar]
- 33.Hur C, Nishioka N S, Gazelle G S. Cost-effectiveness of aspirin chemoprevention for Barrett's esophagus. J Natl Cancer Inst. 2004;96:316–325. doi: 10.1093/jnci/djh039. [DOI] [PubMed] [Google Scholar]
- 34.Inadomi J M, Sampliner R, Lagergren J. et al. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med. 2003;138:176–186. doi: 10.7326/0003-4819-138-3-200302040-00009. [DOI] [PubMed] [Google Scholar]
- 35.Shaheen N J, Inadomi J M, Overholt B F. et al. What is the best management strategy for high grade dysplasia in Barrett's oesophagus? A cost effectiveness analysis. Gut. 2004;53:1736–1744. doi: 10.1136/gut.2003.033837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonnenberg A, Fennerty M B. Medical decision analysis of chemoprevention against esophageal adenocarcinoma. Gastroenterology. 2003;124:1758–1766. doi: 10.1016/s0016-5085(03)00393-7. [DOI] [PubMed] [Google Scholar]
- 37.Vij R, Triadafilopoulos G, Owens D K. et al. Cost-effectiveness of photodynamic therapy for high-grade dysplasia in Barrett's esophagus. Gastrointest Endosc. 2004;60:739–756. doi: 10.1016/s0016-5107(04)02167-4. [DOI] [PubMed] [Google Scholar]
- 38.Sharma V K, Wang K K, Overholt B F. et al. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett's esophagus: 1-year follow-up of 100 patients. Gastrointest Endosc. 2007;65:185–195. doi: 10.1016/j.gie.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 39.Fleischer D E, Overholt B F, Sharma V K. et al. Endoscopic ablation of Barrett's esophagus: a multicenter study with 2.5-year follow-up. Gastrointest Endosc. 2008;68:867–876. doi: 10.1016/j.gie.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Ganz R A, Overholt B F, Sharma V K. et al. Circumferential ablation of Barrett's esophagus that contains high-grade dysplasia: a U.S. Multicenter Registry. Gastrointest Endosc. 2008;68:35–40. doi: 10.1016/j.gie.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 41.Gondrie J J, Pouw R E, Sondermeijer C M. et al. Stepwise circumferential and focal ablation of Barrett's esophagus with high-grade dysplasia: results of the first prospective series of 11 patients. Endoscopy. 2008;40:359–369. doi: 10.1055/s-2007-995567. [DOI] [PubMed] [Google Scholar]
- 42.Gondrie J J, Pouw R E, Sondermeijer C M. et al. Effective treatment of early Barrett's neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy. 2008;40:370–379. doi: 10.1055/s-2007-995589. [DOI] [PubMed] [Google Scholar]
- 43.Shaheen N, Sharma P, Overholt B. et al. A randomized, multicenter, sham-controlled trial of radiofrequency ablation (RFA) for subjects with Barrett’s esophagus (BE) containing dysplasia: interim results of the AIM Dysplasia Trial. Gastroenterology. 2008;134:A37. [Google Scholar]
- 44.Sharma V K, Jae Kim H, Das A. et al. Circumferential and focal ablation of Barrett's esophagus containing dysplasia. Am J Gastroenterol. 2009;104:310–317. doi: 10.1038/ajg.2008.142. [DOI] [PubMed] [Google Scholar]
- 45.Sharma V K, Kim H J, Das A. et al. A prospective pilot trial of ablation of Barrett's esophagus with low-grade dysplasia using stepwise circumferential and focal ablation (HALO system) Endoscopy. 2008;40:380–387. doi: 10.1055/s-2007-995587. [DOI] [PubMed] [Google Scholar]
- 46.Schnell T G, Sontag S J, Chejfec G. et al. Long-term nonsurgical management of Barrett's esophagus with high-grade dysplasia. Gastroenterology. 2001;120:1607–1619. doi: 10.1053/gast.2001.25065. [DOI] [PubMed] [Google Scholar]
- 47.Hameeteman W, Tytgat G N, Houthoff H J. et al. Barrett's esophagus: development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96:1249–1256. doi: 10.1016/s0016-5085(89)80011-3. [DOI] [PubMed] [Google Scholar]
- 48.Weston A P, Sharma P, Topalovski M. et al. Long-term follow-up of Barrett's high-grade dysplasia. Am J Gastroenterol. 2000;95:1888–1893. doi: 10.1111/j.1572-0241.2000.02234.x. [DOI] [PubMed] [Google Scholar]
- 49.Sharma P, Morales T G, Bhattacharyya A. et al. Dysplasia in short-segment Barrett's esophagus: a prospective 3-year follow-up. Am J Gastroenterol. 1997;92:2012–2016. [PubMed] [Google Scholar]
- 50.Weston A P, Badr A S, Hassanein R S. Prospective multivariate analysis of clinical, endoscopic, and histological factors predictive of the development of Barrett's multifocal high-grade dysplasia or adenocarcinoma. Am J Gastroenterol. 1999;94:3413–3419. doi: 10.1111/j.1572-0241.1999.01602.x. [DOI] [PubMed] [Google Scholar]
- 51.Cameron A J, Ott B J, Payne W S. The incidence of adenocarcinoma in columnar-lined (Barrett's) esophagus. N Engl J Med. 1985;313:857–859. doi: 10.1056/NEJM198510033131404. [DOI] [PubMed] [Google Scholar]
- 52.Drewitz D J, Sampliner R E, Garewal H S. The incidence of adenocarcinoma in Barrett's esophagus: a prospective study of 170 patients followed 4.8 years. Am J Gastroenterol. 1997;92:212–215. [PubMed] [Google Scholar]
- 53.O'Connor J B, Falk G W, Richter J E. The incidence of adenocarcinoma and dysplasia in Barrett's esophagus: report on the Cleveland Clinic Barrett's Esophagus Registry. Am J Gastroenterol. 1999;94:2037–2042. doi: 10.1111/j.1572-0241.1999.01275.x. [DOI] [PubMed] [Google Scholar]
- 54.Shaheen N J, Crosby M A, Bozymski E M. et al. Is there publication bias in the reporting of cancer risk in Barrett's esophagus? Gastroenterology. 2000;119:333–338. doi: 10.1053/gast.2000.9302. [DOI] [PubMed] [Google Scholar]
- 55.Spechler S J, Lee E, Ahnen D. et al. Long-term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow-up of a randomized controlled trial. JAMA. 2001;285:2331–2338. doi: 10.1001/jama.285.18.2331. [DOI] [PubMed] [Google Scholar]
- 56.Katz D, Rothstein R, Schned A. et al. The development of dysplasia and adenocarcinoma during endoscopic surveillance of Barrett's esophagus. Am J Gastroenterol. 1998;93:536–541. doi: 10.1111/j.1572-0241.1998.161_b.x. [DOI] [PubMed] [Google Scholar]
- 57.Spechler S J, Robbins A H, Rubins H B. et al. Adenocarcinoma and Barrett's esophagus. An overrated risk? Gastroenterology. 1984;87:927–933. [PubMed] [Google Scholar]
- 58.Weston A P, Badr A S, Hassanein R S. Prospective multivariate analysis of factors predictive of complete regression of Barrett's esophagus. Am J Gastroenterol. 1999;94:3420–3426. doi: 10.1111/j.1572-0241.1999.01603.x. [DOI] [PubMed] [Google Scholar]
- 59.Winters C Jr, Spurling T J, Chobanian S J. et al. Barrett's esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology. 1987;92:118–124. [PubMed] [Google Scholar]
- 60.Corley D A, Levin T R, Habel L A. et al. Surveillance and survival in Barrett's adenocarcinomas: a population-based study. Gastroenterology. 2002;122:633–640. doi: 10.1053/gast.2002.31879. [DOI] [PubMed] [Google Scholar]
- 61.Menke-Pluymers M B, Schoute N W, Mulder A H. et al. Outcome of surgical treatment of adenocarcinoma in Barrett's oesophagus. Gut. 1992;33:1454–1458. doi: 10.1136/gut.33.11.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pera M, Trastek V F, Carpenter H A. et al. Barrett's esophagus with high-grade dysplasia: an indication for esophagectomy? Ann Thorac Surg. 1992;54:199–204. doi: 10.1016/0003-4975(92)91370-o. [DOI] [PubMed] [Google Scholar]
- 63.Peters J H Clark G W Ireland A P et al. Outcome of adenocarcinoma arising in Barrett's esophagus in endoscopically surveyed and nonsurveyed patients J Thorac Cardiovasc Surg 1994108813–821.; discussion 821 – 822 [PubMed] [Google Scholar]
- 64.Streitz J M Jr Andrews C W Jr Ellis F H Jr Endoscopic surveillance of Barrett's esophagus. Does it help? J Thorac Cardiovasc Surg 1993105383–387.; discussion 387 – 388 [PubMed] [Google Scholar]
- 65.Overholt B F, Panjehpour M, Halberg D L. Photodynamic therapy for Barrett's esophagus with dysplasia and/or early stage carcinoma: long-term results. Gastrointest Endosc. 2003;58:183–188. doi: 10.1067/mge.2003.327. [DOI] [PubMed] [Google Scholar]
- 66.Sharma P, Wani S, Weston A P. et al. A randomised controlled trial of ablation of Barrett's oesophagus with multipolar electrocoagulation versus argon plasma coagulation in combination with acid suppression: long term results. Gut. 2006;55:1233–1239. doi: 10.1136/gut.2005.086777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bousamra M 2nd, Haasler G B, Parviz M. A decade of experience with transthoracic and transhiatal esophagectomy. Am J Surg. 2002;183:162–167. doi: 10.1016/s0002-9610(01)00861-3. [DOI] [PubMed] [Google Scholar]
- 68.Lerut T Coosemans W Van Raemdonck D et al. Surgical treatment of Barrett's carcinoma. Correlations between morphologic findings and prognosis J Thorac Cardiovasc Surg 19941071059–1065.; discussion 1065 – 1056 [DOI] [PubMed] [Google Scholar]
- 69.McLarty A J, Deschamps C, Trastek V F. et al. Esophageal resection for cancer of the esophagus: long-term function and quality of life. Ann Thorac Surg. 1997;63:1568–1572. doi: 10.1016/s0003-4975(97)00125-2. [DOI] [PubMed] [Google Scholar]
- 70.Patti M G, Corvera C U, Glasgow R E. et al. A hospital's annual rate of esophagectomy influences the operative mortality rate. J Gastrointest Surg. 1998;2:186–192. doi: 10.1016/s1091-255x(98)80011-5. [DOI] [PubMed] [Google Scholar]
- 71.Zaninotto G, Parenti A R, Ruol A. et al. Oesophageal resection for high-grade dysplasia in Barrett's oesophagus. Br J Surg. 2000;87:1102–1105. doi: 10.1046/j.1365-2168.2000.01470.x. [DOI] [PubMed] [Google Scholar]
- 72.Arrowsmith J B, Gerstman B B, Fleischer D E. et al. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37:421–427. doi: 10.1016/s0016-5107(91)70773-6. [DOI] [PubMed] [Google Scholar]
- 73.Chan M F. Complications of upper gastrointestinal endoscopy. Gastrointest Endosc Clin N Am. 1996;6:287–303. [PubMed] [Google Scholar]
- 74.Hart R, Classen M. Complications of diagnostic gastrointestinal endoscopy. Endoscopy. 1990;22:229–233. doi: 10.1055/s-2007-1010734. [DOI] [PubMed] [Google Scholar]
- 75.Hernandez L V, Jacobson J W, Harris M S. Comparison among the perforation rates of Maloney, balloon, and savary dilation of esophageal strictures. Gastrointest Endosc. 2000;51:460–462. doi: 10.1016/s0016-5107(00)70448-2. [DOI] [PubMed] [Google Scholar]
- 76.Kavic S M, Basson M D. Complications of endoscopy. Am J Surg. 2001;181:319–332. doi: 10.1016/s0002-9610(01)00589-x. [DOI] [PubMed] [Google Scholar]
- 77.Iannettoni M D Vlessis A A Whyte R I et al. Functional outcome after surgical treatment of esophageal perforation Ann Thorac Surg 1997641606–1609.; discussion 1609 – 1610 [DOI] [PubMed] [Google Scholar]
- 78.Montgomery E, Bronner M P, Goldblum J R. et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32:368–378. doi: 10.1053/hupa.2001.23510. [DOI] [PubMed] [Google Scholar]
- 79.Ormsby A H, Petras R E, Henricks W H. et al. Observer variation in the diagnosis of superficial oesophageal adenocarcinoma. Gut. 2002;51:671–676. doi: 10.1136/gut.51.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Provenzale D, Kemp J A, Arora S. et al. A guide for surveillance of patients with Barrett's esophagus. Am J Gastroenterol. 1994;89:670–680. [PubMed] [Google Scholar]
- 81.Provenzale D, Schmitt C, Wong J B. Barrett's esophagus: a new look at surveillance based on emerging estimates of cancer risk. Am J Gastroenterol. 1999;94:2043–2053. doi: 10.1111/j.1572-0241.1999.01276.x. [DOI] [PubMed] [Google Scholar]
- 82.Alikhan M, Rex D, Khan A. et al. Variable pathologic interpretation of columnar lined esophagus by general pathologists in community practice. Gastrointest Endosc. 1999;50:23–26. doi: 10.1016/s0016-5107(99)70339-1. [DOI] [PubMed] [Google Scholar]
- 83.Zaman A, Hapke R, Sahagun G. et al. Unsedated peroral endoscopy with a video ultrathin endoscope: patient acceptance, tolerance, and diagnostic accuracy. Am J Gastroenterol. 1998;93:1260–1263. doi: 10.1111/j.1572-0241.1998.00406.x. [DOI] [PubMed] [Google Scholar]
- 84.de Boer A G, Stalmeier P F, Sprangers M A. et al. Transhiatal vs extended transthoracic resection in oesophageal carcinoma: patients' utilities and treatment preferences. Br J Cancer. 2002;86:851–857. doi: 10.1038/sj.bjc.6600203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weinstein M C, Siegel J E, Gold M R. et al. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]


