Abstract
The lack of consistent definitions and nomenclature across clinical trials of novel devices, drugs, or biologics poses a significant barrier to accrual of knowledge in and across peripheral artery disease therapies and technologies. Recognizing this problem, the Peripheral Academic Research Consortium, together with the U.S. Food and Drug Administration and the Japanese Pharmaceuticals and Medical Devices Agency, has developed a series of pragmatic consensus definitions for patients being treated for peripheral artery disease affecting the lower extremities. These consensus definitions include the clinical presentation, anatomic depiction, interventional outcomes, surrogate imaging and physiological follow-up, and clinical outcomes of patients with lower-extremity peripheral artery disease. Consistent application of these definitions in clinical trials evaluating novel revascularization technologies should result in more efficient regulatory evaluation and best practice guidelines to inform clinical decisions in patients with lower extremity peripheral artery disease.
Keywords: amputation, foot, intermittent claudication, leg, myocardial infarction, stroke
Lower extremity peripheral artery disease (LE-PAD) is a manifestation of systemic atherosclerotic disease, which affects over 8 million Americans (1) and conveys a significant health burden globally (1–3). Although LE-PAD can be asymptomatic and subclinical, it is associated with a reduction in functional capacity and quality of life when symptomatic, and, in its most severe form, is a major cause of limb amputation (1–3). Patients with LE-PAD are at an increased risk for myocardial infarction (MI), stroke, and death (1–5). Given this substantial health burden, LE-PAD is the focus of a number of evolving medical, endovascular, and surgical therapies aimed at improving the limb manifestations of the disease. This proliferation of revascularization devices and therapies has highlighted the need for studies that elucidate the direct mechanistic effect, the impact on systemic outcomes (including death, MI, and stroke), and the overall safety of both individual and combined therapeutic strategies.
Systematic safety and effectiveness evaluations of the clinical utility of LE-PAD revascularization therapies and devices (4,6) require high-quality clinical trials data, both for regulatory approval and for the development of best practice guidelines to inform clinical decisions in patients with LE-PAD. Currently, 1 of the biggest barriers to accrual of knowledge in and across peripheral artery disease (PAD) therapies and technologies is the lack of consistent definitions and nomenclature between clinical trials. Although validated, standardized definitions exist for coronary artery disease endpoints for clinical trials, significant variation exists in data elements used to describe both patients undergoing treatment for LE-PAD and the outcomes for evaluation of treatments. Professional societies, academic research organizations, regulatory agencies, and representatives of the pharmaceutical and device industry have recognized both the lack of and the need for consistent consensus definitions for clinical descriptors, anatomy, surrogate measures, and clinical outcomes as new therapies move into clinical practice. Although these groups have previously proposed standardized definitions in specific PAD populations (7–9), these efforts are recent and await broad application to support existing clinical trials or ongoing registries in LE-PAD patients. Unique needs remain for regulatory evaluation and pivotal trials that can support critical trial processes, such as independent event adjudication, core laboratory analysis, and safety monitoring. The value of consensus definitions across stakeholders for such device evaluation is the basis for the Peripheral Academic Research Consortium (PARC) (10).
In response to the need for public access to consistent definitions for pharmacologic and device trials treating patients with LE-PAD, we initiated the PARC, convening 2 face-to-face meetings on February 2, 2012 and February 1, 2013, at the U.S. Food and Drug Administration (FDA) headquarters in White Oak, Maryland, along with numerous interim tele-conferences and communications. The meetings and processes were modeled after the previous Academic Research Consortium (ARC) meetings in 2006, which aimed to develop standardized definitions for coronary stent clinical trials (11), and subsequent efforts aimed at bleeding (Bleeding Academic Research Consortium [BARC]) (12), and transcatheter aortic valve implantation (Valve Academic Research Consortium [VARC]) (13,14). The express purpose of the PARC effort was to develop pragmatic consensus definitions to be consistently applied in clinical trials of patients with LE-PAD. Unique to PARC was the inclusion of representatives from academia, regulatory bodies (from both Japan and the United States), and industry.
CHALLENGES AND SCOPE OF STANDARDIZED LE-PAD DEFINITIONS
There were many fundamental challenges in creating broadly accepted, pragmatic LE-PAD definitions, establishing in part the basis for the process and the scope of the consensus definitions provided. A central challenge was the scope of topics requiring definitions. Several prior efforts had evaluated segments of the LE-PAD population. The foundational document is the 2012 American College of Cardiology Foundation/American Heart Association Key Data Elements and Definitions of Atherosclerotic Vascular Disease (9), to which the PARC document has integrated several characteristics particular to clinical trials in PAD (15). Other groups involved in nomenclature and data elements that influenced PARC include: the DEFINE group, evaluating definitions in patients undergoing lower-extremity endovascular revascularization (7); a vascular surgical group evaluating objective performance goals and trial design for patients undergoing endovascular treatment for critical limb ischemia (CLI) (8); the proceedings from the Society of Interventional Radiology conference on critical limb ischemia trials and registries; the Inter-Society Consensus for Management of PAD (TASC) (16); and the FDA Clinical Data Interchange Standards Consortium (CDISC) effort to improve the quality and efficiency of cardiovascular trials. These documents provided the foundation and nomenclature for much of the work done by the PARC group. To best integrate such efforts and construct the most pragmatic and genuine consensus in its approach, the PARC initiative actively involved as many representative groups as possible, and reviewed all available “standard” definitions from previous sources.
Additionally, patients with LE-PAD span a clinical spectrum ranging from asymptomatic patients to those with atypical leg symptoms, typical claudication with variable degrees of limitation, or CLI including both rest pain and tissue loss. Across this clinical spectrum, definitions for patients were required that included the accurate elucidation of symptoms, anatomic characterization of disease, definitions for both clinical and imaging short- and long-term measures, and finally, clinical outcomes. In addition to developing definitions applicable across the wide spectrum of PAD syndromes, the definitions would need to be pertinent to existing and developing therapies and procedures for LE-PAD. A pre-meeting survey of all participants established key priorities for the consortium. On the bases of a survey and in-person think tank meetings, the PARC key priorities for definitions included: 1) clinical syndromes; 2) anatomic considerations; 3) surrogate endpoints including physiologic and imaging measures; 4) symptomatic limb endpoints; and 5) other clinical endpoints.
PARC COMPOSITION AND GOALS
As summarized in the ARC charter, the ARC was founded as an informal collaboration including academic research organizations from the United States and Europe, joined by representatives from the FDA and device manufacturers. The initial ARC work product was the development of pragmatic consensus definitions for coronary stent trials (11). Regulatory authorities, manufacturers, and professional societies have universally endorsed the ARC definition for stent thrombosis. This initial ARC effort concomitantly developed a process that was comprehensive and efficient through the broad inclusion of all relevant stakeholders. The value of pragmatic consensus definitions has subsequently been illustrated by the use of the ARC stent thrombosis definition in more than 100 clinical trials involving more than a dozen drug-eluting stent platforms, providing the basis for the ongoing accrual of knowledge about this rare, but catastrophic, safety concern for drug-eluting stent implants (17–20). The ARC process relied upon a transparent, noncompetitive approach to developing endpoint definitions capable of being applied to a wide variety of trial designs or specific devices. A key principle in ARC efforts is that the development of a consensus, in particular endpoint definitions, is ultimately independent of how such definitions are actually applied in any specific clinical trial. The PARC group was formed in keeping with the ARC process and included: representatives from academic research groups from the United States, Japan, and Europe; representatives from vascular medicine, vascular surgery, interventional radiology, and cardiology; industry representatives; the FDA; and the Pharmaceuticals and Medical Devices Agency regulatory authorities from Japan (Online Appendix). The goal of the PARC group was to develop standardized definitions for patients with LEPAD allowing for clinical characterization and evaluation of therapies on the basis of either imaging or clinical outcomes. The approach was to have subgroups of the overall committee review specific endpoints and outcomes as writing groups, with review by the whole group and adoption of final definitions using a consensus process.
PARC DEFINITIONS
Clinical symptoms and syndromesde finitions
Traditionally, both the Fontaine and the Rutherford classification systems have been used to capture information regarding lower extremity symptoms and broadly defined functional limitations of patients with LE-PAD (1,3,21,22). The PARC group established baseline symptom definitions benchmarked to the established definitional schemes. Tables 1 and 2 provide the Fontaine and Rutherford limb symptom classifications and the data elements recommended by the PARC group for capture. It should be noted that PARC consensus was to define patients with atypical symptoms related to PAD as “other exertional leg discomfort associated with physical limitations from PAD.” It is assumed that these patients would have symptoms associated with exertion that would be atypical in nature, that is, either present at rest with worsening during exertion or with significant time to symptom resolution. Symptom-limited walking distance and degree of functional limitation should be ascertained and captured in these patients, along with hemodynamic evidence of PAD. The Fontaine and Rutherford classifications were modified to use descriptive, rather than numeric terms to classify the severity of PAD limb symptoms. If validated, this lexicon will clarify ambiguity when reporting baseline characteristics and outcomes regarding the clinical stage or change in stage of the patients evaluated.
TABLE 1.
Clinical Symptom Classification
| Fontaine Classification |
Rutherford Classification |
||||||
|---|---|---|---|---|---|---|---|
| Stage | Symptoms | ↔ | Proposed PARC Universal Data Elements | ↔ | Grade | Category | Symptoms |
| I | Asymptomatic | Asymptomatic | 0 | 0 | Asymptomatic | ||
| II | Intermittent claudication/other exertional limb symptoms | Mild claudication/limb symptoms (no limitation in walking) | ↔ | 0 | 1 | Mild claudication | |
| IIa | ↔ | Moderate claudication/ limb symptoms (able to walk without stopping >2 blocks or 200 m or 4 min) | 1 | 2 | Moderate claudication | ||
| IIb | Severe claudication/limb symptoms (only able to walk without stopping <2 blocks or 200 m or 4 min) | ↔ | 1 | 3 | Severe claudication | ||
| III | Ischemic rest pain | ↔ | Ischemic rest pain (pain in the distal limb at rest felt to be due to limited arterial perfusion) | ↔ | II | 4 | Ischemic rest pain |
| IV | Ulceration or gangrene | ↔ | Ischemic ulcers on distal leg | ↔ | III | 5 | Ischemic ulceration |
| Ischemic gangrene | ↔ | III | 6 | Ischemic gangrene | |||
↔ = comparable terms.
TABLE 2.
Hemodynamic Definitions for CLI
| Patients With Tissue Loss | Patients With Ischemic Rest Pain |
|---|---|
| Ankle pressure <70 mm Hg | Ankle pressure <50 mm Hg |
| Toe pressure <50 mm Hg | Toe pressure <30 mm Hg |
| TcPO2 <40 mm Hg | TcPO2 <20 mm Hg |
| Skin perfusion pressure <40 mm Hg | Skin perfusion pressure <30 mm Hg (23) |
The PARC group provided hemodynamic support for the definition of CLI. Atypical leg symptoms are symptoms that are worsened by exertion, but that do not meet the classic definition of intermittent claudication. These patients should have objective/confirmed evidence of PAD by noninvasive testing.
CLI = critical limb ischemia; PAD = peripheral arterial disease; PARC = Peripheral Academic Research Consortium; TcPO2 = transcutaneous oxygen pressure.
The PARC group clearly identified the significant limitations of the current Rutherford classification system in comparing patients with CLI across clinical trials. In part, this was felt to be due to the changing demographics of CLI patients, with increased rates of diabetes and renal disease. The Society for Vascular Surgery has proposed a system for classification of patients with threatened limbs aimed at addressing many of the potential determinants of amputation, including wound extent, the degree of ischemia and/or perfusion, and presence and extent of foot infection (wound ischemia foot infection) (24). This recent approach has not yet been validated, but represents an area we feel should be evaluated in CLI trials and considered in future PARC revisions.
Anatomic (lesion and vessel) definitions
The PARC lesion and vessel definitions are presented in Table 3. The PARC group reviewed both the DEFINE group (7) and CDISC anatomic definitions (25) with regards to lesion and target lesion endovascular and surgical revascularization. The definitions are specific for anatomic and lesion characteristics, in contrast to the TASC anatomic classification, which remains clinically available for guidance about revascularization. In defining a “significant” anatomic lesion in the LE-PAD arterial tree, PARC considered options similar to those evaluated in the coronary circulation, including classification within the 50% to 100% stenosis/occluded group. Given the lack of quantifiable data on the differences in visually estimated stenosis and outcomes and the difference in size of LE-PAD vessels, the group recommended an efficient nomenclature that can be used by clinicians and core laboratories: mild (<50% diameter stenosis), moderate (50% to 69%), severe (70% to 99%), and occluded (100%). This system was consistent with prior coronary assessments used per CDISC data element definitions and LE-PAD proposed imaging surrogate endpoints during follow-up. Another significant modification was the definition of a treated or target lesion. Due to the treatment lengths specific to the LEPAD vascular bed, and to ensure coverage for both efficacy and safety, the definition of a treated segment was changed to include 10 mm proximal and distal to the lesion. Finally, Table 3 also provides a consensus system for assessing calcification of LE-PAD vessels.
TABLE 3.
PARC Lesion and Vessel Characteristics and Definitions
| Lesion or Vessel | Term | Definition |
|---|---|---|
| Significant peripheral artery stenosis* | Mild | <50% |
| Moderate | 50%–69% | |
| Severe | 70%–99% | |
| Occluded | 100% | |
| Lesion length | Focal | ≤1 cm |
| Short | >1 and <5 cm | |
| Intermediate | ≥5 and <15 cm | |
| Long | ≥15 cm | |
| Degree of lesion calcification (34,26) | Focal | <180° (1 side of vessel) and less than one-half of the total lesion length |
| Mild | <180° and greater than one-half of the total lesion length | |
| Moderate | ≥180° (both sides of vessel at same location) and less than one-half of the total lesion length | |
| Severe | >180° (both sides of the vessel at the same location) and greater than one-half of the total lesion length | |
| Anatomic level of LE-PAD | Aortoiliac | Aortoiliac (distal limit bottom of pelvic rim in the AP view by angiography or inguinal ligament) |
| Femoropopliteal | Femoropopliteal (distal limit is origin of anterior tibial artery) | |
| Tibialpedal | Tibialpedal (anterior tibial and below including foot arteries) | |
| Aortoiliac segment | Infrarenal abdominal aorta | |
| Common iliac artery | ||
| Internal iliac artery | ||
| External iliac artery | ||
| Femoropopliteal | Common femoral artery | |
| Profunda femoris artery | ||
| Superficial femoral artery | ||
| P1 segment (above knee popliteal artery): from Hunter's canal to proximal edge of patella | ||
| P2 segment: from the proximal part of patella to center of knee joint space | ||
| P3 segment (below knee popliteal artery): from the center of knee joint space to origin of anterior tibial artery | ||
| Tibialpedal | Tibial-peroneal trunk (from the origin of the anterior tibial artery to the bifurcation of the posterior tibial and peroneal artery) | |
| Anterior tibial artery | ||
| Posterior tibial artery | ||
| Peroneal artery | ||
| Plantar pedal loop | ||
| pedal vessel | ||
| PT, DP†‡ | ||
| Target lesion | Any vascular segment treated or attempted to be treated during the trial procedure with the index device. The target lesion is the treated segment including 10 mm proximal and ending 10 mm distal to the index device or therapy (stent, balloon, or atherectomy catheter). | |
| TLR | TLR is any repeat intervention of the target lesions (plus 10 mm proximal and distal to the index device) or surgical bypass of the target vessel performed for restenosis or other complication involving the target lesion. If the target vessel is occluded and bypass is done to another artery below the knee, this should be considered TLR. In the assessment of TLR, angiograms should be assessed by an angiographic core laboratory (if designated) and made available to the clinical endpoints committee for review. | |
| Target vessel | Any vessel (e.g., noncardiac or nonintracranial) that contains the target lesion treated with the study device. The target vessel includes the target lesion as well as the entire length of native vessel upstream and downstream from the target lesion, including side branches. | |
| Target limb | Any symptomatic limb that contains the target lesion and all vessels from aortic bifurcation to the foot. | |
The majority of the anatomic classifications were adapted from Diehm et al. (7).
Lesion stenoses are clinically based on visual angiographic assessments. For clinical trials, lesion stenosis may be evaluated with core-laboratory QCA.
PARC recommends continued efforts to encourage documentation of pedal anatomy in relevant patients.
It is desirable to obtain selective tibial imaging evaluating the vascular supply to tissue at risk with categorization of pedal/arcuate vessels in patients with tissue loss.
DP = dorsalis pedis artery; PT = posterior tibial artery; QCA = quantitative coronary angiography; TLR = target lesion revascularization; other abbreviations as in Table 2.
We recommend DEFINE anatomic groupings modified to include aortoiliac, femoropopliteal, and tibialpedal to define anatomic locations of disease. Table 3 presents the segments with their anatomic borders. Several other groups have developed complex lesion and anatomy assessment below the knee and in the pedal arch, including the angiosome concept (27,28). The PARC group recommends continued research and data capture in relevant patients with distal disease to help inform future efforts. Inherent in the PARC lesion and vessel recommendations is the performance of complete pre- and post-revascularization imaging to assess the presence, extent, and location of atherosclerotic disease in the lower extremity. The PARC group recognizes that the current classification captures lesion stenosis, location, and some information about morphology, but does not describe specific lesion patterns or features such as aneurysm or ulceration.
Acute procedural success
To develop a standardized definition for acute procedural success, the working group considered both the timing of evaluation and the application of the definition across a broad range of possible endovascular and surgical procedures. Acute procedural success needed to encompass technical success as well as freedom from major adverse clinical events. It should be noted for all procedure success definitions that the group believes that, although visual estimation is used for clinical care, quantitative coronary angiography would be preferable in clinical trials.
Our consensus definition is:
Acute technical success for peripheral revascularization is defined as the achievement of a final residual diameter stenosis <30% for stent and <50% for angioplasty or atherectomy by angiography at the end of the procedure (and without flow-limiting arterial dissection or hemodynamically significant translesional pressure gradient <10 mm Hg) for endovascular revascularization or patent vessel or bypass conduit for surgical revascularization (Table 4) (modified from the FDA CDISC definition).
Acute procedural success for peripheral revascularization is defined as both acute technical success and absence of major adverse events (e.g., death, stroke, MI, acute onset of limb ischemia, index bypass graft or treated segment thrombosis, and/or need for urgent/emergent vascular surgery) within 72 h of the index procedure.
TABLE 4.
PARC Acute Technical and Procedural Success
|
Acute Procedural Success
| |||
| Definition of acute procedural success (endovascular and surgical): evidence of both acute technical success and absence of major adverse events (e.g., death, stroke, myocardial infarction, acute onset of limb ischemia, index bypass graft or treated segment thrombosis, and or need for urgent/emergent vascular surgery) within 72 h of the index procedure. | |||
|
Acute Technical Success | |||
| Definition of acute technical success (endovascular and surgical): evidence of successful revascularization as presented in the following text. | |||
| Endovascular revascularization | Angioplasty alone | ≤50% stenosis Absence of flow-limiting dissection or hemodynamically significant translesion gradient |
Confirmed by digital subtraction angiography and/or invasive pressure measurement demonstrating <10 mm Hg gradient |
| Atherectomy alone | ≤50% stenosis Absence of flow-limiting dissection or hemodynamically significant translesion gradient |
Confirmed by digital subtraction angiography and/or invasive pressure measurement demonstrating <10 mm Hg gradient | |
| Stent or stent graft | ≤30% stenosis Absence of flow-limiting dissection or hemodynamically significant translesion gradient |
Confirmed by digital subtraction angiography and/or invasive pressure measurement demonstrating <10 mm Hg gradient | |
| Surgical revascularization | Endarterectomy | Patent native vessel on which operation was performed | Confirmed by at least 1 of the following: • Doppler examination • Digital subtraction angiography • Noninvasive hemodynamic measurement |
| Bypass graft/conduit | Patent graft or conduit | Confirmed by at least 1 of the following: • Doppler examination • Digital subtraction angiography • Noninvasive hemodynamic measurement |
|
Applies to both patients with intermittent claudication/other exertional limb symptoms and patients with critical limb ischemia. Completion angiogram from common femoral artery to pedal/plantar arteries is recommended to exclude acute adverse events. Two angiographic, tangential views of the treated segment are recommended to define acute technical success. A focused examination of the index limb after sheath removal (endovascular) and skin closure (surgical), including pulse examination and presence/absence of Doppler signals, is also recommended. Definition of acute procedures success assumes that other previously defined safety endpoints such as major bleeding or acute renal failure would also be collected and assessed with regards to timing from the procedure.
PARC = Peripheral Academic Research Consortium.
This definition, along with definitions specific to endovascular and surgical procedures, is presented in Table 4. The group defined urgent surgery as generally requiring hospitalization and occurring within 24 h of the index procedure and emergency surgery as needing to be performed without delay. The group retained conventions from the coronary published studies regarding the time point for evaluation of successful revascularization utilizing stent technology compared with balloon or atherectomy technologies. Further research on systemic and limb-related adverse events around revascularization, as well as long-term clinical outcomes, is needed to inform these definitions. The PARC group also felt that special emphasis should be paid to ensuring that both per-protocol and intention-to-treat cohorts were captured and reported in peripheral revascularization trials to ensure that all modes of failure are captured.
The PARC group also defined both clinically driven target vessel revascularization and vessel patency, adapting relevant coronary definitions from CDISC. The definitions are as follows:
Clinically driven LE-PAD revascularization is defined as target lesion revascularization performed due to target lesion diameter stenosis ≥50% and either evidence of clinical or functional ischemia (e.g., recurrent/progressive life-limiting intermittent claudication, claudication unresponsive to medical therapy, CLI) or recurrence of the clinical syndrome for which the initial procedure was performed. Clinically driven target lesion revascularization occurs in the absence of protocol-directed surveillance ultrasound or angiography.
Vessel patency includes the absence of clinically driven target lesion revascularization and/or recurrent target lesion diameter stenosis ≥50% by imaging (e.g., invasive angiography or, most commonly, duplex ultrasonography). If patency data are incorporated within the primary endpoint of a clinical trial, the angiographic images or duplex ultrasonographic images should be assessed by appropriate core laboratories and made available to the clinical endpoints committee for review upon request.
SHORT- AND LONG-TERM SURROGATE ENDPOINTS FOR PROCEDURAL SUCCESS USING IMAGING AND PHYSIOLOGICAL MEASURES
Many imaging and physiologic surrogate endpoints are used for both long- and short-term efficacy and safety assessment in therapies aimed at patients with LE-PAD. We focused our evaluation and definitions on 3 central elements: timing of evaluation, method of evaluation, and the patient's clinical presentation as intermittent claudication (or other exertional symptoms typical of PAD) versus CLI. The timing for evaluation was defined as subacute (from in hospital/within 72 h [whichever comes first] to 30 days post-index procedure) to 3, 6, and 12 months. These time frames were chosen to match stages of interventional site healing and device behavior, and are also consistent with clinically meaningful and common time points of contact with PAD patients following interventions. Multiple modalities were included from physiological pressure measurements, to duplex ultrasonography, and to advanced imaging with computed tomography and cardiac magnetic resonance imaging (Table 5).
TABLE 5.
Short- and Long-Term Surrogate Endpoints for Procedural Success Using Imaging and Physiologic Measures
| Time Point of Evaluation |
|||||
|---|---|---|---|---|---|
| Measurement Technique | Subacute (72 h to 30 days) | 3 Months | 6 Months | 12 Months | Ref. # |
|
Intermittent Claudication
| |||||
| ABI (or TBI) at rest* | Increase in resting ABI or TBI ≥0.10 from pre-procedure value | Resting ABI or TBI ≥0.10 from pre-procedure value | Resting ABI or TBI ≥0.10 from pre-procedure value | Resting ABI or TBI ≥0.10 from pre-procedure value | (7,29) |
| Failure in follow up defined as reduction in ABI or TBI by 0.10 or return to pre-procedure value | (29) | ||||
| Duplex ultrasound† | ≤50% diameter stenosis as defined by peak systolic velocity index (ratio of intrastenotic peak systolic velocity to pre-stenotic velocity) <2.4 | ≤50% diameter stenosis as defined by peak systolic velocity index (ratio of intrastenotic peak systolic velocity to pre-stenotic velocity) <2.4 | ≤50% diameter stenosis as defined by peak systolic velocity index (ratio of intrastenotic peak systolic velocity to pre-stenotic velocity) <2.4 | ≤50% diameter stenosis as defined by peak systolic velocity index (ratio of intrastenotic peak systolic velocity to pre-stenotic velocity) <2.4 | (7) |
| CT/CMR/invasive angiography | ≤50% diameter stenosis or ≤70% area stenosis | ≤50% diameter stenosis or ≤70% area stenosis | ≤50% diameter stenosis or ≤70% area stenosis | ≤50% diameter stenosis or ≤70% area stenosis | (1,7) |
|
Critical Limb Ischemia | |||||
| Ankle (or toe) pressure‡§ | >70 mm Hg (Rutherford 4) >50 mm Hg (Rutherford 5–6) |
>70 mm Hg (Rutherford 4) >50 mm Hg (Rutherford 5–6) |
>70 mm Hg (Rutherford 4) >50 mm Hg (Rutherford 5–6) |
>70 mm Hg (Rutherford 4) >50 mm Hg (Rutherford 5–6) |
(7,24) |
| ∥Duplex ultrasound | ≤50% diameter stenosis as defined by peak systolic velocity index (ratio of intrastenotic peak systolic velocity to pre-stenotic velocity) <2.4 | ≤50% diameter stenosis as defined by peak systolic velocity index (ratio of intrastenotic peak systolic velocity to pre-stenotic velocity) <2.4 | ≤50% diameter stenosis as defined by peak systolic velocity index (ratio of intrastenotic peak systolic velocity to pre-stenotic velocity) <2.4 | ≤50% diameter stenosis as defined by peak systolic velocity index (ratio of intrastenotic peak systolic velocity to pre-stenotic velocity) <2.4 | (7) |
| CT/MRI/invasive angiography | ≤50% diameter stenosis or ≤70% area stenosis | ≤50% diameter stenosis or ≤70% area stenosis | ≤50% diameter stenosis or ≤70% area stenosis | ≤50% diameter stenosis or ≤70% area stenosis | (1,7) |
Patients with ABI ≥1.4 are considered noncompressible and should not be included in analyses of improvements in ABI.
The PARC group felt that the Duplex ultrasound is difficult to reliably obtain in infrageniculate vessels, and patients with CLI may benefit from evaluation of presence or absence of total occlusion of below knee vessels.
Toe pressure and toe-brachial index should be used only in the event of noncompressible vessels.
Ankle (or toe) pressure used instead of ratio for CLI patients as a guide for a threshold level to maintain adequate perfusion, applies to patients treated with either endovascular and surgical therapy.
The PARC group did not define hemodynamic failure in follow-up, as the shorter-term goals of wound healing may have been achieved. The PARC group suggests these time points for evaluation but recommend that analyses and reports include the entire pre-specified reporting window to ensure all possible data and relevant findings are captured.
ABI = ankle brachial index; CT = computed tomography; CMR = cardiac magnetic resonance; TBI = toe-brachial index; other abbreviations as in Table 2.
CLINICAL OUTCOMES AND ENDPOINT DEFINITIONS
Definitions for clinical outcomes in patients with LEPAD constitute 1 of the most complex areas for consensus, in part due to the relevance of any endpoint in the setting of such a heterogeneous range of clinical syndromes. The PARC consensus thus dichotomized patient-level endpoints on the basis of general presentation, for example, intermittent claudication/other exertional limb symptoms versus CLI. The endpoints for intermittent claudication are on the basis of functional improvement. Walking time and/or functional definitions and quality-of-life definitions are presented in Table 6. Specifically, the definition of peak walking time on a treadmill is provided, and this measure was felt to integrate all of the factors that might limit an LE-PAD patient's peak exercise performance. Treadmill testing should use standardized protocols to ensure reproducible results. The PARC consortium did not endorse a specific treadmill protocol. The 6-min walk distance (utilized as a primary outcome in heart failure and pulmonary hypertension studies and validated in the PAD population) is also defined on the basis of the distance walked on an unobstructed course of 50 or 100 feet (15 or 30 m). The 6-min walk test measures the maximal distance walked after 6 min, regardless of whether the patient stops to rest or not; thus, this test can be used to evaluate patients with severe claudication, ischemic rest pain, or limited tissue loss. Like all of the functional measures defined, this endpoint was felt to be clinically meaningful and pragmatically useful, as it both characterizes a patient's limitation at baseline and the response to treatment.
TABLE 6.
PARC Functional/Clinical Outcome Definitions for Patients With Intermittent Claudication and Critical Limb Ischemia
| Intermittent Claudication | Ref. # | |||
|---|---|---|---|---|
| Walking/functional capacity definitions | Peak walking time(s) Assessed using a graded treadmill protocol that records the longest time of exercise limited by maximally tolerated claudication pain. |
Claudication onset time(s) Assessed using a graded treadmill protocol that records the time during exercise at the onset of claudication pain. Defines a clinically relevant endpoint, and may be responsive to treatment effect. |
6-min walk test (feet/min) Assessed on an unobstructed course of 50 or 100 feet. Measures the maximal distance walked after 6 min, regardless of whether or not the patient stops to rest (rest periods are acceptable). |
(1,9,15) |
| Quality of life (recommended assessment tools) | Walking Impairment Questionnaire A validated disease-specific assessment of patient-reported outcomes that quantifies the patient's ability to walk a defined distance, speed, and stairs. |
Peripheral Artery Questionnaire A disease-specific health status questionnaire that quantifies the patient's physical limitations, symptoms, social function, treatment satisfaction, and quality of life. |
(30,31) | |
| Clinical assessments | Change in symptom classification Report the change in symptom classification on the basis of PARC classification |
Clinical failure Need for major repeat revascularization (repeat endovascular intervention, thrombolysis, open bypass, open revision of existing bypass) or lower extremity amputation. |
(9,32) | |
| Critical Limb Ischemia | ||||
| Amputation definitions | Lower extremity amputation Any procedure that results in the removal of bone and tissue from the lower extremity. |
Major amputation Any procedure that results in an amputation at the level of the ankle or above; • Below knee amputation—amputation affecting the tibia at any point below the knee and above the ankle; • Above knee amputation—amputation above the knee, affecting the femur at any level. |
Minor amputation Any procedure that results in an amputation below the ankle, including the foot or toe(s). |
(8,9,32,33) |
| Clinical assessment | Major adverse limb events Above-ankle amputation of the index limb or major repeat revascularization (new bypass graft, jump/interposition graft revision, or thrombectomy/thrombolysis). |
Wound healing Complete epithelialization of an ischemic wound of target limb persistent for at least 14 days. |
Ischemic pain relief Improvement in (or relief of) pain of target limb for at least 2 weeks using visual analogue scale. |
(7,8,24) |
For patients with CLI, definitions were agreed upon for major and minor lower extremity amputation, wound healing, ischemic pain, and major adverse limb events (Table 6). Major amputation was defined as:
Any procedure that results in an amputation at the level of the ankle or above;
Below-knee amputation—amputation affecting the tibia at any point below the knee and above the ankle; or
Above-knee amputation—amputation above the knee, affecting the femur at any level.
Major adverse limb events were defined as an above-ankle amputation of the index limb or major repeat revascularization of the target limb (new bypass graft, jump/interposition graft revision, repeat endovascular therapy, or thrombectomy/thrombolysis). The PARC major adverse limb event (MALE) definition is a modification of the Society for Vascular Surgery definition to include all repeat open and endovascular interventions in the target limb. After discussion, 30 days was empirically chosen as a reasonable and objective time point to assess the early progress of wound healing. Early wound healing was defined as complete epithelialization of an ischemic wound of the target limb that stayed closed for at least 2 weeks, evaluated at 30 days. Other time points may also be appropriate and would be acceptable to the FDA for a study designed to support a marketing application. Hemodynamic measurements were also provided for guidance in CLI. Taken together, the outcome definitions provided for both IC and CLI patients should provide options and standard methods for evaluating a broad range of both therapies and patients.
ADOPTION OF PARC DEFINITIONS IN CLINICAL RESEARCH
The PARC consortium recommends consistent application of these consensus definitions in ongoing and future clinical trials and registries to better and more consistently inform evaluations of both new therapies and technologies and to support continued improvement in correlations between interventions, surrogate mechanistic measures, and clinical outcomes. The consistent use of these definitions support both more efficient regulatory approval of new devices and the post-market development of best practice guidelines by professional society consortia. In an effort to support adoption of these definitions, the PARC group will be working with the standards development community to accelerate electronic data capture of these elements in national registries and electronic health records.
CONCLUSIONS
The PARC initiative has developed a series of pragmatic consensus definitions that include the clinical presentation, anatomic depiction, interventional outcomes, surrogate imaging and physiological follow-up, and clinical outcomes of patients with LE-PAD. Consistent application of these definitions in clinical trials evaluating novel revascularization technologies will result in more efficient regulatory evaluation and best practice guidelines in this rapidly moving field (Central Illustration). Consistent with the ARC charter, this process and the definitions provided rely heavily on consensus and integration of previously developed professional society definitions, with adoption and adaptation to optimize utility for key clinical trial processes, such as independent adjudication, core laboratories, and safety monitoring. As with all ARC initiatives, the process was transparent, was inclusive of stakeholders, and maintained a collaborative focus on LE-PAD. The central priority was the recognition that application of consistent definitions across clinical trials of novel devices, drugs, or biologics is far more informative for the accrual of knowledge about optimal therapy and clinical outcomes than are attempts to create novel, “perfect,” but varying definitions for every individual study.
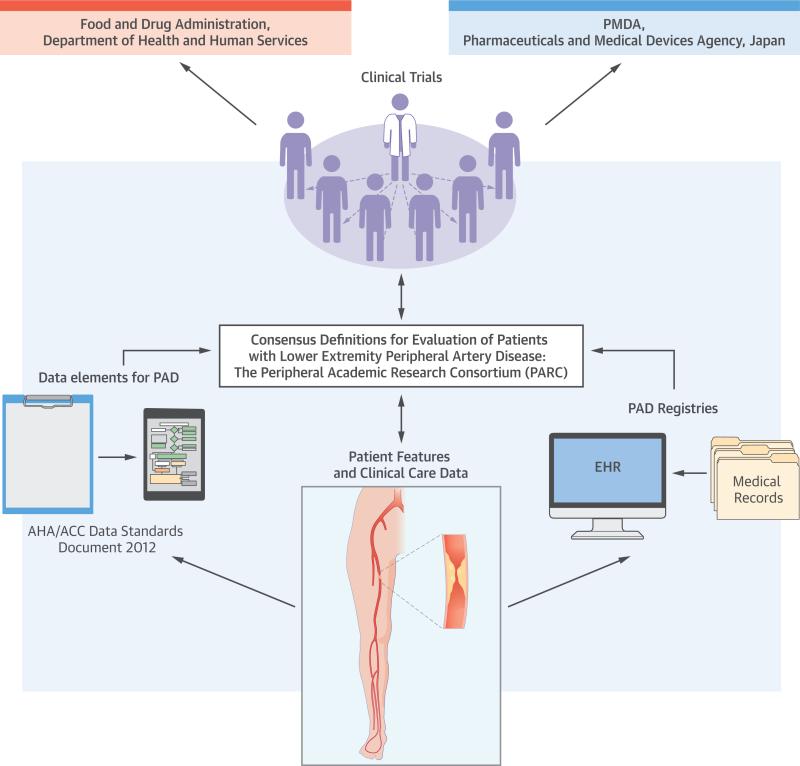
CENTRAL ILLUSTRATION. PARC-PAD Definitions: Consensus Definitions for Evaluation of Patients With Lower Extremity Peripheral Artery Disease: The PARC.
The Peripheral Academic Research Consortium (PARC) included input from both the Food and Drug Administration and the Pharmaceuticals and Medical Devices Agency to develop consensus definitions. Patient features and clinical care data will be entered into patients’ electronic health records (EHRs) used in peripheral artery disease (PAD) registries for clinical trials, and were on the basis of the data elements for PAD in the 2012 American Heart Association/American College of Cardiology (AHA/ACC) data standards document (9). Patient features and clinical care data, data elements, and PAD registries all are used in clinical trials.
Supplementary Material
Acknowledgments
Grants were provided from the industry sponsors and representative listed to the International Society for Cardiovascular Translational Research and the Duke Clinical Research Institute to cover the costs of travel, lodging, and the meeting expenses for academic attendees to the meetings in Silver Spring, Maryland. Dr. Patel has received research grants from Johnson & Johnson Pharmaceutical Research and Development, AstraZeneca, the National Heart, Lung, and Blood Institute, Agency for Healthcare Research and Quality, and Maquet; and has served on the advisory board and/or as a consultant to Bayer Healthcare, Janssen, and Genzyme. Dr. Conte has served as a member of the steering committee for AstraZeneca; and has served as a consultant/advisory board member for Cook Medical and Medtronic. Dr. Dib is the Medical Director of CSI. Dr. Cutlip has received research grants or contracts from Medtronic, Boston Scientific, Abbott Vascular, STENTYS, and Celonova. Dr. Geraghty has served as a consultant and trial principal investigator for Bard/Lutonix; has served as an advisory board member for Bard/Lutonix and Boston Scientific; was on the trial steering committee for Covidien; and has served as a speaker and trial principal investigator for Cook Medical. Dr. Gray has received consultant fees from Cordis, Medtronic, Abbott, Boston Scientific, and WL Gore; and research support from Cordis, Medtronic, Abbott, WL Gore, Mercator, The Medicines Company, and Cardiovascular Systems Incorporated. Dr. Hiatt has received grant support from CPC Clinical Research (a nonprofit affiliate of the University of Colorado), AstraZeneca, Bayer Healthcare, the National Institutes of Health, CSI, DNAVEC, Glaxo-SmithKline, Kyushu University, Pluristem, ReNeuron, and Rigel. Dr. Jaff was a noncompensated advisor to Abbott Vascular, Boston Scientific, Cordis Corporation, Covidien Vascular, and Medtronic Vascular; has served as a board member for VIVA Physicians, a 501(c) 3 not-for-profit education and research organization; and has equity investment in Embolitech, Hotspur, Icon Interventional, PQ Bypass, and Vascular Therapies. Dr. Jones has received research grants from the American Heart Association, AstraZeneca, Boston Scientific, and Bristol-Myers Squibb; and has served as an advisory board member for AstraZeneca. Dr. Lookstein has served as a consultant to Boston Scientific, Bayer Healthcare, and Cordis Corporation. Dr. Mehran has received institutional research grant support from The Medicines Company, Bristol-Myers Squibb/Sanofi, Lilly/Daiichi Sankyo, Regado Biosciences, and STENTYS; has served as a consultant to Abbott Vascular, AstraZeneca, Boston Scientific, Covidien, CSL Behring, Janssen (Johnson & Johnson), Maya Medical, and Merck; has served on the advisory board of Covidien, Janssen Pharmaceuticals, Merck, Sanofi, and Endothelix, Inc.; and has equity/is a shareholder in Endothelix, Inc. Dr. Misra was the Chair for the Data Safety and Monitoring Board for the FLEXSTENT study sponsored by CORDIS (modest grant); and received research grants from the National Institute of Health. Dr. Norgren was a steering committee member/consultant for Otsuka Pharma, AnGes, AstraZeneca, and Novartis. Dr. Olin was on the member steering committee clinical trial on placental stem cells for claudication and medical advisory board for PAD related studies for Pluristem; was on the international steering committee for EUCLID trial and was the site investigator for EUCLID trial for AztraZeneca; was on the steering committee TRA2P trial for Vorapaxar and the medical advisory board for Vorapaxor clinical trials for PAD for Merck; and was on the medical advisory and safety board for Tyrosine Kinase Inhibitors and Cardiovascular Risk for Novartis. Dr. Rundback has served on the clinical events committee for Biotronik and St. Jude; has served as a principal investigator for Covidien and Symbionix; has served as a course director for CSI; and has served as a consultant for Covidien, CSI, Sil Vascular, and Intact Vascular. Dr. Povsic was supported by research grants and received advisory fees from Baxter Healthcare; and has served on the data safety monitoring board of Pluristem, Inc. Dr. Tcheng has served on the advisory board of Philips Medical Systems and Cardiovascular Systems, Inc.; and has received research grants from the Food and Drug Administration. Dr. White has served on the research/advisory board of St. Jude and Neovasc; and has served as the steering committee chair for NCDR CathPVI, as the executive committee co-chair for the BEST trial, and as a member of the steering committee for EUCLID (AstraZeneca). Dr. Wiechmann has served on the advisory boards of Boston Scientific and Bard Peripheral Vascular; has received research funding from The Medicines Company; has served as a consultant to Cordis Corporation, Terumo Medical, and Bard Peripheral Vascular; has received clinical trial support from Cordis Corporation, and Bard Peripheral Vascular; and has an equity interest in PQ Bypass. Dr. Krucoff has received modest consulting fees from Medtronic, CSI, Covidien, Abbott Vascular, and Boston Scientific; and has received significant grant funding from Medtronic, CSI, and Abbott Vascular.
ABBREVIATIONS AND ACRONYMS
- CLI
critical limb ischemia
- LE-PAD
lower extremity peripheral artery disease
- MI
myocardial infarction
- PAD
peripheral artery disease
Footnotes
APPENDIX For a full list of PARC participants, please see the online version of this article.
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Dr. Michael H. Criqui, MD, MPH, has served as Guest Editor for this paper.
REFERENCES
- 1.Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;58:2020–45. doi: 10.1016/j.jacc.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowkes GR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–40. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 3.Tendera M, Aboyans V, Bartelink ML, et al. for the European Stroke Organisation, ESC Committee on Practice Guidelines ESC guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2851–906. doi: 10.1093/eurheartj/ehr211. [DOI] [PubMed] [Google Scholar]
- 4.Jaff MR, Cahill KE, Yu AP, et al. Clinical outcomes and medical care costs among Medicare beneficiaries receiving therapy for peripheral arterial disease. Ann Vasc Surg. 2010;24:577–87. doi: 10.1016/j.avsg.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Subherwal S, Bhatt DL, Li S, et al. Polyvascular disease and long-term cardiovascular outcomes in older patients with non-ST-segment-elevation myocardial infarction. Circ Cardiovasc Qual Outcomes. 2012;5:541–9. doi: 10.1161/CIRCOUTCOMES.111.964379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris TJ, Zafar AM, Murphy TP. Utilization of lower extremity arterial disease diagnostic and revascularization procedures in Medicare beneficiaries 2000-2007. AJR Am J Roentgenol. 2011;197:W314–7. doi: 10.2214/AJR.10.6132. [DOI] [PubMed] [Google Scholar]
- 7.Diehm N, Pattynama PM, Jaff MR, et al. Clinical endpoints in peripheral endovascular revascularization trials: a case for standardized definitions. Eur J Vasc Endovasc Surg. 2008;36:409–19. doi: 10.1016/j.ejvs.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Conte MS, Geraghty PJ, Bradbury AW, et al. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. J Vasc Surg. 2009;50:1462–73. e1–3. doi: 10.1016/j.jvs.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 9.Creager MA, Belkin M, Bluth EI, et al. 2012 ACCF/AHA/ACR/SCAI/SIR/STS/SVM/SVN/SVS key data elements and definitions for peripheral athero-sclerotic vascular disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Peripheral Atherosclerotic Vascular Disease). J Am Coll Cardiol. 2012;59:294–357. doi: 10.1016/j.jacc.2011.10.860. [DOI] [PubMed] [Google Scholar]
- 10.Krucoff MW, Mehran R, van Es GA, et al. The academic research consortium governance charter. J Am Coll Cardiol Intv. 2011;4:595–6. doi: 10.1016/j.jcin.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Cutlip DE, Windecker S, Mehran R, et al. for the Academic Research Consortium Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 12.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 13.Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for Transcatheter Aortic Valve Implantation clinical trials: a consensus report from the Valve Academic Research Consortium. J Am Coll Cardiol. 2011;57:253–69. doi: 10.1016/j.jacc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Kappetein AP, Head SJ, Genereux P, et al. for the Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–54. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Hiatt WR, Goldstone J, Smith SC, Jr, et al. for the American Heart Association Writing Group 1 Atherosclerotic Peripheral Vascular Disease Symposium II: nomenclature for vascular diseases. Circulation. 2008;118:2826–9. doi: 10.1161/CIRCULATIONAHA.108.191171. [DOI] [PubMed] [Google Scholar]
- 16.Norgren L, Hiatt WR, Dormandy JA, et al. for the TASC II Working Group Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 17.Généreux P, Stone GW, Harrington RA, et al. for the CHAMPION PHOENIX Investigators Impact of intra-procedural stent thrombosis during percutaneous coronary intervention: insights from the CHAMPION PHOENIX Trial (Clinical Trial Comparing Cangrelor to Clopidogrel Standard of Care Therapy in Subjects who Require Percutaneous Coronary Intervention). J Am Coll Cardiol. 2014;63:619–29. doi: 10.1016/j.jacc.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Farooq V, Serruys PW, Zhang Y, et al. Short-term and long-term clinical impact of stent thrombosis and graft occlusion in the SYNTAX Trial at 5 years: Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery trial. J Am Coll Cardiol. 2013;62:2360–9. doi: 10.1016/j.jacc.2013.07.106. [DOI] [PubMed] [Google Scholar]
- 19.Serruys PW, Farooq V, Kalesan B, et al. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5-year report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) randomized, noninferiority trial. J Am Coll Cardiol Intv. 2013;6:777–89. doi: 10.1016/j.jcin.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Clinical outcomes with bioabsorbable polymer- versus durable polymer-based drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2014;63:299–307. doi: 10.1016/j.jacc.2013.09.061. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/ AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease). J Am Coll Cardiol. 2006;47:1239–312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg. 2000;31(1 Pt 2):S1–296. [PubMed] [Google Scholar]
- 23.Castronuovo JJ, Jr., Adera HM, Smiell JM, et al. Skin perfusion pressure measurement is valuable in the diagnosis of critical limb ischemia. J Vasc Surg. 1997;26:629–37. doi: 10.1016/s0741-5214(97)70062-4. [DOI] [PubMed] [Google Scholar]
- 24.Mills JL, Sr., Conte MS, Armstrong DG, et al. for the Society for Vascular Surgery Lower Extremity Guidelines Committee The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59:220–34. e1–2. doi: 10.1016/j.jvs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol. 2014 Dec 29; doi: 10.1016/j.jacc.2014.12.018. http://dx.doi.org/10.1016/j.jacc.2014.12.018. [DOI] [PubMed]
- 26.Rocha-Singh KJ, Zeller T, Jaff MR. Peripheral arterial calcification: prevalence, mechanism, detection and clinical implications. Catheter Cardiovasc Interv. 2014;83:E212–20. doi: 10.1002/ccd.25387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klomp HM, Steyerberg EW, Wittens CH, et al. for the ESES Study Group A prognostic model for amputation in critical lower limb ischemia. Vasc Med. 2009;14:109–15. doi: 10.1177/1358863X08098227. [DOI] [PubMed] [Google Scholar]
- 28.Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Brit J Plastic Surg. 1987;40:113–41. doi: 10.1016/0007-1226(87)90185-8. [DOI] [PubMed] [Google Scholar]
- 29.Aboyans V, Criqui MH, Abraham P, et al. for the American Heart Association Council on Peripheral Vascular Disease Council on Epidemiology and Prevention, Council on Clinical Cardiology, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 30.Spertus J, Jones P, Poler S, et al. The peripheral artery questionnaire: a new disease-specific health status measure for patients with peripheral arterial disease. Am Heart J. 2004;147:301–8. doi: 10.1016/j.ahj.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Nead KT, Zhou M, Diaz Caceres R, et al. Walking impairment questionnaire improves mortality risk prediction models in a high-risk cohort independent of peripheral arterial disease status. Circ Cardiovasc Qual Outcomes. 2013;6:255–61. doi: 10.1161/CIRCOUTCOMES.111.000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geraghty PJ, Matsumura JS, Conte MS. Premarket assessment of devices for treatment of critical limb ischemia: the role of Objective Performance Criteria and Goals. J Vasc Surg. 2009;50:1459–61. doi: 10.1016/j.jvs.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 33.Bradbury AW, Adam DJ, Bell J, et al. for the BASIL Trial Participants Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: a description of the severity and extent of disease using the Bollinger angiogram scoring method and the TransAtlantic Inter-Society Consensus II classification. J Vasc Surg. 2010;51:32S–42S. doi: 10.1016/j.jvs.2010.01.075. [DOI] [PubMed] [Google Scholar]
- 34.Dattilo R, Himmelstein SI, Cuff RF. The COMPLIANCE 360 trial: a randomized, prospective, multicenter, pilot study comparing acute and long-term results of orbital atherectomy to balloon angioplasty for calcified femoropopliteal disease. J Invasive Cardiol. 2014;26:355–60. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.