Abstract
Early diagnosis of drug-resistant tuberculosis is critical in order to establish appropriate drug treatment regimens and to prevent the further transmission of resistant strains. Line probe assays (LPAs) are promising rapid diagnostics for use in low income settings, and can be used to screen smear-positive sputum samples for resistance to rifampicin (RIF) and isoniazid (INH) in as little as 1–2 days. Though the diagnostic performance of these assays has been well evaluated, little research has elucidated the true nature of indeterminate LPA results, or assessed the ability of these assays to perform on a wide range of clinical samples
We evaluated the performance of the commercially available Genotype MTBDRplus LPA against conventional MGIT 960 culture and drug susceptibility testing (DST) results for 308 pulmonary (PTB) and 32 extrapulmonary (EPTB) tuberculosis samples. Invalid LPA results (defined as missing the MTB identification band) were obtained for 18 PTB samples, which were excluded from further analysis. The sensitivity and specificity of the Genotype MTBDRplus assay for multidrug-resistant tuberculosis (MDR-TB), based upon the results obtained for the remaining 322 samples, was 95.2% and 95.1%, respectively. 13.7% (40/290) of the PTB samples were not interpretable, or indeterminate, by LPA (defined as having the absence of both wild type and corresponding mutation bands) for INH and/or RIF and were further evaluated by pyrosequencing (PSQ). Contrary to standard LPA interpretation, INH and RIF susceptibility were confirmed by both DST and PSQ in 7.5% (3/40) and 27.5% (11/40) of indeterminate samples, respectively. When LPA test results were not interpretable in this study, PSQ was found to be a valuable and rapid technique to resolve discrepancies.
Keywords: Tuberculosis, Drug Resistance, Pyrosequencing, Heteroresistance, Indeterminate
Introduction
Tuberculosis (TB) remains one of the world’s deadliest communicable diseases. The recent increase of drug-resistant strains, as a result of the ineffective treatment and increased direct transmission of resistant infections, presents an important dilemma for global TB control efforts [1]. The detection of resistance is often delayed when utilizing conventional phenotypic drug susceptibility testing (DST) methods due to the slow growth of Mycobacterium tuberculosis (MTB) in liquid culture. These conventional growth-based diagnostic methods require approximately four weeks to generate results [3]. During this time, patients may be taking medications that are completely ineffective, and risk directly transmitting resistant infections unto others. As such, there is an urgent need for rapid diagnostic tests for drug-resistant TB strains.
MTB acquires drug resistance through the accumulation of resistance-associated mutations on specific genes [2, 3]. The World Health Organization (WHO) has recently approved a commercially available line probe assay (LPA), the Genotype MTBDRplus assay [Hain Lifescience], which detects drug-resistance based upon the presence or absence of these specific resistance-associated mutations [4]. The diagnostic performance of the Genotype MTBDRplus assay is based upon the amplification and genetic analysis of those gene regions known to harbor resistance-associated mutations, as determined by reverse hybridization to wild type and mutated sequences. The assay detects the presence of MTB as well as the most common genetic mutations conferring resistance to RIF (mutations within the rpoB gene) and INH (mutations within the katG gene and inhA promoter) [5, 6], and its diagnostic performance has been well evaluated in pulmonary samples [40]. In this study, the Genotype MTBDRplus diagnostic LPA was thoroughly evaluated for its ability to detect multidrug-resistant TB (MDR-TB) in pulmonary (PTB) and extrapulmonary (EPTB) samples as compared to standard DST methods. Any discrepancies were further evaluated at the molecular level using pyrosequencing (PSQ), a real time sequencing method, in order to comment upon the true nature of indeterminate and discrepant test results.
Materials and Methods
Setting
The study was performed at P. D. Hinduja National Hospital and Medical Research Center, Mumbai, from August 2011– May 2013. The Mycobacteriology laboratory at Hinduja Hospital is accredited through the College of American Pathologists (CAP), the National Accreditation Board for Testing and Calibration Laboratories (NABL), and the Central TB Division, Government of India (CTD, GOI), for performance of liquid culture and DST.
Study Design and Ethics Statement
This single site, cross-sectional study was conducted in order to determine the diagnostic performance of the Genotype MTBDRplus assay for PTB and EPTB samples, in comparison to conventional methodologies. Indeterminate results were further evaluated by pyrosequencing.
The study was approved by the Institutional Review Board (IRB) of P. D. Hinduja National Hospital and Medical Research Center (National Health and Education Society), Mumbai, India. Consent was waived for this study, as the study was to be performed on existing sediments identified only by laboratory-generated numbers and with no traceability back to the patients. All patient details remained confidential.
Sample Collection
A total of 340 consecutive samples were previously collected. Both phenotypic and genotypic testing was conducted on 308 PTB samples (sputum) and 32 EPTB samples (pus, body fluid, biopsy, tissue etc.).
Ziehl-Neelsen Staining
All collected samples were graded as per WHO recommended criteria [7]. EPTB samples selected for the present study were all smear positive, as recommended for MTBDRplus assay.
Sample Processing
All samples were processed by the NALC-NaOH method, as described previously [8]. Final sediments were resuspended in 2ml of PBS (pH= 7.4). Of this suspension, 500 μl was utilized to inoculate a 7ml MGIT tube and 500 μl was used to inoculate solid media (LJ). The remaining sediment was transferred to 1.5ml screw cap tubes for further genotypic analysis. Sediments were stored at −80°C when not in use.
Drug Susceptibility Testing (DST)
Positive liquid MGIT 960 cultures were subjected to further phenotypic DST testing. The drug concentrations used to determine RIF and INH susceptibility were 1μg/mL and 0.1μg/ml, respectively. [28]
Genotypic MTBDRplus Assay
DNA was extracted from 500μl of each decontaminated patient sediment sample. All DNA samples were stored at −80°C when not in use. The Genotype MTBDRplus assay was performed for each sample as per manufacturer’s instructions [9]. The assay contains six control probes: a conjugate control, an amplification control, an MTB complex control, and rpoB, katG and inhA amplification controls. For the detection of INH and RIF resistance, the assay strip contains 21 mutation and wild type probes: eight rpoB wild-type probes (covering codons 505 to 533); the four rpoB mutant probes D516V, D526Y, H526D, and S531L; a katG codon 315 wild-type probe; two katG codon 315 mutant probes with AGC-ACC and AGC-ACA mutations; two inhA wild-type probes covering positions −15 and −16 of the gene regulatory region; and four inhA mutant probes with mutations C→T at position −15, A→G at position −16, T→C at position −8, and T→A at position −8. A detailed test flow chart for this study is shown in Figure 1. Assay results were considered invalid if the control probes were absent, and not interpretable (NI)/indeterminate if the gene loci probe for both the wildtype and mutation probes being assessed was absent. The MTBDRplus assay was not repeated if results were NI/indeterminate.
Figure 1.
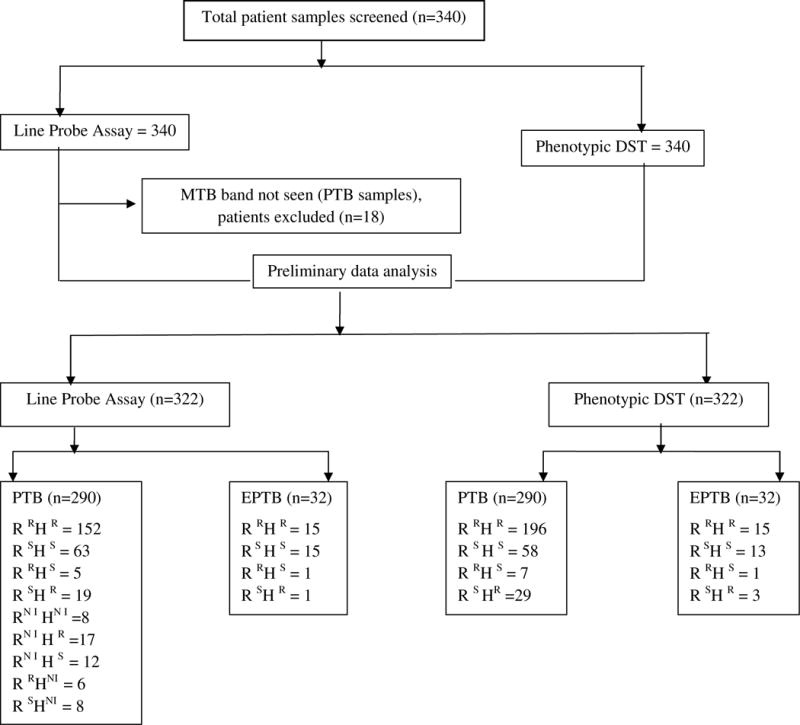
Flow Chart of Sample Testing Process and Phenotypic and Genotypic Testing Results.
MTB= Mycobacterium tuberculosis, DST= drug susceptibility testing, PTB= pulmonary tuberculosis, EPTB= extrapulmonary tuberculosis, R= Rifampicin, H= Isoniazid, R = Resistant, S = Susceptible.
Pyrosequencing (PSQ)
PSQ was performed on all samples found to be NI by MTBDRplus. The same extracted DNA sample, as used for the line probe assay, was used for PSQ. The PSQ assay utilized in this study involved three essential parts: amplification of gene fragments by PCR, capture of biotinylated single-stranded DNA on streptavidin sepharose beads, and sequencing by a commercially available, modified PSQ platform [10]. Our PSQ assay included eight sub-assays: one for identification of MTB and seven for detection of drug resistance mutations. For determination of INH resistance, the assay sequences three loci: katG codons 312–316, the inhA promoter from −4 to −20, and the ahpC promoter from −4 to −23. Mutations in the ahpC promoter are believed to be compensatory to mutations in katG, which cause deficient catalase activities, and are indirectly associated with INH resistance [24, 25]. The molecular target included in the PSQ assay for determination of RIF resistance is the RIF-resistance determining region of the rpoB gene (codons 507–533).
Statistical Analysis
Diagnostic tests were evaluated as per STARD recommendations [11]. Sensitivity and specificity were calculated by comparing phenotypic and molecular test results using Meta Disc software.
Results
Concordance between LPA and DST Results
A total of 322 samples were analyzed by Genotype MTBDRplus assay and phenotypic DST, after excluding 18 samples wherein the MTB band was not detected by the molecular test. The average turnaround time for LPA within the context of our study was 2–3 days (data not shown). Among the 322 samples available for analysis, 290 were PTB and 32 were EPTB. According to phenotypic DST, 65% (211/322) of these were MDR and 22.3% were sensitive to both RIF and INH. Phenotypic monoresistance to RIF and monoresistance to INH was seen for 2.6% (8/322) and 10% (32/322) of samples, respectively.
After excluding three samples failing to detect the MTB band, as above, the genotypic LPA assay found 40 samples to be NI, showing the absence of both wild type and mutation probes for INH and/or RIF resistance detection (Table 1). As such, LPA data was available for both RIF and INH in 282 samples, as shown in Table 2. Out of the total 322 samples evaluated by LPA, 59.2% (180/282) were confirmed to be MDR and 27.6% (78/282) were found to be sensitive to both RIF and INH (Figures 2 and 3). Monoresistance to RIF or INH was found in 1.7% (5/282) and 6.7% (19/282) of samples, respectively, for samples with interpretable genetic data for both drug compounds.
Table 1.
Comparison of Phenotypic DST and PSQ Results for Samples Found to be Not Interpretable (NI)/Indeterminate by Genotype MTBDRplus Assay.

|
R= Resistant
S= Susceptible
NI= Not Interpretable
INH= Isoniazid
RIF= Rifampicin
PSQ= Pyrosequencing
DST= Drug Susceptibility Testing
Table 2.
Comparison of Line Probe Assay (LPA) and Phenotypic DST Results for RIF and INH, excluding Not Interpretable (NI) LPA Results.

|
R= Resistant
S= Susceptible
NI= Not Interpretable
INH= Isoniazid
RIF= Rifampicin
LPA= Line Probe Assay
DST= Drug Susceptibility Testing
Figure 2.

Specificity Forest Plot.
DST= Drug susceptibility testing, LPA= Line Probe Assay, P= pulmonary tuberculosis, EP= extrapulmonary tuberculosis, R= Rifampicin, H= Isoniazid
Figure 3.

Sensitivity Forest Plot.
DST= Drug susceptibility testing, LPA= Line Probe Assay, P= pulmonary tuberculosis, EP= extrapulmonary tuberculosis, R= Rifampicin, H= Isoniazid
Not Interpretable (NI)/Indeterminate Genotypic Test Results
NI/indeterminate results occurred concurrently for RIF and INH in 2.4% (8/322) of tests. RIFNI occurred in 9.6% (29/322) of tests and INHNI occurred in 4.3% (14/322) of tests. Follow-up PSQ analysis showed that, among the eight concurrent RIFNI and INHNI results, relevant resistance-associated mutations were indeed present in 7/8 samples. The remaining sample, though phenotypically resistant for INH, was found to be wild type for relevant gene regions by PSQ. Among the 14 solely INHNI results encountered, PSQ detected mutations in 12/13 samples that were phenotypically resistant to INH, and 1 DST INH-resistant sample was found to be wild type. The one DST INH-susceptible sample was found to be wild type by PSQ. Of the 29 RIFNI encountered, 20 samples were phenotypically resistant to RIF and 9 samples were phenotypically susceptible. PSQ confirmed mutations in all 20 RIF-resistant samples while the 9 RIF-susceptible samples were all found to have wild type rpoB sequences. Compared to phenotypic DST, genotypically discordant results occurred a total of 46 times [INH (27) and RIF (19)], as examined in Table 2.
Detection of Mutations associated with Drug Resistance
The rpoB Mutant 3 (S531L) was the predominant mutation found in PTB (132) and EPTB (18) among the 172 RIF resistant samples evaluated by LPA, as shown in Table 3. For the 200 INH resistant samples evaluated, the katG Mutant 1(315 AGC-ACC) was the predominant mutation identified amongst both PTB (153) and EPTB [18] samples, as shown in Table 4.
Table 3.
LPA Mutation Findings and Distribution for Molecular Evaluation of RIF Susceptibility.
| LPA Result | Mutation | PTB No. of Mutants | EPTB No. of Mutants |
|---|---|---|---|
| Mutant 1 | D516V | 5 | 0 |
| Mutant 2A | H526Y | 2 | 0 |
| Mutant 2B | H526D | 3 | 0 |
| Mutant 3 | S531L | 144 | 18 |
LPA= Line Probe Assay
PTB= Pulmonary Tuberculosis
EPTB= Extrapulmonary Tuberculosis
Table 4.
LPA Mutation Findings and Distribution for Molecular Evaluation of INH Susceptibility.
| LPA Result | Mutation | PTB No. of Mutants | EPTB No. of Mutants |
|---|---|---|---|
| katG Mutant 1 | AGC-315-ACC | 153 | 18 |
| katG Mutant 2 | AGC-315-ACA | 4 | 0 |
| katG Mutant 1 & inhA Mutant 1 | AGC-315-ACC & −15C/T | 16 | 5 |
| inhA Mutant 1 | −15C/T | 0 | 0 |
| inhA Mutant 2 | −16A/G | 0 | 1 |
| inhA Mutant 3A | −8T/C | 0 | 0 |
| inhA Mutant 3B | −8T/A | 3 | 0 |
LPA= Line Probe Assay
PTB= Pulmonary Tuberculosis
EPTB= Extrapulmonary Tuberculosis
Discussion
This study investigated the full performance of the Genotype MTBDRplus assay, assessing both interpretable and non-interpretable results for a range of PTB and EPTB samples. The sensitivity of the assay was found to be suboptimal in the smear negative, culture-positive sputum samples and smear positive EPTB samples evaluated. The sensitivity of the LPA for MDR-TB was 95.29%; with a and specificity was 95.16%. The sensitivity for the detection of INH resistance was 89.29%, with a specificity of 95.95%. However, the Genotype MTBDRplus assay failed to detect 8.05% of INH resistant strains. The sensitivity for detection of RIF resistance in our study was 91.98%, with a specificity of 95.79%, but the assay failed to detect 5.31% of RIF resistant strains. The S531L mutation in the rpoB gene was the most frequently occurring mutation among RIF resistant strains (n=162) [37, 38, 39] as observed in previous studies. For determination of INH resistance, the S315T mutations in the katG gene were by far the most common found in our study (n=196).
Recent studies have found the MTBDRplus assay to be an effective and feasible diagnostic tool for MDR-TB screening in endemic regions [12, 13, 14, 24, 42]. While the sensitivity of the molecular diagnostic test for detection of INH resistance in our study was slightly lower than that for RIF and MDR detection (compared to conventional methods), it was analogous to that of previously reported studies [25, 31, 32]. The slightly lower sensitivity of the MTBDRplus test for INH, as compared to conventional methods, is likely due to the presence of genetic mutations conferring INH resistance that are located outside the katG and inhA promoter gene regions included in the LPA [26, 27, 33, 35]. In the present study, we did not identify any ahpC promoter mutations via PSQ, but other gene regions have been implicated in conferring resistance to INH that were not included in the assay, such as kasA [41]. An alternative explanation for the persistence of discordant results may be the presence of mixed populations of bacteria, consisting of both susceptible and resistant strains, and hetero-resistance [29, 30, 34]. In the present study, 6.2% (18/290) of samples were considered hetero-resistant by LPA, and were phenotypically resistant by MGIT DST. Pyrosequencing confirmed these samples to be genotypically resistant, indicating that observed discrepancies were likely due to signal overdevelopment during LPA, rather than mixed infection. As the MTBDRplus assay is currently only recommended for smear positive samples, having a poor limit of detection for MTB, the detection of heteroresistance is unlikely.
LPA indeterminate have been previously reported to be related to smear- and culture-status of the evaluated samples, and generally occur in 1.4–19.2% of samples, depending on sample type (EPTB v. PTB), [40] with un-interpretable reads only occurring in 6% of smear positive sputum samples upon test repeat [42]. Our study found 12.4% of results to be NI without test repeat. According to the manufacturer’s instructions for reporting of LPA results, samples demonstrating the absence of wild type and corresponding mutation bands (NI/Indeterminate results) are to be reported as resistant. Although this interpretation proved accurate for the majority of INHNI and RIFNI results in our study, other LPA ideterminate results from this study were not as easily resolved, highlighting an important limitation for MTBDRplus performance. In the present study, NI results were proven susceptible by DST and PSQ in a notable 7.5% (3/40) and 27.5% (11/40) of INHNI and RIFNI samples, respectively. Although the molecular basis of these discordant LPA results has not been fully elucidated, we can speculate as to the mechanisms behind test discordance. The occurrence of NI results where the wild type probes failed to hybridize to their corresponding sequences could result from the inaccessibility of the genomic sequence in a given sample, such as when secondary DNA structures are present. This problem has been noted in rpoB hybridization, in particular, and might play a role in the observed high occurrence (27.5%) of NI results proven RIF-susceptible in this study [36].
Conclusions
Although the Genotype MTBDRplus test has proven to be a reliable, rapid and easy diagnostic for the simultaneous detection of RIF and INH resistance in M. tuberculosis, the nuances of LPA performance in regards to the occurrence and interpretation of indeterminate results are still being evaluated. This study has found the conventional interpretation of LPA indeterminate results as resistant to be incorrect 7.5% of the time when assessing INH resistance and 27.5% of the time when assessing RIF resistance. In case of discrepant results, we find that PSQ can be a valuable tool to rapidly evaluate LPA indeterminate results, in lieu of repeating phenotypic DST, as the technology appears to resolve discrepancies in line with phenotypic DST results.
What is the key question?
Does the absence of wild type and corresponding mutation probes on an LPA strip actually indicate resistant strains, as per manufacturer’s instructions?
What is the bottom line?
Clinicians should correlate discrepant LPA results.
Why read on?
To decipher indeterminate LPA results.
Acknowledgments
Funding Body: P. D. Hinduja Hospital and Medical Research Centre
Footnotes
Competing Interests: None to declare
Contributionship statement: CN: Wrote the manuscript and performed LPA, RP: Performed laboratory tests and analyzed data, MS: Performed phenotypic testing, KA: Performed all pyrosequencing, MK: Performed data analysis, SG: Reviewed and revised manuscript, TR: Reviewed manuscript, AC: Reviewed manuscript, AS: Reviewed manuscript, CR: Designed the study and reviewed the manuscript.
References
- 1.WHO. WHO report 2009. Geneva: World Health Organization; 2009. Global tuberculosis control: surveillance, planning, financing. [Google Scholar]
- 2.Wright A, Zignol M, Van Deun A, et al. Epidemiology of antituberculosis drug resistance 2002–07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet. 2009;373(9678):1861–1873. doi: 10.1016/S0140-6736(09)60331-7. [DOI] [PubMed] [Google Scholar]
- 3.Espinal MA, Kim SJ, Suarez PG, et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA: journal of the American Medical Association. 2000;283:2537–2545. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 4.Genotype MTBDRplus product page. Nehren, Germany: Hain Lifescience; http:www.hain-lifescience.de.en/products/microbiology/mycobacteria/genotype-MTBDRplus.html. [Accessed August 15, 2011] [Google Scholar]
- 5.Ling DI, Zwerling AA, Pai M. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis a meta-analysis. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2008;32:1165–1174. doi: 10.1183/09031936.00061808. [DOI] [PubMed] [Google Scholar]
- 6.WHO Policy Statement: molecular line probe assays for rapid screening of patients at risk of multidrug-resistant tuberculosis. Geneva: World Health Organization; 2008. Available at http://www.who.int/tb/features_archive/policy_statement.pdf. [Google Scholar]
- 7.World Health Organization. Treatment of Tuberculosis: Guidelines for national programmes. 2nd. Geneva: 1997. (WHO/TB/97.220). [Google Scholar]
- 8.Rodrigues C, Almedia D, Shenai S, et al. Dedicated decontamination: A necessity to prevent cross-contamination in High throughput Mycobacteriology Laboratories. Indian Journal of Medical Microbiology. 2007;25(1):4–6. doi: 10.4103/0255-0857.31053. [DOI] [PubMed] [Google Scholar]
- 9.Lacoma A, et al. GenoType MTBDRplus assay for molecular detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis strains and clinical samples. J Clin Microbiol. 2008;46:3660–3667. doi: 10.1128/JCM.00618-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bravo LT, Tuohy MJ, Ang C, Destura RV, et al. Pyrosequencing for rapid detection of Mycobacterium tuberculosis resistance to rifampin, isoniazid, and fluoroquinolones. J Clin Microbiol. 2009;47:3985–90. doi: 10.1128/JCM.01229-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bossuyt PM, Reitsma JB, E Bruns D, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Clinical chemistry and laboratory medicine. 2003;41(1):68–73. doi: 10.1515/CCLM.2003.012. [DOI] [PubMed] [Google Scholar]
- 12.Miotto P, Piana F, Cirillo DM, Migliori GB. Genotype MTBDRplus: a further step toward rapid identification of drug-resistant Mycobacterium tuberculosis. J Clin Microbiol. 2008;46(1):393–394. doi: 10.1128/JCM.01066-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnard M, Albert H, Coetzee G, O’Brien R, et al. Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa. Am J Respir Crit Care Med. 2008;177(7):787–792. doi: 10.1164/rccm.200709-1436OC. [DOI] [PubMed] [Google Scholar]
- 14.de Oliveira MM, da Silva Rocha A, Cardoso Oelemann M, et al. Rapid detection of resistance against rifampicin in isolates of Mycobacterium tuberculosis from Brazilian patients using a reverse-phase hybridization assay. J Microbiol Methods. 2003;53(3):335–342. doi: 10.1016/s0167-7012(02)00253-1. [DOI] [PubMed] [Google Scholar]
- 15.Nikolayevskyy VV, Brown TJ, Bazhora YI, et al. Molecular epidemiology and prevalence of mutations conferring rifampicin and isoniazid resistance in Mycobacterium tuberculosis strains from the southern Ukraine. Clin Microbiol Infect. 2007;13(2):129–138. doi: 10.1111/j.1469-0691.2006.01583.x. [DOI] [PubMed] [Google Scholar]
- 16.Cavusoglu C, Turhan A, Akinci P, Soyler I. Evaluation of the Genotype MTBDR assay for rapid detection of rifampin and H resistance in Mycobacterium tuberculosis isolates. J Clin Microbiol. 2006;44(7):2338–2342. doi: 10.1128/JCM.00425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miotto P, Piana F, Penati V, et al. Use of genotype MTBDR assay for molecular detection of rifampin and H resistance in Mycobacterium tuberculosis clinical strains isolated in Italy. J Clin Microbiol. 2006;44(7):2485–2491. doi: 10.1128/JCM.00083-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mäkinen J, Marttila HJ, Marjamaki M, et al. Comparison of two commercially available DNA line probe assays for detection of multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol. 2006;44(2):350–352. doi: 10.1128/JCM.44.2.350-352.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brossier F, Veziris N, Truffot-Pernot C, et al. Performance of the genotype MTBDR line probe assay for detection of resistance to rifampin and H in strains of Mycobacterium tuberculosis with low- and high-level resistance. J Clin Microbiol. 2006;44(10):3659–3664. doi: 10.1128/JCM.01054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vijdea R, Stegger M, Sosnovskaja A, et al. Multidrug-resistant tuberculosis: rapid detection of resistance to rifampin and high or low levels of H in clinical specimens and isolates. Eur J Clin Microbiol Infect Dis. 2008;27(11):1079–1086. doi: 10.1007/s10096-008-0548-9. [DOI] [PubMed] [Google Scholar]
- 21.Hillemann D, Rusch-Gerdes S, Richter E. Evaluation of the GenoType MTBDRplus assay for rifampin and H susceptibility testing of Mycobacterium tuberculosis strains and clinical specimens. J Clin Microbiol. 2007;45(8):2635–2640. doi: 10.1128/JCM.00521-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang WL, Chen HY, Kuo YM, Jou R. Performance assessment of the GenoType MTBDRplus test and DNA sequencing in detection of multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol. 2009;47(8):2520–2524. doi: 10.1128/JCM.02499-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akpaka PE, Baboolal S, Clarke D, et al. Evaluation of methods for rapid detection of resistance to H and rifampin in Mycobacterium tuberculosis isolates collected in the Caribbean. J Clin Microbiol. 2008;46(10):3426–3428. doi: 10.1128/JCM.01455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hillemann D, Weizenegger M, Kubica T, Richter E, Niemann S. Use of the genotype MTBDR assay for rapid detection of rifampin and INH resistance in Mycobacterium tuberculosis complex isolates. J Clin Microbiol. 2005;43(8):3699–3703. doi: 10.1128/JCM.43.8.3699-3703.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling DI, Zwerling AA, Pai M. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2008;32:1165–1174. doi: 10.1183/09031936.00061808. [DOI] [PubMed] [Google Scholar]
- 26.Evans J, Stead MC, Nicol MP, Segal H. Rapid genotypic assays to identify drug-resistant Mycobacterium tuberculosis in South Africa. The Journal of antimicrobial chemotherapy. 2009;63:11–16. doi: 10.1093/jac/dkn433. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Yew WW. Mechanisms of drug resistance in Mycobacterium tuberculosis. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2009;13:1320–1330. [PubMed] [Google Scholar]
- 28.WHO. Guidelines for surveillance of drug resistance in tuberculosis. 4th. Geneva: World Health Organization; 2009. (WHO/HTM/TB/2009422). 2009. [Google Scholar]
- 29.Van Rie A, Victor TC, Richardson M, Johnson R, et al. Reinfection and mixed infection cause changing Mycobacterium tuberculosis drug-resistance patterns. Am J Respir Crit Care Med. 172:636–642. doi: 10.1164/rccm.200503-449OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Deun A, Barrera L, Bastian I, Fattorini L, et al. Mycobacterium tuberculosis strains with highly discordant rifampin susceptibility test results. J Clin Microbiol. 2009;47:3501–6. doi: 10.1128/JCM.01209-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cattamanchi A, Dantes RB, Metcalfe JZ, et al. Clinical characteristics and treatment outcomes of patients with isoniazidmonoresistant tuberculosis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;48:179–185. doi: 10.1086/595689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bwanga F, Hoffner S, Haile M, Joloba ML. Direct susceptibility testing for multi drug resistant tuberculosis: a meta-analysis. BMC Infect Dis. 2009;9:67. doi: 10.1186/1471-2334-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan M, Kalantri S, Flores L, Pai M. A commercial line probe assay for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. BMC Infect Dis. 2005;5:62. doi: 10.1186/1471-2334-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinder H, Mieskes KT, Löscher T. Heteroresistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2001;5:339–345. [PubMed] [Google Scholar]
- 35.Anek-Vorapong R, Sinthuwattanawibool C, Podewils, et al. Validation of the GenoType MTBDRplus assay for detection of MDR-TB in a public health laboratory in Thailand. BMC infectious diseases. 2010;10:123. doi: 10.1186/1471-2334-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng M, Feng S, Luo F, Wang S, Sun X, Zhou X, et al. Visual detection of rpoB mutations in rifampin-resistant Mycobacterium tuberculosis strains by use of an asymmetrically split peroxidase DNAzyme. J Clin Microbiol. 2012;50(11):3443–3450. doi: 10.1128/JCM.01292-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang KC, Yew WW, Zhang Y. A systematic review of rapid drug susceptibility tests for multidrug-resistant tuberculosis using rifampin resistance as a surrogate. Expert Opin Med Diagn. 2009;3:99–122. doi: 10.1517/17530050802665694. http://dx.doi.org/10.1517/17530050802665694. [DOI] [PubMed] [Google Scholar]
- 38.Nebenzahl-Guimaraes H, Jacobson KR, Farhat MR, Murray MB. Systematic review of allelic exchange experiments aimed at identifying mutations that confer drug resistance in Mycobacterium tuberculosis. J Antimicrob Chemother. 2013 Sep 20; doi: 10.1093/jac/dkt358. http://dx.doi.org/10.1093/jac/dkt358. [DOI] [PMC free article] [PubMed]
- 39.World Health Organization (WHO) Rapid implementation of the Xpert MTB/RIF diagnostic test: technical and operational ‘how-to’ practical considerations. World Health Organization; Geneva, Switzerland: 2012. (Report WHO/HTM/TB/2011.2). http://whqlibdoc.who.int/publications/2011/9789241501569_eng.pdf. [Google Scholar]
- 40.Ling DI, Zwerling AA, Pai M. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. Eur Respir J. 2008;32:1165–1174. doi: 10.1183/09031936.00061808. http://dx.doi.org/10.1183/09031936.00061808. [DOI] [PubMed] [Google Scholar]
- 41.Shekar S, Yeo ZX, Wong JCL, Chan MKL, Ong DCT, et al. Detecting Novel Genetic Variants Associated with Isoniazid-Resistant Mycobacterium tuberculosis. PLoS ONE. 2014;9(7):e102383. doi: 10.1371/journal.pone.0102383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raizada N, Sachdeva KS, Chauhan DS, Malhotra B, Reddy K, et al. A Multi-Site Validation in India of the Line Probe Assay for the Rapid Diagnosis of Multi-Drug Resistant Tuberculosis Directly from Sputum Specimens. PLoS ONE. 2014;9(2):e88626. doi: 10.1371/journal.pone.0088626. [DOI] [PMC free article] [PubMed] [Google Scholar]


