Abstract
Objective
POLE mutations in high grade endometrioid endometrial cancer (EEC) have been associated with improved survival. We sought to investigate the prevalence of POLE tumor mutation and its prognostic significance on outcomes and clinical applications in a sub-analysis of women with high grade EEC from a previously described cohort of 544 EEC patients in which POLE mutation status and survival outcomes was assessed.
Materials and Methods
PCR amplification and Sanger sequencing was used to test for POLE mutations in 72 tumors. Associations between POLE mutation, demographic and clinicopathologic features and survival were investigated with Cox proportional hazard models.
Results
POLE mutations were identified in 7/72 (9.7%) grade 3 EECs. No significant differences in the clinicopathologic features between those with POLE mutations and those without were identified. Adjusted for age, a decreased risk of recurrence was suggested in patients with a POLE mutation (aHR=0.37, 95%CI 0.09-1.55), as well as decreased risk of death (aHR=0.19, 95% CI 0.03-1.42).
Conclusions
POLE mutations in tumors of women with grade 3 EEC are associated with a lower risk of recurrence and death, though not statistically significant due to high variability in these estimates. These findings, consistent with recently published combined analyses, support POLE mutation status as a noteworthy prognostic marker and may favor a change in the treatment of women with grade 3 EECs, particularly in those with early stage disease, in which omission of adjuvant therapy and decreased surveillance could possibly be appropriate.
Keywords: POLE mutation, high-grade, endometrial cancer, prognostic marker
Introduction
While endometrial cancer (EC) is the most common gynecologic cancer reported in the developed world1, controversies over adjuvant treatment and surveillance modalities continue to be prominent issues. Despite excellent surgical cure rates with surgery alone, a proportion of women with intermediate and high-risk early stage disease will require adjuvant treatment to decrease risk of recurrence.2 Those with high-risk early stage disease, including high grade tumors (grade 3 endometrioid, serous and clear cell) and/or advanced disease are recommended to undergo adjuvant therapy with chemotherapy with or without radiation. Pathologic characteristics including lymphovascular space invasion, myometrial invasion, grade and stage are used to determine whether adjuvant therapy is appropriate, however the exact criteria for therapy are imperfect, and often leads to under and over treatment of EC patients. The identification of novel molecular markers that could better define the risk of recurrence would benefit patients and guide physicians' recommendations for adjuvant therapy needs and subsequent surveillance recommendations. Despite the identification of many prognostic molecular markers in EC, none have been shown to play a clinically relevant role in the treatment or surveillance of women with EC.3,4
POLE encodes the major catalytic and proofreading subunits of the Polε DNA polymerase enzyme complex.5,6 The proofreading (exonuclease) function locates and replaces erroneous bases in the daughter strand through failed complementary pairing with the parental strand. High fidelity incorporation of bases by POLE, coupled with its proofreading function, ensures a low mutation rate. POLE mutations have shown to increase spontaneous mutation rates contributing to tumorigenesis in yeast and mouse models.7-12 ECs are frequently defective in DNA mismatch repair (MMR). Reduced post-replication surveillance and repair results in a 100-fold increase in somatic mutations in human tumor cell lines.13 Loss of DNA proofreading function in the DNA polymerase ε (POLE) has similarly been shown to be important in tumorigenesis in EC.14,15 Approximately 8% of ECs harbor POLE mutations.16,17
Recent studies have investigated the prognostic importance of POLE mutations in EC and an association with improved survival outcomes has been reported.16,17 As POLE mutations were noted to be more common in grade 3 endometrioid tumors compared to low grade tumors in these analyses, the survival benefit of POLE among those high grade endometrioid tumors specifically has been investigated among smaller cohorts. Meng and colleagues recently reported on the progression free survival (PFS) of high grade endometrioid endometrial cancers (EECs) by describing their data of 53 women with high grade EEC along with The Cancer Genome Atlas (TCGA) data of 49 women with high grade EEC.18 They reported POLE mutations in 8 of 53 tumors (15%), which was comparable to TCGA data, which reported mutations in 8 of 49 tumors (16%). Church and colleagues similarly reported a POLE mutation incidence of 22% (7 of 32) in high grade EEC.17 When combining data with TCGA, Meng et al observed a significantly improved PFS in women with POLE mutations, reporting that none of the patients with a POLE mutation in either cohort experienced a recurrence. The overall survival (OS) benefit was investigated as well, and while Meng et al reported a trend towards improved survival, this was not statistically significant, attributable to the small numbers of patients with POLE mutations. The TCGA data for disease-specific survival was not available; therefore the disease-specific analyses could not be combined with the data from Meng and colleagues. It was reported that among the patients with POLE mutations in the Meng cohort, none died from their disease or any other cause within 5 years of diagnosis.18
Church and colleagues have additionally reported on the significance of POLE mutations in a larger data set, including patients from the PORTEC-1 and -2 studies (n=788), as well as combined data from three additional series (n=628).19 In those women in the PORTEC trials with grade 3 EECs (n=109), 15 patients had POLE mutated tumors, and none of those women experienced a recurrence, compared to 29 of 94 (30.9%) women with POLE wild-type tumors, resulting in a statistically significant improved recurrence-free survival (RFS) (HR=0.11, 95%CI = 0.001-0.84, P=.03). These results, combined with the above-mentioned studies, support the conclusion that POLE mutations are associated with favorable survival outcomes, with the greatest effect seemingly in high grade EECs.
These studies have suggested a significantly impactful prognostic role of predicting improved survival outcomes in women with POLE mutations in high grade EECs. This is a subset of EC patients who have been associated with worse outcomes, and therefore are often advised to undergo adjuvant treatment to minimize progression, recurrence and death from disease. Current data suggests that women with grade 3 EECs with POLE mutations have an improved clinical course, which may guide alternative management and surveillance choices for these women. It is critical that the findings to date be validated in additional populations before any recommendations for care of EC patients with POLE mutations be universally implemented. Our objective in this study is to investigate the prevalence of POLE mutation tumor status and its prognostic significance on survival and recurrence outcomes, specifically in women with high grade EEC from a previously described cohort of 544 EECs, and to discuss the clinical implications that POLE mutations may have in treatment and subsequent surveillance practices.
Materials and Methods
Study Population
Matched EC and normal tissues were prospectively collected at time of hysterectomy by a single academic institution. All research subjects consented to molecular analyses and follow-up (protocols 91-507 and 93-0828). The analyses performed at a different single academic institution were undertaken with IRB approval (2012C0117).
High molecular weight genomic DNA for 544 surgically staged, EECs was analyzed for POLE mutation, of which 72 were categorized as nuclear grade 3. Extensive data are available for all cases. The cohort has been previously described.20-22
Mutation testing
The exonuclease domain of POLE (residues 268-471) was assessed for mutations using PCR amplification and Sanger sequencing. Primers and conditions have been previously described as well as all mutations .23 PCR products were sequenced (ABI Prism BigDye Terminator Cycle Sequencing Kit version 3.1, Applied Biosystems®) at the Nucleic Acid Shared Resource laboratory at the academic institution performing the analyses (http://cancer.osu.edu/research/cancerresearch/sharedresources/na/services/dna_sequencing/pages/index.aspx).
Statistical Analyses
Baseline clinicopathologic and demographic characteristics were described overall and by POLE status, and compared by a chi-square test for categorical covariates or by the Wilcoxon ranksum test for continuous covariates. The method of Kaplan and Meier were used to estimate unadjusted survival distributions for the time to death and time to recurrence. Associations between POLE mutation and the risk of recurrence and risk of death were estimated via Cox proportional hazards models.
Overall survival was defined as time from surgery to death of any cause. Recurrence-free survival was defined as time from surgery to the time of first recurrence of death from disease. For both endpoints patients were censored at the time of last follow-up when no event has occurred.
The assumption proportional hazards were estimated through tests of the Schoenfeld residuals and by visual inspection. All p-values and confidence intervals are reported as two-sided and unadjusted for multiple comparisons. Analyses were performed using SAS software (Version 9.3 of the SAS System for Windows, SAS Institute Inc., Cary, NC, USA) and Stata 13.0 (StataCorp. 2013 Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
Results
Clinical and molecular pathologic features
Our cohort included 72 high grade EECs. The median age was 64.6 years (range 44.3-90.7 years), with a median BMI of 31.7 (18.7-58.2). The majority of patients (65.3%) had early stage disease and over half the patient population underwent adjuvant treatment of some type (chemotherapy, hormone therapy, radiation, or any combination) (Table 1).
Table 1. Clinicopathologic features of study population.
| Grade 3 Endometrioid EC | |
|---|---|
| N | 72 |
| Age (median, range) | 64.6 (44.3-90.7) |
| BMI (median, range) | 31.7 (18.7-58.2) |
| Stage | |
| I/II | 47 (65.3%) |
| III/IV | 24 (33.3%) |
| Unstaged | 1 (1.3%) |
| Deep myometrial invasion (≥50%) | 33 (45.8%) |
| Presence of LVSI | 49 (67.1%) |
| Adjuvant therapy (any) | 41 (56.9%) |
EC: endometrial cancer; BMI: body mass index; LVSI: lymphovascular space invasion.
Among these 72 high grade EECs, 7 tumors harbored missense POLE mutations. The clinicopathologic features of patients with a POLE mutation compared to those with wild type tumors are illustrated in Table 2. No significant differences between these two groups were identified. The median age for those with POLE mutations was slightly younger than the wild type cohort (median age 58.3 years (55.2-87.3) versus 66.5 (44.3-90.7), respectively), with the majority (85.7%) having early stage disease and deep myometrial invasion (83.3%).
Table 2. Clinical and pathologic characteristics of Grade 3 endometrioid ECs by POLE mutation status.
| POLE mutation | POLE Wild type | p-value * | |
|---|---|---|---|
| N | 7 | 65 | |
| Age (median, min-max) | 58.3 (55.2-87.3) | 66.5 (44.3-90.7) | 0.20 |
| Race | 0.99 | ||
| White | 6 (85.7) | 55 (84.6) | |
| Black | 1 (14.3) | 10 (15.4) | |
| BMI (median, min-max) ˆ | 25.8 (18.7-54.2) | 32.3 (19.5-58.2) | 0.18 |
| High stage | 0.41 | ||
| Advanced stage (III/IV) | 1 (14.3) | 23 (35.9) | |
| Early stage (I/II) | 6 (85.7) | 41 (64.1) | |
| Deep myometrial invasion | 5 (83.3%) | 28 (49.1%) | 0.20 |
| (≥50%)ˆˆ | |||
| Presence of LVSI | 4 (57.1%) | 45 (71.4%) | 0.42 |
| Adjuvant therapy (any) | 2 (28.6%) | 39 (60.0%) | 0.13 |
EC: endometrial cancer; BMI: body mass index; LVSI: lymphovascular space invasion.
P-values calculated by Fisher's Exact test for categorical variables and by Wilcoxon rank-sum test for continuous variables.
Available for 63 participants (58 POLE WT and 5 POLE mutation).
Available for 63 participants (57 POLE WT and 6 POLE mutation).
Prognostic significance of POLE mutation in grade 3 EEC
Kaplan-Meier methods and Cox regression models were used to describe associations between POLE mutation and survival endpoints. The RFS curve is shown in Figure 1A. With a median follow up of 38 months, one recurrence among the 7 patients with a POLE tumor mutation was observed. That patient recurred at 23.3 months in the pelvis, compared to 25 recurrences in those patients with a wild type tumor, and she did not die from disease. The 5-year RFS for those with POLE mutations was noted to be 71% (95% confidence interval (CI) 26-92%) compared to 50% (95% CI 37-62%) of those with wild type tumors (aHR=0.37, 95% CI 0.09-1.55, adjusted for patient age). A decreased risk of recurrence is noted in those patients with POLE mutations, after adjusting for age, with a risk of recurrence of approximately one third that of those patients with wild type tumors. Similarly, a trend was suggested for the protective effect of POLE mutation on OS (all cause) (Figure 1B). One non-cancer related death was observed in the POLE mutation group, compared to 39 deaths among the wild type cohort. The 5-year all-cause OS was 86% in the POLE group (95% CI 33-98%) compared to 51% in the wild type group (95% CI 37-63%), after adjusting for age with an adjusted hazard ratio of 0.19 (95% CI 0.03-1.42). This translates to an approximately 80% decreased risk of death in women with a POLE mutation compared to women with wild type tumors.
Figure 1. Kaplan-Meier recurrence-free survival (1A) and all-cause overall survival (1B) by POLEmutational status.
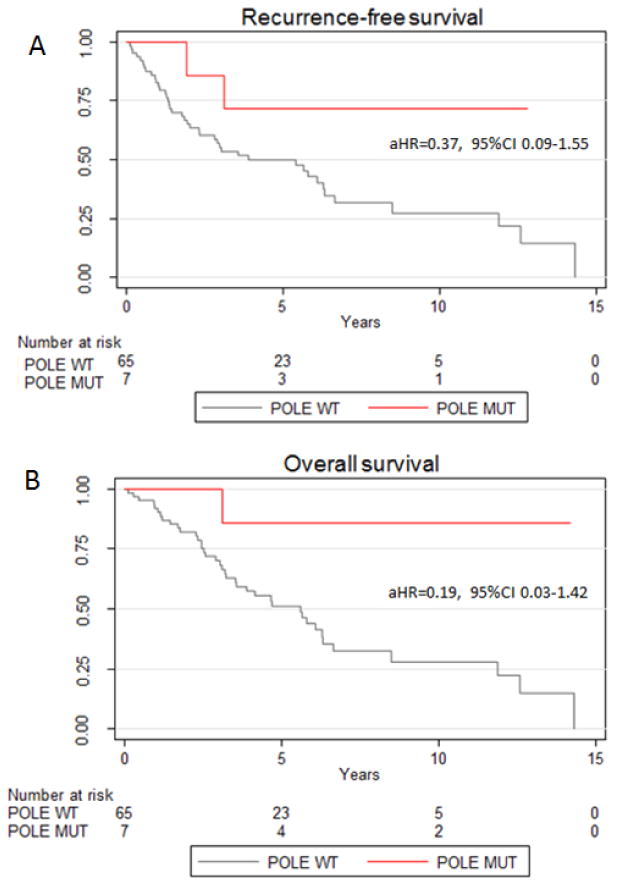
aHR is the age adjusted hazard ratio.
Discussion
In this report we present data on POLE mutation in grade 3 EEC. This analysis was undertaken in response to growing data that POLE mutation in EECs, in high grade tumors in particular, may confer a prognostic survival advantage. We noted a 9.7% POLE mutation rate amongst the 72 grade 3 EECs studied. This is comparable to what has been reported in previous studies, including data reported by TCGA in 16% of grade 3 EECs harbored a POLE mutation,16 data presented by Meng et al (8/53, 15%),18 as well as data published by Church et al,17 citing a 22% POLE prevalence in one series, and 13.7% in the more recent publication including PORTEC-1 and -2 patients.19 Combining our data with the current literature, it appears that POLE mutations are present in approximately 10-20% of grade 3 EECs. This frequency of mutation is noteworthy given that grade 3 tumors, regardless of stage, are associated with a more aggressive tumor behavior, and are regarded as a high-intermediate risk prognostic factor, even in early stage disease.24,25 Therefore, these high grade tumors are typically treated more aggressively (chemotherapy and/or radiation) and patients are often subjected to more rigorous surveillance following treatment based on this histology alone.26
Our survival analyses reveal a strong, yet highly variable, trend towards improved outcomes in both RFS and OS consistent with recent publications.17-19 Given the modest number of grade 3 cases in our study, we did not break down outcome by stage. Our findings are consistent with a survival advantage of those patients with tumors harboring POLE mutations. Meng et al reported on survival outcomes by combining data with TCGA data, and reported a significantly improved PFS advantage in the POLE mutation group in univariate (P=0.025) and multivariate (P=0.01) analyses, such that not one patient with a POLE mutation experienced a recurrence.18 The OS (disease-specific) was shown to have a similar trend towards improved survival, as none of the 8 patients with a POLE mutation died from their disease, however this was not significant after adjusting for age and disease stage. This lack of statistical significance is likely due to the small number of patients in the POLE mutation group (8 patients), which is problematic in our study as well, as only 7 patients had POLE mutated tumors, which contributes to our lack of statistical significance. However, our analyses strongly suggest a significant survival advantage, with a risk of recurrence of roughly one third that of a patient with a wild type tumor, and risk of death is decreased by 80% compared to a patient with a wild type tumor. Church and colleagues reported no recurrences among POLE mutated grade 3 tumors in patients in the PORTEC-1 and -2 cohort, compared to 30.9% recurrence rate among those women with grade 3 tumors in the rest of the subgroup, resulting in a statistically significantly greater RFS (HR=0.11, 95%CI =0.001-0.84, P=.03), as well as no cancer related deaths compared to 26.6% (25/94) EC deaths in the wild-type cohort.19 In our analysis, one patient recurred in the POLE group, compared to 26 of 45 women with wild type tumors. This was a 55-year-old black female who was diagnosed with a stage 1B grade 3 endometrioid EC, who underwent no further therapy. Her POLE mutation was a alanine to proline mutation at position 456 (p.Ala456Pro), a previously described POLE mutation.17 She recurred in a para-aortic lymph node 23.3 months after her diagnosis. She was alive at that date of last contact, 14.4 years from her original diagnosis. Similarly, only one patient died (all-cause), and that patient did not experience a recurrence. This is in comparison to 39 deaths among the women with wild type tumors. It is important to note that our data differs from previous publications by identifying two of seven patients with POLE mutant tumors who experienced either a recurrence or death. While the prognostic significance of POLE suggests improved outcomes, it may not be universal as previously described, and continued investigation of the biology of POLE mutated G3 EECs will be critical to our understanding of the management of this molecular phenotype.
The prognostic role of POLE mutations has been highly anticipated. The initial PFS benefit reported by TCGA suggested an improved PFS, however the data included all grades, and histologies of EC. Since that time, continued investigation has led to the hypothesis that that true survival benefit is seen in those patients with high grade EEC. The benefit of these mutations in other histologies such as serous and clear cell remains inconclusive. Our data strongly trends towards significance for improved survival outcomes, however we endorse that the small number of tumors with POLE mutations limits our power, and therefore our results do not meet traditional levels of statistical significance. Despite this, our data contribute to the existing literature, and support our conclusion that POLE mutations are a significant prognostic marker for improved survival in grade 3 EEC, and should serve to guide management decisions in these patients. A further weakness to our study is the lack of disease-specific death data. As this population often has significant medical comorbidities including obesity that can contribute to all-cause mortality rates, disease-specific mortality data could reinforce our data and results. Unfortunately, this data was not collected.
The accruing data supporting the substantial improvement in recurrence and survival challenges providers to consider omitting adjuvant treatment in this population. However, changes to the current adjuvant treatment trends of radiation and/or chemotherapy for grade 3 EEC with POLE mutations are unlikely to occur without prospective data in a randomized setting. Until such data is collected and matured, consideration of changing surveillance recommendations should be contemplated. There are no prospective studies that have evaluated the role of surveillance in EC follow-up. Based on recommended guidelines and institutional practices, retrospective research and literature reviews encompass the best evidence that is available.27 Despite recommendations from the NCCN and SGO discouraging the routine use of modalities other than symptoms and physical exam in asymptomatic women undergoing surveillance, the use of additional surveillance methods, including CA 125 testing, vaginal cytology, and CT imaging are well documented among current practitioners caring for women with high grade EC.26 Hunn et al recently reported on a multi-institutional retrospective review of 254 women with high grade EC from 2000-2011, with the most common histotype being grade 3 EEC (34%). Vaginal cytology was performed in 72% of the high grade cohort (more frequently in the early stage patients (82%)). CA-125 testing was used in 54% of the population, while CT imaging was used in 67% of patients. Interestingly, all local recurrences and 68% of the loco-regional and distant recurrences were detected by symptoms and physical exam alone. The data support that more intensive surveillance is occurring in women with high grade EC despite recommendations advising against routine screening in this population, which is both costly and often a source of anxiety for many women. In the POLE mutant, grade 3 EEC patient population, the data further supports that such intensive surveillance is not necessary, and should be omitted, as recurrences are unlikely and survival outcomes improved compared to wild-type tumors. As the burden of proof accumulates, surveillance recommendations and practices in asymptomatic women should be limited to symptoms and exam findings in with grade 3 EEC tumors with POLE mutations.
Continued research is critical to elucidate the etiology of the improved survival of POLE mutant EC patients. While the ultra-mutated genome resultant from lack of DNA repair mechanisms has been hypothesized to cause cellular lethality in tumor cells, recent data has also implicated a robust immune response to POLE mutant tumors, which may contribute to or confer the associated favorable prognosis that is seen.28 Bellone and colleagues have recently shown the first in vitro evidence of enhanced recognition of POLE-mutant tumor cells by T cells, with POLE mutated tumors consistently eliciting the combined activation of both helper and cytotoxic T cells, via proliferation of naïve CD4+ and CD8+T cells, tumor specific CD4+ T cells, as well as IFN-γ cytokine secretion.29 This data suggests a robust cytotoxic immune response could play an important role in prognosis in a subset of patients. These data, combined with the fact that POLE mutations lead to ultra-mutated cancers with thousands of neoantigens, suggest that treatment with immune checkpoint inhibitors could be efficacious. PARP inhibitors, for example, which have been shown to cause a moderate elevation in T-bet (a T cell transcription factor playing a fundamental role in coordinating type 1 immune responses) and IFN-γ expression in Th1-skewed CD4(+) T cells in ex vivo studies of mice treated with olaparib30 could have a synergistic effect with the robust immunogenic response seen in POLE mutant tumors. Such hypotheses in this disease site require investigation. Future clinical trials stratifying patients by molecular subtype are critical to determining the appropriate adjuvant treatment plans and surveillance regimens. In conclusion, our findings support tumor POLE mutation status as a noteworthy prognostic marker and may favor a change in the treatment of women with grade 3 EECs, particularly in those with early stage disease, in which omission of adjuvant therapy and decreased surveillance may possibly be appropriate. Continued investigation into the molecular prognostic role of POLE in EC is warranted.
Acknowledgments
Financial disclosure included funding from National Institutes of Health (P30 CA016058), additional funding from the James Comprehensive Cancer Center and Department of Obstetrics and Gynecology at the Ohio State University College of Medicine.
References
- 1.American Cancer Society. Cancer Facts and Figures 2015. Atlanta, Ga: American Cancer Society; 2015. [October 30, 2015]. [Google Scholar]
- 2.Kong A, Johnson N, Kitchener HC, et al. Adjuvant radiotherapy for stage I endometrial cancer. An updated Cochrane systematic review and meta-analysis J Natl Cancer Inst. 2012;104(21):1625–34. doi: 10.1093/jnci/djs374. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Dios DA, Lambrechts D, Coenegrachts L, et al. High-throughput interrogation of PIK3CA, PTEN, KRAS, FBXW7 and TP53 mutations in primary endometrial carcinoma. Gynecol Oncol. 2013;128(2):327–34. doi: 10.1016/j.ygyno.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 4.McIntyre JB, Nelson GS, Ghatage P, et al. PIK3CA missense mutation is associated with unfavorable outcome in grade 3 endometrioid carcinoma but not in serous endometrial carcinoma. Gynecol Oncol. 2014;132(1):188–93. doi: 10.1016/j.ygyno.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Pursell ZF, Isoz I, Lundstrom EB, et al. Yeast DNA polymerase epsilon participates in leading-strand DNA replication. Science. 2007;317(5834):127–30. doi: 10.1126/science.1144067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pursell ZF, Kunkel TA. DNA polymerase epsilon: a polymerase of unusual size (and complexity) Prog Nucleic Acid Res Mol Biol. 2008;82:101–45. doi: 10.1016/S0079-6603(08)00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon M, Giot L, Faye G. The 3′ to 5′ exonuclease activity located in the DNA polymerase delta subunit of Saccharomyces cerevisiae is required for accurate replication. EMBO J. 1991;10(8):2165–70. doi: 10.1002/j.1460-2075.1991.tb07751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldsby RE, Lawrence NA, Hays LE, et al. Defective DNA polymerase-delta proofreading causes cancer susceptibility in mice. Nat Med. 2001;7(6):638–9. doi: 10.1038/88963. [DOI] [PubMed] [Google Scholar]
- 9.Goldsby RE, Hays LE, Chen X, et al. High incidence of epithelial cancers in mice deficient for DNA polymerase delta proofreading. Proc Natl Acad Sci USA. 2002;99(24):15560–5. doi: 10.1073/pnas.232340999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albertson TM, Ogawa M, Bugni JM, et al. DNA polymerase epsilon and delta proofreading suppress discrete mutator and cancer phenotypes in mice. Proc Natl Acad Sci USA. 2009;106(40):17101–4. doi: 10.1073/pnas.0907147106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison A, Bell JB, Kunkel TA, et al. Eukaryotic DNA polymerase amino acid sequence required for 3′----5′ exonuclease activity. Proc Natl Acad Sci USA. 1991;88(21):9473–7. doi: 10.1073/pnas.88.21.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison A, Sugino A. The 3′-5′ Exonucleases of Both DNA-Polymerases Delta and Epsilon Participate in Correcting Errors of DNA-Replication in Saccharomyces-Cerevisiae. Molecular & General Genetics. 1994;242(3):289–296. doi: 10.1007/BF00280418. [DOI] [PubMed] [Google Scholar]
- 13.Parsons R, Li GM, Longley MJ, et al. Hypermutability and mismatch repair deficiency in RER+ tumor cells. Cell. 1993;75:1227–1236. doi: 10.1016/0092-8674(93)90331-j. [DOI] [PubMed] [Google Scholar]
- 14.Briggs S, Tomlinson I. Germline and somatic polymerase ε and δ mutations define a new class of hypermutated colorectal and endometrial cancers. J Pathol Jun. 2013;230(2):148–53. doi: 10.1002/path.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palles C, Cazier J-B, Howarth KM, et al. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet Feb. 2013;45(2):136–44. doi: 10.1038/ng.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Church DN, Briggs SE, Palles C, et al. DNA polymerase epsilon and delta exonuclease domain mutations in endometrial cancer. Hum Mol Genet. 2013;22(14):2820–8. doi: 10.1093/hmg/ddt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng B, Hoang LN, McIntyre JB, et al. POLE exonuclease domain mutation predicts long progression-free survival in grade 3 endometrioid carcinoma of the endometrium. Gynecol Oncol. 2014;134(1):15–9. doi: 10.1016/j.ygyno.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Church DN, Stelloo E, Nout RA, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer J Natl Cancer Inst. 2014;107(1):402. doi: 10.1093/jnci/dju402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zighelboim I, Goodfellow PJ, Gao F, et al. Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J Clin Oncol. 2007;25(15):2042–8. doi: 10.1200/JCO.2006.08.2107. [DOI] [PubMed] [Google Scholar]
- 21.Zighelboim I, Schmidt AP, Gao F, et al. ATR mutation in endometrioid endometrial cancer is associated with poor clinical outcomes. J Clin Oncol. 2009;27(19):3091–6. doi: 10.1200/JCO.2008.19.9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novetsky AP, Zighelboim I, Thompson DM, Jr, et al. Frequent mutations in the RPL22 gene and its clinical and functional implications. Gynecol Oncol. 2013;128(3):470–4. doi: 10.1016/j.ygyno.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Billingsley CC, Cohn DE, Mutch DG, et al. Polymerase ε (POLE) mutations in endometrial cancer: clinical outcomes and implications for Lynch syndrome testing. Cancer. 2015;121(3):386–94. doi: 10.1002/cncr.29046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92(3):744–51. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 25.Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355:1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 26.Hunn J, Tenney ME, Tergas AI, et al. Patterns and utility of routine surveillance in high grade endometrial cancer. Gynecol Oncol. 2015;137(3):485–9. doi: 10.1016/j.ygyno.2015.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salani R, Backes FJ, Fung MF, et al. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am J Obstet Gynecol. 2011;204(6):466–78. doi: 10.1016/j.ajog.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 28.van Gool IC, Eggink FA, Freeman-Mills L, et al. POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res. 2015;21(14):3347–55. doi: 10.1158/1078-0432.CCR-15-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellone S, Centritto F, Black J, et al. Polymerase ε (POLE) ultra-mutated tumors induce robust tumor-specific CD4+ T cell responses in endometrial cancer patients. Gynecol Oncol. 2015;138(1):11–7. doi: 10.1016/j.ygyno.2015.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghonim MA, Pyakurel K, Ibba SV, et al. PARP inhibition by olaparib or gene knockout blocks asthma-like manifestation in mice by modulating CD4(+) T cell function. J Transl Med. 2015;13:225. doi: 10.1186/s12967-015-0583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


