Abstract
Background
Balancing donor and recipient risks in living donor liver transplantation (LDLT) remains an issue of debate. This study assessed the impact of graft selection on outcomes and complications for left lobe (LL) versus right lobe (RL) donors and recipients.
Methods
The medical records of donors and recipients who underwent LDLT at our institution between 2003–2015 were reviewed. For donors, we evaluated graft volume, residual liver volume per standard liver volume, length of hospital stay (LOS), complications, and readmissions. For recipients, we looked at graft and patient survival, graft function at post-operative days (POD) 7 and 14, graft volume, LOS, biliary complications, MELD at transplant, and HCV status.
Results
At five years post-transplant, there were no significant differences in graft survival for LL recipients (86% (95% CI 74–93)) compared with 82% (95% CI 69–89) for RL recipients (p=0.85) or recipient survival (90% vs. 84%, p=0.44). In LL recipients, POD 7 and 14 median INR (1.5 and 1.2, respectively) and total bilirubin (4.6 and 2.7) were significantly greater compared to RL recipients (7 and 14 day INR (1.2, p<0.001; 1.1, p=0.001) and total bilirubin (2.7, p=0.001; 2.1, p=0.05)). LL recipients also had a significantly greater median LOS (14 vs. 10, p=0.008). Median donor LOS was significantly greater for RL donors (7 (IQR 7–8) vs. 7 (IQR 6–7) days, p<0.001).
Conclusion
RL and LL grafts provide comparable long-term outcomes in properly selected donor-recipient pairs and the appropriate use of LL grafts does not impact graft or patient survival at 5 years post-transplant.
INTRODUCTION
In liver transplantation, the use of living donor grafts translates into advantages for the recipient when compared to using grafts from deceased donors, including shorter time-to-transplant and improved waitlist mortality 1, with similar post-transplant survival (83% at 5 years)2. Berg et al. reported a 56% lower risk of death for patients who choose living donor liver transplantation (LDLT) versus patients waiting for deceased donor liver transplantation (DDLT) 2. Despite these encouraging outcomes, only 10% of liver transplants in the United States use grafts from living donors3.
Donor risk is the major concern in LDLT 4. Data from the Adult-to-Adult Living Donor Liver Transplantation (A2ALL) cohort demonstrated that 38% of living donors experience post-operative complications, the majority within the first year of donation 5. However, these outcome data are primarily from right lobe (RL) donors.
Left lobe (LL) grafts are thought to minimize donor risk. Donor mortality in LDLT is estimated at 0.1–1% 1; the Vancouver Forum estimated the global donor mortality rate at 0.1% for LL donors and 0.5% for RL donors 6, but a 2013 world-wide survey of donor death and near-miss events did not demonstrate a difference in mortality between LL and RL donors 7. A recent review of the literature addressing donor morbidity concluded that LL donation results in fewer complications compared with RL donation, particularly with regard to biliary complications 1.
The purpose of this study was to analyze the impact of graft selection (left versus right lobe) on the outcomes of donors and recipients after LDLT at a single United States transplant center.
METHODS
Institutional Review Board approval was obtained prior to the initiation of this study.
Study Population and Data Collection
We conducted a retrospective chart review of donors and recipients undergoing adult to adult LDLT at our institution between January 2003 and April 2015. For both donors and recipients, we obtained basic demographic information and laboratory data (total bilirubin, international normalized ratio (INR)). For donors, we gathered the following additional variables: graft size, length of initial hospital stay (LOS), hospital readmissions, and post-operative complications. For recipients, we evaluated patient and graft survival, graft function at post-operative days (POD) 7 and 14, LOS, biliary complications, need for additional surgery, and operative details including whether portal inflow modulation was required. Standard immune suppression consisted of triple therapy with tacrolimus, mycophenolate mofetil and prednisone, and did not differ based on graft type or over the time course of the study.
Technical Details
Left lobe grafts
Preoperative CT scan defined the liver volumes and hepatic artery anatomy, and MRCP or CT cholangiography defined the biliary anatomy. LL grafts were procured via midline laparotomy. The gallbladder was removed. The hepatic artery and portal vein were identified, and vascular demarcation along Cantlie’s line (from the gallbladder fossa to the space between the middle and right hepatic veins) was identified with a short period of vascular occlusion. This resection plane was the same as that for a right hepatectomy, crossing the distal branches of the middle hepatic vein that extend to the right of the plane, taking the majority of the middle hepatic vein with the future graft. The left portion of the caudate lobe was not included with LL grafts from the start of the data collection until mid-2013, after which the LL of the caudate was included with LL grafts. The bile duct was divided without intraoperative cholangiogram at a point distal to the segment IV duct orifice, and care was taken not to compromise the right posterior hepatic duct if it arose from the left hepatic duct.
For recipient implantation, a venoplasty was performed between the left and middle hepatic veins if there was a size discrepancy or if the middle and left hepatic vein orifices were distinct. The resulting hepatic vein orifice was anastomosed in triangulated fashion to the recipient vena cava. The donor portal vein was typically anastomosed to the recipient left portal. The hepatic artery reconstruction was done using a size-matched, recipient hepatic artery with an end-to-end anastomosis with interrupted suture. For select cases, an operating microscope was used. If there were two arteries, back-bleeding of the smaller artery was tested in the donor. If there was pulsatile back-bleeding the artery was not reconstructed.
The need for portal inflow modification to prevent portal hyper perfusion injury was determined by measuring the portal pressure gradient after reperfusion of the graft. Splenic artery ligation was performed if the portal pressure gradient after reperfusion was > 11 mm Hg as the first graft inflow modification. If the portal pressure was still >11, a portacaval shunt was created between the recipient right portal vein and the vena cava. Biliary reconstruction was generally completed via a duct-to-duct anastomosis or a Roux-en-Y hepaticojejunostomy.
Epidurals were placed in LL donors with patient-controlled epidural analgesia for management of post-operative pain.
Right lobe grafts
Right lobe grafts were procured via a bilateral subcoastal incision. Again, the right hepatic artery and portal vein were occluded to generate a line of demarcation after the gallbladder was removed. The right lobe graft typically included the right hepatic vein, along with distal branches of the middle hepatic vein following Cantlie’s line. The middle hepatic vein almost never required reconstruction. Accessory right hepatic veins > 5mm were reconstructed end-to-side to the vena cava.
The bile duct was divided without intraoperative cholangiogram. RL grafts more frequently presented with multiple bile ducts for reconstruction. The presence of 2 substantial bile ducts required a more complex reconstruction, such as back table reconstruction of ducts that were close in origin, separate duct-to-duct anastomoses to the right and left donor hepatic ducts, or a Roux-en-Y hepaticojejunostomy. Arterial reconstruction was performed in similar fashion to LL grafts. Graft inflow modification was rarely required for RL grafts.
Intravenous narcotics and patient-controlled analgesia was used for management of RL donors.
Statistical Analyses
We described recipient and donor characteristics by LL and RL using proportions and medians (interquartile ranges (IQR)) and evaluated differences using Fisher’s exact and Wilcoxon rank sum tests. Post-transplant patient survival (event defined as a death) and graft survival (event defined as a death or retransplant) and 95% confidence intervals (95% CI) were estimated using the Kaplan-Meier method. Time-to event was measured in years from liver transplant to the time of death, retransplant (for graft survival) or last follow-up (censored). Cox regression evaluated the univariate associations between risk of patient and graft survival with graft function at POD7 and POD14. Early graft dysfunction was defined as INR≥1.6 or total bilirubin ≥ 10 at POD7. The association between early graft dysfunction and recipient (pre-transplant MELD and ascites, transplant ascites and age, HCV, HCC, and gender) and donor (graft volume, gender, gender matching and lobe) characteristics was evaluated using logistic regression. Factors with a univariate p<0.01 were further evaluated in the multivariable analysis. Significance was set at p<0.05.
Length of Stay Modeling
We evaluated the clinical factors associated with recipient and donor LOS. Negative binomial regression was used to investigate associations with recipient LOS as this model accounts for the over-dispersion observed in the data. Linear regression was used to investigate associations with donor LOS as these data followed the normal distribution. For both models, factors with a univariable p<0.1 were evaluated in the multivariable model to determine the independent association with LOS. The final model was selected through backward elimination retaining factors with p<0.05. All analyses were conducted using SAS version 9.4 (Cary, NC) and survival curves were generated using Stata IC 11 (College Station, TX).
RESULTS
Between 2003 and 2015, 136 LDLTs were performed at our institution, with 71 RL and 65 LL grafts. Over the study period, our center shifted to performing mostly LL LDLTs (Figure 1). Seventy-five percent of donors were related to their recipient, and 38% of recipients were diagnosed with Hepatitis C (HCV). Recipients were 56% female with a median age of 57 years and a median LOS of 12 days. Donors were 48% female, median age of 33 years, with a median LOS of 7 days. Right lobe donors were 51% female compared with 44% of left lobe donors (p=0.48). The length of stay for right lobe donors was 7 days (IQR 7–8) and 7 days (IQR 6–7) for left lobe donors (p=0.006). The overall median graft volume was 600 cc: 800 cc for RL grafts and 450 cc for LL grafts. Demographic information for recipients and donors is shown in Tables 1 and 2, respectively. Recipient operative details and post-operative complications are delineated in Tables 3 and 4, respectively.
Figure 1.
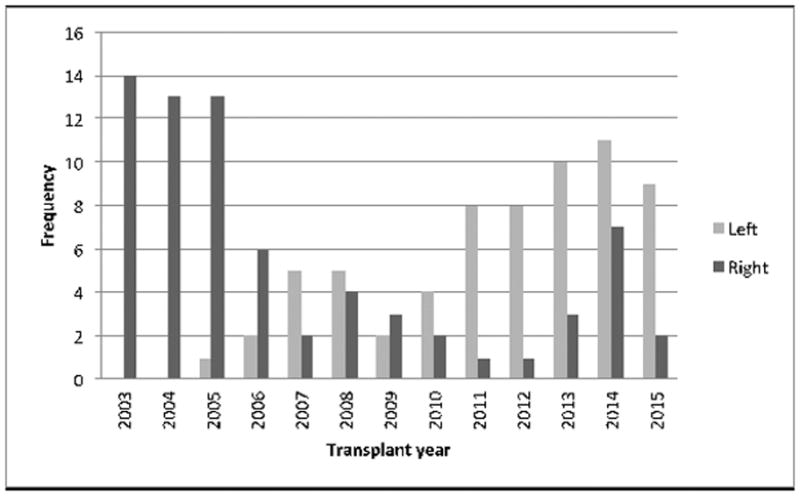
LDLT volume by lobe 2003–2015
Table 1.
Recipient demographics.
| Overall | Right | Left | P-value | |
|---|---|---|---|---|
| Age @ transplant* | 57 (48–63) | 57 (49–65) | 55 (47–62) | 0.34 |
| MELD @ transplant* | 16 (13–20) | 15 (13–20) | 16 (13–19) | 0.74 |
| Gender (% female) | 56% | 44% | 69% | 0.003 |
| HCV (%) | 38% | 41% | 34% | 0.4 |
| Height (cm)* | 170 (160–177) | 175 (163–182) | 165 (160–172) | 0.002 |
| Weight (kg)* | 79 (64–90) | 84 (72–92) | 72 (61–88) | 0.01 |
| BMI* | 26.7 (23.7–29.5) | 26.8 (25–29.5) | 26.3 (22–30) | 0.23 |
| Standard Liver Volume* | 1524 (1347–16667) | 1584 (1438–1684) | 1436 (1303–1631) | 0.01 |
| Graft volume (cc)* | 600 (450–800) | 800 (700–900) | 450 (400–500) | <0.001 |
| Ratio of graft volume:recipient weight* | 0.8 (0.6–1) | 0.99 (0.83–1.16) | 0.58 (0.48–0.74) | <0.001 |
Median (IQR)
Table 2.
Donor demographics.
| Overall | Right | Left | P-value | |
|---|---|---|---|---|
| N | 136 | 71 | 65 | -- |
| Age* | 33 (27–42) | 37 | 31 | 0.001 |
| Gender (% female) | 47% | 51% | 45% | 0.48 |
| Weight (kg)* | 79 (68–89) | 79.5 | 75.8 | 0.86 |
| Height* | 173 (165–180) | 174 | 173 | 0.88 |
| BMI* | 26 (23–28) | 26.8 | 26 | 0.87 |
| Residual liver volume* | 750 (575–1015) | 581 | 1036 | <0.001 |
| EBL* | 325 (250–500) | 400 | 300 | 0.007 |
| Length of stay (days)* | 7 (6–8) | 7 (7–8) | 7 (6–7) | 0.001 |
| Complication | 27 (20%) | 16 (24%) | 11 (17%) | 0.34 |
| Readmission | 25 (19%) | 16 (23%) | 9 (14%) | 0.18 |
| Time to Readmission (days)* | 16 (11–46) | 15 | 64 | 0.01 |
Median (IQR)
Table 3.
Recipient Operative Details.
| Overall | Right | Left | P-value | |
|---|---|---|---|---|
| Splenic Artery Ligation | 32 (24%) | 9 (13%) | 23 (36%) | 0.002 |
| Portocaval Shunt | 19 (14%) | 2 (3%) | 17 (27%) | <0.001 |
| Hepatic Vein Reconstruction | 18 (13%) | 2 (3%) | 16 (25%) | <0.001 |
| Arterial Reconstruction | 33 (24.3%) | 7 (10%) | 26 (40%) | <0.001 |
| Biliary Anastomosis (end to end) | 84 (62%) | 40 (56%) | 44 (68%) | 0.36 |
Table 4.
Recipient Complications.
| Overall | Right | Left | P-value | |
|---|---|---|---|---|
| Length of stay (days)* | 12 (8–17) | 10 (8–16) | 14 (9–17) | 0.008 |
| Additional operation | 58 (43%) | 39 (55%) | 19 (30%) | 0.002 |
| Time to additional operation (days)* | 91 (12–399) | 175 (12–399) | 56 (9–393) | 0.5 |
| Biliary complication requiring reoperation | 46 (34%) | 24 (34%) | 22 (34%) | 0.64 |
Median (IQR)
Graft survival was similar between RL and LL grafts (p=0.69, Figure 2). At five years post-transplant, graft survival for LL recipients was 86% (95% CI 74–92) compared with 82% (95% CI 69–89) for RL recipients (p=0.85). Similarly, no significant difference in patient survival was detected between RL and LL recipients (p=0.81, Figure 3). Five-years post-transplant, recipient survival was 90% (95% CI 77–96) for LL compared with RL survival of 84% (95% CI 72–91). Risk of graft loss and patient death remains similar between RL and LL after adjusting for year of transplant. Donor survival was 100% for both types of donors.
Figure 2.
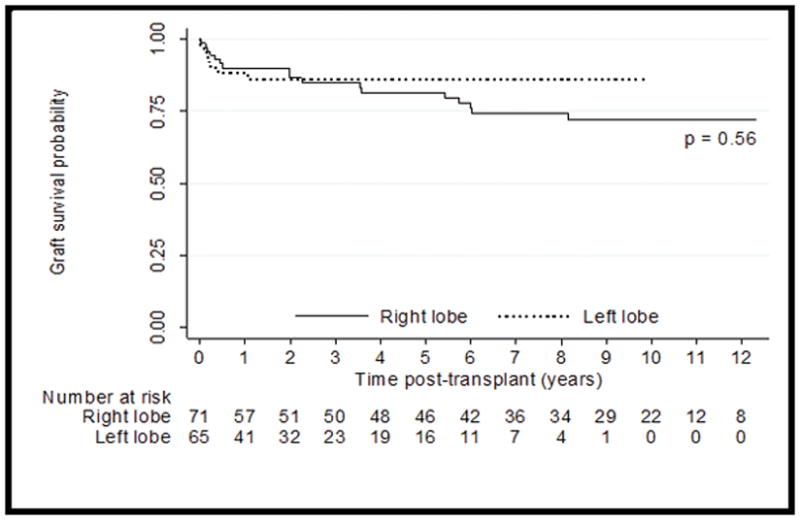
Graft Survival
Figure 3.

Patient Survival
Given that more recent calendar years correspond with both an improvement in graft and patient post-transplant survival and a shift in use of RL to LL grafts at our center, we also assessed differences in graft and patient survival in a more contemporary cohort from 2006 through 2015. There was no difference in graft survival (p=0.60) or patient survival (p=0.73) in this cohort.
Graft function at POD7 and POD14 was evaluated using INR and total bilirubin lab values (Table 5). At POD7, both INR and total bilirubin were significantly greater in LL recipients compared with RL recipients (1.5 vs. 1.2, p<0.001 and 4.6 vs. 2.7, p=0.001, respectively. At POD14, INR and total bilirubin remained significantly greater in LL recipients (1.2 vs. 1.1, p<0.001 and 2.7 vs. 2.1, p=0.049) though the clinical relevance is probably low. In our patient cohort, 32.8% of recipients (n=41, RL=8, LL=33) exhibited early graft dysfunction defined as INR≥1.6 or total bilirubin ≥ 10 at POD7. There was a significantly greater incidence of early graft dysfunction in LL recipients compared to RL recipients (50.8% vs. 13.3%, p<0.001). Recipients who qualified as having early graft dysfunction with both INR and total bilirubin elevations had a significantly greater risk of death (hazard ratio 3.14, p=0.02) and of graft loss (hazard ratio 3.08, p=0.048). In multivariate analysis, the only predictor of early graft dysfunction was graft volume (odds ratio 0.99 (95% CI 0.99–1.00) per 1 unit increase in graft volume, p<0.001). Seventy-three percent (n=99) of recipients were reported to have ascites as a complication of their liver disease prior to transplant, but only 26.5% (n=36) had ascites at the time of transplant. The presence of ascites prior to or at transplant was not associated with early graft dysfunction (p=0.22 and p=0.43, respectively).
Table 5.
Recipient Graft Function at post-op day 7 and 14. Results reported as median (IQR)
| Overall | Right | Left | P-value | |
|---|---|---|---|---|
| INR POD7 | 1.3 (1.2–1.6) | 1.2 (1.1–1.4) | 1.5 (1.3–1.7) | <0.001 |
| Bilirubin POD7 | 3.4 (2.1–7.4) | 2.7 (1.7–5.9) | 4.6 (2.8–10.4) | 0.001 |
| INR POD14 | 1.15 (1.1–1.3) | 1.1 (1–1.2) | 1.2 (1.1–1.4) | 0.001 |
| Bilirubin POD14 | 2.6 (1.3–5.9) | 2.1 (1.1–4.2) | 2.7 (1.5–7.9) | 0.049 |
Forty-three percent of recipients (58/136) underwent one or more surgical procedures following LDLT (55% of RL versus 30% of LL, p=0.002). The majority of re-operations were for biliary complications or hernia repairs. The proportion of RL and LL recipients with re-operations for biliary complications was 34% each (p=0.64). This did not include patients requiring subsequent IR procedures. Four RL patients and four LL patients were re-transplanted during the study period. Additionally, six major vascular complications (five hepatic artery thromboses (three LL, two RL) and one portal vein thrombosis (LL)) occurred.
Nineteen percent of donors (25/136) had one or more hospital readmissions following donation (23% of RL versus 14% of LL, p=0.18). The average time to readmission was 16 days (15 for RL donors, 64 for LL donors, p=0.01) following discharge. Four donors (3LL, 1RL) underwent re-operations: three for wound-related repairs (all LL) and one for a small bowel obstruction (RL). Details of donor complications are presented in Table 6.
Table 6.
Donor Complications
| Tx Year | Lobe | Complication | POD | Grade | Treatment |
|---|---|---|---|---|---|
| 2015 | L | Ileus | 6 | 1 | Conservative |
| 2015 | L | Biloma | 64 | 3 | IR drainage |
| 2014 | L | Wound breakdown | 14 | 3 | Primary closure |
| 2014 | L | Ileus | 4 | 1 | Conservative |
| 2014 | L | Ileus | 5 | 1 | Conservative |
| 2014 | L | Incisional hernia | 300 | 3 | Surgical repair |
| 2013 | L | Bacteremia | 2 | 2 | IV abx |
| 2013 | L | Symptomatic atelectasis | 3 | 1 | Conservative |
| 2012 | L | Recurrent ventral hernia | 131 | 3 | Surgical repair x2 |
| 2008 | R | Biloma | 8 | 3 | Percutaneous drainage |
| 2007 | R | Ileus | 5 | 1 | Conservative |
| 2007 | L | Symptomatic atelectasis | 1 | 1 | Conservative |
| 2006 | R | Pneumonia | 9 | 2 | Antibiotics |
| 2006 | R | Ileus | 5 | 1 | Conservative |
| 2005 | R | Small bowel obstruction | 7 | 4 | Ex-lap w/LOA |
| 2005 | R | Biloma | 4 | 2 | Conservative |
| 2005 | R | Wound infection | 4 | 2 | IV abx |
| 2005 | R | Intraabdominal infection | 30 | 2 | Conservative |
| 2005 | L | Ileus, infection | 14 | 2 | Antibiotics |
| 2005 | R | Biloma | 10 | 3 | Percutaneous drainage |
| 2005 | R | Biloma | 17 | 3 | Percutaneous drainage |
| 2004 | R | Intraabdominal fluid collection | 14 | 2 | Conservative |
| 2004 | R | Biloma | 16 | 3 | Percutaneous drainage |
| 2004 | R | Wound erythema | 3 | 2 | IV abx |
| 2003 | R | Bilious drainage | 3 | 2 | Conservative |
| 2003 | R | Wound infection | 9 | 2 | IV abx |
| 2003 | R | Bile leak | 14 | 3 | ERCP |
Recipient median LOS was significantly greater in LL recipients versus RL recipients (14 (IQR 9–17) vs. 10 (IQR 8–16) days, p=0.008) but was only associated with pre-transplant MELD (p<0.001) in multivariable analyses. Donor LOS was significantly greater for RL donors versus LL donors (7 (IQR 7–8) vs. 7 (IQR 6–7) days, p<0.001) (Figure 4). Donor LOS was associated with several donor characteristics including graft volume, donor age, and residual liver volume. For every 100 cc increase in graft volume, donor LOS increased 0.2 days (p=0.002). Donor age (per 10 year increase) was associated with a 0.4 day increase (p=0.003) in donor LOS.
Figure 4.
Donor length of stay
DISCUSSION
Living donor liver transplantation remains technically challenging and associated with relatively high donor and recipient morbidity. Although the use of LL grafts is attractive for minimizing donor risk, some high volume LDLT centers prefer RL grafts to decrease the risk of graft insufficiency in recipients 8. The definitive effect of graft selection on the tradeoff between donor risk and recipient outcome remains highly debated. The results of this 12-year review of our LDLT outcomes and complications suggest 1) RL and LL grafts provide comparable long-term outcomes in properly selected donor-recipient pairs, 2) the appropriate use of LL grafts does not impact graft or patient survival at 5 years post-transplant among transplant recipients, although LL grafts required graft portal inflow modification more frequently, 3) LL recipients require longer hospital admissions and have greater elevations in liver function tests in the initial postoperative period, and 4) donors of LL grafts have shorter LOS. Of note, there was a trend towards a higher complication rate in RL donors, and the analysis is not powered to demonstrate a difference in donor mortality between LL and RL donors.
A review of twelve years of United Network of Organ Sharing (UNOS) data compared graft and patient survival among recipients of RL and LL grafts from living donors. The vast majority of transplants included in this review (94.6%) were RL transplants. Patient survival was not significantly different between recipients of right and left lobes (p=0.06), however graft survival after RL transplant was better than after LL transplant (p=0.004) and LL LDLT was associated with a more than doubled risk of graft failure (hazard ratio=2.39). Our outcome data showed no significant difference in patient and graft survival between RL and LL recipients, with 90% patient survival in LL graft recipients at 5 years post-transplant. These exceptional LDLT outcomes may be attributed to a variety of factors including surgeon and center experience 9, surgical technique innovation with portal modulation 9, patient selection, and excellent postoperative care.
A single Japanese center compared outcomes after RL and LL LDLT and found that LL donors had decreased morbidity and that recipient survival in RL versus LL recipients did not differ by MELD score or graft-to-recipient weight ratio (GRWR), however they did not report patient and graft survival according to the lobe received 10. Similarly, A2ALL has reported acceptable outcomes in LDLT but has not stratified graft and recipient survival by graft laterality 11. Our data also show acceptable outcomes following LDLT and specifically demonstrates that neither graft nor patient survival differs in recipients of LL and RL grafts.
In the ongoing debate around LL versus RL LDLT, the major concern regarding LL grafts is that smaller graft size leads to recipient graft dysfunction. Portal inflow modulation techniques and proper patient selection have partially mitigated this issue, but the concern still remains. Recent A2ALL data demonstrated that 16–19% of LDLT recipients experienced segmental graft dysfunction, defined as serum bilirubin >10 mg/dl or INR >1.6 on post-transplant day 7 12. Predictors of recipient graft dysfunction included increased pre-operative MELD, lower graft weight to recipient weight ratio, decreased reperfusion arterial flow and increased portal reperfusion pressure, and receiving a LL graft. Interestingly, however, graft weight was not a significant predictor of graft dysfunction, nor was it associated with graft failure at 90 days post-transplant. The authors concluded that segmental graft dysfunction is likely a complex and multifactorial process that cannot be fully accounted for by graft size alone. These numbers suggest that LL recipients are more likely to exhibit early graft dysfunction, but there does not appear to be a long-term impact on graft or patient survival. The comparable 5-year graft and patient survival rates at our center underscore the conclusion that excellent long-term recipient outcomes can still be achieved using small LL grafts.
Importantly, our donor survival was 100%. Our donor complication rate was 20%, with a biliary complication rate of 5.8% and with 3% of donors (n=4) requiring an additional operation. We recently conducted a review of the literature published between 2006–2015 on complications following living donor hepatectomy and found an overall complication rate of 20.5% with 21% of donors requiring additional operations and a biliary complication rate of 6.6% 13. Our overall complication rate is similar to that reported in the literature, but the need for re-operation in our donors and the incidence of biliary complications is less. We discontinued the placement of surgical drains at the cut edge of the donor liver in 2013, so any post-operative fluid collections now require IR drainage. We observed a high incidence of ileus in our donor population, the majority of which occurred in LL donors. Our use of epidural pain management in LL donors was aimed to reduce the use of narcotics and opioid-induced ileus, but delayed return of bowel function continues to remain a major issue in donor morbidity.
This study has several limitations. First, it is a retrospective review of single-center data that may not extrapolate to other centers. Our sample size is not as large as other outcomes studies, however the majority of studies comparing RL and LL transplants are disproportionately weighted towards one graft type and our study is well-balanced. Second, center and surgeon experience have been identified as factors that significantly improve outcomes following LDLT. As our center and surgeons gained experience, our LDLT volume shifted to consist largely of LL transplants. It is possible that this may contribute to the excellent outcomes we observed in LL recipients. The operations associated with left and right lobe donation are going to be different and differences related to anesthetic management, operative technique, including incisions and post-operative pain management will influence any retrospective study. Other confounders including post-operative care and follow-up and changes in surgical technique over the study period may also contribute to our results.
CONCLUSION
This study demonstrates that patient and graft survival was not significantly different between recipients of LL versus RL grafts. LL recipients required graft inflow modification more frequently, had greater elevations in liver function tests at 7 and 14 days post-operatively, and required a longer initial hospital LOS.
ABBREVIATIONS
- A2ALL
Adult-to-Adult Living Donor Liver Transplantation
- DDLT
Deceased donor liver transplantation
- IQR
Interquartile ranges
- LDLT
Living donor liver transplantation
- LL
Left lobe
- LOS
Length of stay
- RL
Right lobe
- RV:SLV
Ratio of residual liver volume to standard liver volume
Footnotes
The authors declare no conflicts of interest.
Hillary Braun : research design, writing of paper, performance of research, data analysis
Jennifer Dodge: writing of paper, data analysis
Garrett Roll; writing of paper
Chris Freise : writing of paper
Nancy Ascher : writing of paper
John Roberts : research design, writing of paper, data analysis
Contributor Information
Hillary J. Braun, Email: Hillary.braun@gmail.com.
Jennifer L. Dodge, Email: Jennifer.dodge@ucsf.edu.
Garrett R. Roll, Email: garrett.roll@ucsf.edu.
Chris E. Freise, Email: chris.freise@ucsf.edu.
Nancy L. Ascher, Email: nancy.ascher@ucsf.edu.
John P. Roberts, Email: john.roberts@ucsf.edu.
References
- 1.Roll GR, Parekh JR, Parker WF, et al. Left hepatectomy versus right hepatectomy for living donor liver transplantation: shifting the risk from the donor to the recipient. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2013 May;19(5):472–481. doi: 10.1002/lt.23608. [DOI] [PubMed] [Google Scholar]
- 2.Berg CL, Merion RM, Shearon TH, et al. Liver transplant recipient survival benefit with living donation in the model for endstage liver disease allocation era. Hepatology. 2011 Oct;54(4):1313–1321. doi: 10.1002/hep.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trotter JF. Challenges in living donor liver transplantation. Clinics in liver disease. 2014 Aug;18(3):651–660. doi: 10.1016/j.cld.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Hertl M, Cosimi AB. Living donor liver transplantation: how can we better protect the donors? Transplantation. 2007 Feb 15;83(3):263–264. doi: 10.1097/01.tp.0000250676.71656.39. [DOI] [PubMed] [Google Scholar]
- 5.Ghobrial RM, Freise CE, Trotter JF, et al. Donor morbidity after living donation for liver transplantation. Gastroenterology. 2008 Aug;135(2):468–476. doi: 10.1053/j.gastro.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barr ML, Belghiti J, Villamil FG, et al. A report of the Vancouver Forum on the care of the live organ donor: lung, liver, pancreas, and intestine data and medical guidelines. Transplantation. 2006 May 27;81(10):1373–1385. doi: 10.1097/01.tp.0000216825.56841.cd. [DOI] [PubMed] [Google Scholar]
- 7.Cheah YL, Simpson MA, Pomposelli JJ, Pomfret EA. Incidence of death and potentially life-threatening near-miss events in living donor hepatic lobectomy: a world-wide survey. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2013 May;19(5):499–506. doi: 10.1002/lt.23575. [DOI] [PubMed] [Google Scholar]
- 8.Lee SG. A Complete Treatment of Adult Living Donor Liver Transplantation: A Review of Surgical Technique and Current Challenges to Expand Indication of Patients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014 Oct 30; doi: 10.1111/ajt.12907. [DOI] [PubMed] [Google Scholar]
- 9.Ikegami T, Shirabe K, Soejima Y, et al. Strategies for successful left-lobe living donor liver transplantation in 250 consecutive adult cases in a single center. Journal of the American College of Surgeons. 2013 Mar;216(3):353–362. doi: 10.1016/j.jamcollsurg.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki J, Iida T, Mizumoto M, et al. Donor morbidity in right and left hemiliver living donor liver transplantation: the impact of graft selection and surgical innovation on donor safety. Transplant international : official journal of the European Society for Organ Transplantation. 2014 Nov;27(11):1205–1213. doi: 10.1111/tri.12414. [DOI] [PubMed] [Google Scholar]
- 11.Olthoff KM, Merion RM, Ghobrial RM, et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL Consortium. Annals of surgery. 2005 Sep;242(3):314–323. doi: 10.1097/01.sla.0000179646.37145.ef. discussion 323–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pomposelli JJ, Goodrich JP, Emond JC, et al. Segmental Graft Dysfunction After Live Donor Liver Transplantation: It’s Not All About Size. A Report from the A2ALL Cohort Study. 2014 Submitted to American Journal of Transplantation. [Google Scholar]
- 13.HJB, NLA, GRR, JPR Biliary Complications Following Living Donor Hepatectomy. Transplantation Reviews. 2015 doi: 10.1016/j.trre.2016.07.003. Under Review. [DOI] [PubMed] [Google Scholar]



