Abstract
We investigated the contributions of direct and indirect T cell antigen recognition pathways to the immune response to porcine antigens in naïve baboons and baboon recipients of pig xenografts. In naïve baboons, in vitro culture of peripheral blood T cells with intact pig cells (direct xenorecognition pathway) or pig cell sonicates and baboon antigen-presenting cells (indirect xenorecognition pathway) induced the activation and expansion of xenoreactive T cells producing proinflammatory cytokines, IL-2 and γIFN. Primary indirect xenoresponses were mediated by pre-existing memory T cells, whose presence is not typically observed in primary alloresponses. Next, baboons were conditioned with a non-myeloablative regimen prior to short-term immunosuppression and transplantation of xenogeneic peripheral blood progenitor cells and a kidney or heart or pancreatic islets from a miniature swine. All transplants were rejected acutely within 30 days after their placement. Post-transplantation, we observed an inhibition of the direct xenoresponse, but a significant expansion of indirectly activated pro-inflammatory T cells. These results suggest that additional treatment to suppress indirect T cell immunity in primates may be required to achieve tolerance of pig xenografts through hematopoietic chimerism.
Introduction
The current shortage of deceased human organs available for transplantation precludes treatment of many patients who would benefit from a transplant. This stresses the need for strategies to accomplish transplantation of xenogeneic organs, tissues and cells. Based upon breeding characteristics as well as physiological and size features, miniature swine have been chosen as a potential source of organs for humans (1, 2). However, experimental models using nonhuman primates as recipients of swine tissues have revealed the post-transplant development of vigorous and immediate immune responses mediated by natural antibodies directed against carbohydrates and proteins of the donor endothelium (1, 2). Concomitant activation of the complement cascade is associated with destruction of the endothelium, tissue injury, inflammation and activation of other components of pro-inflammatory innate and adaptive immunity. These responses lead to hyperacute rejection of xenografts associated with coagulopathy, destruction of the organ endothelium and rapid loss of graft function. Actually, uncontrolled activation of coagulation cascades can leads to a Disseminated Intravascular Coagulation (DIC) in the entire host (3).
Swine expressing human complement pathway regulatory proteins (hCPRPs) (4) or lacking the α1,3-galactosyltransferase gene (GalT-KO) (5) have been genetically engineered. These modifications have significantly extended the survival of pig xenografts in non-human primates from a few days to several weeks. Nevertheless, in the presence of conventional immunosuppression, these xenotransplants succumb to a combination of acute cellular and humoral rejection referred to as “delayed xenograft rejection” which displays some features of acute cellular rejection of fully allogeneic transplants (6, 7). These findings suggest that immune-based strategies, including mixed hematopoietic chimerism (8) and costimulation blockade (9), which can achieve T and B cell immune tolerance in allotransplantation, may be effective at further extending the survival of such genetically-modified xenografts.
Primary in vitro xenospecific responses by T helper cells of equal or even greater magnitude as compared to alloresponses have been reported (10–12). Yamada et al. have documented both direct and indirect alloresponses by CD4+ T cells in a human anti-porcine primary mixed lymphocyte reaction (MLR) model (13). In addition, studies by Markmann suggest the key role of indirect antigen recognition in the immune response of rat T cells to MHC-deficient mouse xenografts (14). Therefore, both direct and indirect antigen presentation pathways are involved in the development of T cell xenoresponses. Consequently, evaluation of the contributions of each of these pathways to rejection of xenografts is required for the design of tolerance protocols in xenotransplantation.
In this study, we used an ELISPOT assay to detect and quantify direct and indirect T cell xenoresponses in naïve baboons and baboon recipients of porcine peripheral blood progenitor cells combined with pancreatic islets or a heart or kidney transplant. In naïve baboons, the direct xenoresponse was low in comparison to that commonly observed in allogeneic mixed lymphocyte reactions, while substantial frequencies of activated T cells recognizing xenoantigens in an indirect fashion were detected. In transplanted baboons treated with our chimerism protocol, the direct xenoresponse was abrogated while significant numbers of pro-inflammatory T cells reacting to xenoantigens indirectly were still detected. The implications of these findings for the design of immune therapies in xenotransplantation are discussed.
Materials and Methods
Animals
Baboons (Papio anubis, n=6) of both sexes, of known ABO blood group and of body weight 10–14 kg (Biological Resources, Houston, TX), were used as recipients. Massachusetts General Hospital (MGH) miniature swine inbred for the major histocompatibility complex (MHC) (n=3), of blood group O, and of cc or dd swine leukocyte antigen (SLA) haplotype, 2–4 months old, 18–40 kg of body weight (bred in our animal facility) served as donors of peripheral blood progenitor cells (PBPCs), islets or kidneys. Care of animals was in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health. Protocols were approved by the MGH Subcommittee on Research Animal Care.
Mobilization and collection of leukocytes (PBPCs) from pigs, conditioning and pig PBPC injections and islet and organ transplantation in baboons
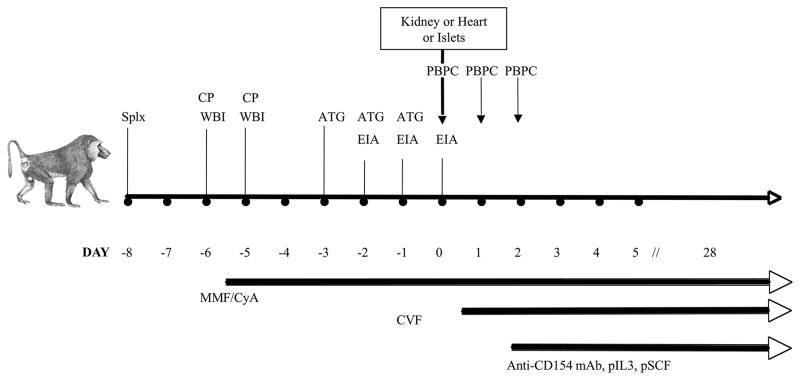
These procedures have all been described in detail previously (15, 16). Briefly, Mobilization and collection of porcine leukocytes (peripheral blood progenitor cells, PBPCs) was carried out by treating pigs with recombinant hematopoietic growth factors (porcine IL-3 at 100 microg/kg/d s.c. (BioTransplant, Inc. Charlestown, MA); and porcine stem cell factor at 100 microg/kg/d s.c. (BioTransplant, Inc) daily from days 0 to 22, and human granulocyte colony stimulating factor at 10 microg/kg/d s.c. (Amgen, Thousand Oaks, CA) from days 5 to 22. Leukapheresis was performed on various days between days 3 to 23 using a Cobe Spectra apheresis machine (Cobe, Lakewood, CO). After collection, the pig PBPCs were washed and after red blood lysis, were frozen with 5% DMSO. The total number of PBPCs administered to each baboon was 3 ×1010 cells/kg. Pig pancreatic islets were prepared as previously described (15, 16) and 14,000 islet equivalents/kg were infused into a colic venous tributary of the portal vein of the recipient baboon on day 0, and some aliquots were injected subcutaneously for subsequent biopsies. At the same time, one baboon received a combination of porcine PBPCs and pancreatic islets; the two other baboons received PBPCs along with a kidney or heart xenotransplant, as reported elsewhere (15, 16) (Fig. 1).
Figure 1. Conditioning and combined bone marrow/organ transplantation.
Pre-transplantation of pig islets or kidney (day 0), all baboons were splenectomized (Splx), and went through extracorporeal immunoadsorption (EIA). In addition, they underwent whole body irradiation (WBI, 2 × 150 Gy) and thymic irrdiation (700 cGy) and were treated with cyclophosphamide (CP), thymoglobulin (ATG), cobra venom factor (CVF), mycophenolate mofetil (MMF) and cyclosporin A (CyA). The monkeys were infused thrice with pig peripheral blood progenitor cells (PBPCs), starting at the time of kidney or islet transplantation. Finally, two days after transplantation, the monkeys were treated with porcine IL-3, anti-CD154 mAbs and porcine stem cell factor (pSCF). All treatments were ceased 28 days after kidney/islet transplantation.
Measurement of xenoresponses by ELISPOT
ELISPOT plates (Polyfiltronics, Whatman, PA) were coated with the following antibodies: anti-human γIFN, clone MD1 (Biosource International, Camarillo, CA) (4 ug/ml), anti-IL-2 mAbs (4 ug/ml, clone 5334.21, R&D Systems, Minneapolis, MN), anti-IL-4 mAbs (clone 8D4-8, Pharmingen, San Diego, CA) (5 ug/ml) and anti-IL-5 mAbs (5 ug/ml, clone TRFK-5, Pharmingen). After washing twice with sterile PBS, the plates were blocked for 2 h with sterile PBS containing 1% bovine serum albumin (BSA) and washed 3 times with sterile PBS. PBMCs (0.6×106) in 200 ul of RPMI medium containing 10% fetal calf serum (FCS) were placed in each well with 0.6×106 irradiated swine PBMCs (direct alloresponse) or swine sonicates (indirect alloresponse). For T memory cell ELISPOT assays, naïve and memory T cells (TMEM) were FACS-sorted using CD45RA and CD62L markers (Naïve: CD45RA+CD62Lhigh, TMEM: CD45RA−CD62L+/−) and cultured (5×105 cells/well) for 48 h with irradiated self-antigen presenting cells (APCs) and pig sonicates. Next, the cells were cultured for 24 h (IL-2) or 48 h (IL-4, IL-5, γIFN at 37° C in 5% CO2. After washing, the following biotinylated anti-lymphokine detection antibodies were added for 4–5 h: anti-human γIFN (4 ug/ml, 51–1890KZ, Pharmingen), anti-IL-2 (3 μg/ml, BAF 202, R&D Systems), anti-IL-4 (2 μg/ml, 51–1850KZ, Pharmingen), and anti-IL-5 (2 μg/ml, 51–1852KZ, Pharmingen) mAbs. To assess binding of biotinylated antibodies, streptavidin-horseradish peroxidase (1:2000 in PBS 0.025% Tween for 1.5 h at room temperature, Vector, Burlingame, CA) was used. The plates were developed and the resulting spots were counted on a computer-assisted ELISPOT image analyzer (Cellular Technology, Cleveland, OH).
Preparation of sonicates
Stimulator PBMCs were suspended at 3 × 107 cells/ml in AIM-V containing 0.5 % FCS, and sonicated with 10 pulses of 1 second each. The resulting suspension was frozen in a dry ice/ethanol bath, thawed at room temperature and centrifuged at 300g for 10 minutes to remove remaining intact cells.
Results
Xenograft survival
Pig PBPCs were injected between days 0 to 2 (3 ×1010 cells/kg) and no pig cells were detected in the peripheral blood by flow cytometry after day 6 (15). Following islet transplantation, a subcutaneous biopsy performed on day 14 showed some residual cells that stained for insulin, but none on day 28. The site of implantation was infiltrated with CD86+ cells, indicating the presence of macrophages associated with rejection (16). The pig kidney removed on day 11 exhibited histological signs of humoral rejection (16). The anti-Gal antibody response showed a return to base line for IgG and IgM within 30 days post-transplantation. However, after cessation of all immunosuppressive therapy, including anti-CD154 mAb, a 4-fold increase of IgG over baseline was observed, as reported previously (17).
Direct and indirect xenoresponses in naïve monkeys
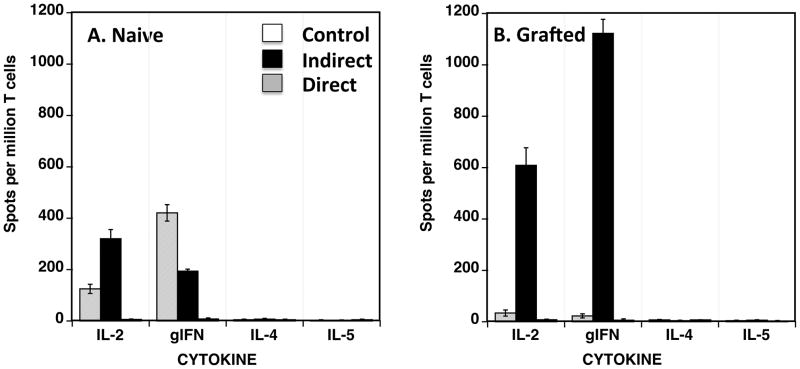
Significant numbers of cells secreting proinflammatory cytokines were detected following in vitro exposure of peripheral blood T cells from naïve baboons to intact pig APCs (direct alloreactivity). This response was primarily mediated by T cells secreting IL-2 (mostly CD4+ T cells) while a few T cells producing γIFN (mostly CD8+ T cells) were also detected (Figure 2A). No xenoreactive T cells producing type 2 cytokines (IL-4 and IL-5) were detected (Figure 2A). The presence of such primary direct xenoresponse in vitro indicates that baboon T cells can recognize intact porcine MHC molecules in a manner similar to that regularly observed in a classical allogeneic mixed lymphocyte reaction (18, 19). No T cell response was observed in the absence of xenogeneic APCs.
Figure 2. Direct and indirect xenoresponses of baboon PBMCs.
Peripheral blood mononuclear cells (PBMCs) from naïve (Panel A) and transplanted (Panel B) baboons were isolated and cultured in vitro with intact xenogeneic pig irradiated PBMCs (direct xenoresponse, grey bars) or sonicates from pig PBMCs (indirect xenoresponse, black bars). Baboon PBMCs cultured with medium only were used as controls (white bars). The frequencies of PBMCs producing type 1 cytokines (γIFN- and IL-2) or type 2 cytokines (IL-4 and IL-5) were determined by ELISPOT. The results are expressed as numbers of cytokine-producing cells per million PBMCs. The results are representative of three baboons tested individually.
High frequencies of IL-2- and γIFN-producing cells were detected among T cells from naïve baboons cultured in the presence of autologous APCs and pig sonicates (Fig. 2A) (indirect alloreactivity). Unlike its direct counterpart, this type of response was dominated by T cells producing γIFN. This shows that naïve baboons that have apparently never been exposed to pig cells can mount a pro-inflammatory indirect xenoresponse. Such primary in vitro indirect reactivity is not typically observed in naïve laboratory mice and naïve monkeys exposed to allogeneic sonicates or peptides (18, 19). This feature is presumably due to a lack of alloreactive memory T cells (TMEM) recognizing allo-MHC determinants indirectly prior to transplantation.
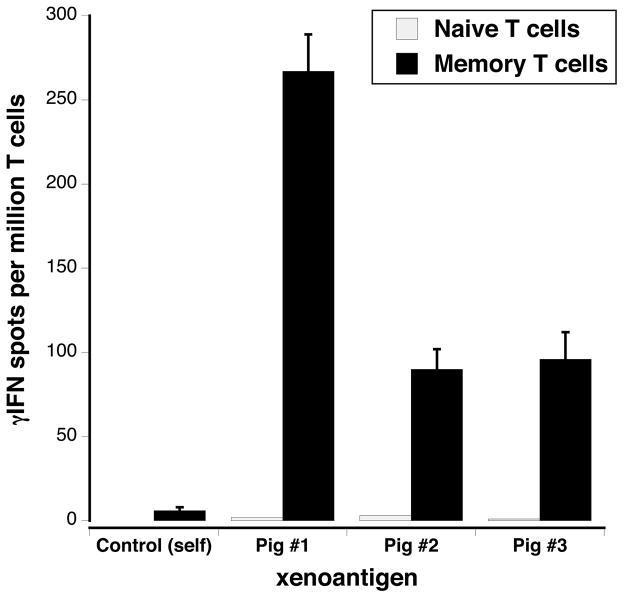
Our xeno-MLR results suggested that naïve baboons display TMEM capable of recognizing swine antigens through the indirect pathway. To test this, we sorted naïve T cells and TMEM from the PBMCs of two naïve baboons using CD45RA and CD62L markers (Naïve: CD45RA+CD62Lhigh, TMEM: CD45RA−CD62L+/−). Next, each T cell subset was tested for its ability to secrete γIFN following incubation with irradiated self-APCs and sonicates derived from three different swine, prepared as previously described (18). We detected significant numbers of TMEM activated through indirect xenorecognition while no responses were observed with naïve T cells (Figure 3) or with TMEM cultured with control self-sonicates (data not shown). Therefore, naïve baboons displayed significant frequencies of TMEM recognizing swine antigens indirectly prior to any xenotransplantation.
Figure 3. Indirect T cell responses of memory T cells from naïve baboons.
Memory T cells (TMEMs) from naïve baboons were sorted by FACS using CD45RA and CD62L markers. The frequencies of γIFN-producing memory T cells recognizing pig xenoantigens in an indirect fashion were determined by ELISPOT. The results are expressed as numbers of cytokine-producing cells per million TMEMs.
Indirect but not direct xenoresponses in baboons with pig grafts
Next, we investigated the direct and indirect xenoreactive T cell responses in baboons with xenografts. Baboon PBMCs were collected 50–60 days after placement of a porcine kidney, heart or pancreatic islets and conditioning, PBPC injections, immunosuppression and costimulation blockade using anti-CD40L monoclonal antibodies (Fig. 1). We observed an abrogation of the pro-inflammatory direct xenoresponse (Figure 2B). In contrast, a marked expansion of T cells activated indirectly and producing IL-2 and γIFN cytokines was recorded (Figure 2B). Of note, the frequencies of T cells mounting indirect xenoresponses (>1000 cells/million T cells) were much higher than those regularly observed in rodents and large animals receiving an allogeneic skin graft or a vascularized solid organ allotransplant (18, 20).
Discussion
Potent direct and indirect xenoresponses to pig antigens were observed in naïve baboons. This result confirms previous observations with mouse and primate T cells stimulated in vitro with swine APCs or autologous APCs and processed forms of swine antigens (10, 13, 21). Our study shows that the baboons’ primary indirect xenoresponses against pig antigens were mediated essentially by memory T cells. This differs from indirect T cell alloreactivity, which is not regularly observed in naïve animals (18, 19). This difference may be due to the multitude of xenoantigens but not alloantigens that can potentially serve as a source of determinants for indirect responsiveness. Consequently, in naive monkeys, such pre-existing xenoreactive TMEM (capable of indirect recognition) may be generated through exposure of naïve T cells to microbial antigens containing determinants mimicking xenodeterminants i.e. in a fashion similar to that observed with direct alloreactivity (heterelogous immunity) (22–25).
Post-transplantation, while no direct xenoresponses were detectable (Figure 2), we observed a significant expansion of activated pro-inflammatory T cells responding to xenoantigen determinants in an indirect fashion. The absence of direct xenoresponse by T cells probably results from immunosuppression by calcineurin inhibitors and anti-CD40L mAbs. In addition, this may be associated with the rapid loss of donor bone marrow-derived passenger leukocytes, which represent the main source of “professional APCs” for presentation of intact xeno-MHC antigens to recipient T cells. On the other hand, indirect xenoresponses may be perpetuated by the continuous presence of recipients’ professional APCs processing and presenting xenopeptides bound to host MHC molecules. It is noteworthy that previous studies by Sawyer et al have shown that T cells activated through indirect allorecognition are resistant to calcineurin inhibitor treatment (26). Recently, we have observed that anti-CD40L mAbs (MR1) fail to suppress efficiently indirect alloresponses in mice (27). Altogether, this suggests that maintenance of indirect T cell xenoreactivity could be due partly to T cell resistance to calcineurin inhibitors and anti-CD40L mAb-mediated costimulation blockade.
The high level and persistence of indirect xenoreactivity observed in transplanted baboons may also reflect the vigorous nature of innate immune responses triggered by xenotransplantation. Actually, both CD47-SIRPalpha and Toll-like receptor pathways have been shown to mediate activation of macrophages by xenoantigens in the absence of T cells (28). It has been reported that macrophages can phagocytose xenogeneic red blood cells (RBCs) without any T cell help; a process depending on expression of species-specific CD47 on RBCs (28). Likewise, O’Connell’s group have shown that Toll-like receptors are highly activated on macrophages during xenograft, but not allograft, rejection, although MyD88-deficient mice were still able to reject islet xenografts (29). Therefore, it is conceivable that potent innate immune responses resulting from xenotransplantation may promote indirect xenoresponses by T cells by enhancing and perpetuating antigen uptake, processing and presentation by recipient macrophages and other APCs. This implies that targeting the innate immune response by gene therapy, such as the generation of human CD47 transgenic pigs, might represent a useful strategy to attenuate indirect xenoreactivity by T cells.
In summary, our study shows that direct xenoreactivity by T cells could be successfully harnessed by therapy aimed towards the induction of mixed hematopoietic chimerism combined with costimulation blockade and conventional immunosuppression. In contrast, these treatments failed to suppress indirect T cell responses to swine antigens processed and presented by recipient APCs. This suggests that successful prevention of acute cellular rejection in pig-to-primate xenotransplantation is likely to require treatments suppressing indirect xenoresponses by T cells.
Acknowledgments
This work was supported by NIH grants U01 AI068642, R21 AI074844, and U19 AI090959 to David K. C. Cooper, NIH P01AI045897 to David H. Sachs and, R21AI100278 and R03AI094235 to Gilles Benichou.
Abbreviations
- APC
antigen-presenting cell
- DIC
disseminated intravascular coagulation
- hCPRP
human complement pathway regulatory protein
- MLR
mixed lymphocyte reaction
- MGH
Massachusetts General Hospital
- MHC
major histocompatibility complex
- PBPC
peripheral blood progenitor cell
- SLA
swine leukocyte antigen
- TMEM
memory T cell
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Ekser B, Ezzelarab M, Hara H, van der Windt DJ, Wijkstrom M, Bottino R, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379(9816):672–683. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 2.Sachs DH, Sykes M, Yamada K. Achieving tolerance in pig-to-primate xenotransplantation: reality or fantasy. Transpl Immunol. 2009;21(2):101–105. doi: 10.1016/j.trim.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ierino FL, Kozlowski T, Siegel JB, Shimizu A, Colvin RB, Banerjee PT, et al. Disseminated intravascular coagulation in association with the delayed rejection of pig-to-baboon renal xenografts. Transplantation. 1998;66(11):1439–1450. doi: 10.1097/00007890-199812150-00006. [DOI] [PubMed] [Google Scholar]
- 4.Byrne GW, McCurry KR, Martin MJ, McClellan SM, Platt JL, Logan JS. Transgenic pigs expressing human CD59 and decay-accelerating factor produce an intrinsic barrier to complement-mediated damage. Transplantation. 1997;63(1):149–155. doi: 10.1097/00007890-199701150-00027. [DOI] [PubMed] [Google Scholar]
- 5.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299(5605):411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen G, Sun H, Yang H, Kubelik D, Garcia B, Luo Y, et al. The role of anti-non-Gal antibodies in the development of acute humoral xenograft rejection of hDAF transgenic porcine kidneys in baboons receiving anti-Gal antibody neutralization therapy. Transplantation. 2006;81(2):273–283. doi: 10.1097/01.tp.0000188138.53502.de. [DOI] [PubMed] [Google Scholar]
- 7.Houser SL, Kuwaki K, Knosalla C, Dor FJ, Gollackner B, Cheng J, et al. Thrombotic microangiopathy and graft arteriopathy in pig hearts following transplantation into baboons. Xenotransplantation. 2004;11(5):416–425. doi: 10.1111/j.1399-3089.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 8.Kurtz J, Shaffer J, Lie A, Anosova N, Benichou G, Sykes M. Mechanisms of early peripheral CD4 T-cell tolerance induction by anti-CD154 monoclonal antibody and allogeneic bone marrow transplantation: evidence for anergy and deletion but not regulatory cells. Blood. 2004;103(11):4336–4343. doi: 10.1182/blood-2003-08-2642. [DOI] [PubMed] [Google Scholar]
- 9.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381(6581):434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 10.Moses RD, Pierson RN, 3rd, Winn HJ, Auchincloss H., Jr Xenogeneic proliferation and lymphokine production are dependent on CD4+ helper T cells and self antigen-presenting cells in the mouse. J Exp Med. 1990;172(2):567–575. doi: 10.1084/jem.172.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swain SL, Dutton RW, Schwab R, Yamamoto J. Xenogeneic human anti-mouse T cell responses are due to the activity of the same functional T cell subsets responsible for allospecific and major histocompatibility complex-restricted responses. J Exp Med. 1983;157(2):720–729. doi: 10.1084/jem.157.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alter BJ, Bach FH. Cellular basis of the proliferative response of human T cells to mouse xenoantigens. J Exp Med. 1990;171(1):333–338. doi: 10.1084/jem.171.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada K, Sachs DH, DerSimonian H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol. 1995;155(11):5249–5256. [PubMed] [Google Scholar]
- 14.Markmann JF, Campos L, Bhandoola A, Kim JI, Desai NM, Bassiri H, et al. Genetically engineered grafts to study xenoimmunity: a role for indirect antigen presentation in the destruction of major histocompatibility complex antigen deficient xenografts. Surgery. 1994;116(2):242–248. discussion 248–249. [PubMed] [Google Scholar]
- 15.Buhler L, Awwad M, Treter S, Chang Q, Basker M, Alwayn IP, et al. Pig hematopoietic cell chimerism in baboons conditioned with a nonmyeloablative regimen and CD154 blockade. Transplantation. 2002;73(1):12–22. doi: 10.1097/00007890-200201150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Buhler L, Deng S, O’Neil J, Kitamura H, Koulmanda M, Baldi A, et al. Adult porcine islet transplantation in baboons treated with conventional immunosuppression or a non-myeloablative regimen and CD154 blockade. Xenotransplantation. 2002;9(1):3–13. doi: 10.1034/j.1399-3089.2002.1o044.x. [DOI] [PubMed] [Google Scholar]
- 17.Buhler L, Yamada K, Kitamura H, Alwayn IP, Basker M, Appel JZ, 3rd, et al. Pig kidney transplantation in baboons: anti-Gal(alpha)1-3Gal IgM alone is associated with acute humoral xenograft rejection and disseminated intravascular coagulation. Transplantation. 2001;72(11):1743–1752. doi: 10.1097/00007890-200112150-00007. [DOI] [PubMed] [Google Scholar]
- 18.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162(1):352–358. [PubMed] [Google Scholar]
- 19.Nadazdin O, Boskovic S, Murakami T, O’Connor DH, Wiseman RW, Karl JA, et al. Phenotype, distribution and alloreactive properties of memory T cells from cynomolgus monkeys. Am J Transplant. 2010;10(6):1375–1384. doi: 10.1111/j.1600-6143.2010.03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Illigens BM, Yamada A, Fedoseyeva EV, Anosova N, Boisgerault F, Valujskikh A, et al. The relative contribution of direct and indirect antigen recognition pathways to the alloresponse and graft rejection depends upon the nature of the transplant. Human Immunology. 2002;63(10):912–925. doi: 10.1016/s0198-8859(02)00449-4. [DOI] [PubMed] [Google Scholar]
- 21.Yamada K, Sachs DH, DerSimonian H. Direct and indirect recognition of pig class II antigens by human T cells. Transplantation proceedings. 1995;27(1):258–259. [PubMed] [Google Scholar]
- 22.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111(12):1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169(7):3686–3693. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 24.Brehm MA, Markees TG, Daniels KA, Greiner DL, Rossini AA, Welsh RM. Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infections. J Immunol. 2003;170(8):4077–4086. doi: 10.4049/jimmunol.170.8.4077. [DOI] [PubMed] [Google Scholar]
- 25.Burrows SR, Silins SL, Khanna R, Burrows JM, Rischmueller M, McCluskey J, et al. Cross-reactive memory T cells for Epstein-Barr virus augment the alloresponse to common human leukocyte antigens: degenerate recognition of major histocompatibility complex-bound peptide by T cells and its role in alloreactivity. Eur J Immunol. 1997;27(7):1726–1736. doi: 10.1002/eji.1830270720. [DOI] [PubMed] [Google Scholar]
- 26.Sawyer GJ, Dalchau R, Fabre JW. Indirect T cell allorecognition: a cyclosporin A resistant pathway for T cell help for antibody production to donor MHC antigens. Transpl Immunol. 1993;1(1):77–81. doi: 10.1016/0966-3274(93)90063-e. [DOI] [PubMed] [Google Scholar]
- 27.Kant CD, Akiyama Y, Tanaka K, Shea S, Connolly SE, Germana S, et al. Primary vascularization of allografts governs their immunogenicity and susceptibility to tolerogenesis. J Immunol. 2013;191(4):1948–1956. doi: 10.4049/jimmunol.1202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, VerHalen J, Madariaga ML, Xiang S, Wang S, Lan P, et al. Attenuation of phagocytosis of xenogeneic cells by manipulating CD47. Blood. 2007;109(2):836–842. doi: 10.1182/blood-2006-04-019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi S, Wang Y, Chandra AP, O’Hara JM, Wu J, Ouyang L, et al. Requirement of MyD88 for macrophage-mediated islet xenograft rejection after adoptive transfer. Transplantation. 2007;83(5):615–623. doi: 10.1097/01.tp.0000253759.87886.39. [DOI] [PubMed] [Google Scholar]