Abstract
Background
Uncontrolled hemorrhage from vessel injuries within the torso remains a significant source of prehospital trauma mortality. Resuscitative endovascular balloon occlusion of the aorta can effectively control non-compressible hemorrhage, but this minimally invasive technique relies heavily upon imaging not available in the field. Our goal was to develop morphometric roadmaps to enhance the safety and accuracy of fluoroscopy-free endovascular navigation of hemorrhage control devices.
Methods
Three-dimensional reconstructions of computed tomography angiography scans from n=122 trauma patients (mean age 47±24 years, range 5-93 years, 64 Male/58 Female) were used to measure centerline distances from femoral artery access sites to the major aortic branch artery origins. Morphometric roadmap equations were created using multiple linear regression analysis to predict distances to the origins of the major arteries in the chest, abdomen and pelvis using torso length, demographics, and risk factors as independent variables. A 40-mm long occlusion balloon was then virtually deployed targeting Zones 1 and 3 of the aorta using these equations. Balloon placement accuracy was determined by comparing predicted versus actual measured distances to the target zone locations within the aortas from the database.
Results
Torso length and age were the strongest predictors of centerline distances from femoral artery access sites to the major artery origins. Male gender contributed to longer distances while diabetes and smoking were associated with shorter distances. Hypertension, dyslipidemia and coronary artery disease had no effect. Using morphometric roadmaps, virtual occlusion balloon placement accuracy was 100% for Zone 3 of the aorta, compared to 87% accuracy when using torso length alone.
Conclusion
Morphometric roadmaps demonstrate potential for improving the safety and accuracy of fluoroscopy-free aortic occlusion balloon delivery. Continued development of minimally invasive hemorrhage control techniques hold promise to improve prehospital mortality for patients with noncompressible exsanguinating torso injuries.
Level of evidence
Diagnostic, level III.
Keywords: REBOA, morphometric roadmaps, vascular anatomy, CTA reconstructions, noncompressible hemorrhage, endovascular, balloon
INTRODUCTION
Unintentional injuries in the United States are the leading cause of death in people younger than 45 years old and are the fourth leading cause of death in all age groups overall1. Exsanguinating noncompressible torso hemorrhage is a major contributor to these mortalities2,3 and a large portion of injured victims die before reaching a hospital. Limiting blood loss is the only way to avoid the cascade of complications associated with massive hemorrhage from trauma, but methods for controlling noncompressible truncal hemorrhage outside of an operating room are not readily available4.
The emergence of endovascular techniques to repair aortic aneurysms using stent grafts has resulted in the development of large diameter, compliant aortic balloons5–7, and the re-emergence of resuscitative endovascular balloon occlusion of the aorta (REBOA) for hemorrhage control. First described in 1954 by Hughes5 in the Korean Conflict, occlusion balloons have been shown to be clinically effective in managing shock due to ruptured aortic aneurysms, one of the most commonly encountered clinical conditions causing noncompressible torso hemorrhage6,8. Recent reports have also demonstrated the utility of aortic balloon occlusion for hemorrhage control in severely injured trauma patients9–13, post-partum hemorrhage14, and massive gastrointestinal bleeding15, describing how in many instances, temporary balloon occlusion may provide a reasonable alternative to conventional open surgical aortic exposure and vessel clamping for initial hemorrhage control and blood pressure stabilization8,16–18. Though most of these reports involve care in hospital settings, endovascular methods may be the only viable prehospital options capable of maintaining cardiac and cerebral perfusion in austere settings, such as on battlefields or in remote rural areas4.
One major limitation of current occlusion balloon technology is the heavy reliance on non-portable fluoroscopic imaging methods19 to navigate and inflate the occlusion balloon proximal to the site of injury. There is considerable variation in human vascular anatomy making radiological guidance useful for visualization of the vessel and device while navigating through complex, aged, and diseased vasculatures. This requirement for heavy imaging equipment has been detrimental for the potential extended application of REBOA technology to treating exsanguinating hemorrhage in the field. The goal of this work was to build demographics-based morphometric statistical roadmaps of distances from common femoral artery access sites to the main branches of the aorta for trauma patients of all age groups and both genders. The morphometric roadmaps were then applied to high resolution trauma patient anatomies to simulate fluoroscopy-free balloon navigation based on the intracavitary balloon catheter insertion distance from the femoral artery access site predicted by the morphometric roadmap equation.
METHODS
Following IRB approval, n=122 thin-section, contrast-enhanced chest-abdomen-pelvis computed tomography angiograms (CTA) were retrieved from our institution's trauma database encompassing both genders (64 Male / 58 Female) and a wide variation in age (mean age 47±24 years, range 5-93 years). Patients with systolic blood pressures <90 mmHg and patients with Injury Severity Scores (ISS) ≥25 were excluded from the study to remove the effects of hypotensive vessel diameter reduction, although hypotension likely has less effect on length due to vessel tethering. Scans of patients with aneurysms and injuries to the vessel segments of interest were also excluded. All scans were obtained with a 1.25 mm slice thickness and a resolution of 512 × 512 pixels. The entire arterial tree including the aorta, its branches, and the more distal junctional arteries supplying the extremities, were reconstructed20 using Mimics software (Materialize Co). As some arterial segments have densities similar to the surrounding tissues, all segmentations were verified by a vascular surgeon to ensure proper selection of vessel boundaries.
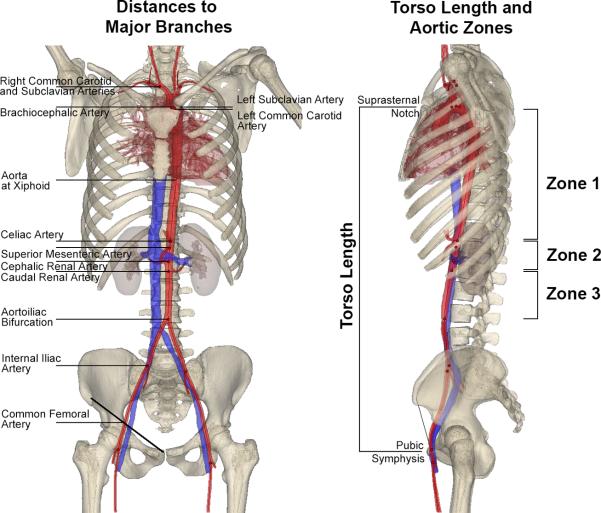
After reconstruction, vessel centerlines were fit to the arterial volumes and then distances from typical common femoral artery access sites to the major aortic branches were measured along the centerline (Figure 1). Torso length was measured as the vertical distance between the pubic symphysis and suprasternal notch. Demographics (age, gender, body mass index [BMI]) and cardiovascular risk factors (hypertension [HTN], diabetes mellitus [DM], dyslipidemia, coronary artery disease [CAD] and smoking history [never, former, current]) were extracted from the medical records that were linked with each CTA. BMI was calculated as weight (kg)/height2 (m2).
Figure 1.
Measurement of distances to the major aortic branches (left) in each of the three aortic zones (right) in a representative 21-year-old female patient.
Multiple linear regression analysis was performed with SPSS v22 (IBM, Armonk, New York) using torso length, age, gender, BMI, HTN, DM, dyslipidemia, CAD, and smoking history parameters as independent variables predicting distances from the common femoral artery access site to the major branch arteries. Stepwise linear regression was used to determine statistically significant predictors. A variable was entered into the model when the significance level of its F value was less than 0.05. Both unstandardized and standardized beta coefficients were determined for each model, and model quality was assessed with adjusted R2.
A combination of multiple linear regression models predicting distances from the common femoral artery access sites to each of the major aortic branches (Figure 1) based on torso length, demographics, and risk factors constituted a morphometric roadmap equation. The output value determined by the equation represents the balloon catheter insertion length along the centerline to get from the femoral artery access site to the center of the target zone. A 40mm-long CODA (Cook Medical) balloon was then hypothetically inserted the predicted length to deliver the virtual occlusion balloon to Zone 1 and Zone 3 of the aorta (Figure 1).
Placement accuracy was assessed by comparing predictions made using morphometric roadmaps to the actual measurements of distance made along the aortic centerline. This comparison allowed determination of whether the entirety of the 40mm-long occlusion balloon would be contained within the target zone of the aorta when advanced the predicted distance towards the heart along the centerline from the common femoral artery access point.
To compare our results with previous data21,22, a single measure of torso length was also utilized to guide simulated balloon placement. Regression models were built using torso length as the independent variable with distances from the common femoral artery access site to the upper and lower borders of Zones 1 and 3 calculated as the dependent variables.
RESULTS
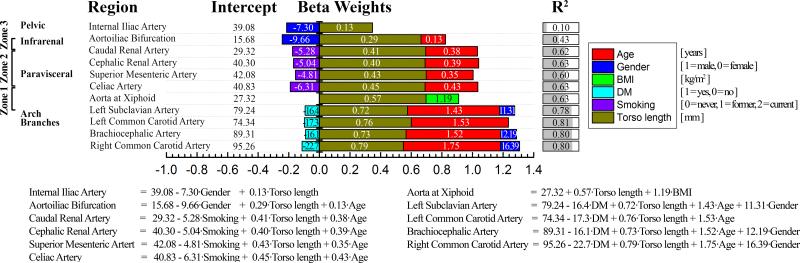
Morphometric roadmap equations of distances from the common femoral artery access sites to the major aortic branches are presented in Figure 2. The bar graphs represent standardized beta weights that demonstrate the influence of each parameter on distance measurement, while numeric values inside the bars are unstandardized beta weights used in the multiple linear regression models. To calculate distances from the common femoral artery access sites to each of the major aortic branch artery origins for a specific subject, one needs to sum the values of intercept and beta weights multiplied by the corresponding torso length, demographic or risk factor variables. For example, the distance from the right common femoral artery access site to the left subclavian artery (in mm) can be calculated as: 79.24 – 16.4·Diabetes Mellitus [0=no, 1=yes] + 0.72·Torso length [mm] + 1.43·Age [years] + 11.31·Gender [1=Male, 0=Female] and the combination of these factors explain 78% of the variation in the predicted distance. Thus in a 50-year-old male with no diabetes and a torso length of 500 mm, this distance is 522 mm, while in the presence of diabetes this distance is decreased by 16.4 mm to a total of 506 mm. For comparison, the same vessel segment in a 20-year old male with the same torso length is only 479 mm, but increases to 551 mm in a 70-year old male; more than a 15% difference in distance over 50 years of aging. Though Figure 2 allows calculation of distances for a variety of specific demographic and risk factor characteristics, Table 1 summarizes the mean distances measured in each age group.
Figure 2.
Morphometric roadmap equations for distances (mm) from the right common femoral artery access site to the major artery branches in the chest, abdomen and pelvis predicted by patient torso length, demographics and risk factors. Unstandardized beta weights for the multiple linear regression models are provided with numeric values within the bars, while the length of the colored bars is made using standardized weights and represents the influence of each parameter on the distance measurement. BMI = Body Mass Index, DM = Diabetes Mellitus.
Table 1.
Age-group mean distances from the common femoral artery access site to the major aortic branch artery origins. Data are presented as average [mm] ± Standard Deviation [mm].
| Distance [mm] from the common femoral artery access site to: | Age Groups, years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5-9 | 10-19 | 20-29 | 30-39 | 40-49 | 50-59 | 60-69 | 70-79 | >80 | ||
| Internal Iliac Artery | 75±10 | 100±15 | 101±16 | 99±10 | 113±16 | 106±16 | 102±13 | 104±24 | 103±13 | |
|
Zone 3
Zone 2 Zone 1 |
Aortoiliac Bifurcation | 114±10 | 167±10 | 174±16 | 167±15 | 170±16 | 173±18 | 172±20 | 173±20 | 169±17 |
| Caudal Renal Artery | 175±20 | 251±14 | 260±16 | 259±17 | 256±14 | 269±20 | 267±25 | 272±29 | 265±21 | |
| Cephalic Renal Artery | 180±18 | 258±13 | 268±17 | 264±18 | 262±15 | 275±18 | 270±24 | 277±27 | 275±17 | |
| Superior Mesenteric Artery | 190±18 | 272±14 | 280±14 | 277±17 | 276±19 | 288±22 | 282±27 | 288±30 | 285±23 | |
| Celiac Artery | 200±18 | 287±15 | 293±15 | 292±18 | 288±20 | 303±23 | 304±32 | 304±33 | 305±21 | |
| Aorta at Xiphoid | 250±21 | 360±31 | 360±30 | 358±24 | 358±29 | 364±33 | 373±46 | 354±34 | 353±29 | |
| Left Subclavian Artery | 344±30 | 495±19 | 507±25 | 516±30 | 519±28 | 553±32 | 552±41 | 560±56 | 564±44 | |
| Left Common Carotid Artery | 355±33 | 507±18 | 518±23 | 529±30 | 539±27 | 570±31 | 568±44 | 580±51 | 580±40 | |
| Brachiocephalic Artery | 359±33 | 512±19 | 526±23 | 536±30 | 541±29 | 574±34 | 572±43 | 584±57 | 588±42 | |
| Right Common Carotid Artery | 393±35 | 558±25 | 575±23 | 586±33 | 592±32 | 626±45 | 628±53 | 643±60 | 648±44 | |
Torso length and age were the strongest predictors of distance from the common femoral artery access site to all major artery branches in the chest, abdomen and pelvis. Male gender contributed to longer distances to the arch branches, while the presence of DM was associated with shorter distances. Smoking was associated with shorter distances to the infrarenal and visceral branch arteries. HTN, dyslipidemia and CAD had no effect on distances from the femoral artery access sites.
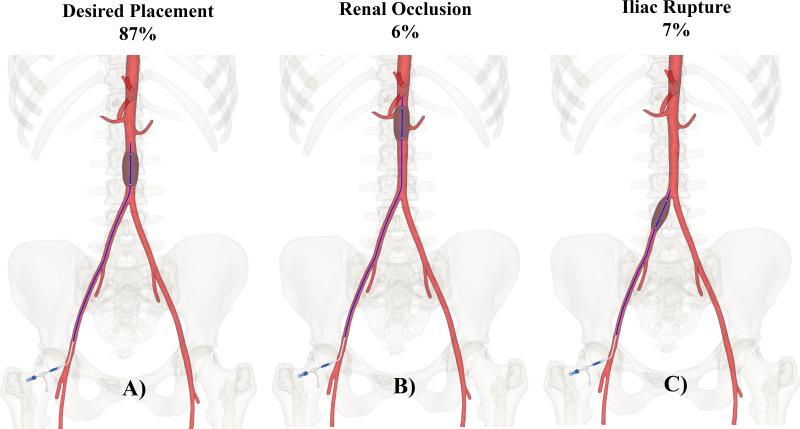
In all patients, including older subjects with tortuous vasculatures, use of morphometric roadmap equations produced accurate placement of the virtual 40mm occlusion balloon entirely within the target Zones 1 and 3. A single measure of torso length also resulted in 100% accurate placement of the occlusion balloon into target Zone 1; however in Zone 3 use of torso length alone resulted in misplacement of the occlusion balloon in 13% of subjects (Figure 3). Specifically, in 6% of subjects the balloon was placed too proximal, with renal artery obstruction (mean age of this group 30±31 years, range 6-88 years), while in 7% of subjects the balloon was not advanced far enough along the centerline and would be inflated in the iliac artery (mean age of this group 57±21 years, range 28-82 years). Overall, inclusion of demographics and risk factors into the statistical model improved the prediction of distances from the common femoral artery access site to the major aortic branches by almost two fold compared to when a single measure of torso length was used as an independent variable21.
Figure 3.
Desired placement of the occlusion balloon in target Zone 3 (A), and misplacement with overshooting (B), and undershooting (C) potentially resulting in renal occlusion or iliac rupture. Percentages are provided for cases when torso length is used as a single predictive variable.
DISCUSSION
REBOA is a minimally invasive technique capable of controlling major intracavitary hemorrhage in hospital and non-hospital settings23. The basic technique was initially described over 50 years ago, but only relatively recently have research, technology and clinical experience converged to create more refined endovascular techniques and devices that quickly and safely provide percutaneous mechanical control of major vessel injuries through remote peripheral arteries. As the endovascular revolution in vascular surgery has spurred the development of smaller and more complex catheter-based diagnostic and treatment modalities, wire and catheter skills training have diffused into most general surgery training programs. Thus, with development of appropriate enabling technologies and enhanced training of hospital and pre-hospital providers, REBOA is poised to challenge the formidable problem of hemorrhagic shock due to catastrophic non-compressible hemorrhage. Contemporary experimental large animal models of hemorrhagic shock demonstrate improvements in hemodynamic and biochemical parameters with REBOA compared to traditional open surgical management24. In humans, Brenner et al18 and Moore et al25 recently described several patients treated successfully with REBOA, documenting the largest clinical experiences in the United States. More extensive experience from other centers around the globe show definite promise for the utility of REBOA, including during the prehospital phase of care, but also raise concerns about both the safety and efficacy of the technique, necessitating more research, development and clinical experience9,10,26.
Stannard27 initially described the optimal target anatomical zones for REBOA. Occlusion within Zone 1 is suggested for patients with abdominal exsanguination and circulatory collapse. Zone 3 occlusion provides flow control for patients with exsanguinating pelvic and inguinofemoral junctional hemorrhage. Zone 2 is the paravisceral aorta, and is currently a zone to avoid with balloon occlusion. Inflation of the balloon outside of Zones 1 or 3 can result in complications, such as iliac artery rupture or occlusion of critical side branches, such as the renal arteries. To navigate endovascular devices through complex vessel anatomies, conventional approaches currently rely heavily on non-portable imaging methods19, primarily fluoroscopy. Recent studies21 in a narrow population of young male trauma patients (mean age 28±4 years) have demonstrated relatively reliable vessel distance measurement predictions based solely on bony landmark locations. Using these easily obtained measurements and newly developed experimental technology28,29, the feasibility of fluoroscopy-free REBOA has been suggested. Our current study focused on expanding the restricted patient populations of prior research to include all age groups and both genders, with simulation of occlusion balloon placement within each of the two target zones of the aorta in all subjects.
Our results demonstrate great potential for accurate placement of the occlusion balloon within target Zones 1 and 3 of the aorta in all age groups and both genders employing morphometric roadmap equation guidance. When using torso length alone, misplacement of the occlusion balloon out of target Zone 3 was observed in 13% of patients. In younger patients, with straighter and shorter vasculatures, balloon catheter insertion distance was usually overestimated and the device landed in the paravisceral aorta (Zone 2), hypothetically occluding the renal arteries. In older subjects, typically with longer vasculatures due to higher tortuosity, the distance was mostly underestimated and the balloon landed in the iliac artery, potentially resulting in iliac artery rupture and exacerbation of catastrophic bleeding. When using morphometric roadmap equations, accurate placement of the balloon in both Zones 1 and 3 was achieved in 100% of subjects.
Our results are in agreement with anatomical data reported in previous studies20,30,31 describing inferior migration of the aortoiliac bifurcation and increased arterial tortuosity and length with aging. However, underestimation and overestimation of distances using torso length alone were observed in both young and old subjects. This demonstrates the importance of using morphometric roadmap equations for balloon catheter insertion distance prediction in subjects of all ages, including young patients. The effects of clinical risk factors on balloon accuracy were small compared to the effects of demographics. This is reassuring, as information on clinical risk factors is frequently not immediately available from critically injured trauma patients. Nevertheless, DM and smoking history contributed to shorter aortic trees and if such information is readily available, it may be incorporated to increase the accuracy of fluoroscopy-free balloon placement. Analysis of larger numbers of patients from even more diverse patient groups may define additional factors important in anatomical predictions, such as race.
Though there is some utility in using simulation to determine balloon catheter placement accuracy, confirmation of these results should be performed in physical models of identical anatomies to evaluate the fidelity of our approach. The increased complexity of a physical model may lead to emergent behaviors of the device or guidance systems that are not predicted by simulation. Further work will clarify the applicability of more complex electronic simulators to accurately portray REBOA for trainee education and for endovascular occlusion device development. Our morphometric roadmap equations are based on simple input parameters but do require detailed calculations not likely to be easily performed in the chaotic trauma-care environment. We envision these morphometric roadmap equations and calculations to be incorporated into easy to use software on portable, handheld electronic devices that would assist with control of non-compressible hemorrhage in situations where imaging capabilities are limited. While newer techniques and devices are being developed, the table of mean distances from the common femoral artery access site to the major aortic branch artery origins for patients in all age groups could be used as estimated balloon catheter insertion distances in emergency rooms and critical care units.
Fluoroscopy-free placement of endovascular devices has been performed for decades. Swan-Ganz catheters have long been guided into pulmonary arteries based on pressure waveforms, and bedside inferior vena cava filters have been safely placed, based only on pre-intervention CT scan data32. Fluoroscopy-free REBOA is fundamentally similar to these procedures and its use in mortally wounded patients with few other options should be intensely investigated. As the technique of REBOA evolves, methods that enhance the safety and applicability of fluoroscopy-free REBOA could help open the door to increasing the use of endovascular hemorrhage control techniques on battlefields and in remote rural areas where our bleeding patients require the most assistance. The continued accumulation of endovascular trauma clinical experience, along with rapidly evolving minimally invasive technologies, should allow the techniques to become more widely used for exsanguinating patients that have few other options for survival.
ACKNOWLEDGEMENTS
Research reported in this publication was supported in part by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Numbers R01 HL125736 and F32 HL124905. The authors also wish to acknowledge the Charles and Mary Heider Fund for Excellence in Vascular Surgery and the MARC U*STAR Program for their support.
Footnotes
DISCLOSURE
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
J.M. and A.K. conceived, designed, and collected data for this study. J.M., W.P., M.A., A.S., K.T., N.P., A.D., and A.K. contributed to analysis and interpretation. J.M. and A.K. performed statistical analyses and wrote the article. J.M., W.P., M.A., A.S., K.T., N.P., A.D., and A.K. critically revised and gave final approval of the article. J.M. and A.K. obtained funding and declare overall responsibility for this work.
REFERENCES
- 1.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control . Natl Cent Inj Prev Control. Centers Dis Control Prev; 2009. Web-based Injury Statistics. Reporting system (WISQARS). [Google Scholar]
- 2.Dorlac WC, DeBakey ME, Holcomb JB, Fagan SP, Kwong KL, Dorlac GR, Schreiber MA, Persse DE, Moore FA, Mattox KL. Mortality from isolated civilian penetrating extremity injury. J Trauma. 2005;59:217–22. doi: 10.1097/01.ta.0000173699.71652.ba. [DOI] [PubMed] [Google Scholar]
- 3.Kauvar DS, Lefering R, Wade CE. Impact of Hemorrhage on Trauma Outcome: An Overview of Epidemiology, Clinical Presentations, and Therapeutic Considerations. J Trauma. 2006;60:S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 4.Kerby JD, Cusick M V. Prehospital emergency trauma care and management. Surg Clin North Am. 2012;92:823–41. vii. doi: 10.1016/j.suc.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Hughes CW. Use of an intra-aortic balloon catheter tamponade for controlling intra-abdominal hemorrhage in man. Surgery. 1954;36:65–8. [PubMed] [Google Scholar]
- 6.Arthurs Z, Starnes B, See C, Andersen C. Clamp Before You Cut: Proximal Control of Ruptured Abdominal Aortic Aneurysms Using Endovascular Balloon Occlusion: Case Reports. Vasc Endovascular Surg. 2006;40:149–155. doi: 10.1177/153857440604000210. [DOI] [PubMed] [Google Scholar]
- 7.Low RB, Longmore W, Rubinstein R, Flores L, Wolvek S. Preliminary report on the use of the Percluder occluding aortic balloon in human beings. Ann Emerg Med. 1986;15:1466–9. doi: 10.1016/s0196-0644(86)80945-3. [DOI] [PubMed] [Google Scholar]
- 8.Mayer D, Pfammatter T, Rancic Z, Hechelhammer L, Wilhelm M, Veith FJ, Lachat M. 10 Years of Emergency Endovascular Aneurysm Repair for Ruptured Abdominal Aortoiliac Aneurysms: Lessons Learned. Ann Surg. 2009;249:510–5. doi: 10.1097/SLA.0b013e31819a8b65. [DOI] [PubMed] [Google Scholar]
- 9.Norii T, Crandall C, Terasaka Y. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score-adjusted untreated patients. J Trauma Acute Care Surg. 2015;78:721–728. doi: 10.1097/TA.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 10.Irahara T, Sato N, Moroe Y, Fukuda R, Iwai Y, Unemoto K. Retrospective study of the effectiveness of Intra-Aortic Balloon Occlusion (IABO) for traumatic haemorrhagic shock. World J Emerg. Surg. 2015;10:1–6. doi: 10.1186/1749-7922-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delamare L, Crognier L, Conil J- M, Rousseau H, Georges B, Ruiz S. Treatment of intra-abdominal haemorrhagic shock by Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). Anaesth Crit Care Pain Med. 2015;34:53–55. doi: 10.1016/j.accpm.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Martinelli T, Thony F, Decléty P, Sengel C, Broux C, Tonetti J, Payen J-F, Ferretti G. Intra-aortic balloon occlusion to salvage patients with life-threatening hemorrhagic shocks from pelvic fractures. J Trauma. 2010;68:942–948. doi: 10.1097/TA.0b013e3181c40579. [DOI] [PubMed] [Google Scholar]
- 13.Miura F, Takada T, Ochiai T, Asano T, Kenmochi T, Amano H, Yoshida M. Aortic Occlusion Balloon Catheter Technique Is Useful for Uncontrollable Massive Intraabdominal Bleeding After Hepato-Pancreato-Biliary Surgery. J Gastrointest. Surg. 2006;10:519–522. doi: 10.1016/j.gassur.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Søvik E, Stokkeland P, Storm BS, Asheim P, Bolås O. The use of aortic occlusion balloon catheter without fluoroscopy for life-threatening post-partum haemorrhage. Acta Anaesthesio Scand. 2012;56:388–93. doi: 10.1111/j.1399-6576.2011.02611.x. [DOI] [PubMed] [Google Scholar]
- 15.Karkos CD, Bruce I a, Lambert ME. Use of the intra-aortic balloon pump to stop gastrointestinal bleeding. Ann Emerg Med. 2001;38:328–331. doi: 10.1067/mem.2001.114408. [DOI] [PubMed] [Google Scholar]
- 16.Mehta M, Taggert J, Darling RC, Chang BB, Kreienberg PB, Paty PSK, Roddy SP, Sternbach Y, Ozsvath KJ, Shah DM. Establishing a protocol for endovascular treatment of ruptured abdominal aortic aneurysms: outcomes of a prospective analysis. J Vasc Surg. 2006;44:1–8. doi: 10.1016/j.jvs.2006.02.057. discussion 8. [DOI] [PubMed] [Google Scholar]
- 17.Karkov CD, Harkin DW, Giannakou A, Gerassimidis TS. Mortality After Endovascular Repair of Ruptured Abdominal Aortic Aneurysms: A systematic Review and Meta Analysis. Arch Surg. 2009;144:770–778. doi: 10.1001/archsurg.2009.132. [DOI] [PubMed] [Google Scholar]
- 18.Brenner ML, Moore LJ, DuBose JJ, Tyson GH, McNutt MK, Albarado RP, Holcomb JB, Scalea TM, Rasmussen TE. A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J Trauma Acute Care Surg. 2013;75:506–11. doi: 10.1097/TA.0b013e31829e5416. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien P, Cox M. Stents in tents: endovascular therapy on the battlefields of the global war on terror. J Surg Radiol. 2011 [Google Scholar]
- 20.Kamenskiy A, Miserlis D, Adamson P, Adamson M, Knowles T, Neme J, Koutakis P, Phillips N, Pipinos I, MacTaggart J. Patient demographics and cardiovascular risk factors differentially influence geometric remodeling of the aorta compared with the peripheral arteries. Surgery . 2015;158(6):1617–27. doi: 10.1016/j.surg.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stannard A, Morrison JJ, Sharon DJ, Eliason JL, Rasmussen TE. Morphometric analysis of torso arterial anatomy with implications for resuscitative aortic occlusion. J Trauma Acute Care Surg. 2013;75:S169–S172. doi: 10.1097/TA.0b013e31829a098d. [DOI] [PubMed] [Google Scholar]
- 22.Morrison JJ, Stannard A, Midwinter MJ, Sharon DJ, Eliason JL, Rasmussen TE. Prospective evaluation of the correlation between torso height and aortic anatomy in respect of a fluoroscopy free aortic balloon occlusion system. Surgery. 2014;155:1044–1051. doi: 10.1016/j.surg.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 23.Mundasad S. Balloon surgery stops fatal bleeding at roadside. BBC News; [August 18, 2015]. Web AddressJournal http//www.bbc.com/news/health-27868418. 2014. [Google Scholar]
- 24.White JM, Cannon JW, Stannard A, Markov NP, Spencer JR, Rasmussen TE. Endovascular balloon occlusion of the aorta is superior to resuscitative thoracotomy with aortic clamping in a porcine model of hemorrhagic shock. Surgery. 2011;150:400–9. doi: 10.1016/j.surg.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Moore LJ, Brenner M, Kozar RA, Pasley J, Wade CE, Baraniuk MS, Scalea T, Holcomb JB. Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J Trauma Acute Care Surg. 2015;79:523–532. doi: 10.1097/TA.0000000000000809. [DOI] [PubMed] [Google Scholar]
- 26.Biffl WL, Fox CJ, Moore EE. The role of REBOA in the control of exsanguinating torso hemorrhage. J Trauma Acute Care Surg. 2015;78:1054–1058. doi: 10.1097/TA.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 27.Stannard A, Eliason JL, Rasmussen TE. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct for hemorrhagic shock. J Trauma. 2011;71:1869–72. doi: 10.1097/TA.0b013e31823fe90c. [DOI] [PubMed] [Google Scholar]
- 28.Scott DJ, Eliason JL, Villamaria C, Morrison JJ, Houston R, Spencer JR, Rasmussen TE. A novel fluoroscopy-free, resuscitative endovascular aortic balloon occlusion system in a model of hemorrhagic shock. J Trauma Acute Care Surg. 2013;75:122–128. doi: 10.1097/ta.0b013e3182946746. [DOI] [PubMed] [Google Scholar]
- 29.Franklin C. Fluoroscopy-Independent Balloon Guided Occlusion Catheter and Methods. 2014:A1. US 2014/0243873.
- 30.Sugawara J, Hayashi K, Yokoi T, Tanaka H. Age-associated elongation of the ascending aorta in adults. JACC Cardiovasc Imaging. 2008;1:739–48. doi: 10.1016/j.jcmg.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Wolf YG, Tillich M, Lee W a, Rubin GD, Fogarty TJ, Zarins CK. Impact of aortoiliac tortuosity on endovascular repair of abdominal aortic aneurysms: evaluation of 3D computer-based assessment. J Vasc Surg. 2001;34:594–9. doi: 10.1067/mva.2001.118586. [DOI] [PubMed] [Google Scholar]
- 32.Srivastava A, Troop B, Peick A, Kanne A. Inferior vena cava filter placement at bedside using computed tomography scan information: a new technique for accurate deployment. Am J Surg. 2016;211:172–178. doi: 10.1016/j.amjsurg.2015.08.017. [DOI] [PubMed] [Google Scholar]