Abstract
Intercellular communication mediated by exosomes, nano-sized extracellular vesicles, is crucial for preserving vascular integrity and in the development of cardiovascular and other diseases. As natural carriers of signal molecules, exosomes released from sources such as blood cells, endothelial cells, immune cells, smooth muscle cells, etc., can modify a multitude of cellular bioactivities. They do so by shuttling lipids, proteins and nucleic acids between donor and recipient cells while circulating in body fluids and in the extracellular space. A recent surge of interest in the field of exosomal biology is in part due to the recognition that the molecules they carry can act as facilitators of both pathogenesis but can also initiate protective and rescue signaling. This mini-review describes current knowledge on exosome function in health and disease including cardiovascular disease.
“Notice the small things: The rewards are inversely proportional” -Liz Vassey
Introduction
Cell derived nano-sized (30–100nm) vesicles termed exosomes are continuously released from most cell types and have been identified in all biological fluids and in the medium of cultured cells in vitro.
Intercellular communication mediated by extracellular vesicles is crucial for preserving vascular integrity and in the development of cardiovascular and other diseases. As natural carriers of signal molecules, exosomes released from sources such as blood cells, endothelial cells, immune cells, smooth muscle cells, etc., can modify a multitude of cellular bioactivities. They do so by shuttling lipids, proteins and nucleic acids between donor and recipient cells while circulating in body fluids and in the extracellular space. A recent surge of interest in the field of exosomal biology is in part due to the recognition that the molecules they carry can act as facilitators of both pathogenesis but can also initiate protective and rescue signaling. Exosomes have been described to modulate immune-regulatory processes, tumor escape mechanisms and to mediate both regenerative and degenerative processes.
To date, a large number of reports dealing with circulating exosomes and their cargos defines them to be a potential source of valuable diagnostic and prognostic biomarkers and holds a great therapeutic potential to serve as vehicles for targeted therapy for cardiovascular and other diseases.
Biogenesis of exosomes and sorting of cargo
While other microparticles such as apoptotic bodies and microvesicles form through blebbing of dying cells and outward budding of plasma membrane, respectively, exosomes are unique in their biogenesis and release. Exosomes are formed through the inward budding of endosomal membranes in areas particularly enriched in lipid rafts. Formation and secretion of such vesicles requires enzymes and ATP and can be both ESCRT (endosomal sorting complexes required for transport) dependent and independent (1–3). They accumulate and are enriched in endosomes called multi vesicular bodies (MVBs), from which they are released into extracellular space and are taken up by neighboring cells or transported though body fluids to remote locations. They contain lipids, proteins and various types of nucleic acids such as mRNA, miRNA and lncRNA. Beside the molecular components involved in exosome generation, secretion, transport and uptake common to all exosomes such as tetraspanins (CD9, CD63,CD81), membrane proteins Annexins and Flotillin, heat shock proteins HSP70, HSP90 (4), exosomes also contain unique molecules specific to their cells of origin. It is this type, variety and abundance of donor cell specific cargo that is a topic of extensive studies, in hopes to shed light on their potential application for development of diagnostic and therapeutic tools. The exact pathways which regulate the packing of exosomal content do not appear to be a random collection of cytoplasmic proteins and nucleic acids from donor cells. Valadi et al 2007 (5) identified many exosomal components not expressed in the cytoplasm of donor cells, indicating a highly regulated and selective cargo sorting mechanism that delivers and guides specific intracellular miRNAs into exosomes (6). Similar conclusions were made based on the observation that miRNA profiles of exosomes and their parent cells can significantly differ from one another (7, 8). Interestingly, the profiles of exosomes from same cell types can be significantly altered based on the cell’s physiological state during synthesis. Quantity and quality of constitutively secreted exosomes is regulated by the physiology of the donor cells as well as by stimuli such as calcium, mitogens, cytokines and stress (9). A new online database, ExoCarta (10), has been established and provides a continuously growing list of molecules identified in exosomes.
Transport and targeting
Detailed mechanisms of exosome trafficking, to near and far destinations, targeting to specific organs, tissues and cells as well as the modes of internalization are still not well understood. It is known that secreted exosomes can either be rapidly internalized by neighboring cells or travel via body fluids to deliver their informational cargo to distant cells or possibly remove it from the organism. Despite the lack knowledge of detailed mechanisms concerning exosomal interaction with target cells, some reports suggest that lipid rafts and exosomal membrane proteins are directly responsible for their trafficking, mode of interaction with cells and dictate their uptake specificity. This is evidenced by reports that the disruption of lipid rafts, annexins or in general any treatment of exosomes to interfere with their transmembrane receptors results in uptake inhibition. Suggested pathways of exosome trafficking and routes and dynamics of exosome-cargo internalization has been extensively reviewed in (11, 12).
Emerging evidence suggests that exosomes released from cells are exploited as natural vehicles for the delivery of membrane tethered signaling molecules and membrane enclosed nucleic acids. They have been shown to be instrumental in modulating the environment and function of targeted cells. Increased interest in the exosomal cargo studies, especially the miRNA, has uncovered their important role in modulating of genetic exchange between cells and modification of cellular function. Thus, they are investigated for the role they play in physiology and pathology of cancers, neurodegenerative and autoimmune disorders, immune responses and cardiovascular pathologies.
Recent studies emphasize the importance of exosome-based communication within the cardiovascular system (13), especially between stem and cardiac cells and the exchange between the heart and the bone marrow compartments (14–16)
Isolation
Isolation and purification of exosomes for both characterization and diagnostics as well as for therapeutic purposes has not been without controversy. Given the small size of the vesicles, which lies below the resolution threshold of light imaging and flow cytometry detection, indirect characterization, albeit not quantitative, has to be employed. They can be enriched and identified with antibodies specific to exosomal membrane markers (4). Furthermore, electron microscopy can be used to visualize and identify exosomes based on their relative size and morphology. Dynamic light scattering (DLS) method can be used as it determines the scatter of laser light passing through the sample. Other approaches, aimed at quantifying exosomes, such as protein concentration measurements are indirect approximations at best, considering that the range of sizes and cargo load can differ substantially based on a multitude of parameters. Isolation techniques for obtaining a pure exosome preparation are based on their unique size and density, distinguishing them from other circulating particles. They mostly involve sequential high velocity centrifugations and gradient separations as well as antibody capture and chemical precipitation. Additionally, affinity and size exclusion chromatography have also found their applications. (17–19)
Use of exosomes/Applications
Biomarkers
Due to their ability to circulate freely in all biological fluids of the body and provide a protective shuttle mechanism for their cargo, exosomes can be collected non-invasively and with minimal risk to patients. As their cargo has been described to differ between different physiological and pathological conditions, their composition can be assessed for identification as potential disease related biomarkers. Their unique pathological signatures have already been identified for numerous cancers and neuropathologies such as prostate (20) ovarian (21) and colorectal cancer (22, 23) and Alzheimer’s disease (24). Even though it is unlikely that a single biomarker will be identified to diagnose myocardial ischemia and or necrosis, circulating miRNA profiles seem to be altered in cardiovascular pathologies, holding promise for early detection and successful intervention (25–29).
Therapeutics
Among other exosomal cargo constituents mRNA and miRNA have been shown to effectively regulate/influence the gene expression and physiology of targeted cells. miRNAs play a pivotal role in cell proliferation, differentiation and apoptosis as well as their pathological dysregulations. Despite the progress in the efficient delivery of miRNAs as gene therapy agents, miRNAs have a very short half-life in circulation and are very susceptible to degradation. However, miRNAs contained in exosomes have a significantly extended shelf life, are protected from enzymatic degradation and can be delivered to target cells without the loss of activity. Cell based therapy for cardiovascular disorders experiences only limited success due to cell retention and engraftment issues as well as potential immunogenicity. Exosome based treatment options however, provide cell free therapeutic candidates that offer to provide comparable benefits. One of the most active areas of translational research is to identify the most effective cell type and environment for generation of most effective cardio-protective and cardio-regenerative exosomes. (15, 30, 31)
Vehicles for drug delivery
Unlike stem cell based therapy, application of engineered exosomes with defined targets and cargos promises a more efficient and direct method of gene therapy delivery. Following the natural path of exosome biogenesis and targeted transport it is feasible to assume that certain stimuli could activate cells to manufacture exosomes beneficial for a range of therapeutic applications. Thus, strides are being made to manipulate exosome producing donor cells in order to enhance their beneficial characteristics. Engineering of custom exosomes can be achieved indirectly by cell manipulations through: stress such as hypoxic preconditioning (15), genetic modifications (32) and epigenetic reprogramming (33) or by transducing donor cells with appropriate vectors to make them stably overexpress and package the intended cargo. Additionally, a direct route of cargo packaging can be used by transfecting or electroporating exosomes with the desired molecular candidates. Regardless of their natural or engineered origin, exosome-based therapeutics can be introduced through intravenous and intraperitoneal injections or through local intra-tissue injections for delivery of targeted therapy (1, 34, 35)
Exosomes in cardiovascular health and disease
Intercellular communication mediated by extracellular vesicles is crucial for preserving vascular integrity and in the development of cardiovascular and other diseases. In cancer, exosomes have been described to possess the ability to pass on malignancy from cells to cell and shape the environment by stimulating blood vessel formation, while concomitantly inhibiting immune response to the tumor. (55–57) Similarly, they have also been shown to shuttle viral particles from cell to cell. (58, 59)
In recent years, many published reports suggest that in addition to cancers and neurodegenerative disorders, major cardiovascular and metabolic pathologies like coronary artery disease, myocardial infarction, heart failure, and diabetes are highly influenced by the exosome directed transfer of molecules. It has been demonstrated that exosomally delivered mRNAs and miRNAs are translated and regulate gene expression of acceptor cells influencing their biology. They contribute to the maintenance of cardiovascular and arterial homeostasis as well as their pathologies and play a functionally significant role in processes such as immune response, tumor progression, proliferation and apoptosis (5, 36, 37, 60).
A continuously growing body of evidence implicates exosome delivered miRNA cargo as major contributors to both the maintenance of cardiovascular and arterial homeostasis as well as progression of pathologies due to their regulatory role in gene expression. Their varied cargo depends on cells of origin and the micro-environmental stimuli which act upon them. Among a long list of investigated miRNAs and their targets thus far (which have been reviewed more extensively in other publications (38–40)), some show modified expression and or activity based on the patho-physiological conditions.
During coronary artery disease, under pro-inflammatory conditions such as atherosclerosis, exosomes derived from vascular endothelial cells, smooth muscle cells, macrophages and other circulating immune cells contribute to communication of the status of inflammation (38, 41, 42) possibly directing cellular responses to a changing environment. Rautou et al 2011, describes a correlation between exosomes released from atherosclerotic plaques and stimulation of endothelial cell proliferation, increased adhesion molecule expression and inflammatory cell recruitment, leading promotion of plaque development (43). miR-126 shuttled by endothelial cell (EC) exosomes showed ability to direct accelerated re-endotheliazation (44). Thus its repressed expression in exosomes from diabetic patients and EC cells cultured under hyperglycemic conditions (44) could explain its importance to cell and tissue preservation. miR-146 was shown to contribute to reduced atherogenesis (45), whereas miR-302a inhibition lead to reduced atherosclerotic plaque size, increased plaque stability and reduced inflammation. miR-26a was shown to counteract endothelial cell apoptosis (46). A study of ECs cultured in high glucose environment, showed increased production of miR-503, which transferred to vascular pericytes resulted in their impaired migration and proliferative behavior (47). These studies demonstrate how only a few components of the exosomal cargo can have a profound impact on development and progression of a pathology.
Beyond the hostile high glucose environment, another stress stimulus is “shear stress”, which has been identified as a physiological contributor to altered exosome and exosomal cargo shedding and information exchange by ECs. Identifying them as potential therapeutic targets for modulating the progression of atherosclerosis. Their impact on atherosclerotic development involve increased endothelial inflammation, higher permeability of the endothelial barrier, cell cycle arrest and enhanced monocyte adhesion to the endothelium. (48, 49) Conversly, Essandoh K et al (2015) (54), show that blocking of exosome release can be protective against sepsis induced inflammatory response and cardiac dysfunction.
Acute myocardial infarction (MI) is characterized by a sudden occlusion of the coronary vessel and results in cardiac cell death due to ischemia. Studies investigating potential therapies revealed that exosomes released from cardiac progenitor cells (CPCs) and embryonic stem cells (ESCs) have the potential to regulate and enhance cardiac regeneration capabilities (50). Studies reported that CPCs secrete pro-regenerative exosomes during hypoxia and thus contribute to preserved cardiac output after MI as well as contribute to decreased profibrotic gene expression in activated fibroblasts. These findings indicate that hypoxia triggers a regenerative response, during which CPCs transfer antifibrotic miRNAs to fibroblasts, counteract cardiomyocyte apoptosis, enhance angiogenesis thus preserving LV ejection fraction (15, 30). Additionally, Lai et al 2010 characterized mesenchymal stem cell derived exosome treatment applied to the ischemia reperfusion injury model and reported preserved cardiac function and decreased infarct size. (51) Cardiosphere derived exosomes have also been shown to enhance the proliferative and angiogenic effects of cardiomyocytes (31)
Cardiac remodeling due to prolonged stress due to coronary artery disease, atrial fibrillation, and hypertension leads to hypertrophy of cardiomyocytes, fibrosis of the heart muscle and ultimately heart failure. The fibroblast secreted exosomes enriched in miR-21 have been shown to act as paracrine signaling mediators inducing cardiomyocyte hypertrophy. (13)
In (52) miR-590 and miR199a have been shown to reprogram adult CMs to proliferate. Exosomes have been described to promote angiogenesis, transform fibroblasts to myofibroblasts, and transform mesenchymal stem cells to myofibroblasts. In the heart exosomes have been documented to play important roles in the maintenance of cardiac homeostasis as well as in the pathogenesis of heart disease (1, 13, 40, 53). These reports identify exosome-based intercellular cargo exchange as a fundamental contributor to the preservation, and/or detriment of the heart muscle post ischemic injury. Thus, suggesting an enormous interventional potential by regulating the exchange miRNA based communication.
Challenges and Problems
Despite a large body of evidence suggesting that exosome based cell communication feature could be exploited for both diagnostic and therapeutic purposes, the mechanisms of cargo selection, cargo loading and vesicle targeting are still largely unknown. Most of all however, type of active molecules contributing to recipient cell’s alteration of physiology have to be investigated. Despite the functional impact of exosomes on cell homeostasis, proliferation, senescence, transformation etc. the open question remains of specifically which exosome-contained molecules influence their recipient cells.
miRNAs are so far the most studied cargo molecules. Considering a large number and variety of protein, lipid and nucleic acid components trafficked via exosomes, it is likely that a combination of these components could have a more potent influence on cells as they might act in concert and affect multiple a multitude of signaling pathways.
Conclusions
The capacity of exosomes in circulation to maintain stability and provide protection for a wide range of cargo including mRNAs, miRNAs and a variety of other less investigated biomolecules such as proteins, enzymes, molecular chaperones and signaling molecules (6), makes this subset of extracellular microvesicles a promising therapeutic tool for treatment of cardiovascular and other pathologies. Being biocompatible, low immunogenic and having the ability to permeate though biological barriers (61) allows for distribution of viable and effective cargo and gives hope for their potential application as vehicles for delivery of therapeutics. Continued investigations will contribute to increased understanding of spatio-temporal relations of exosomal synthesis and release and targeting and hold promise for the development of new cell-free and individualized, patient specific therapies of the future.
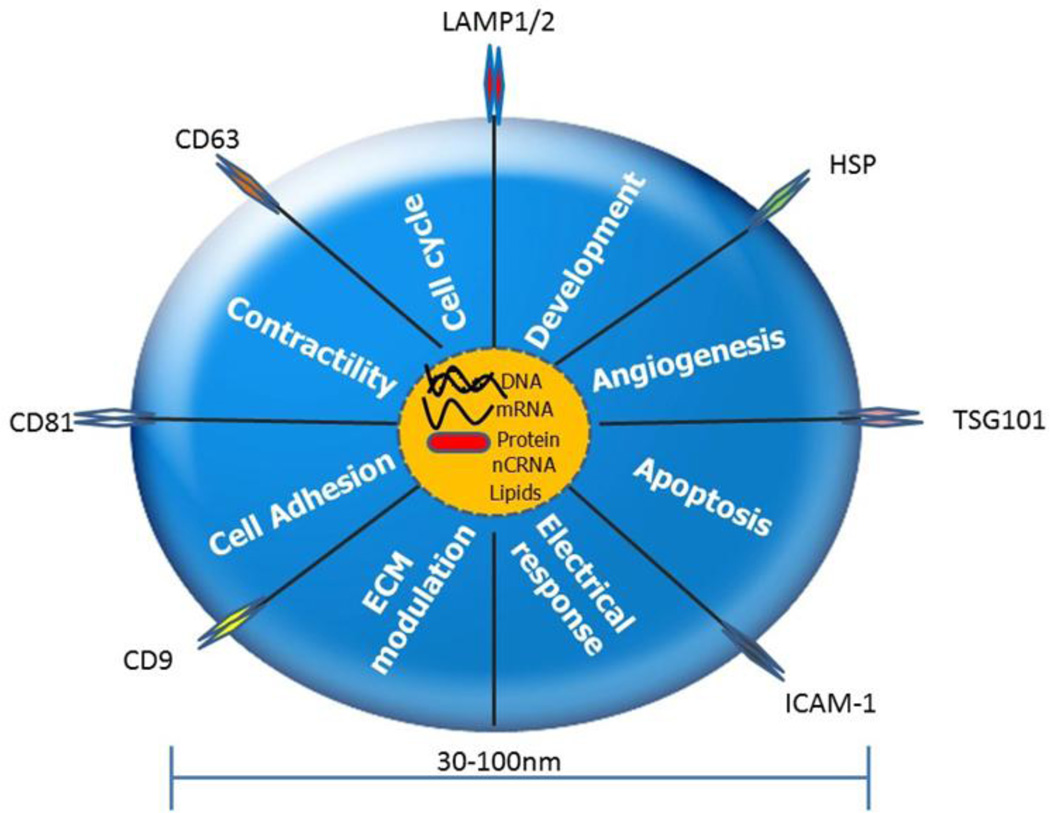
Fig. 1. Exosomes impact the physiology of the cells, tissues and organs.
Exosomes carry and deliver messenger RNAs (mRNAs), noncoding RNA (nCRNAs), proteins, lipids and participate in cellular functions viz., cell adhesion, extracellular matrix (ECM) modulation, electrical response, apoptosis, angiogenesis, development
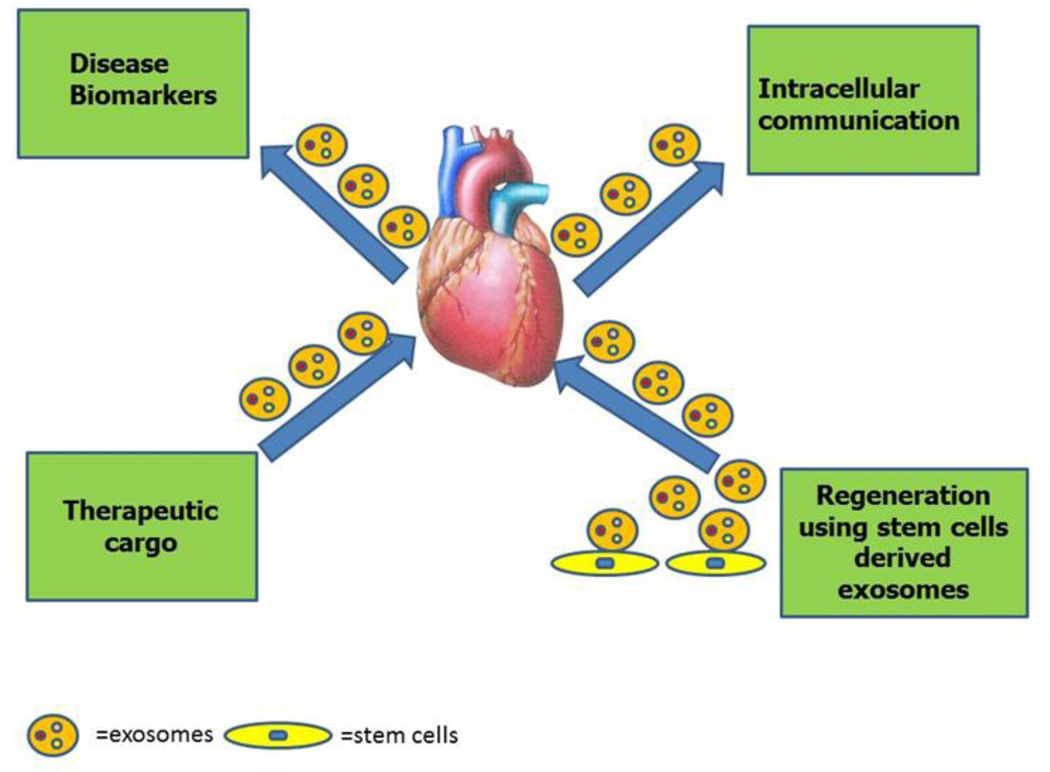
Fig. 2. Applications of exosomes in cardiovascular health and disease.
Exosomes can be isolated from patients’ bio-fluids to identify biomarkers. Exosome can be used as a cargo to carry therapeutic regimens. Exosome mediated intracellular communication between different cardiovascular cells (cardiomyocytes, endothelial cells and fibroblasts) can be studied to further understand cardiovascular disease. Stem cells derived exosomes can be used to as a therapeutic regimen for cardiovascular disease.
Table-1.
Studies reporting exosomes in cardiovascular research.
| S. No | Source of exosomes |
Exosomal content |
Outcome | Reference |
|---|---|---|---|---|
| 1 | Rat Cardiac Fibroblasts | miR-21 | Cardiomyocyte hypertrophy | (13) |
| 2 | Rat cardiac progenitor cells | miR-15b, miR-17, miR-20a, miR-103, miR-199a, miR-210, miR-292 | Improved cardiac function and reduced fibrosis | (15) |
| 3 | Human serum | P53 responsive miRNA | Predictive biomarker for heart failure | (27) |
| 4 | Human cardiac progenitor cells | miR-210, miR-132, and miR-146a-3p | Reduced apoptosis, enhanced angiogenesis and improved left ventricular ejection fraction | (30) |
| 5 | Human Cardiosphere derived cells | miR-146a | Reduced apoptosis, enhanced angiogenesis and promoted cardiomyocyte regeneration | (31) |
| 6 | Human Endothelial cells | miR-126 | Promote vascular endothelial repair | (44) |
| 7 | Mouse EC | miR-503 | miR-503 regulates pericyte-endothelial crosstalk in microvascular diabetic complications | (47) |
| 8 | Embryonic stem cells | miR-294 | Reduced apoptosis, enhanced angiogenesis and cardiac progenitor cell activation and cardiomyocyte proliferation | (50) |
| 9 | Human Mesenchymal stem cells | Protein | Preserved cardiac function and reduced infarct size post cardiac ischemia/reperfusion injury | (51) |
| 10 | Mouse model of sepsis | Not applicable | Blockade of exosome generation in sepsis dampens the sepsis-triggered inflammatory response and thereby, improves cardiac function and survival. | (54) |
Acknowledgments
Funding Source:
Work described in this manuscript was in part supported by National Institute of Health grants HL091983, HL126186, HL053354 and HL108795 to RK and by American Heart Association Postdoctoral Grant 15POST22720022 to VNSG
References
- 1.Cervio E, Barile L, Moccetti T, Vassalli G. Exosomes for Intramyocardial Intercellular Communication. Stem Cells Int. 2015;2015:482171. doi: 10.1155/2015/482171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013 Dec 15;126(Pt 24):5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 3.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 4.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012 Jul;1820(7):940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007 Jun;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 6.Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, Perez-Hernandez D, Vazquez J, Martin-Cofreces N, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoorvogel W. Resolving sorting mechanisms into exosomes. Cell Res. 2015 May;25(5):531–532. doi: 10.1038/cr.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Li S, Li L, Li M, Guo C, Yao J, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015 Feb;13(1):17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Loughlin AJ, Woffindale CA, Wood MJ. Exosomes and the emerging field of exosome-based gene therapy. Curr Gene Ther. 2012 Aug;12(4):262–274. doi: 10.2174/156652312802083594. [DOI] [PubMed] [Google Scholar]
- 10.Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J Mol Biol. 2015 Oct 3; doi: 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014 Aug 4;3 doi: 10.3402/jev.v3.24641. 10.3402/jev.v3.24641. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian T, Zhu YL, Hu FH, Wang YY, Huang NP, Xiao ZD. Dynamics of exosome internalization and trafficking. J Cell Physiol. 2013 Jul;228(7):1487–1495. doi: 10.1002/jcp.24304. [DOI] [PubMed] [Google Scholar]
- 13.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014 May;124(5):2136–2146. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 2014 Feb 18;9(2):e88685. doi: 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, et al. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res. 2015 Jan 16;116(2):255–263. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong SG, Lee WH, Huang M, Dey D, Kodo K, Sanchez-Freire V, et al. Cross talk of combined gene and cell therapy in ischemic heart disease: role of exosomal microRNA transfer. Circulation. 2014 Sep 9;130(11 Suppl 1):S60–S69. doi: 10.1161/CIRCULATIONAHA.113.007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Momen-Heravi F, Balaj L, Alian S, Mantel PY, Halleck AE, Trachtenberg AJ, et al. Current methods for the isolation of extracellular vesicles. Biol Chem. 2013 Oct;394(10):1253–1262. doi: 10.1515/hsz-2013-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lozano-Ramos I, Bancu I, Oliveira-Tercero A, Armengol MP, Menezes-Neto A, Del Portillo HA, et al. Size-exclusion chromatography-based enrichment of extracellular vesicles from urine samples. J Extracell Vesicles. 2015 May 28;4:27369. doi: 10.3402/jev.v4.27369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greening DW, Xu R, Ji H, Tauro BJ, Simpson RJ. A protocol for exosome isolation and characterization: evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol Biol. 2015;1295:179–209. doi: 10.1007/978-1-4939-2550-6_15. [DOI] [PubMed] [Google Scholar]
- 20.Overbye A, Skotland T, Koehler CJ, Thiede B, Seierstad T, Berge V, et al. Identification of prostate cancer biomarkers in urinary exosomes. Oncotarget. 2015 Oct 6;6(30):30357–30376. doi: 10.18632/oncotarget.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang MK, Wong AS. Exosomes: Emerging biomarkers and targets for ovarian cancer. Cancer Lett. 2015 Oct 10;367(1):26–33. doi: 10.1016/j.canlet.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Matsumura T, Sugimachi K, Iinuma H, Takahashi Y, Kurashige J, Sawada G, et al. Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. Br J Cancer. 2015 Jul 14;113(2):275–281. doi: 10.1038/bjc.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogata-Kawata H, Izumiya M, Kurioka D, Honma Y, Yamada Y, Furuta K, et al. Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS One. 2014 Apr 4;9(4):e92921. doi: 10.1371/journal.pone.0092921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem. 2012 Feb 3;287(6):3842–3849. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sluijter JP, van Rooij E. Exosomal microRNA clusters are important for the therapeutic effect of cardiac progenitor cells. Circ Res. 2015 Jan 16;116(2):219–221. doi: 10.1161/CIRCRESAHA.114.305673. [DOI] [PubMed] [Google Scholar]
- 26.Khalyfa A, Gozal D. Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. J Transl Med. 2014 Jun 10;12 doi: 10.1186/1479-5876-12-162. 162,5876-12-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumoto S, Sakata Y, Suna S, Nakatani D, Usami M, Hara M, et al. Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ Res. 2013 Jul 19;113(3):322–326. doi: 10.1161/CIRCRESAHA.113.301209. [DOI] [PubMed] [Google Scholar]
- 28.Wang R, Li N, Zhang Y, Ran Y, Pu J. Circulating microRNAs are promising novel biomarkers of acute myocardial infarction. Intern Med. 2011;50(17):1789–1795. doi: 10.2169/internalmedicine.50.5129. [DOI] [PubMed] [Google Scholar]
- 29.Recchioni R, Marcheselli F, Olivieri F, Ricci S, Procopio AD, Antonicelli R. Conventional and novel diagnostic biomarkers of acute myocardial infarction: a promising role for circulating microRNAs. Biomarkers. 2013 Nov;18(7):547–558. doi: 10.3109/1354750X.2013.833294. [DOI] [PubMed] [Google Scholar]
- 30.Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. 2014 Sep 1;103(4):530–541. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 31.Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014 May 8;2(5):606–619. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romagnoli GG, Zelante BB, Toniolo PA, Migliori IK, Barbuto JA. Dendritic Cell-Derived Exosomes may be a Tool for Cancer Immunotherapy by Converting Tumor Cells into Immunogenic Targets. Front Immunol. 2015 Jan 19;5:692. doi: 10.3389/fimmu.2014.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ailawadi S, Wang X, Gu H, Fan GC. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim Biophys Acta. 2015 Jan;1852(1):1–11. doi: 10.1016/j.bbadis.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014 Feb;35(7):2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 35.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011 Apr;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 36.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012 Jan 19;119(3):756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittelbrunn M, Sanchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012 Apr 18;13(5):328–335. doi: 10.1038/nrm3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfeifer P, Werner N, Jansen F. Role and Function of MicroRNAs in Extracellular Vesicles in Cardiovascular Biology. Biomed Res Int. 2015;2015:161393. doi: 10.1155/2015/161393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahoo S, Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ Res. 2014 Jan 17;114(2):333–344. doi: 10.1161/CIRCRESAHA.114.300639. [DOI] [PubMed] [Google Scholar]
- 40.Emanueli C, Shearn AI, Angelini GD, Sahoo S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vascul Pharmacol. 2015 Aug;71:24–30. doi: 10.1016/j.vph.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diehl P, Fricke A, Sander L, Stamm J, Bassler N, Htun N, et al. Microparticles: major transport vehicles for distinct microRNAs in circulation. Cardiovasc Res. 2012 Mar 15;93(4):633–644. doi: 10.1093/cvr/cvs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chistiakov DA, Orekhov AN, Bobryshev YV. Extracellular vesicles and atherosclerotic disease. Cell Mol Life Sci. 2015 Jul;72(14):2697–2708. doi: 10.1007/s00018-015-1906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rautou PE, Leroyer AS, Ramkhelawon B, Devue C, Duflaut D, Vion AC, et al. Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ Res. 2011 Feb 4;108(3):335–343. doi: 10.1161/CIRCRESAHA.110.237420. [DOI] [PubMed] [Google Scholar]
- 44.Jansen F, Yang X, Hoelscher M, Cattelan A, Schmitz T, Proebsting S, et al. Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation. 2013 Oct 29;128(18):2026–2038. doi: 10.1161/CIRCULATIONAHA.113.001720. [DOI] [PubMed] [Google Scholar]
- 45.Fish JE, Cybulsky MI. ApoE attenuates atherosclerosis via miR-146a. Circ Res. 2015 Jun 19;117(1):3–6. doi: 10.1161/CIRCRESAHA.115.306733. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Qin W, Zhang L, Wu X, Du N, Hu Y, et al. MicroRNA-26a prevents endothelial cell apoptosis by directly targeting TRPC6 in the setting of atherosclerosis. Sci Rep. 2015 Mar 24;5:9401. doi: 10.1038/srep09401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caporali A, Meloni M, Nailor A, Mitic T, Shantikumar S, Riu F, et al. p75(NTR)-dependent activation of NF-kappaB regulates microRNA-503 transcription and pericyte-endothelial crosstalk in diabetes after limb ischaemia. Nat Commun. 2015 Aug 13;6:8024. doi: 10.1038/ncomms9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vion AC, Ramkhelawon B, Loyer X, Chironi G, Devue C, Loirand G, et al. Shear stress regulates endothelial microparticle release. Circ Res. 2013 May 10;112(10):1323–1333. doi: 10.1161/CIRCRESAHA.112.300818. [DOI] [PubMed] [Google Scholar]
- 49.Son DJ, Kumar S, Takabe W, Kim CW, Ni CW, Alberts-Grill N, et al. The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nat Commun. 2013;4:3000. doi: 10.1038/ncomms4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015 Jun 19;117(1):52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010 May;4(3):214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 52.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012 Dec 20;492(7429):376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 53.Das S, Halushka MK. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc Pathol. 2015 Jul-Aug;24(4):199–206. doi: 10.1016/j.carpath.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Essandoh K, Yang L, Wang X, Huang W, Qin D, Hao J, et al. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim Biophys Acta. 2015 Nov;1852(11):2362–2371. doi: 10.1016/j.bbadis.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clayton A. Cancer cells use exosomes as tools to manipulate immunity and the microenvironment. Oncoimmunology. 2012 Jan 1;1(1):78–80. doi: 10.4161/onci.1.1.17826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Epple LM, Griffiths SG, Dechkovskaia AM, Dusto NL, White J, Ouellette RJ, et al. Medulloblastoma exosome proteomics yield functional roles for extracellular vesicles. PLoS One. 2012;7(7):e42064. doi: 10.1371/journal.pone.0042064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol. 2011 Sep;33(5):441–454. doi: 10.1007/s00281-010-0234-8. [DOI] [PubMed] [Google Scholar]
- 58.Marleau AM, Chen CS, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. 2012 Jun 27;10 doi: 10.1186/1479-5876-10-134. 134,5876-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chahar HS, Bao X, Casola A. Exosomes and Their Role in the Life Cycle and Pathogenesis of RNA Viruses. Viruses. 2015 Jun 19;7(6):3204–3225. doi: 10.3390/v7062770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ibrahim A, Marban E. Exosomes: Fundamental Biology and Roles in Cardiovascular Physiology. Annu Rev Physiol. 2015 Nov 30; doi: 10.1146/annurev-physiol-021115-104929. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boukouris S, Mathivanan S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin Appl. 2015 Apr;9(3–4):358–367. doi: 10.1002/prca.201400114. [DOI] [PMC free article] [PubMed] [Google Scholar]