Abstract
Background & Aims
Understanding HCV transmission among people who inject drugs (PWID) is important for designing prevention strategies. This study investigated whether HCV infection among younger injectors occurs from few or many transmission events from older injectors to younger injectors among PWID in Vancouver, Canada.
Methods
HCV antibody-positive participants at enrolment or follow-up (1996-2012) were tested for HCV RNA and sequenced (Core-E2). Time-stamped phylogenetic trees were inferred using Bayesian Evolutionary Analysis Sampling Trees (BEAST). Association of age with phylogeny was tested using statistics implemented in the software Bayesian Tip Significance (BaTS) testing. Factors associated with clustering (maximum cluster age: five years) were identified using logistic regression.
Results
Among 699 participants with HCV subtype 1a, 1b, 2b and 3a infection (26% female, 24% HIV+): 21% were younger (<27 years), and 10% had recent HCV seroconversion. When inferred cluster age was limited to <5 years, 15% (n=108) were in clusters/pairs. Although a moderate degree of segregation was observed between younger and older participants, there was also transmission between age groups. Younger age (<27 vs. >40, AOR: 3.14, 95% CI:1.54, 6.39),HIV (AOR: 1.97, 95%-CI: 1.22, 3.18) and subtype 3a (AOR: 2.12, 95% CI: 1.33, 3.38) were independently associated with clustering.
Conclusions
In this population of PWID from Vancouver, HCV among young injectors was seeded from many transmission events between HCV-infected older and younger injectors. Phylogenetic clustering was associated with younger age and HIV. These data suggest that HCV transmission among PWID is complex, with transmission occurring between and among older and younger PWID.
Keywords: injection drug use, HCV, phylogenetics, transmission, age, molecular epidemiology, HIV
INTRODUCTION
Globally, the prevalence of hepatitis C virus (HCV) infection among people who inject drugs (PWID) is 50-80% [1]. HCV incidence remains high, particularly among young PWID [2-5]. A better understanding of HCV transmission among PWID is important for the design of HCV prevention strategies, including treatment as prevention. Traditional epidemiological approaches for evaluating factors associated with HCV infection are limited in that they measure factors associated with acquisition of infection, but may not reflect factors associated with transmission of infection.
Viral phylogenies provide an opportunity to infer ancestral relationships between observed sequence data [6]. A phylogeny is represented by a tree structure containing tips (observed data), nodes (inferred ancestors) and branches (connections between tips and nodes)[6]. Phylogenetic methods approximate the full transmission chain of an outbreak based on the available data, with additional information such as clinical or behavioural data informing the dynamics of infection and outcomes in the population (Reviewed in [7]). Bayesian Markov Chain Monte Carlo (MCMC) inference accounts for uncertainty during the process of phylogenetic reconstruction by determining the probability of the tree given the data and modifiable model parameters.
Phylogenetic studies provide an opportunity to investigate underlying transmission patterns that cannot be determined through epidemiological studies (e.g. HIV) [8, 9]. In one cohort of PWID in Vancouver, Canada, younger age, HIV co-infection, HCV seroconversion and recent syringe borrowing were independently associated with HCV phylogenetic clustering [10]. In another cohort of young PWID in Vancouver, methamphetamine injecting was shown to be associated with HCV clustering [11].
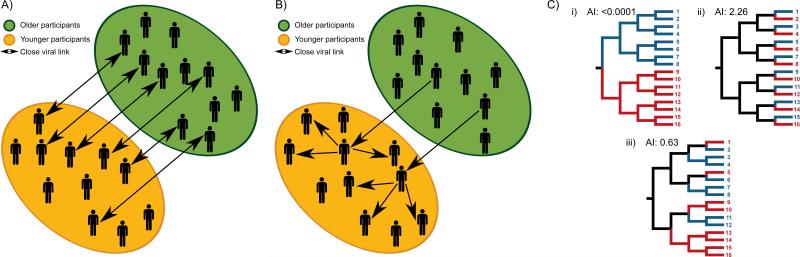
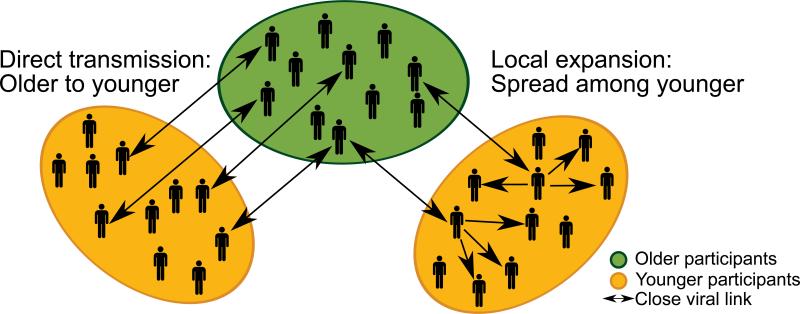
The primary aim of this study was to investigate whether HCV infection in young injectors are seeded from many transmission events between HCV-infected older injectors and younger injectors (Fig. 1A), or whether HCV infection in young injectors is seeded from few transmission events from HCV-infected older injectors (Fig. 1B) in two prospective cohorts of PWID in Vancouver, Canada. Second, this study aimed to assess factors associated with phylogenetic clustering.
Fig 1. Potential modes of HCV transmission among people who inject drugs.
This study sought to evaluate whether: (A) HCV infection in young injectors is seeded by many transmission events between HCV-infected older injectors and younger injectors, or (B) HCV infection in young injectors is seeded by few transmission events from HCV-infected olderinjectors with further transmission among younger injectors. (C) Evaluation of phylogeny-trait correlation by Association Index (AI) illustrates the distribution of participant characteristics on the inferred phylogeny. The correlation between the phylogeny and traits may be high (i), low (ii) or moderate (iii). Permission obtained from Elsevier B.V. © Parker, J. et al. (2008) Correlating viral phenotypes with phylogeny: Accounting for phylogenetic uncertainty Infection, Genetics and Evolution doi: 10.1016/j.meegid.2007.08.001
MATERIALS AND METHODS
Study population and design
The At Risk Youth Study (ARYS) and the Vancouver Injection Drug Users Study (VIDUS) are open prospective community-recruited cohorts of PWID in Vancouver, Canada. Detailed sampling and recruitment procedures for these cohorts have been described elsewhere [12-14]. Beginning in May 1996, active PWID (i.e., those who reported injecting drugs in the previous month) were recruited into VIDUS [13]. Beginning in September 2005, street-involved youth (aged 14 and 26 years with use of illicit drugs in the past 30 days) were recruited into ARYS [14]. Participants must have resided in the Greater Vancouver region and provided written informed consent.
For the current study, all participants who: 1) were HCV antibody-positive at enrolment; or 2) demonstrated recent HCV seroconversion (defined by an HCV antibody negative test at enrolment followed by an HCV antibody positive test at a subsequent study visit) between May 1996 and December 2012 and with an available sample for HCV RNA testing and sequencing were eligible for inclusion. The University of British Columbia/Providence Health Care Research Ethics Board approved this study.
Study assessments
At enrolment and semi-annually, participants completed an interviewer-administered questionnaire. Data on socio-demographic characteristics, as well as information pertaining to drug use patterns and risk behaviours were collected. Among anti-HCV positive participants, questionnaire data from the first anti-HCV antibody positive visit was utilized (i.e. at baseline for those with HCV infection at baseline, and at the first follow-up visit following infection among those with recent HCV seroconversion). Nurses collected blood samples for HIV and HCV serology, and also provided basic medical care and referrals to appropriate health care services. Participants received $30 CAD for each study visit.
HCV RNA testing and sequencing
As previously described, HCV RNA was quantified using an in-house PCR (limit of detection: 200 IU/ml), and amplification and sequencing of a 1,514bp fragment of the HCV genome covering Core, Envelope-1, hypervariable region-1 and beginning of Envelope-2 (E2) was attempted on all samples with detectable HCV RNA [15]. Purified amplicons were sequenced using the Sanger method and sequence chromatograms processed using RECall [16]. HCV subtype for each sequence obtained was determined using the COMET online tool [17].
Phylogenetic analysis
Bayesian inference
Among participants with HCV subtypes 1a, 2b, and 3a, phylogenetic trees of the Core-E2 fragment were estimated using the Bayesian Markov Chain Monte Carlo (MCMC) approach implemented in BEAST v1.8.1, as previously described [18]. The substitution rates of the Core-E2 region were estimated using a genomic partition model from an independent set of HCV isolates that exhibit strong temporal signal [19], with the rate estimate subsequently applied as a strong prior distribution on the molecular clock rate. The nucleotide substitution, molecular clock and coalescent priors are further described in Supplementary Information. Bayesian MCMC convergence and effective sampling sizes were assessed using Tracer v1.6, and a maximum clade credibility (MCC) inferred from the posterior set of trees in TreeAnnotator v1.8.1 [20], both with a 10% burn-in of measured states.
Associating traits with inferred tree topology
Participant demographic and clinical characteristics were compared with the inferred phylogenies to identify traits that might correlate with the phylogenetic tree topology (Fig. 1C). By assigning particular characteristics to each participant and subsequently reconstructing the states of ancestral nodes in the phylogeny, we can determine whether a trait is more likely to be shared by participants whose viruses are closely related than would be expected by chance alone (i.e. when compared to randomly distributed characteristics on the same phylogeny) [21]. To account for phylogenetic uncertainty, the posterior set of trees was randomly subsampled to provide 1000 representative trees and 100 state randomisations were performed to provide a statistical significance test of the null distribution [21]. For the Association Index statistic, an observed-to-expected ratio provides a measure of the degree of association, with a value of 0 being complete association and values above ≥1 showing no association.
Phylogenetic clustering
Pairs (n=2) or clusters(n≥3) were identified if the most common recent ancestor of the participants (inferred) was less than 5 years in the past, using a custom-made JAVA script (ClusterByTime; Dr Jayna Raghwani, http://evolve.zoo.ox.ac.uk/Evolve/ClusterByTime.html). Since the script analyses a posterior set of trees, and not a single MCC tree, phylogenetic uncertainty is explicitly considered when identifying clusters by age. Results were compared to a 1.5% nucleotide genetic distance (excluding hypervariable region-1) threshold using Cluster Picker [22], and sensitivity analysis performed for both tMRCA and genetic distance limits. Further details are in Supplementary Information.
Statistical analyses
Descriptive analyses were performed to characterise the study sample according to the following strata: being in a pair or cluster (n≥2 participants), or neither. Participant characteristics in these categories were compared using Chi-squared, Fisher's exact and Kruskal-Wallis tests (as appropriate).
Unadjusted logistic regression analyses were used to identify factors associated with pair/cluster membership. Hypothesized factors were determined a priori on the basis of factors previously shown to be associated with HCV clustering and/or HCV acquisition. These factors included: sex [2]; younger age [23]; duration of injecting [24], cohort, recent HCV seroconversion [25]; and HIV status (positive vs. negative) [5]. In multivariate logistic regression analyses, two fully adjusted models containing all variables were assessed, with either age or duration of injecting included (given the potential for collinearity between these variables).
Statistically significant differences were assessed at p<0.05; all p-values are two-sided. All analyses were performed using STATA software (version 12.1; StataCorp L.P., College Station, Texas, USA).
RESULTS
Participant characteristics
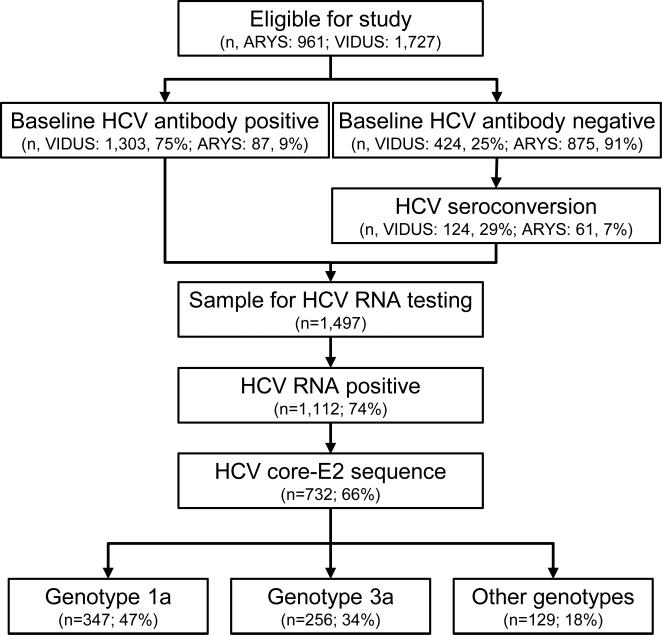
In total, 2,688 participants from the ARYS (n=961) and VIDUS (n=1,727) cohorts were eligible for inclusion (Fig. 2). At enrolment, 52% (1,390 of 2,688) were HCV antibody positive. Among participants who were HCV antibody negative at enrolment (n=1299), 185 participants demonstrated recent HCV seroconversion during follow-up, and were therefore eligible for inclusion.
Fig. 2.
Participant disposition flow-chart.
Among 1,497 HCV antibody positive participants with available samples for HCV RNA testing, 74% (1,112 of 1,497) had detectable HCV RNA. PCR amplification and Sanger sequencing of the Core-E2 segment was obtainable for 76% (845 of 1,112) and 66% (732 of 1,112) of participants with detectable HCV RNA, respectively. Low HCV RNA level (<10,000) and inadequate sample volume have previously been associated with an inability to obtain a Core-E2 segment among participants with detectable HCV RNA in this study [10].
Participant characteristics among those with available HCV sequencing (n=732) are shown in Table 1. Recent HCV seroconversion was observed in 10% (n=76) and HIV coinfection in 23% (n=166) of participants. HCV genotype distribution was: 1a: 48% (n=347), 1b: 6% (n=44), 2a: 3% (n=20), 2b: 7% (n=52), 3a: 35% (n=256), 4a: <1% (n=4), 6a: 1% (n=8), 6e: <1% (n=1).
Table 1.
Characteristics of participants in the VIDUS and ARYS cohorts with HCV Core-E2 genotype result, stratified by age at enrolment/HCV seroconversion.
| Characteristics | Total n=699 | Age <27 n=150 | Age ≥27 n=549 | p-value |
|---|---|---|---|---|
| Female sex (vs. male sex) | 179 (26%) | 54 (36%) | 125 (23%) | 0.001 |
| High school education or higher (vs. less than high school) | 165 (24%) | 62 (41%) | 103 (19%) | <0.001 |
| Unstable housing (vs. stable)† | 494 (71%) | 110 (73%) | 384 (70%) | 0.419 |
| Years injecting (median (Q1, Q3)) | 13 (6, 23) | 5 (3, 8) | 16 (8, 24) | |
| Duration of injecting (years) | <0.001 | |||
| <3 | 78 (11%) | 37 (25%) | 41 (7%) | |
| 3-10 | 213 (30%) | 88 (59%) | 125 (23%) | |
| 10-20 | 187 (27%) | 19 (13%) | 168 (31%) | |
| ≥20 | 221 (32%) | 6 (4%) | 215 (39%) | |
| Currently enrolled in methadone treatment | 89 (13%) | 15 (10%) | 74 (14%) | 0.262 |
| Downtown East Side residence † | 394 (56%) | 52 (35%) | 342 (62%) | <0.001 |
| Jail† | 100 (14%) | 32 (21%) | 68 (12%) | 0.006 |
| Syringe borrowing† | 271 (39%) | 51 (34%) | 220 (40%) | 0.176 |
| Crack use† | 195 (28%) | 66 (44%) | 129 (24%) | <0.001 |
| Cocaine injecting† | 556 (80%) | 90 (60%) | 466 (85%) | <0.001 |
| Heroin injecting† | 490 (70%) | 106 (71%) | 384 (70%) | 0.864 |
| Speedball injecting† | 264 (38%) | 43 (29%) | 221 (40%) | 0.009 |
| Methamphetamine injecting† | 51 (7%) | 39 (26%) | 12 (2%) | <0.001 |
| ARYS cohort | 65 (9%) | 61 (41%) | 4 (<1%) | <0.001 |
| Recent HCV seroconversion | 73 (10%) | 39 (26%) | 34 (6%) | <0.001 |
| HIV infection† | 165 (24%) | 22 (15%) | 143 (26%) | 0.004 |
| HCV subtype | 0.223 | |||
| 1a | 347 (50%) | 73 (49%) | 274 (50%) | |
| 1b | 44 (6%) | 6 (4%) | 38 (7%) | |
| 2b | 52 (7%) | 8 (5%) | 44 (8%) | |
| 3a | 256 (37%) | 63 (42%) | 193 (35%) |
In last six months. Missing data: ^ 1 participant, * 2 participants, # 6 participants.
Phylogenetic clustering
Phylogenetic analysis was performed on a total of 699 Core-E2 sequences from participants with 1a, 1b, 2b and 3a infection, comprising the majority (95%) of the cohort. Among those analysed, 22% (n=150) were younger (aged <27 years), while 10% (n=73) had recent HCV seroconversion.
A total of 108 (15%) participants were related, with 87 in pairs (2 participants, n=44 pairs, 12%) and 21 in clusters (≥3 participants, n=6 clusters, 3%) if the most common recent ancestor of the participants (inferred) was less than 5 years in the past (Table 2). Pairs/clusters ranged from two to six participants (median: 2). Sensitivity analysis varying the time to most common recent ancestor and genetic distance thresholds demonstrated high similarity, with agreement of 93.7-95.4% and kappa scores of 0.74-0.83 (Supplementary Information).
Table 2.
Phylogenetic clustering by age among PWID in Vancouver, Canada.
| 5 year tMRCA threshold (n=699) | |
|---|---|
| Number related | 108 (15%) |
| Median cluster size (range) | 2 (2-6) |
| Pairs (n=87*) | |
| Older only | 58/87 (67%) |
| Younger with older | 16/87 (18%) |
| Younger only | 14/87 (16%) |
| Clusters (n=21) | |
| Older only | 9/21 (43%) |
| Younger with older | 12/21 (57%) |
| Younger only | 0/21 (0%) |
| Among younger (n=28*) | |
| One younger with older | 8/28 (29%) |
| Two younger with older | 7/28 (25%) |
| Younger only | 14/28 (50%) |
One participant, aged <27 years, with HCV infection at baseline, was identified in two separate pairs.
Phylogenetic clustering by age, recent HCV seroconversion and HIV co-infection
Among the identified pairs, the majority of participants were older participants (aged >27 years) paired with other older participants (58/87, 67%). Meanwhile 18% of participants in pairs (16/87) were in pairs contained a younger and an old participant, and 16% of participants in pairs (14/87) were in pairs containing only younger participants. One younger participant was identified in two separate pairs: once with another younger participant and once with an older participant. Fifty-seven percent (12 of 21) of the participants in clusters were in a cluster containing a mix of younger and older participants, with the remaining (9 of 21, 43%) being in clusters containing only older participants. There were no clusters containing only younger PWID in clusters defined by tMRCA. Similar outcomes were identified when clusters membership was defined as maximum genetic distance <1.5% (Supplementary Table 3).
Among the 28 younger PWID in transmission pairs/clusters, half (14 of 28, 50%) were in pairs containing only younger PWID, with 15 in pairs/clusters also containing older PWID. One younger participant was identified in two separate pairs, with another younger participant and with an older participant.
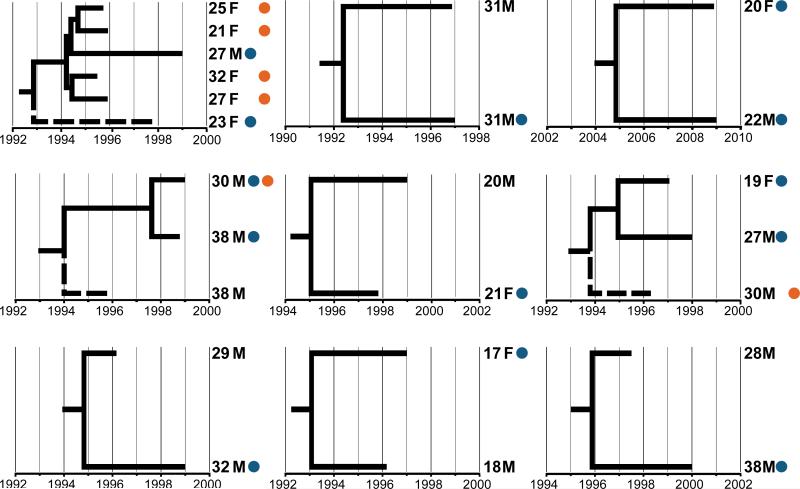
Representative time scaled phylogenies of pairs/clusters containing at least one participant with recent HCV seroconversion with time to most common recent ancestor less than five years (Fig. 3) show intermixing of age, gender, recent HCV seroconversion, and HIV coinfection among participants in the clusters.
Fig. 3. Representative time scaled phylogenies of subclusters containing at least one participant with recent HCV seroconversion with time to most common recent ancestor less than five years.
Dashed lines represent low frequency cluster members, while tips are annotated with participant age, gender, recent HCV seroconversion status (blue circle) and HIV status (orange circle).
Similar information on phylogenetic clustering for people with recent HCV seroconversion and HIV co-infection is shown in Supplementary Table 2. The minority of participants in pairs had recent HCV seroconversion (15%), while almost half (45%) were co-infected with HIV.
Assessing traits associated with inferred phylogeny
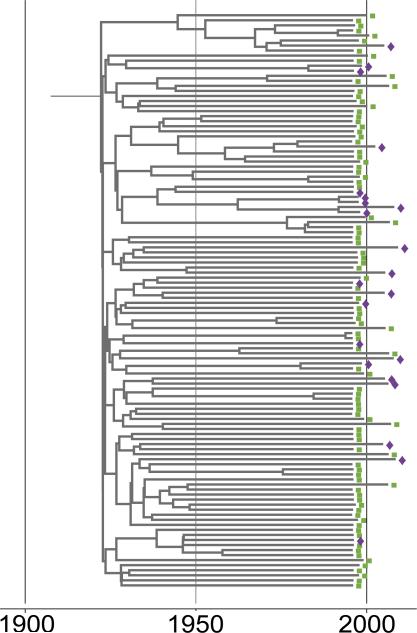
As shown in Fig. 4, the distribution of younger age across the phylogeny appeared disperse, without large-scale local expansions among younger participants or those with recent HCV infection.
Fig. 4. Subsection of an inferred Bayesian common ancestor tree of VIDUS and ARYS participants with HCV subtype 1a infection.
Tips are annotated with younger (<27 years, purple diamond) and older (>27 years, green square) age.
Given this, the distribution of participant characteristics on the tips of the inferred phylogeny was compared to that expected by chance (i.e. when compared to randomly distributed characteristics on the same phylogeny). For participants with subtype 1a, 2b and 3a (Table 3) infection, the null hypothesis of no association between patient characteristic and phylogenetic structure was rejected for younger (vs. older) participants (using the Association Index statistic), suggesting that viruses from younger participants were more likely to be closely related to viruses from other younger participants than to older participants, and vice versa (Fig. 1C). Similarly, viruses from participants in the ARYS study were more likely to be closely related to viruses from other ARYS participants than viruses from VIDUS participants, and conversely. For participants with subtype 1a infection, HIV co-infection was significant by Association Index (Table 3).
Table 3.
Phylogeny-trait association test of participant characteristic distribution among people who inject drugs in Vancouver, Canada using BaTS
| Characteristic | Number | Association Index |
|||
|---|---|---|---|---|---|
| Observed (95% CI) | Expected (95% CI) | Ratio | p-value | ||
| Subtype 1a (n=347) | |||||
| Age <27 years (vs. >27 years) | 73 (21%) | 11.0 (9.9, 12.1) | 13.3 (11.7, 14.9) | 0.83 | 0.010 |
| Female (vs. Male) | 82 (24%) | 15.2 (14.1, 16.3) | 14.5 (13.3, 15.9) | 1.05 | 0.750 |
| Inject <3 years (vs. ≥3 years) | 36 (10%) | 7.7 (6.8, 8.6) | 7.4 (6.4, 8.2) | 1.04 | 0.680 |
| ARYS Cohort (vs. VIDUS) | 27 (8%) | 5.1 (4.4, 5.8) | 5.8 (4.9, 6.7) | 0.88 | 0.110 |
| Recent HCV seroconversion (vs. HCV infection at baseline) | 36 (10%) | 6.7 (5.8, 7.6) | 7.3 (6.3, 8.4) | 0.92 | 0.170 |
| HIV co-infection (vs. not) | 92 (27%) | 12.7 (11.5, 13.9) | 15.7 (14.3, 17.2) | 0.81 | 0.010 |
| Subtype 1b (n=44) | |||||
| Age <27 years (vs. >27 years) | 6 (14%) | 1.1 (0.9, 1.4) | 1.1 (0.6, 1.5) | 1.03 | 0.55 |
| Female (vs. Male) | 11 (25%) | 1.5 (1.1, 1.9) | 1.8 (1.2, 2.4) | 0.8 | 0.14 |
| Inject <3 years (vs. ≥3 years) | 6 (14%) | 1.1 (0.9, 1.3) | 1.1 (0.6, 1.5) | 0.95 | 0.43 |
| ARYS Cohort (vs. VIDUS) | 2 (5%) | 0.4 (0.4, 0.5) | 0.4 (0.1, 0.7) | 0.97 | 0.37 |
| Recent HCV seroconversion (vs. HCV infection at baseline) | 2 (5%) | 0.2 (0.1, 0.4) | 0.4 (0.1, 0.7) | 0.49 | 0.08 |
| HIV co-infection (vs. not) | 12 (27%) | 1.6 (1.3, 1.8) | 1.9 (1.2, 2.6) | 0.83 | 0.27 |
| Subtype 2b (n=52) | |||||
| Age <27 years (vs. >27 years) | 8 (15%) | 0.5 (0.4, 0.8) | 1.5 (1.0, 2.0) | 0.36 | <0.001 |
| Female (vs. Male) | 10 (19%) | 2.1 (1.7, 2.4) | 1.8 (1.1, 2.3) | 1.19 | 0.770 |
| Inject <3 years (vs. ≥3 years) | 7 (13%) | 1.5 (1.2, 1.7) | 1.4 (1.0, 1.7) | 1.11 | 0.680 |
| ARYS Cohort (vs. VIDUS) | 3 (6%) | 0.1 (0.1, 0.1) | 0.6 (0.3, 0.9) | 0.09 | <0.001 |
| Recent HCV seroconversion (vs. HCV infection at baseline) | 6 (12%) | 0.8 (0.7, 1.0) | 1.2 (0.7, 1.6) | 0.70 | 0.080 |
| HIV co-infection (vs. not) | 13 (25%) | 2.7 (2.3, 3.2) | 2.1 (1.5, 2.8) | 1.28 | 0.930 |
| Subtype 3a (n=256) | |||||
| Age <27 years (vs. >27 years) | 73 (29%) | 9.2 (8.2, 10.2) | 12.0 (10.6, 13.7) | 0.77 | <0.001 |
| Female (vs. Male) | 76 (30%) | 12.2 (11.1, 13.3) | 12.1 (10.6, 13.8) | 1.00 | 0.570 |
| Inject <3 years (vs. ≥3 years) | 34 (13%) | 7.3 (6.7, 8.1) | 6.7 (5.8, 7.8) | 1.09 | 0.810 |
| ARYS Cohort (vs. VIDUS) | 33 (13%) | 5.1 (4.4, 5.8) | 6.5 (5.8, 7.3) | 0.79 | <0.001 |
| Recent HCV seroconversion (vs. HCV infection at baseline) | 29 (11%) | 5.5 (4.9, 6.1) | 5.9 (5.0, 6.8) | 0.94 | 0.260 |
| HIV co-infection (vs. not) | 48 (19%) | 8.3 (7.4, 9.2) | 8.9 (7.7, 10.1) | 0.94 | 0.210 |
BaTS: Bayesian Tip-association Significance test; CI: Confidence interval; ARYS: At-Risk Youth Study; VIDUS: Vancouver Injection Drug User Study
The Association Index ratio was modest for all characteristics found to be significant, with values ranging 0.36 – 0.88 (Table 3). Concordance between Association Index and an alternative measure of association, Parsimony Score, was high across the subtypes and characteristics (Supplementary Table 3).
Factors associated with phylogenetic clustering
In analyses adjusting for age (Table 4), factors independently associated with pair/cluster membership included younger age, HIV co-infection, and subtype 3a infection. In analyses adjusting for duration of injecting (Supplementary Table 4b), factors independently associated with pair/cluster membership included shorter duration of injecting, HIV co-infection, and subtype 3a infection. The results were similar when cluster membership was defined by a maximum genetic distance threshold of 1.5% (Supplementary Table 4a-f).
Table 4.
Factors associated with phylogenetic pair/cluster membership among people who inject drugs in Vancouver, Canada.
| Characteristic | No cluster (n=591) | Cluster (n=108) | p-value | Unadjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|---|---|---|
| Age quartiles | 0.005 | ||||||
| <27 | 123 (21%) | 27 (25%) | 2.20 (1.17, 4.12) | 0.014 | 3.14 (1.54, 6.39) | 0.002 | |
| 27 – 34 | 155 (26%) | 42 (39%) | 2.71 (1.51, 4.85) | 0.001 | 2.70 (1.48, 4.91) | 0.001 | |
| 35 – 39 | 123 (21%) | 20 (19%) | 1.63 (0.83, 3.17) | 0.154 | 1.74 (0.88, 3.44) | 0.108 | |
| >40 | 190 (32%) | 19 (18%) | 1.00 | - | 1.00 | - | |
| Female (vs. Male) | 149 (25%) | 30 (28%) | 0.574 | 1.14 (0.72, 1.81) | 0.574 | 0.87 (0.53, 1.41) | 0.564 |
| Duration injecting (years) | <0.001 | ||||||
| <3 | 55 (9%) | 23 (21%) | 4.45 (2.26, 8.75) | <0.001 | |||
| 3-10 | 172 (29%) | 41 (38%) | 2.53 (1.42, 4.53) | 0.002 | |||
| 10-20 | 162 (27%) | 25 (23%) | 1.64 (0.87, 3.08) | 0.124 | |||
| >20 | 202 (34%) | 19 (18%) | 1.00 | - | |||
| ARYS Cohort (vs. VIDUS) | 58 (10%) | 7 (6%) | 0.273 | 0.64 (0.28, 1.44) | 0.277 | 0.39 (0.15, 1.00) | 0.049 |
| Recent HCV seroconversion (vs. HCV infection at baseline) | 60 (10%) | 13 (12%) | 0.556 | 1.21 (0.64, 2.29) | 0.556 | 1.45 (0.72, 2.93) | 0.302 |
| HIV co-infection (vs. not) | 128 (22%) | 37 (34%) | 0.005 | 1.89 (1.21, 2.94) | 0.005 | 1.97 (1.22, 3.18) | 0.005 |
| HCV subtype | 0.039 | ||||||
| 1a | 307 (52%) | 40 (37%) | 1.00 | - | 1.00 | - | |
| 1b | 36 (6%) | 8 (7%) | 1.71 (0.74, 3.93) | 0.21 | 2.13 (0.90, 5.03) | 0.086 | |
| 2b | 43 (7%) | 9 (8%) | 1.61 (0.73, 3.54) | 0.24 | 1.67 (0.75, 3.76) | 0.212 | |
| 3a | 205 (35%) | 51 (47%) | 1.91 (1.22, 2.99) | 0.005 | 2.12 (1.33, 3.38) | 0.002 |
Note: Participants were classified as in a cluster if the time to most common recent ancestor of a potential cluster was less than five years
OR: Odds ratio; CI: Confidence interval; ARYS: At-Risk Youth Study; VIDUS: Vancouver Injection Drug User Study
Full model adjusted for age, sex, cohort of enrolment, recent HCV seroconversion, HIV infection, and HCV subtype;
DISCUSSION
This study characterises the molecular epidemiology of HCV among two cohorts of PWID recruited between 1996 and 2012 in Vancouver, Canada. HCV infection among young injectors (aged <27 years) was seeded from many transmission events between HCV-infected older injectors and younger injectors. Phylogenetic association by age and cohort was observed in this population. The proportion of participants identified in a pair/cluster less than five years old was 15%, with younger age, shorter duration of injecting and HIV coinfection independently associated with phylogenetic clustering. Treatment as prevention strategies will likely require broad scale-up across both younger and older PWID populations to prevent both transmission and HCV-related liver disease. Further, these phylogenetic methodologies can be applied to emerging HCV epidemics among PWID in other settings.
Transmission of HCV infection occurred both within and between younger and older PWID in this study. When examining participant age within the inferred phylogeny, younger participants were dispersedly spread among older PWID, with little evidence that HCV transmission was confined by age. The Association Index statistic showed an intermediate level of phylogenetic structure, without complete separation of young and old into two groups. Grouping of sequences by age group suggests that viruses from younger participants are more likely to be closely related to viruses from other younger participants than randomly distributed across the phylogeny. Transmission within age groups in more common than among age classes, but the latter does occur. Further, within individual clusters, it is apparent that younger participants are equally as likely to cluster with older PWID as with other younger PWID. As participants were categorised by age based on the ARYS definition of youth (i.e. <27 years old), the effect of co-clustering among age groups may be overestimated if participants were close to the stratification point (e.g. if a participant aged just less than 27 years co-clustered with a participant just older than 27 years).
Younger injectors and those new to injecting are at greatest risk of acquiring HCV infection [26]. Initiation to injecting is complex and multifaceted, with transition associated with homelessness [27, 28], previous drug use patterns [28, 29], survival sex work [30], sexual or physical abuse [31], and social and injecting networks [32]. Similar factors are associated with HCV acquisition, in addition to female gender [2], HIV infection [2], requiring help injecting [33], having a partner who injects [33], and borrowing injecting equipment [23]. However, infection with HCV has been shown to be associated with initiating injection drug use with someone at least five years older [34]. Further, younger PWID may be introduced into high-risk injecting practices by older injectors, are more likely to require assistance injecting, and more likely to share injecting equipment [35]. The high degree of intermixing within clusters and low phylogenetic association between younger and older PWID in this study suggest that the HCV epidemic in this setting consisted of many different HCV transmission events from older to younger PWID. This is in comparison to an HCV epidemic where there might be few HCV transmission events from older to younger PWID, with subsequent ongoing transmission among younger PWID.
Overall, 15% of participants were in pairs or clusters when the inferred cluster was no older than five years, consistent with a genetic distance clustering threshold of 1.5%. This methodology for defining phylogenetic clusters resulted in a lower proportion of clustering than genetic-distance based methods [10, 36, 37]. However, it more accurately reflects the dynamics of transmission by incorporating phylogenetic uncertainty and utilising the molecular clock from a Bayesian Monte Carlo Markov Chain analysis. A similar methodology was implemented in studying HIV network formation [38], albeit without subsampling from the posterior set of trees to account for phylogenetic uncertainty. Determining cluster membership by age of the inferred cluster may provide an alternate method for the assessment of clustering in the context of transmission studies.
Factors associated with phylogenetic clustering in this study included younger age, shorter duration since injecting initiation, HIV co-infection, and subtype 3a infection. Younger age and HIV infection have been shown to be associated with acquisition of HCV infection [2, 39] and phylogenetic clustering [10] in previous studies. This may be attributed to increased injecting risk behaviours among these sub-populations. Among young PWID in Vancouver, people with HIV/HCV co-infection were more likely to report syringe borrowing and greater than daily cocaine injecting than those with HCV alone [40]. The finding that a shorter duration since initiation of injecting was also associated with clustering is not surprising, given that younger PWID have a shorter duration since initiating injecting compared to older PWID. Phylogenetic clustering has also been shown to be associated with social and injecting networks, with cluster membership correlating with reported injecting relationships [25].
This study has a number of limitations. The VIDUS and ARYS cohorts had differences in inclusion and exclusion criteria. To help mitigate potential cohort effects, all regression-based analyses were adjusted for cohort of enrolment. However, it was crucial to combine ARYS and VIDUS participants in this study so that transmission events among PWID after 2005 were included. Second, the VIDUS and ARYS studies are not random samples of the eligible population and the findings may not be generalizable to the broader Vancouver injection drug-using population or other urban settings. There also may be un-sampled additional parties involved in the transmission cluster and direction of HCV transmission cannot be determined. The naturally expected phylogenetic distribution of traits such as younger age and shorter duration injecting may not be random, on account of their probable recent infection and thus propensity for clustering. Further simulation of transmission and within-host dynamics may be required to further understand this effect. Third, the utilisation of PCR to amplify HCV RNA may introduce bias in the selection of participants given the nature of the methodology and the potential to insufficiently detect variant strains of the virus (including mixed infections). Lastly, information on all behaviours were collected by self-report and may be subject to response biases.
The promise of HCV treatment as a potential prevention strategy has been driven by modeling studies suggesting that HCV treatment for PWID can lead to substantial reductions in HCV prevalence and reduce transmission [41-43]. Given the complex dynamics of HCV transmission among PWID demonstrated in this study, HCV treatment as prevention strategies that target both young PWID (at greater risk of transmission) and older PWID (at greatest risk of HCV-related liver disease progression) may result in prevention of both new HCV infections and HCV-related advanced liver disease [44, 45]. While the findings from this study could be used to inform dynamics mathematical models of HCV transmission among PWID, further prospective interventional studies are also needed to evaluate optimal HCV treatment as prevention strategies.
In conclusion, this study of PWID from Vancouver demonstrates that HCV transmission among PWID is complex and multifaceted, with transmission occurring both between and within older and younger PWID. These data suggest that, despite an increased risk of HCV transmission among younger PWID, it is likely that HCV prevention strategies will require broad coverage among both young and old PWID.
Supplementary Material
Acknowledgements
The authors thank the study participants for their contribution to the research, current and past researchers and staff, with particular thanks to T. Kerr, Z. Brumme, V. Montoya, C. Woods, and S. Dobrer for their assistance with this project. Funding for this study was provided by the National Institutes of Health (NIH) (VIDUS-R01DA011591, U01DA038886; R03DA033851-01) and the Canadian Institutes of Health (CIHR) (HHP-67262, RAA-79918, HES-115697; MOP-125948).
Financial Support: Funding for this study was provided by the National Institutes of Health (NIH) (VIDUS-R01DA011591, U01DA038886; R03DA033851-01) and the Canadian Institutes of Health (CIHR) (HHP-67262, RAA-79918, HES-115697; MOP-125948). NIH and CIHR had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The Kirby Institute is funded by the Australian Government Department of Health and Ageing. The views expressed in this publication do not necessarily represent the position of the Australian Government. JG is supported by a National Health and Medical Research Council (NHMRC) Career Development Fellowship. VDL is supported by a Scholar Award from the Michael Institute for Health Research and a New Investigator Award from CIHR. M-JM is supported by fellowships from the Michael Smith Foundation for Health Research and the Canadian Institutes of Health Research. BDLM is supported by an Avenir Award (No. 1DP2DA040236) from the National Institute on Drug Abuse. KH is supported by the CIHR New Investigator Award (201412MSH-340060-196922). JM is supported by the British Columbia Ministry of Health and through an Avant-Garde Award (No. 1DP1DA026182) from the National Institute of Drug Abuse (NIDA), at the US National Institutes of Health (NIH). JM has also received financial support from the International AIDS Society, United Nations AIDS Program, World Health Organization, National Institutes of Health Research-Office of AIDS Research, National Institute of Allergy & Infectious Diseases, The United States President's Emergency Plan for AIDS Relief (PEPfAR), UNICEF, the University of British Columbia, Simon Fraser University, Providence Health Care and Vancouver Coastal Health Authority. GD is supported by an NHMRC Practitioner Research Fellowship. This research was undertaken, in part, thanks to funding from the Canada Research Chairs program through a Tier 1 Canada Research Chair in Inner City Medicine which supports EW.
List of abbreviations in the order of appearance
- HCV
hepatitis C virus
- PWID
people who inject drugs
- NSP
needle and syringe programs
- OST
opioid substitution therapy
- HIV
human immunodeficiency virus
- ARYS
At Risk Youth Study
- VIDUS
Vancouver Injection Drug Users Study
- RNA
ribonucleic acid
- PCR
polymerase chain reaction
- E2
envelope-2
- MCMC
Markov Chain Monte Carlo
- BaTS
Bayesian association of tip significance
- tMRCA
time to most common recent ancestor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest/Disclosures: JG is a consultant/advisor and has received research grants from Abbvie, Bristol Myers Squibb, Gilead Sciences and Merck. JM has received grants from Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare. MK receives research grants from Merck, Gen-Probe (Hologic), Siemens and Roche. GD is a consultant/advisor and has received research grants from Abbvie, Bristol Myers Squibb, Gilead, Merck, Janssen and Roche. OP has received speakers fees from Gilead.
Author's contributions:
KH is one of the principal investigators of the VIDUS study. KD is the principal investigator for the ARYS study. BJ, TA, MK, AP, and JG designed this sub-study with input from AO, RH, GD, GM, JR and OP. BJ, AO, and FL performed all laboratory work. BJ had access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the results. BJ performed the statistical analyses with input from JG, TA, JR, AP and VDL. BJ wrote the first draft of the article with input from JG, AP, and TA. All authors critically reviewed the first draft of the article and approved the final version to be submitted.
REFERENCES
- 1.Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrick DM, Tyndall MW, Cornelisse PG, Li K, Sherlock CH, Rekart ML, et al. Incidence of hepatitis C virus infection among injection drug users during an outbreak of HIV infection. Canadian Medical Association Journal. 2001;165:889–895. [PMC free article] [PubMed] [Google Scholar]
- 3.Hagan H, Pouget ER, Williams IT, Garfein RL, Strathdee SA, Hudson SM, et al. Attribution of hepatitis C virus seroconversion risk in young injection drug users in 5 US cities. The Journal of infectious diseases. 2010;201:378–385. doi: 10.1086/649783. [DOI] [PubMed] [Google Scholar]
- 4.Kim C, Kerr T, Li K, Zhang R, Tyndall MW, Montaner JS, et al. Unstable housing and hepatitis C incidence among injection drug users in a Canadian setting. BMC Public Health. 2009;9:270. doi: 10.1186/1471-2458-9-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grebely J, Lima VD, Marshall BDL, Milloy MJ, DeBeck K, Montaner J, et al. Declining Incidence of Hepatitis C Virus Infection among People Who Inject Drugs in a Canadian Setting, 1996-2012. PloS One. 2014:9. doi: 10.1371/journal.pone.0097726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Z, Rannala B. Molecular phylogenetics: principles and practice. Nat Rev Genet. 2012;13:303–314. doi: 10.1038/nrg3186. [DOI] [PubMed] [Google Scholar]
- 7.Hartfield M, Murall CL, Alizon S. Clinical applications of pathogen phylogenies. Trends in Molecular Medicine. 2014;20:394–404. doi: 10.1016/j.molmed.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Lewis F, Hughes GJ, Rambaut A, Pozniak A, Leigh Brown AJ. Episodic Sexual Transmission of HIV Revealed by Molecular Phylodynamics. PLoS Med. 2008;5:e50. doi: 10.1371/journal.pmed.0050050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner BG, Roger M, Routy J-P, Moisi D, Ntemgwa M, Matte C, et al. High Rates of Forward Transmission Events after Acute/Early HIV-1 Infection. J Infect Dis. 2007;195:951–959. doi: 10.1086/512088. [DOI] [PubMed] [Google Scholar]
- 10.Jacka B, Applegate T, Krajden M, Olmstead A, Harrigan PR, Marshall BD, et al. Phylogenetic clustering of hepatitis C virus among people who inject drugs in Vancouver, Canada. Hepatology. 2014;60:1571–1580. doi: 10.1002/hep.27310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham EB, Jacka B, DeBeck K, Applegate TL, Harrigan PR, Krajden M, et al. Methamphetamine injecting is associated with phylogenetic clustering of hepatitis C virus infection among street-involved youth in Vancouver, Canada. Drug & Alcohol Dependence. 2015;152:272–276. doi: 10.1016/j.drugalcdep.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tyndall MW, Currie S, Spittal P, Li K, Wood E, O'Shaughnessy MV, et al. Intensive injection cocaine use as the primary risk factor in the Vancouver HIV-1 epidemic. AIDS (London, England) 2003;17:887–893. doi: 10.1097/00002030-200304110-00014. [DOI] [PubMed] [Google Scholar]
- 13.Strathdee SA, Patrick DM, Currie SL, Cornelisse PG, Rekart ML, Montaner JS, et al. Needle exchange is not enough: lessons from the Vancouver injecting drug use study. AIDS (London, England) 1997;11:F59–65. doi: 10.1097/00002030-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Wood E, Stoltz JA, Montaner JS, Kerr T. Evaluating methamphetamine use and risks of injection initiation among street youth: the ARYS study. Harm Reduct J. 2006;3:18. doi: 10.1186/1477-7517-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamoury F, Jacka B, Bartlett S, Bull RA, Wong A, Amin J, et al. The influence of Hepatitis C Virus genetic region on phylogenetic clustering analysis. PloS One. 2015 doi: 10.1371/journal.pone.0131437. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods CK, Brumme CJ, Liu TF, Chui CK, Chu AL, Wynhoven B, et al. Automating HIV drug resistance genotyping with RECall, a freely accessible sequence analysis tool. Journal of clinical microbiology. 2012;50:1936–1942. doi: 10.1128/JCM.06689-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Struck D, Perez Bercoff D, Devaux C, Schmit JC. 8th European HIV Drug Resistance Workshop. Sorrento, Italy: 2010. COMET: A Novel approach to HIV-1 subtype prediction. [Google Scholar]
- 18.Bradshaw D, Raghwani J, Jacka B, Sacks-Davis R, Lamoury F, Down I, et al. The importance of social networks in HCV transmission amongst HIV-infected gay and bisexual men in Australia. AIDS (London, England) Submitted. [Google Scholar]
- 19.Gray R, Parker J, Lemey P, Salemi M, Katzourakis A, Pybus O. The mode and tempo of hepatitis C virus evolution within and among hosts. BMC Evolutionary Biology. 2011;11:131. doi: 10.1186/1471-2148-11-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heled J, Bouckaert R. Looking for trees in the forest: summary tree from posterior samples. BMC Evolutionary Biology. 2013;13:221. doi: 10.1186/1471-2148-13-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker J, Rambaut A, Pybus OG. Correlating viral phenotypes with phylogeny: Accounting for phylogenetic uncertainty. Infection, Genetics and Evolution. 2008;8:239–246. doi: 10.1016/j.meegid.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Ragonnet-Cronin M, Hodcroft E, Hue S, Fearnhill E, Delpech V, Brown AJ, et al. Automated analysis of phylogenetic clusters. BMC Bioinformatics. 2013;14:317. doi: 10.1186/1471-2105-14-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy E, Alary M, Morissette C, Leclerc P, Boudreau JF, Parent R, et al. High hepatitis C virus prevalence and incidence among Canadian intravenous drug users. Int J STD AIDS. 2007;18:23–27. doi: 10.1258/095646207779949880. [DOI] [PubMed] [Google Scholar]
- 24.Maher L, Jalaludin B, Chant KG, Jayasuriya R, Sladden T, Kaldor JM, et al. Incidence and risk factors for hepatitis C seroconversion in injecting drug users in Australia. Addiction (Abingdon, England) 2006;101:1499–1508. doi: 10.1111/j.1360-0443.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- 25.Sacks-Davis R, Daraganova G, Aitken C, Higgs P, Tracy L, Bowden S, et al. Hepatitis C virus phylogenetic clustering is associated with the social-injecting network in a cohort of people who inject drugs. PloS One. 2012;7:e47335. doi: 10.1371/journal.pone.0047335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagan H, Thiede H, Des Jarlais DC. Hepatitis C virus infection among injection drug users: survival analysis of time to seroconversion. Epidemiology. 2004;15:543–549. doi: 10.1097/01.ede.0000135170.54913.9d. [DOI] [PubMed] [Google Scholar]
- 27.Feng C, DeBeck K, Kerr T, Mathias S, Montaner J, Wood E. Homelessness Independently Predicts Injection Drug Use Initiation Among Street-Involved Youth in a Canadian Setting. Journal of Adolescent Health. 2013;52:499–501. doi: 10.1016/j.jadohealth.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy É, Haley N, Leclerc P, Cédras L, Blais L, Boivin J-F. Drug injection among street youths in montreal: Predictors of initiation. Journal of Urban Health. 2003;80:92–105. doi: 10.1093/jurban/jtg092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Werb D, Kerr T, Buxton J, Shoveller J, Richardson C, Montaner J, et al. Crystal methamphetamine and initiation of injection drug use among street-involved youth in a Canadian setting. Canadian Medical Association Journal. 2013;185:1569–1575. doi: 10.1503/cmaj.130295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller CL, Pearce ME, Moniruzzaman A, Thomas V, Christian CW, Schechter MT, et al. The Cedar Project: risk factors for transition to injection drug use among young, urban Aboriginal people. Canadian Medical Association Journal. 2011;183:1147–1154. doi: 10.1503/cmaj.101257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood E, Stoltz J-A, Zhang R, Strathdee SA, Montaner JSG, Kerr T. Circumstances of first crystal methamphetamine use and initiation of injection drug use among high-risk youth. Drug and Alcohol Review. 2008;27:270–276. doi: 10.1080/09595230801914750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neaigus A, Gyarmathy VA, Miller M, Frajzyngier VM, Friedman SR, Des Jarlais DC. Transitions to injecting drug use among noninjecting heroin users - Social network influence and individual susceptibility. Jaids-J Acq Imm Def. 2006;41:493–503. doi: 10.1097/01.qai.0000186391.49205.3b. [DOI] [PubMed] [Google Scholar]
- 33.Miller CL, Johnston C, Spittal PM, Li K, LaLiberté N, Montaner JSG, et al. Opportunities for prevention: Hepatitis C prevalence and incidence in a cohort of young injection drug users. Hepatology. 2002;36:737–742. doi: 10.1053/jhep.2002.35065. [DOI] [PubMed] [Google Scholar]
- 34.Garfein RS, Doherty MC, Monterroso ER, Thomas DL, Nelson KE, Vlahov D. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1998;18:S11–S19. doi: 10.1097/00042560-199802001-00004. [DOI] [PubMed] [Google Scholar]
- 35.De P, Cox J, Boivin J-F, Platt RW, Jolly AM. The importance of social networks in their association to drug equipment sharing among injection drug users: a review. Addiction (Abingdon, England) 2007;102:1730–1739. doi: 10.1111/j.1360-0443.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- 36.Pilon R, Leonard L, Kim J, Vallee D, De Rubeis E, Jolly AM, et al. Transmission Patterns of HIV and Hepatitis C Virus among Networks of People Who Inject Drugs. PloS One. 2011:6. doi: 10.1371/journal.pone.0022245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hope VD, Hickman M, Ngui SL, Jones S, Telfer M, Bizzarri M, et al. Measuring the incidence, prevalence and genetic relatedness of hepatitis C infections among a community recruited sample of injecting drug users, using dried blood spots. J Viral Hepat. 2011;18:262–270. doi: 10.1111/j.1365-2893.2010.01297.x. [DOI] [PubMed] [Google Scholar]
- 38.Leigh Brown AJ, Lycett SJ, Weinert L, Hughes GJ, Fearnhill E, Dunn DT. Transmission Network Parameters Estimated From HIV Sequences for a Nationwide Epidemic. The Journal of infectious diseases. 2011;204:1463–1469. doi: 10.1093/infdis/jir550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagan H, Des Jarlais DC, Stern R, Lelutiu-Weinberger C, Scheinmann R, Strauss S, et al. HCV Synthesis Project: Preliminary analyses of HCV prevalence in relation to age and duration of injection. International Journal of Drug Policy. 2007;18:341–351. doi: 10.1016/j.drugpo.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 40.Miller CL, Wood E, Spittal PM, Li K, Frankish JC, Braitstein P, et al. The future face of coinfection: prevalence and incidence of HIV and hepatitis C virus coinfection among young injection drug users. J Acquir Immune Defic Syndr. 2004;36:743–749. doi: 10.1097/00126334-200406010-00012. [DOI] [PubMed] [Google Scholar]
- 41.Martin NK, Vickerman P, Foster GR, Hutchinson SJ, Goldberg DJ, Hickman M. Can antiviral therapy for hepatitis C reduce the prevalence of HCV among injecting drug user populations? A modeling analysis of its prevention utility. Journal of hepatology. 2011;54:1137–1144. doi: 10.1016/j.jhep.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 42.Martin NK, Vickerman P, Grebely J, Hellard M, Hutchinson SJ, Lima VD, et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58:1598–1609. doi: 10.1002/hep.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hellard M, Rolls DA, Sacks-Davis R, Robins G, Pattison P, Higgs P, et al. The impact of injecting networks on hepatitis C transmission and treatment in people who inject drugs. Hepatology. 2014;60:1861–1870. doi: 10.1002/hep.27403. [DOI] [PubMed] [Google Scholar]
- 44.Grebely J, Matthews GV, Lloyd AR, Dore GJ. Elimination of Hepatitis C Virus Infection Among People Who Inject Drugs Through Treatment as Prevention: Feasibility and Future Requirements. Clinical Infectious Diseases. 2013;57:1014–1020. doi: 10.1093/cid/cit377. [DOI] [PubMed] [Google Scholar]
- 45.Martin NK, Vickerman P, Dore GJ, Hickman M. The hepatitis C virus epidemics in key populations (including people who inject drugs, prisoners and MSM): the use of direct-acting antivirals as treatment for prevention. Current opinion in HIV and AIDS. 2015;10:374–380. doi: 10.1097/COH.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.