Abstract
Background
Studies of liver donors’ psychosocial outcomes focus on the short-term and rely largely on quality-of-life measures not specific to donation. We sought to examine long-term donation effects on three psychosocial domains: perceived physical, emotional, and socioeconomic outcomes.
Methods
Individuals donating 3–10 years previously at nine centers were eligible for telephone surveys. Survey responses were examined descriptively. Cluster analysis was used to identify distinct donor groups based on response profiles across psychosocial domains. Potential predictors of response profiles were evaluated with regression analysis.
Results
517 donors (66%) participated (M=5.8 years postdonation, SD=1.9). 15%–48% of donors endorsed current donation-related physical health problems and concerns, and 7%–60% reported socioeconomic concerns (e.g., insurance difficulties, financial expenditures). However, on average, donors experienced high psychological growth, and 90% felt positively about donation. Cluster analysis revealed five donor groups. One group showed high psychological benefit, with little endorsement of physical or socioeconomic concerns (15% of donors). Four groups showed less favorable profiles, with varying combinations of difficulties. The largest such group showed high endorsement of physical concerns and financial expenditures, and only modest psychological benefit (31% of donors). Men and non-Hispanic whites were most likely to have unfavorable response profiles (p<.01). Compared with donors aged 19–30, older donors were less likely to have unfavorable profiles; these differences were significant for donors aged >40–50 (p’s<.008).
Conclusions
Even many years postdonation, donors report adverse physical and socioeconomic effects, but positive emotional effects as well. Identification of response profiles and predictors may improve targeting of postdonation surveillance and care.
Introduction
The protection of living donors’ well-being and the prevention of negative consequences of donation are among the highest priorities in transplantation.1–5 Well-being extends beyond medical outcomes to encompass psychosocial outcomes, including perceived physical symptoms, emotional well-being, socioeconomic concerns, and general (non-donation-specific) health-related quality-of-life (HRQOL).1,4,6–12 Moreover, there is increasing recognition that it is insufficient to consider these outcomes in only the first few years postdonation; donors require long-term follow-up to identify any late-term sequelae.5,6,10,11 One of the foremost goals of the Adult to Adult Living Donor Liver Transplantation Cohort Study (A2ALL) was to collect long-term data to provide critical information to donors and transplant programs, and to inform the development of interventions to optimize donor outcomes.
To date, the literature on liver donors’ psychosocial outcomes pertains almost exclusively to the first several years postdonation.6,8,11–23 This literature has relied on generic, non-donation-specific, HRQOL assessments (e.g., Short-Form-36 [SF-36]), with findings indicating that donors’ well-being, on average, meets or exceeds that of the general population.6–8,12,14–16,18–21 Additionally, donors almost uniformly express no regret about donation, and report deep gratification at helping another person.15,17,19,23 Nevertheless, some single-center studies suggest that significant percentages of liver donors experience psychosocial difficulties postdonation. For example, up to 33% of donors report poorer health postdonation,15–17 up to 50% worry about lasting health effects,24,25 and over 25% have financial hardships, with prominent concerns about current and future insurance status.16,19,26 Whether these problems persist, worsen, or resolve after the first few years postdonation is largely unknown. In addition, the elevated rates of these problems are reported in the same literature—and sometimes within the same study—that also reports that liver donors’ generic HRQOL meets or exceeds that of the general population.6,12,20,27,28 This suggests that generic measures may be insensitive for living donors and should be used primarily as adjuncts to more sensitive, specific assessment of donors’ potential problems.6,12,29,30
Evidence beyond the first few years postdonation is scarce. Within A2ALL, data from donors enrolled during the early years of the project (A2ALL-1, 2004–2009) showed that, on average, generic HRQOL assessed with the SF-36 exceeds normative levels, even many years postdonation.31 Although A2ALL-1 included donors who were up to 11 years postdonation, sample attrition was relatively high beyond 1–3 years postdonation. A small single-center US study,32 and a large, single-center study in Japan reported similar findings of high SF-36 scores in donors with a median or mean, respectively, of ~7 years postdonation.32,33 These reports did not examine in detail donor concerns about the impact of donation on their physical, emotional, or socioeconomic well-being.
In the present study, we surveyed liver donors who donated 3–10 years previously at centers participating in A2ALL-2 (2009–2014). We assessed psychosocial outcomes in three domains: perceived physical health and symptoms related to donation; emotional well-being and response to donation; and socioeconomic concerns arising from donation. The survey also assessed generic HRQOL. Key goals were not only to provide descriptive information regarding these outcomes from a large multicenter cohort, but to examine whether demographic factors, donation-related clinical factors (e.g., surgical complications), and time since donation predicted psychosocial and HRQOL outcomes.
Materials and Methods
Study design
The A2ALL-2 consortium consists of nine US and Canadian transplant centers (see Acknowledgements). Six centers had participated in A2ALL-1. Three new centers joined the consortium in 2009. The present cross-sectional long-term follow-up survey was conducted with donors from all nine centers. All centers followed the medical/psychosocial evaluation and exclusion criteria for living donor selection in current US national policy.3 (Although the policy was enacted after study participants donated, center members led and participated in writing the policy, which relied on their experience already applying those criteria.)
Respondents
Eligible donors were aged ≥18, spoke English, and were 3–10 years postdonation (i.e., donation occurred between 2002–2009). The 3-year minimum was selected given our focus on long-term psychosocial outcomes. We set an upper limit of 10 years because individuals donating before 2002 did so during an era when many of the centers were developing their expertise in living liver donor surgery. Thus, these early donors’ experiences and psychosocial outcomes may differ from more recent donors.
Procedure
The study was approved by the Institutional Review Boards and Privacy Boards of the University of Michigan Data Coordinating Center and each participating center. At the six centers with continuing A2ALL participation, all of whom had previously established enrolled cohorts of their donors, all donors donating between 2002–2009 were telephoned by their center’s study coordinator to obtain informed consent. At the three centers new to the A2ALL consortium, all individuals donating between 2002–2009 were sent a letter describing the study and allowing them to opt out of it. If they did not opt out, they were called to obtain consent.
At all centers, donors not reached by telephone (after at least three attempts), were sent a letter describing the study. Coordinators used internet-based search strategies developed by the survey team (MAD, AFD, ZB) to obtain updated contact information as needed.
If donors consented, their contact information was forwarded to the survey team, who assigned trained interviewers to complete the 30–45 minute assessments using computer-assisted telephone interview (CATI) methods.34,35 The CATI approach ensures that interviewers use consistent wording, and it minimizes missing data because a response (or reason for no response) is required before additional items are asked.35
Trained study personnel at each center reviewed donors’ medical records for clinical information, as described below.
Measures
We assessed donation-specific psychosocial variables in three domains, plus general HRQOL. Measures are fully described in the Supplementary File, Table S1). We selected published measures that have been used in prior living donor studies and, for multi-item rating scales, have established psychometric properties (described in sources cited with each measure).
With regard to perceived physical health symptoms and concerns, donors completed the 10 abdominal symptom items from the Checklist of Donation-Related Physical Symptoms (focused on the past month.13,23,36 Donors answered two additional items about whether they had donation-related medical problems, and whether they could not perform some physical activities as well as before donation.36–38 For each item, they described any problems/limitations. We also utilized four items assessing current and future donation-related health worries.37–40
In the domain of emotional well-being and adjustment, donors completed the 10-item Posttraumatic Growth Inventory-Short Form (PTGI-SF)41 to assess whether they experienced positive life changes (e.g., learned more about personal strengths) due to their donation. They completed the 10-item Better Person Scale,37 assessing feelings of being a better person for having donated. Donors answered two individual items assessing psychological reactions to donation:37 whether they would now make the same decision to donate, and what their overall feelings were about their donation. Finally, if donors knew that their recipient was deceased, they rated how guilty or responsibility they felt for the death.37
For socioeconomic concerns, we utilized items modeled after those employed in other samples.38,42 Donors were asked whether they had incurred unreimbursed donation-related medical and nonmedical costs, whether donation-related financial costs had been burdensome, whether they had changed jobs due to the donation; and whether their personal income had been permanently affected by donation. Finally, they reported whether they had had problems since donation either keeping or getting new health insurance and/or life insurance.
Respondents’ generic HRQOL, not specific to donation, was assessed with the SF-36, version 2.43,44 It assesses 8 domains (general health, physical functioning, vitality, bodily pain, role limitations due to physical health, role limitations due to emotional health, social functioning, mental health). It also yields two summary measures (physical and mental component scores).
Demographics, including relationship to the recipient and recipient vital status, were obtained during survey interviews. Medical records data were retrieved on donor body mass index (BMI) at evaluation, donation date, length of post-surgical hospitalization, and medical complications and related rehospitalizations during the first year postdonation.
Analyses
We used descriptive statistics to examine demographic and clinical characteristics, and responses to donation-specific and generic HRQOL measures. Demographic comparisons between survey respondents and nonrespondents were made using t tests and chi-squared tests. Donors’ SF-36 scores were compared with (a) general population norms using one-sample t tests, and (b) a healthy reference group, namely a middle-aged cohort with no self-reported chronic diseases from the National Health Measurement Study (NHMS) cohort,45,46 using two-sample t tests.
Because we acquired a wide array of donation-specific measures, we performed a multivariable descriptive analysis across these measures to determine whether donors could be categorized into discrete groups that each showed a unique profile or pattern of outcomes that donors attributed to donation. For this purpose, we employed hierarchical agglomerative cluster analysis.47,48 (Items asked only if recipients were deceased were not included because few donors were asked these items; SF-36 measures were not included because they did not pertain to donation-specific perceptions.)
Cluster analysis sorts individuals into groups, each with members similar to each other but different from members of other groups. We employed the widely-used unweighted pair-group method, with arithmetic averages and squared Euclidean distance coefficients.47 Standard criteria were applied (based on amalgamation coefficient change as clustering proceeded) to judge when the optimal number of clusters was reached. Standard cross-validation techniques were applied to determine whether the cluster solution was stable and, hence, likely to be generalizable.47,48 Thus, the sample was divided randomly in half, and the cluster solution obtained in one subsample was compared with that in the other subsample to determine whether the same clusters were identified.
We evaluated potential demographic and clinical predictors of: (a) donation-specific response profile (i.e., cluster group membership); (b) donor response to recipient death; and (c) generic HRQOL (composite summary scores). For each outcome, logistic regression models were fit, and the final, most parsimonious model was selected using best subset selection.49 Before regression analyses, we determined that model assumptions were met.50 Missing data on the predictors were imputed using IVEware.51 The extent of missing data is noted in Table 1 (footnote a).
Table 1.
Background characteristics of respondents and nonrespondents
| Characteristica | Respondents (N=517) | Nonrespondents (N=271)b | Comparison | |
|---|---|---|---|---|
| Testc | p | |||
| Demographic | ||||
| Female sex, % (n) | 53.2 (275) | 46.9 (127) | 2.85 | .091 |
| Age at donation, years, % (n) | 25.03 | <.001 | ||
| 19–30 | 23.0 (119) | 37.6 (102) | ||
| >30–40 | 26.7 (138) | 28.0 (76) | ||
| >40–50 | 30.0 (155) | 22.9 (62) | ||
| >50–61 | 20.3 (105) | 11.4 (31) | ||
| Age at survey, years, % (n) | 24.88 | <.001 | ||
| 23–30 | 9.1 (47) | 18.5 (50) | ||
| >30–40 | 24.6 (127) | 29.5 (80) | ||
| >40–50 | 27.9 (144) | 27.7 (75) | ||
| >50–68 | 38.5 (199) | 24.4 (66) | ||
| Race/ethnicity, % (n) | 16.61d | <.001 | ||
| White/European | 87.6 (453) | 75.4 (150) | ||
| Hispanic/white | 4.6 (24) | 10.6 (21) | ||
| Black/African | 2.1 (11) | 2.5 (5) | ||
| Asian | 2.3 (12) | 7.5 (15) | ||
| Other | 3.3 (17) | 4.0 (8) | ||
| Years since donation, M (SD) | 5.8 (1.9) | 6.0 (1.8) | 1.98 | .048 |
| range | 3–10 | 3–10 | ||
| Relation to transplant recipient, % (n), donor is: | 0.59e | .893 | ||
| First-degree relative | ||||
| Parent | 3.7 (19) | 1.5 (4) | ||
| Adult child | 31.3 (162) | 25.8 (70) | ||
| Sibling | 23.4 (121) | 8.8 (24) | ||
| Spouse/partner | 7.7 (40) | 16.0 (26) | ||
| Other relative | ||||
| Biological relative | 6.2 (32) | 5.2 (14) | ||
| Nonbiological relative | 10.8 (56) | 4.4 (12) | ||
| Unrelated | 16.8 (87) | 14.8 (24) | ||
| Geographic region of transplant center, % (n)f | 7.61 | .022 | ||
| East | 32.3 (167) | 24.4 (66) | ||
| Midwest | 58.6 (303) | 62.0 (168) | ||
| West | 9.1 (47) | 13.7 (37) | ||
| Education at survey, % (n) | --- | --- | ||
| ≤ high school | 20.9 (108) | --- | ||
| vocational or some college | 22.9 (118) | |||
| college graduate | 36.2 (187) | |||
| postgraduate | 20.0 (103) | |||
| Married or had long-term partner at survey, % (n) | 70.5 (364) | --- | --- | --- |
| Employment status at survey, % (n) | --- | --- | --- | |
| Employed full-time | 76.8 (395) | |||
| Employed part-time | 10.3 (53) | |||
| Unemployed | 9.7 (50) | |||
| Retired | 3.1 (16) | |||
|
| ||||
| Clinical donation-related | ||||
| BMI at evaluation, M (SD) | 26.6 (4.2) | --- | --- | --- |
| Postdonation length of hospital stay, days, median (IQR) | 7.0 (6.0–8.0) | --- | --- | --- |
| Post-operative complications in first year postdonation, highest Clavien grade, % (n)g | --- | --- | --- | |
| None | 71.3 (263) | |||
| Grade 1 | 8.9 (33) | |||
| Grade 2 | 19.2 (71) | |||
| Grade 3 | 0.5 (2) | |||
| Hospital readmission due to complications in first year postdonation, % yes (n) | 2.2 (8) | --- | --- | --- |
| Recipient vital status at follow-up (donor report), % deceased (n) | 17.6 (91) | --- | --- | --- |
| Months postdonation that recipient death occurred, M (SD) | 30.3 (27.7) | --- | --- | --- |
| Months before survey that recipient death occurred, M (SD) | 45.5 (24.7) | --- | --- | --- |
BMI, body mass index; IQR, interquartile range; M, mean; SD, standard deviation
Respondent and nonrespondent groups had complete data on each variable with the following exceptions: 72 nonrespondents were missing data on race/ethnicity, 109 nonrespondents were missing data on relation to recipient, 1 respondent each was missing data on education and recipient vital status at follow-up (donor report), 3 respondents were missing data on marital status, 6 respondents were missing data on employment, 10 respondents were missing data on BMI and postdonation length of hospital stay, 148 respondents were missing data on post-operative complications and hospital readmissions. The latter 148 respondents did not return to their centers for follow-up care through the complete first year postdonation. Although the 3 centers new to A2ALL (see Methods section for description of study design) were more likely to report missing data on post-operative complications and readmissions, there was no statistically significant association of donor center (comparing the 3 new to 6 continuing centers) with either complications (exact p=.280) or readmissions (exact p=.471).
The 271 nonrespondents included 59 donors lost to follow-up, 92 donors located but who did not respond to requests to participate in the survey, and 120 donors who refused to participate in the survey.
Chi squared test for proportions; t test for continuous variables.
Due to small cell sizes, categories of Hispanic, Black, Asian, and Other were collapsed into one category before statistical testing.
Due to small cell sizes, categories of parent, child and sibling were collapsed to reflect “first degree relatives,” and categories of other biologic relative and other nonbiologic relative were collapsed to reflect “other relatives” before statistical testing.
Sample sizes on a center-by-center basis are too small to permit separate analyses; centers are grouped by region. Experience in living donation, i.e., year of inception of center programs and number of living donors contributed to the present study, are included in parentheses following each center: centers in the East included Columbia University (1998, 24), Lahey Clinic and Medical Center (1998, 108), University of Pennsylvania (1999, 11), and Virginia Commonwealth University (1998, 24); Centers in the Midwest included University of Pittsburgh (1999, 115), Northwestern University (1999, 37), and University of Toronto (2000, 151); Centers in the West included University of Colorado (1997, 29), and University of California at San Francisco (2000, 18).
Results
Sample description
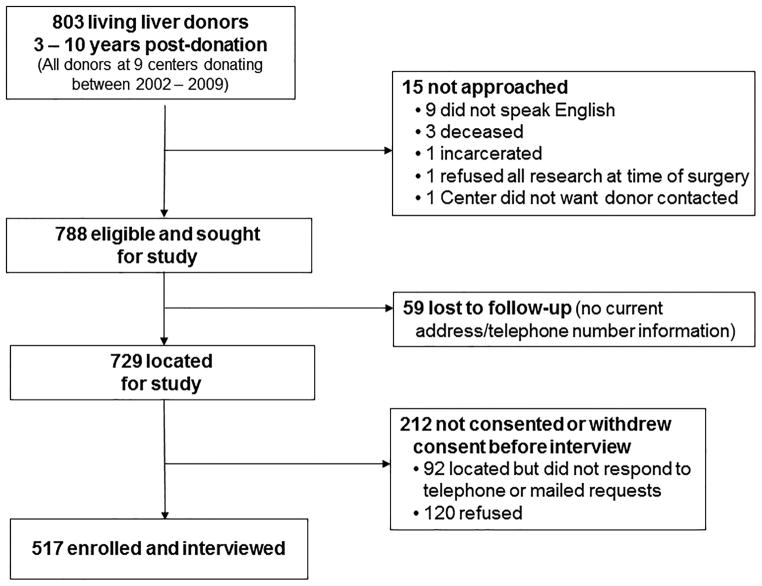
Recruitment is shown in Figure 1. Of 803 donors donating between 2002–2009, 15 (1.9%) were not approached. A total of 517 donors were enrolled and interviewed, yielding a response rate of 66% of 788 eligible donors, and 71% of the 729 donors who were located.
Figure 1.
Study flowchart of interview accrual.
Table 1 displays demographic and clinical characteristics for respondents and—when available—for nonrespondents. Respondents were relatively evenly split by sex, aged 19–61 at donation, predominantly non-Hispanic whites, and educated beyond high school. A majority were first-degree relatives or spouses of recipients. The donation hospitalization was generally about 1 week. A majority of donors experienced no complications or related rehospitalizations during the first year postdonation. At survey, donors averaged almost 6 years postdonation.
Compared with nonrespondents, respondents were older and more likely to be non-Hispanic whites. Years since donation and regional location of donors’ centers differed significantly between groups, but the differences were relatively small. There were no significant differences for sex or relationship to the transplant recipient.
Description of individual donation-specific outcomes and HRQOL
Donation-specific psychosocial status
Responses on measures in the three domains are shown in Table 2. Within the domain of physical symptoms and health concerns, donors currently experienced an average of almost three of 10 assessed symptoms. The most frequently endorsed symptoms included numbness around the surgical site (69% of donors), decreased stomach tone (50%), low back pain (36%), and itching around the surgical site (33%).
Table 2.
Patient-reported donation-specific psychosocial outcomes: physical health symptoms and concerns, emotional well-being and adjustment, and socioeconomic concerns (n=517 donors)
| Domain | Descriptive statistic | |
|---|---|---|
| Physical health symptoms and concerns | ||
| 1. No. of current physical symptoms attributed to donation,a | M (SD) | 2.9 (2.1) |
| median (IQR) | 3 (1.0 – 4.0) | |
| 2. Have current donation-related medical problems, % yes (n) | 15.1 (78) | |
| 3. Unable to do some physical activities as well since donation,b % yes (n) | 21.6 (111) | |
| 4. Health-related worries related to donation, % yes (n) | ||
| Worried about the physical health effects of donation | 25.2 (130) | |
| Worried about current health | 27.1 (140) | |
| Worried about future negative effects on healthc | 30.7 (157) | |
| Worried about never feeling physically 100% againb | 17.1 (88) | |
| Any of the four areas of worry endorsed | 47.8 (247) | |
|
| ||
| Emotional well-being and response to donation | ||
| 1. Posttraumatic Growth Inventory score (0=low, 50=high),b M (SD) | 25.8 (13.4) | |
| 2. Better Person Scale score (0=low, 10=high), M (SD) | 5.1 (2.5) | |
| 3. Would make the same decision to donation again,b % agree (n) | 90.9 (468) | |
| 4. Overall feelings about donation,f % somewhat to very positive (n) | 90.3 (463) | |
| 5. If donor knew recipient was deceased (n=91), | ||
| Feel guilty about this outcome (1=not at all, 10=very much),a | M (SD) | 2.1 (2.2) |
| median (IQR) | 1 (1–2) | |
| Feel responsible for this outcome (1=not at all, 10=very much),a | M (SD) | 2.1 (2.2) |
| median (IQR) | 1 (1–2) | |
|
| ||
| Socioeconomic concerns | ||
| 1. Incurred costs related to donation, % yes (n) | ||
| Incurred medical costsb | 18.1 (93) | |
| Incurred nonmedical costsd | 57.6 (296) | |
| Incurred any medical or nonmedical costs | 60.5 (311) | |
| 2. Donation-related costs were a burden,b % yes (n) | 15.1 (78) | |
| 3. Changed jobs due to donation,b % yes (n) | 7.4 (38) | |
| 4. Personal income permanently affected by donation:e | income reduced, % (n) | 6.6 (34) |
| income increased, % (n) | 2.1 (11) | |
| no change, % (n) | 91.2 (469) | |
| 5. Have had problems getting or keeping insurance since donation, % yes (n) | ||
| Problems getting or keeping health insurancee | 6.4 (33) | |
| Problems getting or keeping life insurance since donationf | 6.8 (35) | |
| Problems with either type of insurance endorsedf | 11.3 (58) | |
IQR, interquartile range; M, mean; No., number; SD, standard deviation
1 case was missing data.
2 cases were missing data.
6 cases were missing data.
4 cases were missing data.
3 cases were missing data.
5 cases were missing data.
Table 2 shows that 15% of donors reported current donation-related medical problems. The most common problems were hernias (described by 22% [n=17] of 78 donors reporting current problems), gastrointestinal/digestive issues (nausea, fat and food intolerances, 22% [17/78]), chronic diarrhea (10% [8/78]), and problems with scar tissue and adhesions (9% [7/78]).
Twenty-two percent of donors reported that they could not do some physical activities as well as before donation (Table 2). Donors’ descriptions of these limitations pertained primarily to abdominal exercises or activities requiring abdominal strength (noted by 47% [n=52] of the 111 donors reporting current limitations), vigorous physical activity (21% [23/111]), and lifting significant weight at work or in sports (19% [21/111]).
Finally, 17% to 31% of donors reported health worries due to donation, with concern about future health being most frequently endorsed (Table 2). Overall, 48% endorsed worry on at least one of these items.
Concerning emotional outcomes of donation, donors’ average posttraumatic growth level and Better Person Scale scores were at approximately the scales’ midpoints. Over 90% of donors stated that they would make the same decision to donate again, and most had positive overall feelings about donating. Among 91 donors reporting that their recipient was deceased, low mean levels of guilt or responsibility for the death were endorsed.
Within the socioeconomic domain, up to 58% of donors had donation-related financial expenditures, and 15% reported that the expenses were burdensome. Some donors reported having had to change jobs, and some had permanent income reductions due to donation. Overall, 11% experienced health and/or life insurance problems due to donation.
Generic HRQOL
Table 3 shows mean SF-36 subscale and summary scores. Relative to US population norms (M=50, SD=10), donors reported significantly better HRQOL on all measures (p’s<.001). Relative to a middle-aged national cohort with no self-reported chronic diseases (NHMS),45,46 donors were similar or better on most physical HRQOL measures (with the exception of bodily pain) and similar or worse on emotional HRQOL measures. However, in all cases, there were small between-group absolute differences of a few scale points or less (minimal clinically important difference, 5 points, i.e., 0.5 SD from normative mean55). Using this clinically significant cut point (0.5 SD from the US normative mean),55 we also classified donor HRQOL scores as poor (>5 points below the normative mean), average (within 5 points), or superior (>5 points above the normative mean). Most donors had at least average scores (Table 3). However, 7.5% to 15.9% of donors showed poor scores, with the greatest percentage of poor scores on the Mental Component Summary (MCS).
Table 3.
SF-36 distributions of scores in the study sample (n=517 donors) vs. normative and healthy national sample comparators.
| SF-36 measure | Donors (n=517)
|
NHMS healthy cohort (n=868)a
|
Donors with poor, average, or superior scores relative to the normative mean in the general population of 50, % (n)
|
||||
|---|---|---|---|---|---|---|---|
| Meanb | SD | Meanb | SD | Poor (>5 points below) | Average (within 5 points) | Superior (>5 points above) | |
| Physical functioning | 54.3 | 6.0 | 54.8 | 5.7 | 7.5 (39) | 31.7 (164) | 60.7 (314) |
| Role – physical | 53.7c | 7.1 | 53.3 | 6.4 | 9.7 (50) | 17.8 (92) | 72.5 (374) |
| Bodily pain | 54.2* | 10.1 | 55.2* | 7.5 | 15.1 (78) | 22.8 (118) | 62.1 (321) |
| Vitality | 54.0* | 9.6 | 57.5* | 8.1 | 15.5 (80) | 31.9 (165) | 52.6 (272) |
| General health | 55.1* | 8.7 | 53.9c* | 7.4 | 11.8 (61) | 25.5 (132) | 62.7 (324) |
| Social functioning | 52.8* | 7.9 | 53.8* | 6.6 | 11.4 (59) | 17.6 (91) | 71.0 (367) |
| Role – emotional | 52.4c | 7.7 | 53.2d | 6.8 | 14.0 (72) | 11.8 (61) | 74.2 (383) |
| Mental health | 53.4* | 8.4 | 56.2* | 7.7 | 14.7 (76) | 27.9 (144) | 57.4 (297) |
| Physical component summary | 54.6c | 7.4 | 54.0d | 5.6 | 8.1 (42) | 27.7 (143) | 64.1 (331) |
| Mental component summary | 52.5c* | 8.7 | 55.1d* | 7.3 | 15.9 (82) | 32.9 (170) | 51.2 (264) |
NHMS, National Health Measurement Study; SD, standard deviation; SF-36, Short-Form-36
In a 2006 national sample of the noninstitutionalized US population aged 35–89 (n=3522)46 who completed telephone assessments of health-related quality of life, we selected all persons with the same upper age limit and same BMI range as in the living donor sample, and excluded all persons with any of 11 self-reported chronic diseases (coronary heart disease, stroke, diabetes, arthritis, eye disease, sleep disorder, respiratory disease, depression, ulcer, thyroid disease, back pain).
Scores were transformed to have a mean of 50 and an SD of 10 in the general population (i.e., norm-based scoring43). Higher scores indicate better HRQOL for all measures. All mean scores for the sample are significantly higher than SF-36 version 2 norms for the US general population (one-sample t-tests, all p values < .001). We used norm-based scoring in these analyses because it enables more accurate understanding of relative differences between subscale scores, e.g., whether scores on a given subscale are truly more favorable than scores on another subscales.43 Norm-based scoring is recommended by the scale designers.43 Because some studies of living donor HRQOL report 0–100 scoring rather than norm-based scoring, Table S2 in the Supplementary File provides donors’ scores computed with 0–100 scoring in order to allow comparison to other published living donor cohorts.
1 case was missing data.
2 cases were missing data.
Comparison of donors and NHMS healthy cohort members, 2-sample t tests, p < .05.
Group profiles across donation-specific psychosocial measures
The cluster analysis examined whether donors could be categorized into discrete groups, each showing a unique profile of outcomes attributed to donation. The analysis included all donation-specific measures with sufficient variability (i.e., items on which >90% of donors’ answers fell into one response category were excluded). A 5-cluster solution was obtained. These clusters are shown in Table 4, along with responses from donors in each cluster group to the psychosocial measures. To facilitate interpretation, within each row of the table, dark shading indicates the group with the highest score or percentage; light shading indicates the group with the lowest score or percentage. The test statistics (rightmost column, Table 4) indicate variables on which the groups significantly differ; these characteristics were most influential in the analysis.
Table 4.
Groups identified by cluster analysis as showing distinctive patterns of responses to patient-reported donation-specific psychosocial outcomes, n=507 donors with complete data on all variables. For each psychosocial characteristic, darker shading indicates group with highest percentage or highest mean; lighter shading indicates group with lowest percentage or lowest mean
| Domain | (1) | (2) | (3) | (4) | (5) | Test Statistica | p |
|---|---|---|---|---|---|---|---|
| Highest psychological benefit/ | High psychological benefit/ | Some psychological benefit/ | Low psychological benefit/ | Lowest psychological benefit/ | |||
| Mostly Low physical and socioeconomic concerns (n=74) | Some physical and socioeconomic concerns (n=109) | Highest physical concern/ Some socioeconomic concerns (n=158) | Mostly Low physical and socioeconomic concerns (n=109) | Some physical and socioeconomic concerns (n=57) | |||
|
|
|
|
|
||||
| Physical health symptoms and concerns | |||||||
| No. of current physical symptoms attributed to donation, M (SD) | 2.7 (2.2) | 2.9 (2.1) | 3.3 (2.2) | 2.4 (1.9) | 2.9 (2.2) | 2.45b | .045 |
| Have current donation-related medical problems, % yes (n) | 10.8 (8) | 12.8 (14) | 17.1 (27) | 14.7 (16) | 15.8 (9) | 1.97 | .771 |
| Unable to do some physical activities as well since donation, % yes (n) | 18.9 (14) | 22.9 (25) | 23.4 (37) | 22.9 (25) | 15.8 (9) | 1.98 | .740 |
| Any health-related worries related to donation, % yes (n) | 37.8 (28) | 49.5 (54) | 57.0 (90) | 34.9 (38) | 52.6 (30) | 16.21 | .003 |
|
|
|
|
|
||||
| Emotional well-being and response to donation | |||||||
| Posttraumatic Growth Inventory score (0=low, 50=high), M (SD) | 45.7 (3.0) | 36.2 (2.7) | 25.9 (3.8) | 13.7 (3.6) | 3.2 (2.5) | 1987.81 | <.001 |
| Better Person Scale score (0=low, 10=high), M (SD) | 6.4 (2.5) | 6.2 (2.2) | 5.0 (2.3) | 4.1 (2.0) | 3.5 (2.5) | 23.02 | <.001 |
|
|
|
|
|
||||
| Socioeconomic concerns | |||||||
| Incurred costs related to donation, % yes (n) | 51.4 (38) | 55.0 (60) | 69.6 (110) | 62.4 (68) | 54.4 (31) | 10.51 | .033 |
| Donation-related costs were a burden, % yes (n) | 17.6 (13) | 15.6 (17) | 15.2 (24) | 9.2 (10) | 21.1 (12) | 4.96 | .291 |
| Have had problems getting or keeping health/life insurance since donation, % yes (n) | 10.8 (8) | 10.1 (11) | 10.1 (16) | 10.1 (11) | 19.3 (11) | 4.21 | .379 |
M, mean; SD, standard deviation
F test for continuous variables; x2 for dichotomous variables.
log transformed to normalize distribution prior to statistical test; means are presented in the original units to facilitate interpretation.
Cluster group 1 consists of 74 donors showing the highest psychological benefit from donation and generally low physical and socioeconomic concerns (first column, Table 4): they had the highest mean emotional well-being scores (dark shading), the lowest percentage of members with current donation-related medical problems, and the lowest percentage who incurred donation-related costs (light shading). They also had relatively low percentages of other physical and socioeconomic problems. In contrast, group 5 includes 57 donors with the lowest psychological benefit, plus at least some physical and socioeconomic concerns: they had low mean emotional well-being scores, they had some physical symptoms and concerns (although these were not as prominent as for some other groups), and they were most likely to report burdensome donation costs and insurance difficulties.
The labels describing each of the remaining three groups similarly characterize their distinctive profiles of benefits and concerns. Of these groups, group 3 (n=158 donors) had the highest physical concerns. They were the most likely to have physical symptoms and problems, and to have incurred donation-related financial expenses. They had relatively modest psychological benefit. Finally, group 2 (n=109) showed a profile of relatively high psychological benefit (mean scores approached those for the highest psychological benefit group), with a mixed pattern of physical and socioeconomic concerns. Group 4 (n=109) showed relatively lower psychological benefit, but they also had relatively low physical and socioeconomic concerns.
Cross-validation analyses replicated the pattern of clusters found in the complete sample: a 5-cluster solution was optimal in each replication; 77% of donors in one subsample, and 74% in the second subsample, were classified into the same cluster as in the full sample. This provides strong evidence for cluster stability.47,48
Predictors of donation-specific response profiles, donor reaction to recipient death, and poor generic HRQOL
Regression analysis results are shown in Table 5. Significant predictors of membership in the five response profiles (cluster groups) include sex, race/ethnicity, and age at donation. Using the most favorable profile group as the referent (group 1: high psychological benefit/mostly low physical and socioeconomic concerns), men were 2.45 to 6.23 times more likely to fall into other (less favorable) profile groups. Men were at highest risk (odds ratio[OR]=6.23) of falling into the group with the lowest psychological benefit. Non-Hispanic whites were also more likely to fall into the less favorable profile groups. They showed the highest odds of falling into the group with the highest physical concerns (group 3, OR=7.61). Finally, compared with donors aged 19–30, all older age groups were at reduced risk of falling into the less favorable profile groups; this risk reduction relative to the youngest (referent) group was largest and statistically significant for donors aged >40 to 50. No other significant demographic or clinical predictors emerged, even in sensitivity analyses that further examined individual center effects and effects linked to donor relationship to recipient (Table 5, footnote a).
Table 5.
Predictors of three outcomes: (a) Respondent profiles on donation-specific psychosocial measures among all donors; (b) guilt/ responsibility among donors whose recipient was deceased; and (c) poor HRQOL on the SF-36 Mental Component Score among all donors
| Predictora | Respondent profiles on donation-specific psychosocial measures (n=507)
|
Any guilt or responsibility for recipient’s death (n=91) | Poor HRQOL, Mental Component Summary Score (n=516) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Highest psych. benefit/ | 2. High psych. benefit/ | 3. Some psych. benefit/ | 4. Low psych. benefit/ | 5. Lowest psych. benefit/ | |||||||||||||||||
| Mostly Low physical and socioeconomic cost | Some physical and socioeconomic cost | High physical cost/ Some socioeconomic cost | Mostly Low physical and socioeconomic cost | Some physical cost/ High socioeconomic cost | |||||||||||||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| Male sex | ref | 2.45 | 1.24, 4.86 | .010 | 3.54 | 1.84, 6.81 | <.001 | 4.44 | 2.23, 8.81 | <.001 | 6.23 | 2.84,13.69 | <.001 | --- | --- | --- | --- | --- | --- | ||
| White/European | ref | 3.19 | 1.45, 7.05 | .004 | 7.61 | 3.21,18.03 | <.001 | 5.58 | 2.27,13.73 | <.001 | 6.35 | 2.06,19.57 | <.001 | --- | --- | --- | --- | --- | --- | ||
| Age at donation | |||||||||||||||||||||
| 19–30 | ref | ref | ref | ref | ref | --- | --- | --- | --- | --- | --- | ||||||||||
| >30–40 | ref | 0.66 | 0.26, 1.67 | .380 | 0.95 | 0.38, 2.38 | .907 | 0.72 | 0.27, 1.89 | .499 | 0.38 | 0.12, 1.17 | .090 | ||||||||
| >40–50 | ref | 0.25 | 0.10, 0.60 | .002 | 0.31 | 0.13, 0.74 | .008 | 0.29 | 0.11, 0.71 | .007 | 0.26 | 0.09, 0.71 | .008 | ||||||||
| >50–61 | ref | 0.44 | 0.15, 1.26 | .127 | 0.67 | 0.24, 1.96 | .441 | 0.79 | 0.28, 2.26 | .660 | 0.54 | 0.17, 1.74 | .301 | ||||||||
| Years since donation | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 0.76 | 0.61, 0.97 | .025 | --- | --- | --- |
| Length of stay after donation surgery | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 1.14 | 1.03, 1.27 | .009 |
CI, confidence interval; HRQOL, health-related quality of life; OR, odds ratio; psych., psychological; ref, reference; SF-36, Short-Form-36
Other variables that were examined but did not meet criteria for inclusion in final models (all considered with the categorical or continuous variable formats noted in Table 1 unless otherwise noted: education, relationship to recipient (first degree relative, spouse, other relative, unrelated), geographic region where center was located, donor BMI at evaluation, post-operative complications (grade 2+ vs. grade ≤1), whether recipient was deceased, years since recipient died, time from donation to recipient death. Hospital readmission was not included as a potential predictor because very few donors were rehospitalized, and readmissions are included in the Clavien grading of complications. In addition, sensitivity analyses were performed in order to explore whether, aside from geographic region where a center was located, there might be individual center differences on the three outcomes. No sizable or statistically significant center differences emerged, either when center was considered alone in relation to each outcome variable, or when center was included in the multivariable modeling. Moreover, the inclusion of individual center in the multivariable modeling also did not change the effects that were identified as statistically significant in the regression models. Similarly, sensitivity analyses were performed in order to explore whether a finer-grained classification of relationship to recipient (using all categories in Table 1) might reveal associations with the outcomes in either univariate or multivariable modeling. No sizeable or statistically significant effects emerged and inclusion of the finer-grained relationship variable in the multivariable modeling did not affect the statistical significance of the associations reported in the table above.
To examine predictors of donor reaction to recipient death, we created a composite outcome reflecting whether any feelings of guilt or responsibility were endorsed (i.e., donors scored >1 on either scale) because the original variables were extremely skewed. Regression results indicated that greater time since donation was associated with reduced risk of such feelings (Table 5). No other significant predictors emerged.
The final regression examined predictors of poor SF-36 MCS scores. (The Physical Component Summary [PCS] was not considered because >90% of the sample scored in the average to superior range.) One predictor emerged: a longer hospital stay postdonation significantly increased the likelihood of a poor MCS score (Table 5).
Discussion
Ours is the first study to consider both a range of psychosocial outcomes that living liver donors attributed to donation, as well as generic HRQOL in the long-term years after donation. We assembled one of the largest cohorts to date, and the multicenter study design enhances our findings’ potential generalizability.
Chief among our results is evidence that substantial percentages of donors report donation-specific concerns, particularly regarding physical health and socioeconomic consequences of donation. This is despite the facts that (a) donors’ average, generic HRQOL levels exceed general population normative levels and (b) are similar to healthy comparison groups—findings typical in living donor populations8,14–16,18–21,24,32,33,56 Thus, even 3–10 years postdonation, 15% to 48% of donors reported current donation-related medical problems, physical activity limitations, and worries about current and future health. Although donors may have mistakenly attributed their medical problems to donation, many problems represent typical postdonation difficulties (e.g., incisional hernias, gastrointestinal problems associated with gallbladder removal,57 which accompanies liver donation), suggesting that donors are indeed experiencing donation-related issues. Similarly, the physical limitations noted by 22% of donors are consistent with having undergone abdominal surgery. Furthermore, from a socioeconomic perspective, many donors reported not only personal financial costs arising from donation, but in some cases, job changes, permanent income reductions, and problems obtaining or keeping health or life insurance due to donation. These types of experiences may serve as major disincentives to donation, and the development of strategies to mitigate them is paramount to ensure that living donation remains a viable option for future transplant candidates.58–63
At the same time, however, many donors in our sample had positive psychological outcomes. Consistent with a large literature,15,17,19,23,37,64–66 donors overwhelmingly endorsed positive feelings about the donation and would make the same decision to donate again. Similar to another recent study in living kidney and liver donors,67 our sample reported average levels of personal psychological growth from the experience comparable to those in populations exposed to other psychologically and/or physically stressful events.68–73 They were equally or more likely than other types of donors to feel that they were “better persons” for having donated.74–76
How can we reconcile or integrate these disparate perceptions of donation impact across domains of psychosocial well-being? Our cluster analysis results suggest an integration, showing that there are distinct donor groups, identifiable based on their response profiles across the multiple domains. Five different groups emerged. One group showed very high psychological benefit with low levels of physical health or socioeconomic concerns. Clearly, this profile is most desirable, and 15% of donors fell into this group. The remaining 85% fell into four groups, each of which reflected less than optimal combinations of degree of psychological benefit versus degrees of physical or socioeconomic concerns. Particularly noteworthy is the largest single group emerging in the analysis: donors who were the most likely to have physical health concerns in every area assessed, had incurred socioeconomic costs, and showed only modest psychological benefit (31% of the sample).
We believe that the cluster analysis results are clinically significant: care providers routinely seek to integrate multiple, often apparently disparate patient responses into a meaningful whole. In the context of living donation, these providers are vitally concerned with understanding and predicting the overall cost-benefit profile a donor is likely to achieve. Patient-reported outcomes, based on donor perceptions of the consequences of donation, are critical to understanding relative costs and benefits. But previous donor studies fail to empirically integrate data collected on multiple patient-reported outcomes. Our findings suggest what may be typical response profiles across three psychosocial dimensions in liver donors in the long-term postdonation.
Equally critical are our results regarding predictors of which donors were most likely to fall into certain cluster groups. Although we considered time since donation plus a range of demographic and clinical factors assessed either pre-donation, perioperatively, or during the first year postdonation, only three demographic characteristics emerged as significant risk factors. Thus, men and non-Hispanic whites were more likely to fall into cluster groups with less than optimal combinations of psychological benefit versus physical and socioeconomic concerns. Older donors (aged >30, particularly those in the >40 to 50 age group) were at reduced risk of falling into these groups. Conversely, the donor group with the highest psychological benefits and generally low physical and socioeconomic concerns was most likely to consist of women, racial/ethnic minorities, and older donors—namely those aged >40 to 50. These results are an important addition to the larger literature examining living donors’ psychosocial outcomes: this literature has yielded mixed, contradictory findings concerning whether and the direction in which demographic factors, such as those we identified, predict donor psychosocial outcomes6—perhaps because the outcomes have been considered piecemeal rather than in terms of complete response profiles. Given our descriptive findings that important effects exist, future research is needed to explore why certain demographic groups would be less likely to report favorable profiles of benefits vs. concerns.
In addition to examining psychosocial outcome profiles across all donors, we examined donor responses concerning recipient death. We observed very low average levels of perceived guilt or responsibility for the death. In fact, the greater the time since donation, the less likely donors were to experience these feelings, regardless of how long it had been since the death and how soon posttransplant it occurred. We do not believe this indicates that recipient death fails to affect donors; as we and others have previously found,7,29 donors typically assert that, while they may experience profound grief, they take great comfort knowing they did everything possible to help their recipient.
Our study has important limitations. First, the study was cross-sectional; we could not examine whether donors’ psychosocial status or general HRQOL changed from pre- to postdonation. However, our psychosocial outcomes were donation-specific and could not logically be assessed predonation, and we also compared general HRQOL to that of both normative data and a healthy national survey sample to put our sample in perspective. Second, we studied adult-to-adult donors from North American centers; generalizability to pediatric settings, and whether our findings concerning, for example, financial issues would generalize to other geographical regions is unknown. Nevertheless, we know of no cross-continent comparisons of long-term psychosocial outcomes. Third, we observed several demographic differences between survey responders and nonresponders; this also may reduce our findings’ generalizability. However, our response rate was higher than in previous large long-term HRQOL follow-up studies after liver donation.31,33 Fourth, a considerable time gap existed between donation and the survey. Although we have information on factors such as complications during the first year postdonation, a time period during which donors were followed closely by their centers, our donors’ interim psychosocial and medical status until the point of follow-up is unknown. This precluded us from examining long-term psychosocial status in relation to other factors across the entire postdonation period. Fifth, there are other potentially important risk factors for donor psychosocial outcomes that we did not assess, including predonation ambivalence about donating; family social supports; donor psychiatric status and the development of depressive or anxiety disorders postdonation; and surviving recipients’ well-being.6,7 Finally, we did not assess non-donor comparison groups to ascertain results unique to our donors. However, donation-specific outcomes are not relevant to comparison groups, and we marshalled both normative and healthy population-level comparative data for our generic HRQOL assessments.
Despite limitations, our study provides important information regarding the range of psychosocial outcomes observed across multiple dimensions in living liver donors assessed up to 10 years postdonation. The identification of specific response profiles across dimensions may be useful clinically: findings that basic, easily assessed demographic risk factors predict which donors may fall into less favorable psychosocial outcome profiles may allow for better targeting of both (a) clinical surveillance and care, and (b) additional education both pre- and postdonation regarding donation-related risks and potential health concerns. Specifically, our findings suggest that men, non-Hispanic white donors, and younger donors should be targeted for surveillance and heightened education. Future work is needed to determine whether the observed response profiles generalize to other cohorts, and whether there are other prominent patterns that we did not uncover. In addition, a richer array of risk factors, in a cohort followed prospectively, may allow for identification of additional characteristics that affect the likelihood of donor psychosocial difficulties in the years after liver donation, and could further inform donor education and clinical care.
Supplementary Material
Acknowledgments
3. Funding
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases through cooperative agreements (grants U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62494, U01-DK62498, U01-DK62531, U01-DK62536, U01-DK85515, U01-DK85563, and U01-DK85587).
The following individuals were instrumental in the planning and conduct of this study at each of the participating institutions:
Columbia University Medical Center, New York, NY (DK62483): PI: Jean C. Emond, MD; Co-Is: Robert S. Brown, Jr., MD, MPH, James Guarrera, MD, FACS, Benjamin Samstein, MD, Elizabeth Verna, MD, MS; Study Coordinators: Theresa Lukose, PharmD, Connie Kim, BS, Tarek Mansour, MB BCH, Joseph Pisa, BA, Jonah Zaretsky, BS.
Lahey Hospital & Medical Center, Burlington, MA (DK85515): PI: Elizabeth A. Pomfret, MD, PhD, FACS; Co-Is: Christiane Ferran, MD, PhD, Fredric Gordon, MD, James J. Pomposelli, MD, PhD, FACS, Mary Ann Simpson, PhD; Study Coordinators: Erick Marangos, Agnes Trabucco, BS, MTASCP.
Northwestern University, Chicago, IL (DK62467): PI: Michael M.I. Abecassis, MD, MBA; CoIs: Talia B. Baker, MD, Zeeshan Butt, PhD, Laura M. Kulik, MD, Daniela P. Ladner, MD, Donna M. Woods, PhD; Study Coordinators: Patrice Al-Saden, RN, CCRC, Tija Berzins, Amna Daud, MD, MPH, Elizabeth Rauch, BS, Teri Strenski, PhD, Jessica Thurk, BA, MA, Erin Wymore, BA, MS, CHES.
University of California San Francisco, San Francisco, CA (DK62444): PI: Chris E. Freise, MD, FACS; Co-I: Norah A. Terrault, MD, MPH; Study Coordinators: Alexandra Birch, BS, Dulce MacLeod, RN.
University of Colorado, Aurora, CO (DK62536): PI: James R. Burton, Jr., MD; Co-Is: Gregory T. Everson, MD, FACP, Michael A. Zimmerman, MD; Study Coordinator: Jessica Fontenot, BS.
University of Michigan Health System, Ann Arbor, MI (DK62498): PI: Robert M. Merion, MD, FACS; DCC Staff: Yevgeniya Abramovich, BA, Charlotte A. Beil, MPH, Carl L. Berg, MD, Abby Brithinee, BA, Tania C. Ghani, MS, Brenda W. Gillespie, PhD, Beth Golden, BScN, Margaret Hill-Callahan, BS, LSW, Lisa Holloway, BS, CCRC, Terese A. Howell, BS, CCRC, Anna S.F. Lok, MD, Monique Lowe, MSI, Anna Nattie, BA, Gary Xia, BA.
University of Pennsylvania, Philadelphia, PA (DK62494): PI: Kim M. Olthoff, MD; Co-Is: Abraham Shaked, MD, PhD, David S. Goldberg, MD, Karen L. Krok, MD, Mark A. Rosen, MD, PhD, Robert M. Weinrieb, MD; Study Coordinators: Debra McCorriston, RN, Mary Shaw, RN, BBA.
University of Pittsburgh, Pittsburgh, PA (DK85587): PI: Abhinav Humar, MD; Co-Is: Andrea F. DiMartini, MD, Mary Amanda Dew, PhD, Mark Sturdevent, MD; Study Coordinators: Megan Basch, RN, Sheila Fedorek, RN, CCRC, Leslie Mitrik, BS.
University of Toronto, Toronto, ON, CA (DK85563): PI: David Grant, MD, FRCSC; Co-Is: Oyedele Adeyi, MD, FCAP, FRCPC, Susan Abbey, MD, FRCPC, Hance Clarke, MSc, MD, FRCPC, Susan Holtzman, PhD, Joel Katz, CRC, PhD, Gary Levy, BSc, FRCPC, MD, Nazia Selzner, MD, PhD; Study Coordinators: Kimberly Castellano, BSc, Andrea Morillo, BM, BCh, Erin Winter, BSc.
Virginia Commonwealth University - Medical College of Virginia Campus, Richmond, VA (DK62531): PI: Adrian H. Cotterell, MD, FACS; Co-Is: Robert A. Fisher, MD, FACS, Ann S. Fulcher, MD, Mary E. Olbrisch, PhD, ABPP, R. Todd Stravitz, MD, FACP; Study Coordinators: April Ashworth, RN, BSN, Joanne Davis, RN, Sarah Hubbard, Andrea Lassiter, BS, Luke Wolfe, MS.
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: Edward Doo, MD, James E. Everhart, MD, MPH, Jay H. Hoofnagle, MD, Stephen James, MD, Patricia R. Robuck, PhD, Averell H. Sherker, MD, FRCPC, Rebecca J. Torrance, RN, MS.
Heather Van Doren, MFA, coordinating senior editor with Arbor Research Collaborative for Health, provided editorial assistance on this manuscript.
ABBREVIATIONS
- A2ALL
Adult to Adult Living Donor Liver Transplantation Cohort Study
- BMI
body mass index
- CATI
computer-assisted telephone interview
- CI
confidence interval
- HRQOL
health-related quality of life
- IQR
interquartile range
- M
mean
- MCS
Mental Component Summary
- OR
odds ratio
- PCS
Physical Component Summary
- SD
standard deviation
- SF 36
Short-Form-36
Footnotes
1. Contributions to the work
Mary Amanda Dew: research conceptualization, design, data collection, data analysis, interpretation of analysis, manuscript preparation, revision of the manuscript, final approval of the manuscript
Andrea F. DiMartini: research conceptualization, design, data collection, interpretation of analysis, manuscript preparation, revision of the manuscript, final approval of the manuscript
Daniela P. Ladner: research conceptualization, design, interpretation of analysis, revision of the manuscript, final approval of the manuscript
Mary Ann Simpson: research conceptualization, interpretation of analysis, manuscript preparation, revision of the manuscript, final approval of the manuscript
Elizabeth A. Pomfret: research conceptualization, interpretation of analysis, manuscript preparation, revision of the manuscript, final approval of the manuscript
Brenda W. Gillespie: design, data analysis, interpretation of analysis, manuscript preparation, revision of the manuscript, final approval of the manuscript
Robert M. Merion: research conceptualization, design, interpretation of analysis, revision of the manuscript, final approval of the manuscript
Jarcy Zee: data analysis, interpretation of analysis, manuscript preparation, revision of the manuscript, final approval of the manuscript
Abigail R. Smith: data analysis, interpretation of analysis, revision of the manuscript, final approval of the manuscript
Susan Holtzman: research conceptualization, interpretation of analysis, revision of the manuscript, final approval of the manuscript
Averell H. Sherker: interpretation of analysis, revision of the manuscript, final approval of the manuscript
Robert Weinrieb: interpretation of analysis, revision of the manuscript, final approval of the manuscript
Robert A. Fisher: research conceptualization, interpretation of analysis, revision of the manuscript, final approval of the manuscript
Jean C. Emond: research conceptualization, interpretation of analysis, revision of the manuscript, final approval of the manuscript
Chris E. Freise: research conceptualization, interpretation of analysis, revision of the manuscript, final approval of the manuscript
James R. Burton, Jr.: interpretation of analysis, revision of the manuscript, final approval of the manuscript
Zeeshan Butt: research conceptualization, design, data collection, data analysis, interpretation of analysis, manuscript preparation, revision of the manuscript, final approval of the manuscript
2. Disclosures
The authors declare no conflicts of interest.
This study was presented in part at the 13th annual meeting of the American Transplant Congress, Seattle, WA, May 19, 2013.
This is publication number #31 of the Adult to Adult Living Donor Liver Transplantation Cohort Study.
References
- 1.Barr ML, Belghiti J, Villamil FG, et al. A report of the Vancouver Forum on the care of the live organ donor: Lung, liver, pancreas, and intestine data and medical guidelines. Transplantation. 2006;81(10):1373–1385. doi: 10.1097/01.tp.0000216825.56841.cd. [DOI] [PubMed] [Google Scholar]
- 2.Brown RS, Jr, Higgins R, Pruett TL. The evolution and direction of OPTN oversight of live organ donation and transplantation in the United States. Am J Transplant. 2009;9(1):31–34. doi: 10.1111/j.1600-6143.2008.02433.x. [DOI] [PubMed] [Google Scholar]
- 3.Organ Procurement and Transplantation Network/United Network for Organ Sharing (OPTN/UNOS) [Last accessed 12/9/15];OPTN Policies, Policy 14: Living donation. Updated 12/1/15. http://optn.transplant.hrsa.gov/governance/policies/
- 4.Pruett TL, Tibell A, Alabdulkareem A, et al. The ethics statement of the Vancouver Forum on the live lung, liver, pancreas, and intestine donor. Transplantation. 2006;81(10):1386–1387. doi: 10.1097/01.tp.0000214976.36526.e3. [DOI] [PubMed] [Google Scholar]
- 5.Simpson MA, Pomfret EA. Checking the harness: Safety for living liver donors. Liver Transplant. 2012;18(Suppl 2):S15–S19. doi: 10.1002/lt.23525. [DOI] [PubMed] [Google Scholar]
- 6.Dew MA, Zuckoff A, DiMartini AF, et al. Prevention of poor psychosocial outcomes in living organ donors: From description to theory-driven intervention development and initial feasibility testing. Prog Transplant. 2012;22(3):280–293. doi: 10.7182/pit2012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dew MA, Switzer GE, DiMartini AF, Myaskovsky L, Crowley-Matoka M. Psychosocial aspects of living organ donation. In: Tan HP, Marcos A, Shapiro R, editors. Living Donor Organ Transplantation. NY: Taylor and Francis; 2007. pp. 7–26. [Google Scholar]
- 8.DuBay DA, Holtzman S, Adcock L, et al. Adult right-lobe living liver donors: Quality of life, attitudes and predictors of donor outcomes. Am J Transplant. 2009;9(5):1169–1178. doi: 10.1111/j.1600-6143.2009.02614.x. [DOI] [PubMed] [Google Scholar]
- 9.Holtzman S, Adcock L, Dubay D, et al. Financial, vocational, and interpersonal impact of living liver donation. Liver Transplant. 2009;15(11):1435–1442. doi: 10.1002/lt.21852. [DOI] [PubMed] [Google Scholar]
- 10.Jowsey SG, Schneekloth TD. Psychosocial factors in living organ donation: Clinical and ethical challenges. Transplant Rev. 2008;22(3):192–195. doi: 10.1016/j.trre.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Miller-Matero LR, Eshelman A, Paulson D, et al. Beyond survival: How well do transplanted livers work? A preliminary comparison of standard-risk, high-risk, and living donor recipients. Clin Transplant. 2014;28(6):691–698. doi: 10.1111/ctr.12368. [DOI] [PubMed] [Google Scholar]
- 12.Parikh ND, Ladner DP, Abecassis MM, Butt Z. Quality of life for donors after living donor liver transplantation: A review of the literature. Liver Transplant. 2010;16(12):1352–1358. doi: 10.1002/lt.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan SC, Liu CL, Lo CM, Lam BK, Lee EW, Fan ST. Donor quality of life before and after adult-to-adult right live donor liver transplantation. Liver Transplant. 2006;12(10):1529–1536. doi: 10.1002/lt.20897. [DOI] [PubMed] [Google Scholar]
- 14.Feltrin A, Pegoraro R, Rago C, et al. Experience of donation and quality of life in living kidney and liver donors. Transpl Int. 2008;21(5):466–472. doi: 10.1111/j.1432-2277.2007.00632.x. [DOI] [PubMed] [Google Scholar]
- 15.Humar A, Carolan E, Ibrahim H, et al. A comparison of surgical outcomes and quality of life surveys in right lobe vs. left lateral segment liver donors. Am J Transplant. 2005;5(4 Pt 1):805–809. doi: 10.1111/j.1600-6143.2005.00767.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim-Schluger L, Florman SS, Schiano T, et al. Quality of life after lobectomy for adult liver transplantation. Transplantation. 2002;73(10):1593–1597. doi: 10.1097/00007890-200205270-00012. [DOI] [PubMed] [Google Scholar]
- 17.Kusakabe T, Irie S, Ito N, Kazuma K. Feelings of living donors about adult-to-adult living donor liver transplantation. Gastroenterol Nurs. 2008;31(4):263–272. doi: 10.1097/01.SGA.0000334032.48629.c0. [DOI] [PubMed] [Google Scholar]
- 18.Jin SG, Xiang B, Yan LN, et al. Quality of life and psychological outcome of donors after living donor liver transplantation. World J Gastroenterol. 2014;18(2):182–187. doi: 10.3748/wjg.v18.i2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karliova M, Malagó M, Valentin-Gamazo V, et al. Living-related liver transplantation from the view of the donor: A 1-year follow-up survey. Transplantation. 2002;73(11):1799–1804. doi: 10.1097/00007890-200206150-00017. [DOI] [PubMed] [Google Scholar]
- 20.Kroencke S, Nashan B, Fischer L, Erim Y, Schulz KH. Donor quality of life up to two years after living donor liver transplantation: A prospective study. Transplantation. 2014;97(5):582–589. doi: 10.1097/01.TP.0000438206.04348.b2. [DOI] [PubMed] [Google Scholar]
- 21.Schulz KH, Kroencke S, Beckmann M, et al. Mental and physical quality of life in actual living liver donors versus potential living liver donors: A prospective, controlled, multicenter study. Liver Transplant. 2009;15(12):1676–1687. doi: 10.1002/lt.21917. [DOI] [PubMed] [Google Scholar]
- 22.Walter M, Bronner E, Pascher A, et al. Psychosocial outcome of living donors after living donor liver transplantation: A pilot study. Clin Transplant. 2002;16(5):339–344. doi: 10.1034/j.1399-0012.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- 23.Verbesey JE, Simpson MA, Pomposelli JJ, et al. Living donor adult liver transplantation: A longitudinal study of the donor’s quality of life. Am J Transplant. 2005;5(11):2770–2777. doi: 10.1111/j.1600-6143.2005.01092.x. [DOI] [PubMed] [Google Scholar]
- 24.Trotter JF, Talamantes M, McClure M, et al. Right hepatic lobe donation for living donor liver transplantation: Impact on donor quality of life. Liver Transplant. 2001;7(6):485–493. doi: 10.1053/jlts.2001.24646. [DOI] [PubMed] [Google Scholar]
- 25.Walter M, Papachristou C, Pascher A, et al. Impaired psychosocial outcome of donors after living donor liver transplantation: A qualitative case study. Clin Transplant. 2006;20(4):410–415. doi: 10.1111/j.1399-0012.2006.00464.x. [DOI] [PubMed] [Google Scholar]
- 26.Yang RC, Thiessen-Philbrook H, Klarenbach S, Vlaicu S, Garg AX. Insurability of living organ donors: A systematic review. Am J Transplant. 2007;7(6):1542–1551. doi: 10.1111/j.1600-6143.2007.01793.x. [DOI] [PubMed] [Google Scholar]
- 27.Erim Y, Beckmann M, Valentin-Gamazo C, et al. Quality of life and psychiatric complications after adult living donor liver transplantation. Liver Transplant. 2006;12(12):1782–1790. doi: 10.1002/lt.20907. [DOI] [PubMed] [Google Scholar]
- 28.Sondenaa K, Gondolesi GE, Roayaie S, Goldman JS, Hausken T, Schwartz ME. Functional abdominal complaints occurred frequently in living liver donors after donation. Scand J Gastroenterol. 2011;46(5):611–615. doi: 10.3109/00365521.2010.537685. [DOI] [PubMed] [Google Scholar]
- 29.Crowley-Matoka M, Siegler M, Cronin DC., II Long-term quality of life issues among adult-to-pediatric living liver donors: A qualitative exploration. Am J Transplant. 2004;4(5):744–750. doi: 10.1111/j.1600-6143.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- 30.Jay CL, Butt Z, Ladner DP, Skaro AI, Abecassis MM. A review of quality of life instruments used in liver transplantation. J Hepatol. 2009;51(5):949–959. doi: 10.1016/j.jhep.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ladner DP, Dew MA, Forney S, et al. Long-term quality of life after liver donation in the Adult to Adult Living Donor Liver Transplantation Cohort Study (A2ALL) J Hepatol. 2015;62(2):346–353. doi: 10.1016/j.jhep.2014.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Humphreville VR, Radosevich DM, Humar A, et al. Longterm health-related quality of life after living donor liver transplantation. Liver Transplant. 2015;22(1):53–62. doi: 10.1002/lt.24304. [DOI] [PubMed] [Google Scholar]
- 33.Takada Y, Suzukamo Y, Oike F, et al. Long-term quality of life of donors after living donor liver transplantation. Liver Transplant. 2012;18(11):1343–1352. doi: 10.1002/lt.23509. [DOI] [PubMed] [Google Scholar]
- 34.Kempf AM, Remington PL. New challenges for telephone survey research in the twenty-first century. Ann Rev Public Health. 2007;28:113–126. doi: 10.1146/annurev.publhealth.28.021406.144059. [DOI] [PubMed] [Google Scholar]
- 35.Lavrakas PJ. Telephone survey methods: Sampling, selection, and supervision. Newbury Park, CA: Sage; 1993. [Google Scholar]
- 36.Dew MA, DiMartini AF, DeVito Dabbs AJ, et al. Preventive intervention for living donor psychosocial outcomes: Feasibility and efficacy in a randomized controlled trial. Am J Transplant. 2013;13(10):2672–2684. doi: 10.1111/ajt.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmons RG, Simmons RL, Marine SK. Gift of life: The effect of organ transplantation on individual, family, and societal dynamics. New Brunswick, NJ: Transaction Books; 1987. [Google Scholar]
- 38.DiMartini A, Porterfield K, Fitzgerald MG, et al. Psychological profiles of living liver donors and post-donation outcomes. In: Weimar W, Bos MA, van Busschbach JJ, editors. Organ transplantation: Ethical, legal and psychological aspects. Towards a common European policy. Lengerich, Germany: Pabst Science Publishers; 2008. pp. 216–220. [Google Scholar]
- 39.Switzer GE, Goycoolea JM, Dew MA, Graeff EC, Hegland J. Donating stimulated peripheral blood stem cells vs bone marrow: Do donors experience the procedures differently? Bone Marrow Transplant. 2001;27(9):917–923. doi: 10.1038/sj.bmt.1703011. [DOI] [PubMed] [Google Scholar]
- 40.DiMartini A, Cruz R, Dew MA, et al. Motives and decision making of potential living liver donors: Comparisons between gender, relationships and ambivalence. Am J Transplant. 2012;12(1):136–151. doi: 10.1111/j.1600-6143.2011.03805.x. [DOI] [PubMed] [Google Scholar]
- 41.Cann A, Calhoun LG, Tedeschi RG, et al. A short form of the Posttraumatic Growth Inventory. Anxiety, Stress, Coping. 2010;23(2):127–137. doi: 10.1080/10615800903094273. [DOI] [PubMed] [Google Scholar]
- 42.Holtzman S, Adcock L, Dubay DA, et al. Financial, vocational, and interpersonal impact of living liver donation. Liver Transplant. 2009;15(11):1435–1442. doi: 10.1002/lt.21852. [DOI] [PubMed] [Google Scholar]
- 43.Ware JE, Kosinski M, Bjorner JB, Turner-Bowker DM, Gondek B, Maruish ME. User’s manual for the SF-36v2 Health survey. 2. Lincoln, RI: Quality Metric; 2007. [Google Scholar]
- 44.Ware JE., Jr . SF-36 health survey update. In: Maruish ME, editor. The use of psychological testing for treatment planning and outcomes assessment. 3. Vol. 3. Hillsdale, NJ: Lawrence Erlbaum; 2004. pp. 693–718. [Google Scholar]
- 45.Fryback DG, Dunham NC, Palta M, et al. US Norms for Six Generic Health-Related Quality-of-Life Indexes From the National Health Measurement Study. Med Care. 2007;45(12):1162–1170. doi: 10.1097/MLR.0b013e31814848f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fryback Dennis G. United States National Health Measurement Study, 2005–2006. Ann Arbor, MI: Inter-university Consortium for Political and Social Research [distributor]; 2009. Jun 23, ICPSR23263-v1. http://doi.org/10.3886/ICPSR23263.v1 or http://www.icpsr.umich.edu/icpsrweb/ICPSR/studies/23263?searchSource=find-analyze-home&sortBy=&q=national+health+measurement+study. [Google Scholar]
- 47.Everitt BS, Landau S, Leese M, Stahl D. Cluster analysis. 5. Chichester, UK: John Wiley & Sons; 2011. [Google Scholar]
- 48.Romesburg C. Cluster analysis for researchers. Morrisville, NC: Lulu Press; 2004. [Google Scholar]
- 49.Harrell FE. Regression modeling strategies: With applications to linear models, logistic regression, and survival analysis. New York: Springer-Verlag; 2001. [Google Scholar]
- 50.Tabachnick BG, Fidell LS. Using multivariate statistics. 6. New York: Pearson; 2013. [Google Scholar]
- 51.Raghunathan TE, Solenberger PW, John Van Hoewyk J. IVEware: Imputation and Variance Estimation Software User Guide. Survey Methodology Program, Survey Research Center, Institute for Social Research, University of Michigan; Ann Arbor, MI: 2002. [Google Scholar]
- 52.Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholescystectomy. Surgery. 1992;111(5):518–526. [PubMed] [Google Scholar]
- 53.Clavien PA, Camargo CA, Jr, Croxford R, Langer B, Levy GA, Greig PD. Definition and classification of negative outcomes in solid organ transplantation. Application in liver transplantation. Ann Surg. 1994;220(2):109–120. doi: 10.1097/00000658-199408000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abecassis MM, Fisher RA, Olthoff KM, et al. Complications of living donor hepatic lobectomy: A comprehensive report. Am J Transplant. 2012;12(5):1208–1217. doi: 10.1111/j.1600-6143.2011.03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 56.Clemens K, Boudville N, Dew MA, et al. The long-term quality of life of living kidney donors: A multicenter cohort study. Am J Transplant. 2011;11(3):463–469. doi: 10.1111/j.1600-6143.2010.03424.x. [DOI] [PubMed] [Google Scholar]
- 57.Lamberts MP, Lugtenberg M, Rovers MM, et al. Persistent and de novo symptoms after cholecystectomy: a systematic review of cholecystectomy effectiveness. Surgl Endosc. 2013;27(3):709–718. doi: 10.1007/s00464-012-2516-9. [DOI] [PubMed] [Google Scholar]
- 58.Delmonico FL, Martin D, Domínguez-Gil B, et al. Living and deceased organ donation should be financially neutral acts. Am J Transplant. 2015;15(5):1187–1191. doi: 10.1111/ajt.13232. [DOI] [PubMed] [Google Scholar]
- 59.Fisher JS, Butt Z, Friedewald J, et al. Between scylla and charybdis: Charting an ethical course for research into financial incentives for living kidney donation. Am J Transplant. 2015;15(5):1180–1186. doi: 10.1111/ajt.13234. [DOI] [PubMed] [Google Scholar]
- 60.LaPointe Rudow D, Hays R, et al. Consensus conference on best practices in live kidney donation: Recommendations to optimize education, access, and care. Am J Transplant. 2015;15(4):914–922. doi: 10.1111/ajt.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pomfret EA. Life insurability of the right lobe live liver donor. Liver Transplant. 2005;11(7):739–740. doi: 10.1002/lt.20476. [DOI] [PubMed] [Google Scholar]
- 62.Salomon DR, Langnas AN, Reed AI, Bloom RD, Magee JC, Gaston RS for the AST/ASTS Incentives Workshop Group (IWG) AST/ASTS workshop on increasing organ donation in the United States: Creating an “arc of change” from removing disincentives to testing incentives. Am J Transplant. 2015;15(5):1173–1179. doi: 10.1111/ajt.13233. [DOI] [PubMed] [Google Scholar]
- 63.Sickand M, Cuerden MS, Klarenbach SW, et al. Reimbursing live organ donors for incurred non-medical expenses: A global perspective on policies and programs. Am J Transplant. 2009;9(12):2825–2836. doi: 10.1111/j.1600-6143.2009.02829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fehrman-Ekholm I, Brink B, Ericsson C, Elinder CG, Dunér F, Lundgren G. Kidney donors don’t regret: Follow-up of 370 donors in Stockholm since 1964. Transplantation. 2000;69(10):2067–2071. doi: 10.1097/00007890-200005270-00016. [DOI] [PubMed] [Google Scholar]
- 65.Reichman TW, Fox A, Adcock L, et al. Anonymous living liver donation: Donor profiles and outcomes. Am J Transplant. 2010;10(9):2099–2104. doi: 10.1111/j.1600-6143.2010.03244.x. [DOI] [PubMed] [Google Scholar]
- 66.Sharp J, McRae A, McNeill Y. Decision making and psychosocial outcomes among living kidney donors: A pilot study. Prog Transplant. 2010;20(1):53–57. doi: 10.1177/152692481002000109. [DOI] [PubMed] [Google Scholar]
- 67.Rudow DL, Iacoviello BM, Charney D. Resilience and personality traits among living liver and kidney donors. Prog Transplant. 2014;24(1):82–90. doi: 10.7182/pit2014448. [DOI] [PubMed] [Google Scholar]
- 68.Blix I, Hansen MB, Birkeland MS, Nissen A, Heir T. Posttraumatic growth, posttraumatic stress and psychological adjustment in the aftermath of the 2011 Oslo bombing attack. Health Qual Life Outcomes. 2013;11:160. doi: 10.1186/1477-7525-11-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lelorain S, Tessier P, Florin A, Bonnaud-Antignac A. Posttraumatic growth in long term breast cancer survivors: Relation to coping, social support and cognitive processing. J Health Psychol. 2012;17(5):627–639. doi: 10.1177/1359105311427475. [DOI] [PubMed] [Google Scholar]
- 70.Leung YW, Alter DA, Prior PL, et al. Posttraumatic growth in coronary artery disease outpatients: Relationship to degree of trauma and health service use. J Psychosom Res. 2012;72(4):293–299. doi: 10.1016/j.jpsychores.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lowe SR, Manove EE, Rhodes JE. Posttraumatic stress and posttraumatic growth among low-income mothers who survived Hurricane Katrina. J Consult Clin Psychol. 2013;81(5):877–889. doi: 10.1037/a0033252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sanders A, Szymanski K. Siblings of people diagnosed with a mental disorder and posttraumatic growth. Community Mental Health J. 2013;49(5):554–559. doi: 10.1007/s10597-012-9498-x. [DOI] [PubMed] [Google Scholar]
- 73.Sheikh AI. Posttraumatic growth in the context of heart disease. J Clin Psychol Med Settings. 2004;11(4):265–273. [Google Scholar]
- 74.Butterworth VA, Simmons RG, Bartsch G, Randall B, Schimmel M, Stroncek DF. Psychosocial effects of unrelated bone marrow donation: Experiences of the National Marrow Donor Program. Blood. 1993;81(7):1947–1959. [PubMed] [Google Scholar]
- 75.Corley MC, Elswick RK, Sargeant CC, Scott S. Attitude, self-image, and quality of life of living kidney donors. Nephrol Nurs J. 2000;27(1):43–50. [PubMed] [Google Scholar]
- 76.Molassiotis A, Holroyd E. Assessment of psychosocial adjustment in Chinese unrelated bone marrow donors. Bone Marrow Transplant. 1999;24(8):903–910. doi: 10.1038/sj.bmt.1702000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.