Abstract
Non-alcoholic fatty liver disease (NAFLD) is a common liver disease that ranges from simple steatosis to non-alcoholic steatohepatitis (NASH). So far, the underlying mechanism remains poorly understood. Here we show that hepatic carboxylesterase 2 (CES2) is markedly reduced in NASH patients, diabetic db/db mice and high fat diet (HFD)-fed mice. Restoration of hepatic CES2 expression in db/db or HFD-fed mice markedly ameliorates liver steatosis and insulin resistance. In contrast, knockdown of hepatic CES2 causes liver steatosis and damage in chow or Western diet-fed C57BL/6 mice. Mechanistically, we demonstrate that CES2 has TG hydrolase activity. As a result, gain of hepatic CES2 function increases fatty acid oxidation and inhibits lipogenesis whereas loss of hepatic CES2 stimulates lipogenesis by inducing ER stress. We further show that loss of hepatic CES2 stimulates lipogenesis in a sterol regulator element-binding protein 1 (SREBP-1)-dependent manner. Finally, we show that hepatocyte nuclear factor 4α (HNF4α) plays a key role in controlling hepatic CES2 expression in diabetes, obesity or NASH.
Conclusions
The current study indicates that CES2 plays a protective role in the development of NAFLD. Targeting the HNF4α-CES2 pathway may be useful for treatment of NAFLD.
Keywords: Carboxylesterase 2, lipolysis, steatosis, lipogenesis and HNF4α
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common, chronic liver disease worldwide. NAFLD initiates from simple steatosis, which may progress to non-alcoholic steatohepatitis (NASH), liver cirrhosis and liver carcinoma. So far, the mechanism underlying the pathogenesis of human NAFLD is poorly understood (1).
NAFLD is often associated with conditions that cause increased lipogenesis, including diabetes, obesity and insulin resistance. Inhibition of lipogenic pathways prevents liver steatosis in diabetic or diet-induced obese animals (2, 3), suggesting that uncontrolled lipogenesis contributes to the development of NAFLD in animals. Nonetheless, the contribution of lipogenesis to human NAFLD remains to be determined.
Recent studies have identified defective lipolysis as an important mechanism underlying the pathogenesis of human NAFLD (4). Patients with NAFLD have reduced expression of adipose triglyceride lipase (ATGL) (5). Mutations in human ATGL cause neutral lipid storage disease with myopathy (6). A common single nucleotide polymorphism in PNPLA3 (rs738409; PNPLA3I148M), a membrane-bound lipase, is associated with human liver steatosis but not with other metabolic disease (7–9), and causes hepatic triglyceride (TG) accumulation in mice (10). A deficiency in hepatic Atgl (11, 12) or carboxylesterase 1 (Ces1) (13), two of the hepatic TG lipases, also induces liver steatosis in mice. These data underscore the importance of lipolysis to hepatic TG homeostasis. Nonetheless, hepatic Ces1 expression is induced in diabetic mice (13).
In this report, we show that carboxylesterase 2 (CES2), a highly abundant esterase in both the liver and intestine, is reduced in the livers of NASH patients, diabetic db/db mice and high fat diet (HFD)-induced obese mice. Restoration of hepatic Ces2 expression in db/db or HFD-fed mice improves liver steatosis and insulin sensitivity whereas loss of hepatic Ces2 results in liver steatsosis and liver damage in both chow and Western diet-fed mice. Over-expression of human CES2 has similar beneficial effects. We further show that CES2 has TG hydrolase (TGH) activity and regulates hepatic TG levels by modulating lipolysis, endoplasmic reticulum (ER) stress and lipogenesis. Finally, we show that hepatocyte nuclear factor 4α (HNF4α), whose expression is markedly reduced in NASH, diabetes and obesity (14), regulates CES2 expression in vivo. Our data suggest that the HNF4α-CES2 pathway plays an important role in the pathogenesis of NAFLD.
Experimental Procedures
Human liver tissues, mice and diets
C57BL/6 mice, db/db mice and Srebp1−/−, Hnf4αfl/fl mice were purchased from the Jackson Laboratories (Bar Harbor, Maine, USA). Liver-specific Hnf4α−/− mice (L-Hnf4α−/−) were generated by crossing albumin-Cre mice with Hnf4αfl/fl mice. For high fat diet (HFD) feeding, C57BL/6J mice were fed a chow diet or an HF diet (60% kcal from fat; Research Diets, New Brunswick, NJ, USA) for 12 weeks. For Western diet (WD) feeding, C57BL/6J mice were fed a chow diet or a WD diet (42% kcal fat/0.2% cholesterol; Harlan Laboratories, Madison, WI, USA) for 2 weeks. Unless otherwise stated, male mice were used and all mice were fasted for 5–6 h prior to euthanization. Human liver samples were obtained from the Liver Tissue Cell Distribution System at University of Minnesota (14). All the animal experiments were approved by the Institutional Animal Care and Use Committee at Northeast Ohio Medical University (NEOMED). The use of human tissue samples were approved by the Institutional Review Board at NEOMED.
Western blot assays
Western blot assays were performed using whole liver lysates (15) or nuclear extracts of the liver samples (13, 16) as described previously.
Lipid and glucose analysis
Approximately 100 mg liver tissue was homogenized in methanol and lipids were extracted in chloroform/methanol (2:1 v/v) as described (17). Triglycerides, cholesterol, glucose and β-hydroxybutyrate in the liver or plasma were analyzed using commercially available kits.
VLDL secretion
The VLDL secretion study was performed as described (18).
Hepatic de novo lipogenesis
Mice were fasted for 4 h and then injected intraperitoneally with 2H2O (30 μl/g). After 4 h, liver and plasma were snap-frozen in liquid nitrogen. The newly synthesized triglycerides were measured by mass spectrometry at the MMPC in Case Western Reserve University.
Recombinant CES2 protein expression and purification
Full-length Ces2 cDNA was cloned to pET28c (Novagen, USA) and the CES2 protein was expressed in BL21/DE3 bacteria. CES2 protein was purified with Ni-NTA super agarose (Invitrogen, USA) and verified by Western blot assays. p-nitrophenyl butyrate (PNPB) (Sigma-Aldrich, USA) or [3H]triolein (Perkin Elmer, Hopkinton, MA) is used to measure its esterase or TGH activity.
TGH activity assays
Detailed information on TGH activity assays can be found in Supplementary Information.
Cell culture, transient transfection, and luciferase assays
Mouse primary hepatocytes were isolated and cultured as described (14). Four hours post plating, primary mouse hepatocytes were infected with adenoviruses for 48 h before harvest. HepG2 cells were cultured in DMEM containing 10% FBS. Transient transfections were performed in triplicate as described (19). Detailed information can be found in Supplementary Information.
Chromatin immunoprecipitation (ChIP) assays
ChIP assays were performed using liver lysates and HNF4α antibody as described previously (13, 18). qPCR was performed to amplify a region containing a known DR-1 element located within the proximal promoter of Ces1.
Fatty acid oxidation
Primary hepatocytes were cultured in DMEM containing 10% FBS in 12-well dishes and infected with either Ad-empty or Ad-Ces2. Fatty acid oxidation was performed using [3H]palmitate as substrate as described (13, 20).
Pulse-chase analysis of fatty acid release
To measure triglyceride hydrolysis, mouse primary hepatocytes were transduced with Ad-empty or Ad-Ces2 at an MOI of 20 in triplicate after attachment. Detailed information on the pulse-chase study can be found in Supplementary Information.
Oil red O staining and H & E staining
Liver and white adipose tissue were fixed in 4% formalin and then embedded in OCT or paraffin. Oil red O staining or HE staining was performed as descried (21).
Oxygen consumption and CO2 production
Mice fed a high fat diet were put in the Comprehensive Lab Animal Monitoring System (CLAMS). Food intake, oxygen consumption, CO2 production and heat production were determined as described previously (22).
Fatty acid analysis
Hepatic total free fatty acids were measured using a kit from Wako Chemicals USA (Richmond, VA). Hepatic fatty acid composition was analyzed by gas chromatography-mass spectrometry. Detailed information can be found in the Supplementary Information.
Statistical analysis
Statistical significance was analyzed using unpaired Student t test or ANOVA (GraphPad Prisim, CA). All values are expressed as mean±SEM. Differences were considered statistically significant at P<0.05.
Results
Hepatic CES2 expression is markedly reduced in db/db or HFD-fed mice and NASH patients
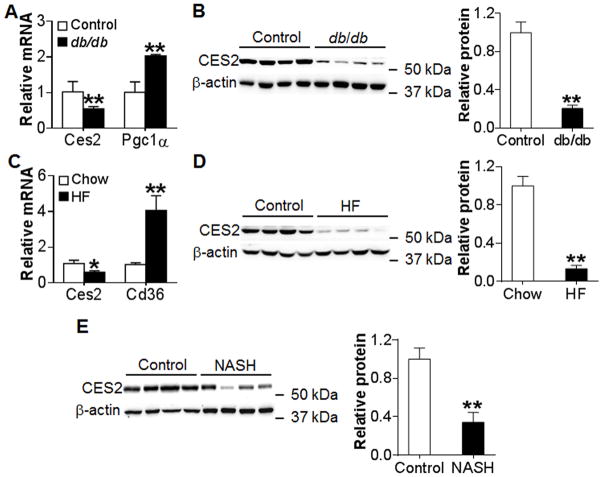
To investigate whether CES2 is associated with metabolic disorders, we examined hepatic CES2 expression in genetically obese db/db mice, HFD-fed obese mice and NASH patients. In both db/db mice (Figure 1A and 1B) and HFD-fed mice (Figure 1C and 1D), hepatic Ces2 mRNA level was reduced by 50% (Figure 1A and 1C) and CES2 protein level was reduced by 80–85% (Figure 1B and 1D). As expected, these mice had severe liver steatosis (Figure S1A and S1B). In human NASH patients, hepatic CES2 protein level was reduced by >60% (Figure 1E). In addition, hepatic CES2 protein level was also reduced by ~ 50% in patients with simple steatosis (Figure S1C and S1D). Thus, the data of Figure 1 indicate that hepatic CES2 is markedly reduced in diabetic db/db or HFD-fed mice and patients with NAFLD.
Figure 1. Hepatic CES2 expression is down-regulated in db/db mice, HFD-fed mice and NASH patients.
(A) Hepatic mRNA level in db/db mice (n=4). Pgc1α serves as a positive control. (B) Hepatic protein levels in db/db mice (left panel) and relative CES2 protein level (right panel) (n=4). (C) Hepatic mRNA levels in HFD-fed mice (n=6–9). Cd36 serves as a positive control. (D) Hepatic protein levels in HFD-fed mice (left panel) and relative CES2 protein level (right panel) (n=6–9). (E) Hepatic protein level in NASH patients (left panel) and relative CES2 protein level (right panel) (n=8). *P<0.05, **P<0.01
Restoration of hepatic CES2 expression in db/db mice alleviates fatty liver and improves glucose tolerance
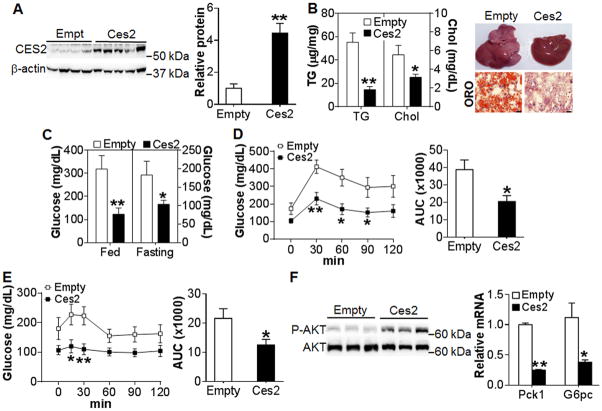
To determine whether CES2 plays a role in metabolism, we generated an adenovirus expressing Ces2. Over-expression of Ces2 in the liver of C57BL/6J mice had no effect on body weight (Figure S2A) but reduced hepatic TG levels by >30% (Figure S2C–S2D), suggesting that hepatic Ces2 may regulate TG metabolism. Since hepatic Ces2 expression was reduced by 5 fold in db/db mice (Figure 1B), we over-expressed Ces2 in db/db mice by 4 fold to recapitulate hepatic Ces2 expression (Figure 2A). Restoration of Ces2 expression reduced body weight (Figure S3A) and hepatic TG and cholesterol levels by 73% and 45%, respectively (Figure 2B, left panel), but had no effect on plasma TG or cholesterol level (Figure S3B and S3C). Oil red O staining of liver sections further confirmed a drastic reduction in neutral lipid accumulation (Figure 2B, right panel). The improvement in hepatic TG homeostasis was not associated with any change in whole body energy metabolism or activity (Figure S3D–S3G).
Figure 2. Restoration of hepatic Ces2 expression in db/db mice ameliorates metabolic disorder.
(A–F) db/db mice were injected i.v. with Ad-empty or Ad-Ces2 (n=7). After 13 days, mice were euthanized. (A) Hepatic protein levels (left panel) and relative CES2 protein level (right panel). (B) Hepatic TG and total cholesterol (TC) levels (left panel) and representative liver images (top) and oil red O (ORO) staining (bottom) of liver sections (right panel). Scale bars, 50 μm. (C) Postprandial glucose and fasting glucose levels on day 13. (D) GTT (left panel) and area under the curve (AUC) of GTT (right panel). (E) ITT (left panel) and AUC of ITT (right panel). (F) Hepatic phospho-AKT(Ser-473) level (left panel) and mRNA level (right panel). *P<0.05, **P<0.01
Restoration of hepatic Ces2 expression also markedly reduced both postprandial (319±58 vs 123±26 mg/dL) and fasting (173±33mg/dL vs 104±13 mg/dL) blood glucose levels (Figure 2C), and improved remarkably both glucose tolerance (Figure 2D) and insulin sensitivity (Figure 2E), with the area under the curve (AUC) being reduced by 49% (Figure 2D, right panel) and 47% (Figure 2E, right panel), respectively. Consistent with improved glucose homeostasis, Ces2 over-expression increased hepatic phosphor-AKT level (Figure 2F, left panel) and inhibited hepatic gluconeogenic genes phosphoenolpyruvate carboxykinase (Pck1) and glucose 6-phosphotase (G6pc) by >60% (Figure 2E, right panel). Together, the data of Figure 2 indicate that restoration of hepatic Ces2 expression markedly ameliorates hepatic steatosis and insulin resistance in db/db mice.
Restoration of Ces2 expression in HFD-fed mice reduces adiposity and improves glucose and lipid homeostasis
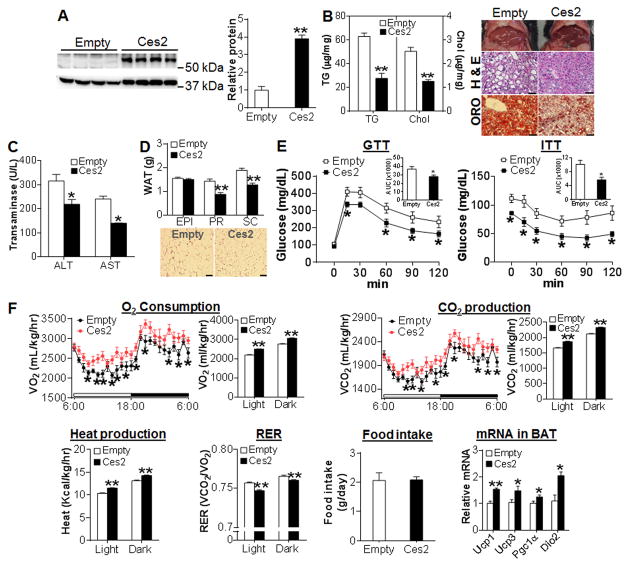
HFD feeding mimics the development of obesity and insulin resistance in humans. Since hepatic Ces2 expression is reduced by 6 fold in HFD-fed mice (Figure 1D), we also determined whether restoration of hepatic Ces2 in HFD-fed mice had similar effects as observed in db/db mice. When Ces2 expression was recapitulated by 4 fold in HFD-fed mice (Figure 3A), there was a significant reduction in body weight (Figure S4A) and liver weight (Figure S4B). Restoration of Ces2 expression also reduced hepatic TG and cholesterol levels by 60% and 50%, respectively (Figure 3B, left panel) and significantly improved liver morphology and neutral lipid accumulation (Figure 3B, right panel). As a result, restoration of Ces2 expression significantly reduced plasma ALT and AST levels (Figure 3C).
Figure 3. Restoration of hepatic Ces2 expression in HFD-fed mice improves metabolic disorder and energy expenditure.
(A–F) C57BL/6J mice were fed an HFD for 3 months and then injected i.v. with Ad-empty or Ad-Ces2 (n=10). After 14 days, mice were sacrificed. (A) Hepatic protein levels (left panel) and relative CES2 protein level (right panel). (B) Hepatic TG and cholesterol levels (left panel) as well as representative liver image (top), H & E staining (middle) and oil red O staining (bottom) of liver sections (right panel). Scale bars, 50 μm. (C) Plasma ALT and AST levels. (D) White adipose tissue (WAT) weight (top panel) and H & E staining of adipocytes (lower panel). EPI, epididymal fat. PR, perirenal fat. SC, subcutaneous fat. Scale bars, 100 μm. (E) GTT (left panel) and ITT (right panel). AUC of GTT or ITT is also shown in the right upper corner. (F) CLAMS was used to determine 24-h O2 consumption, CO2 production, heat generation, RER and food intake. In addition, mRNA levels of thermogenic genes in brown adipose tissue (BAT) was also determined. *P<0.05, **P<0.01
Restoration of Ces2 expression also reduced both the perirenal and subcutaneous fat mass by 20%–30% (Figure 3D, top panel) and the adipocyte size by 65% (Figure 3D, lower panel). Consistent with such observations, restoration of Ces2 expression significantly improved both glucose tolerance (Figure 3E, left panel) and insulin sensitivity (Figure 3E, right panel). Similar to what was observed in db/db mice, Ces2 over-expression did not affect plasma TG, cholesterol or free fatty acid (FFA) levels in HFD-fed mice (Figure S4C–S4E). Thus, restoration of hepatic Ces2 expression improves hepatic lipid and glucose homeostasis in both db/db mice and HFD-fed mice.
Restoration of hepatic Ces2 expression in HFD mice increases energy expenditure
The finding that restoration of hepatic CES2 expression improves glucose and lipid homeostasis prompted us to determine whether CES2 affects energy expenditure. In HFD-fed mice, restoration of Ces2 expression increased oxygen consumption, CO2 production and heat generation but reduced the respiration quotient rate (RER) in both light and dark cycles with no change in food intake (Figure 3F) or activity (Figure S4F). Consistent with increased energy expenditure, genes involved in thermogenesis, including Pgc1α, Ucp1, Ucp3 and Dio2, were significantly up-regulated in brown adipose tissue (BAT) (Figure 3F). Thus, hepatic Ces2 regulates energy expenditure and adiposity through, at least partly, inducing thermogenesis in BAT.
Ces2 inhibits de novo lipogenesis (DNL)
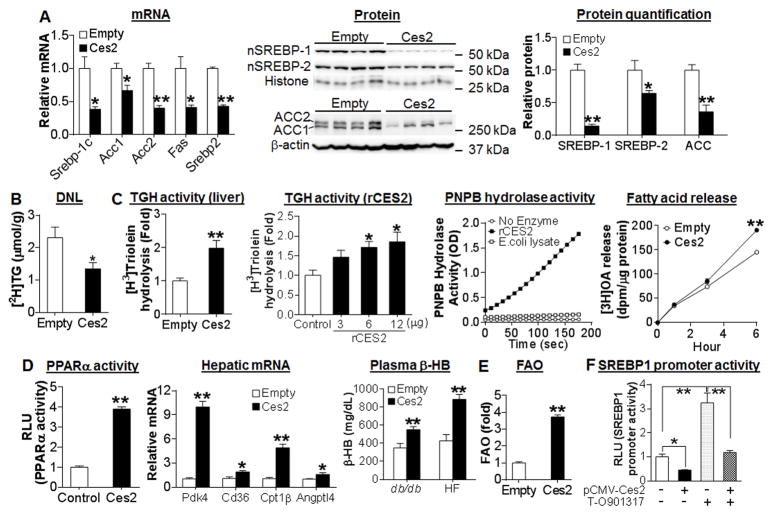
The finding that gain of Ces2 function lowers hepatic TG levels in chow-fed wild-type mice, db/db mice and HFD-fed mice led us to investigate the underlying mechanism. Hepatic TG level is regulated by very low-density lipoprotein (VLDL) secretion, TG synthesis and TG hydrolysis. Ces2 overexpression had no effect on hepatic ApoB or MTP expression (Figure S5A) or VLDL secretion (Figure S5B). In contrast, Ces2 over-expression significantly inhibited hepatic mRNA levels of lipogenic genes, including Srebp1c, Acc1, Acc2, Fas and Srebp-2 by 30%–60% in chow-fed C57BL/6 mice (Figure 4A, left panel), db/db (Figure S5C) or HFD-fed C57BL/6 mice (Figure S5D). Consistent with the reduced mRNA expression, hepatic protein levels of mature (nuclear) SREBP-1, mature SREBP-2, ACC1 and ACC2 were reduced by 48–73% following Ces2 over-expression (Figure 4A, middle and right panels; Figure S5E). In addition, over-expression of Ces2 also reduced phosphorylated eIF2α (P-eIF2α) level by 50% (Figure S5F), suggesting a role of hepatic CES2 in alleviating endoplasmic reticulum (ER) stress. To determine whether CES2 regulates lipogenesis in vivo, mice were injected with heavy water (2H2O) to label newly synthesized lipids. In agreement with the changes in gene expression, newly synthesized TG was reduced by ~ 50% in the liver of mice over-expressing Ces2 (Figure 4B). Thus, over-expression of hepatic CES2 inhibits lipogenesis in vivo.
Figure 4. CES2 inhibits lipogenesis and promotes fatty acid oxidation.
(A) Left panel: hepatic mRNA levels in C57BL/6J mice (n=6). Middle panel: hepatic nuclear proteins in C57BL/6J mice (top) or db/db mice (bottom) (n=4). Right panel: protein levels in the middle panel were quantified. “nSREBP”, nuclear SREBP. (B) Newly synthesized TG ([2H]TG) in the liver of C57BL/6J mice (n=5). DNL, de novo lipogenesis. (C) Microsomes were isolated from C57BL/6J mice and triglyceride hydrolase activity (TGH) was determined using [3H]Triolein as substrate (n=6) (left panel). Recombinant mouse CES2 (rCES2) protein was also used to determine TGH activity using [3H]Triolein as substrate or esterase activity using PNPB as substrate (n=3) (middle panels). In addition, pulse-chase study was performed by incubation of freshly isolated primary hepatocytes with [3H]oleic acid (OA) for 5 h (pulse) followed by chase for indicated time (n=3) (right panel). (D) HepG2 cells were co-transfected with pGL3-3×PPRE-luc, pCMV-β-gal, pCMV-Ces2 or pCMV-empty and relative luciferase activity units (RLU) were determined after 36 h (n=6) (left panel). Hepatic mRNA levels of PPARα target genes in C57BL/6J mice (n=6) (middle panel) and plasma β-hydroxybutyrate (β-HB) level in db/db mice (n=7) or HFD-fed mice (n=5–6) (right panel) were determined. (E) Fatty acid oxidation (FAO) in primary hepatocytes was determined using [3H]palmitic acid as substrate (n=6). (F) HepG2 cells were co-transfected with pGL3-SREBP-1-luc, CMV-β-gal, CMV-Ces2 or CMV-empty in the presence or absence of the LXRα ligand T-O901317 (10 μM). After 36 h, RLU was determined. *P<0.05, **P<0.01
CES2 induces FAO and inhibits SREBP1 activity by regulating TG hydrolysis
It is likely that CES2 may have TG hydrolase (TGH) activity that in turn mediates its metabolic effects. To determine whether CES2 has TGH activity, we first utilized liver lysates and [3H]triolein as substrate. Over-expression of hepatic Ces2 increased TGH activity by >2 fold (Figure 4C, left panel), suggesting that CES2 may be a TG hydrolase. To test the latter hypothesis, we investigated whether CES2 had TGH activity in vitro using CES2 protein purified from E. Coli. The purified recombinant CES2 (rCES2) protein showed a high capacity to hydrolyze both [3H]triolein and p-nitrophenyl butyrate (PNPB) (Figure 4C, middle panels). Consistent with the TG hydrolase activity, over-expression of CES2 in primary hepatocytes significantly increased free fatty acid (FFA) release in the pulse-chase study (Figure 4C, right panel). Thus, our data demonstrate that CES2 has TGH activity both in vitro and in vivo.
FFAs released from TG hydrolysis may function as a signaling molecule by activating peroxisome proliferator-activated receptor α (PPARα), a nuclear receptor that regulates fatty acid oxidation (FAO). Indeed, over-expression of Ces2 increased PPARα activity in transient transfection assays (Figure 4D, left panel) and hepatic mRNA levels of PPARα target genes, including Pdk4, Cd36, Cpt1b and Angptl4 (Figure 4D, middle panel). Consistent with the increased PPARα activity, plasma level of β-hydroxybutyrate (β-HB), an indicator of hepatic FAO, was significantly increased in both db/db and HFD-fed mice over-expressing Ces2 (Figure 4D, right panel). In addition, Ces2 over-expression also increased FAO by 4 fold in isolated primary hepatocytes (Figure 4E). Thus, over-expression of CES2 increases PPARα activity and induces FAO in vitro and in vivo.
FFAs have also been reported to inhibit SREBP-1c transcription through antagonizing liver X receptor (LXR) transcriptional activity or directly suppressing the processing of SREBP-1c (23, 24). As shown in Figure 4F, over-expression of Ces2 in HepG2 cells inhibited the Srebp-1c promoter activity by >55% in the presence or absence of the LXR agonist T-0901317. In agreement with the role of CES2 in inhibiting lipogenesis and promoting FAO, over-expression of Ces2 in db/db or HFD-fed mice reduced hepatic FFA levels by >50% (Figure S5G and S5H). Analysis of hepatic fatty acid composition by gas chromatography-mass spectrometry (GC-MS) showed that all the major fatty acids, including C14:0, C16:0, C16:1, C18:0, C18:1, C18:2, C20:4 and C22:6, were significantly reduced by Ces2 over-expression (Table 1). Taken together, the data of Figure 4 and Figure S5 demonstrate that over-expression of CES2 reduces hepatic TG levels likely through enhancing lipolysis and subsequent induction of FAO and inhibition of lipogenesis.
Table 1.
Hepatic fatty acid composition in mice that over-express or are deficient in Ces2
| Adenovirus | Fatty acid composition (μg/mg)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| C14:0 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C20:4 | C22:6 | |
| Ad-empty | 0.03±0.00 | 15.41±0.78 | 0.20±0.02 | 2.42±0.13 | 23.12±1.48 | 14.50±0.67 | 4.35±0.25 | 1.94±0.07 |
| Ad-Ces2 | 0.02±0.00* | 7.20±0.34* | 0.11±0.01* | 2.05±0.05* | 8.87±0.52* | 6.01±0.20* | 3.43±0.13* | 1.62±0.03* |
| Ad-shLacZ | 0.02±0.00 | 5.93±0.38 | 0.28±0.05 | 1.70±0.06 | 5.03±0.86 | 3.99±0.27 | 2.78±0.07 | 0.97±0.03 |
| Ad-shCes2 | 0.03±0.00* | 8.07±0.56* | 0.23±0.03 | 2.54±0.13* | 13.33±1.39* | 5.67±0.24* | 2.71±0.11 | 0.87±0.01* |
P<0.05 Ad-Ces2 vs Ad-empty (HFD-fed mice; n=8) or Ad-shCes2 vs Ad-shLacZ (chow-fed mice; n=8)
Overexpression of human CES2 in HFD-fed mice exhibits beneficial effects on glucose and lipid metabolism
Human and mouse CES2 proteins share 71% identify in amino acid homology. The data of Figure 1 show that CES2 expression is down-regulated by >60% in NASH patients. Thus, we investigated whether human CES2 had similar functions as mouse Ces2. Over-expression of human CES2 in HFD-fed mice (Figure S6A) had no effect on plasma TG (Figure S6B) or cholesterol (Figure S6C) level but reduced hepatic TG level by 40% (Figure S6D) and mRNA levels of lipogenic genes (Figure S6E). In addition, human CES2 over-expression increased hepatic β-HB level (Figure S6F), reduced plasma glucose level (Figure S6G) and improved glucose tolerance (Figure S6H). Thus, human CES2 and mouse Ces2 have similar effects on lipid and glucose homeostasis.
Knockdown of hepatic Ces2 causes liver steatosis and liver damage
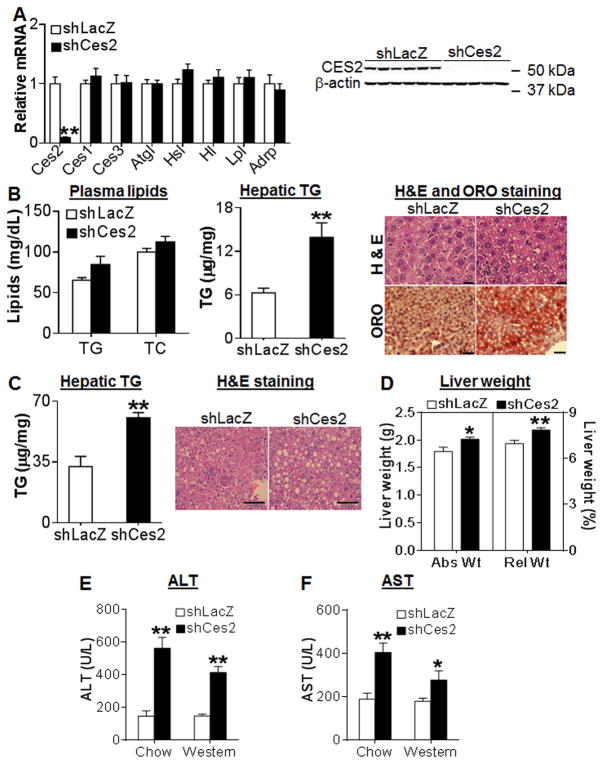
Since hepatic CES2 is reduced in diabetes, obesity and NASH by >65%, we then asked whether hepatic Ces2 deficiency affects lipid and/or glucose metabolism. Over-expression of an shRNA against Ces2 (shCes2) in chow-fed C57BL/6 mice reduced hepatic Ces2 mRNA (Figure 5A, left panel) and protein (Figure 5A, right panel) by >90%, without affecting other TG hydrolases (Ces1, Ces3, Atgl, Hsl) or Adrp in the liver (Figure 5A, left panel). Hepatic Ces2 knockdown had no effect on plasma TG or cholesterol level (Figure 5B) or hepatic cholesterol level (Figure S7A), but increased hepatic TG level by >2 fold (Figure 5B, middle panel), which was also confirmed by both H & E staining and oil red O (ORO) staining of liver sections (Figure 5B, right panel). When C57BL/6J mice were fed a Western diet, loss of hepatic Ces2 also resulted in a 2-fold increase in hepatic TG level (Figure 5C, left panel), accompanied by massive lipid droplet accumulation (Figure 5C, right panel) and increased liver weight (Figure 5D). Finally, loss of hepatic Ces2 in either chow or Western diet-fed mice significantly increased plasma levels of ALT (Figure 5E) and AST (Figure 5F). Thus, hepatic CES2 is essential for both maintaining normal hepatic TG levels and protecting against liver damage.
Figure 5. CES2 knockdown causes liver steatosis and liver damage in C57BL/6J mice.
(A and B) Chow-fed C57BL/6J mice were injected i.v. with Ad-shLacZ or Ad-shCes2 (n=7–8). (A) Hepatic mRNA (left panel) and protein (right panel) levels. (B) Plasma TG and cholesterol levels (left panel), hepatic TG level (middle panel) and representative H & E staining (top) or oil red O staining (bottom) of liver sections (right panel). Scale bars, 20 μm (H & E staining) or 50 μm (ORO staining). (C and D) C57BL/6J mice were fed a Western diet for 2 weeks, followed by i.v. injection of Ad-shLacZ or Ad-shCes2 (n=7–8). (C) Hepatic TG level (left panel) and representative H & E staining of liver sections (right panel). Scale bars, 100 μm. (D) Liver weight was presented as absolute liver weight (Abs Wt) and relative liver weight to whole body weight (Rel Wt; %). (E and F) Plasma ALT (E) and AST (F) levels. *P<0.05, **P<0.01
Loss of hepatic CES2 induces lipogenesis by inducing SREBP-1
To understand the mechanism underlying liver steatosis in Ces2-deficient mice, we first determined whether VLDL secretion was affected. Ces2 deficiency had no effect on hepatic ApoB or MTP protein expression (Figure S7B) or VLDL secretion (Figure S7C). Hepatic genes involved in FAO were unchanged (Figure S7D). Consistent with the latter finding, Ces2 knockdown did not affect FAO in primary hepatocytes (Figure S7E) or plasma β-HB level (Figure S7F).
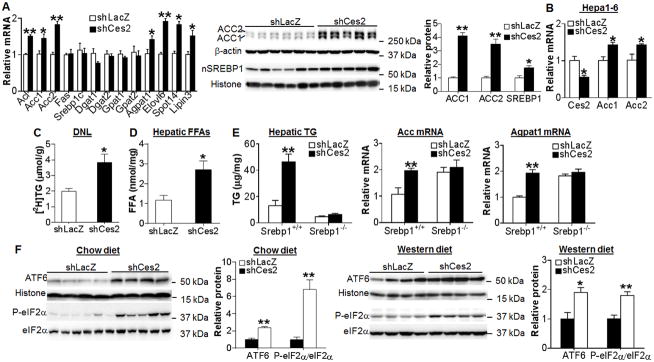
Interestingly, loss of Ces2 significantly induced the mRNA levels of some lipogenic genes, including Acl, Acc1, Acc2, Agpat1, Spot14, Elovl6 and lipin3 (Figure 6A, left panel), and the protein levels of ACC1, ACC2 and mature SREBP1 by 4 fold, 3.5 fold and 2 fold, respectively (Figure 6A, middle and right panels). Knockdown of Ces2 in Hepa1-6 cells also induced Acc1 and Acc2 expression (Figure 6B), suggesting that regulation of Acc1 and Acc2 by Ces2 is cell autonomous. Consistent with the increased level of lipogenic genes, Ces2 knockdown induced the rate of hepatic TG synthesis by 2 fold (Figure 6C) and hepatic FFA level by 2.6 fold (Figure 6D). Analysis of fatty acid composition by GC-MS indicated that C14:0, C16:0, C18:0, C18:1 and C18:2 fatty acids were significantly increased in Ces2-deficient mice (Table 1). The increase in hepatic fatty acid levels was accompanied by unchanged whole body energy metabolism or activity (Figure S7G–S7J). These data demonstrate that loss of hepatic CES2 stimulates lipogenesis.
Figure 6. CES2 knockdown increases lipogenesis by inducing SREBP-1 expression and ER stress.
(A) Hepatic mRNA (left panel) and protein (middle and right panels) levels in chow-fed C57BL/6 mice (n=7–8). (B) Hepa1-6 cells were infected with Ad-shLacZ or Ad-shCes2 at an MOI of 10. After 30 h, mRNA levels were quantified (n=3). (C) Newly synthesized TG ([2H]TG) in the livers of C57BL/6J mice infected with Ad-shLacZ or Ad-shCes2 (n=5). (D) Hepatic free fatty acid (FFA) level in C57BL/6J mice (n=7–8). (E) Srebp1−/− and Srebp1+/+ mice were injected i.v. with Ad-shLacZ or Ad-shCes2 (n=5–7). After 7 days, hepatic levels of TG (left panel), Acc mRNA (middle panel) and Agpat1 mRNA (right panel) were determined. (F) Hepatic protein levels in chow-fed C57BL/6J mice (n=4–6) (left 2 panels) or Western diet-fed C57BL/6J mice (n=7) (right 2 panels). *P<0.05, **P<0.01
The data of Figure 6A suggest that SREBP1 may play a critical role in the development of steatosis in Ces2-deficient mice. To test this hypothesis, we knocked down Ces2 in the liver of Srebp1+/+ mice and Srebp1−/− mice. As expected, knockdown of Ces2 increased hepatic TG level by 2.8 fold in Srebp1+/+ mice; however, this increase was blunted in Srebp1−/− mice (Figure 6E, left panel). Similarly, knockdown of Ces2 induced SREBP target genes, such as Acc, Agpat1 (Figure 6E, middle and right panels), Lipin3, Acl, Elovl6 and Spot14 (Figure S8A–S8D), in Srebp1+/+ mice but not in Srebp1−/− mice. Although SREBP-1 is important for Ces2 signaling, SREBP-1 did not regulate Ces2 expression (Figure S8E). Finally, knockdown of Ces2 had no effect on hepatic total cholesterol level in either Srebp1+/+ mice or Srebp1−/− mice (Figure S8F). Together, these data demonstrate that loss of CES2 increases lipogenesis and hepatic TG level through induction of hepatic SREBP1 expression.
Loss of hepatic CES2 induces ER stress
The data of Figure 6A–6C show that loss of hepatic CES2 increases mature SREBP1 protein but not Srebp1 mRNA level, suggesting that loss of hepatic CES2 stimulates SREBP processing. CES2 is an ER-localized protein. Since CES2 has TG hydrolase activity (Figure 4), loss of CES2 may cause TG accumulation in ER, which in turn triggers ER stress. Under normal conditions, GRP78 (BiP) binds to IRE1, PERK, and ATF6 to prevent their activation. Loss of hepatic Ces2 significantly induced nuclear ATF6 and p-eIF2α levels in both chow diet-fed mice (Figure 6F, left 2 panels) and Western diet-fed mice (Figure 6F, right 2 panels). In contrast, loss of hepatic CES2 had no effect on GRP78 or p-IRE1 level (Figure S9). Elevated ER stress has been shown to induce SREBP processing (25, 26) whereas inhibition of the PERK pathway represses SREBP1 activation and lipogenesis (27). Therefore, loss of hepatic CES2 increases mature SREBP1 expression and lipogenesis likely through inducing ER stress.
Hepatic CES2 is regulated by HNF4α
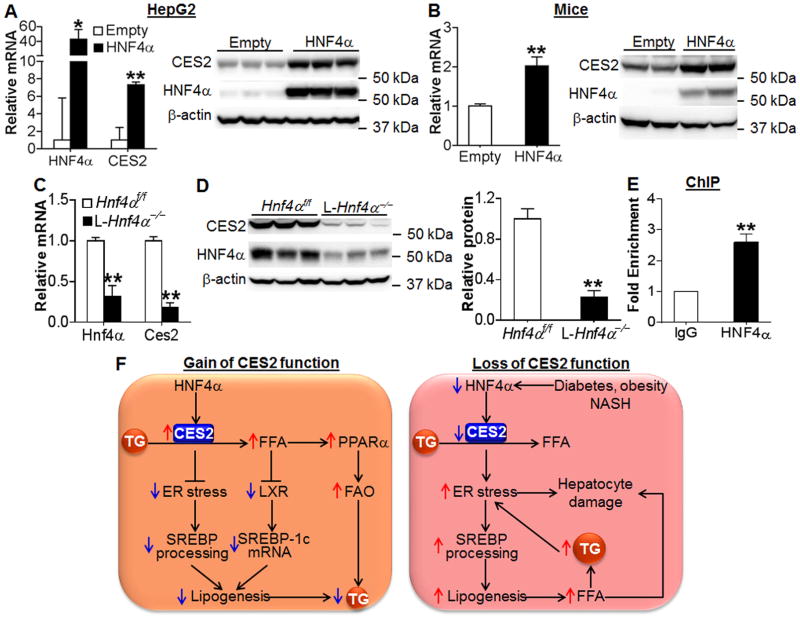
Our data have shown that hepatic CES2 is protective against the development of NAFLD. Therefore, it is important to determine the mechanism by which hepatic CES2 is reduced in NASH, diabetes and obesity. Our recent work show that hepatocyte nuclear factor 4α (HNF4α) is reduced by 70–80% in the livers of both NASH patients and db/db or HFD-fed mice (14). HNF4α is a nuclear hormone receptor that regulates the expression of genes involving in bile acid, lipid, glucose and xenobiotic drug metabolism. Thus, we investigated whether HNF4α regulates CES2 expression in vitro and in vivo. Over-expression of HNF4α significantly induced human CES2 mRNA and protein expression in HepG2 cells (Figure 7A). Similarly, HNF4α over-expression also induced mouse Ces2 mRNA and/or protein levels in AML12 cells or Hepa1-6 cells (Figure S10A) and in C57BL/6 mice (Figure 7B). In contrast, Ces2 mRNA and protein levels were reduced by >80% in liver-specific Hnf4α−/− (L-Hnf4α−/−) mice (Figure 7C and 7D). Consistent with the change in CES2 mRNA level, over-expression of HNF4α also induced human CES2 gene promoter activity (Figure S10B). Finally, chromatin immunoprecipitation (ChIP) assays showed that HNF4α protein bound to the proximal promoter of the Ces1 gene (Figure 7E). Together, these data demonstrate that hepatic CES2 is a downstream target of HNF4α and that the decrease in hepatic CES2 expression may result, at least in part, from reduced HNF4α expression in NASH, diabetes or obesity.
Figure 7. Hepatic CES2 is regulated by HNF4α.
(A) HepG2 cells were infected with Ad-Empty or Ad-HNF4α. After 48 h, mRNA (left panel) and protein (right panel) levels were determined (n=3). (B) C57BL/6J mice were injected i.v. with Ad-Empty or Ad-HNF4α (n=6 per group). After 7 days, Ces2 mRNA (left panel) and protein (right panel) levels were determined. (C and D) Hepatic mRNA (C) and protein (D, left panel) levels were determined and relative CES2 protein level quantified (D, right panel) in liver-specific Hnf4α−/− mice (L-Hnf4α−/−) and their control mice (Hnf4αf/f) (n=5). (E) ChIP assays were performed using liver lystates (n=4). (F) A central role for hepatic CES2 in regulating TG homeostasis. CES2 is a TG hydrolase and is regulated by HNF4α. CES2 hydrolyzes TG to release FFA, thus reducing ER stress, inhibiting LXR activity and augmenting PPARα activity. As a result, SREBP transcription/processing is repressed and FAO is increased, leading to reduced lipogenesis and TG levels in the liver. Under the conditions of metabolic stress (diabetes, obesity and NASH), hepatic HNF4α and CES2 expression is reduced, leading to increased TG accumulation and ER stress. The increased ER stress can cause hepatocyte damage and increased SREBP processing, resulting in elevated lipogenesis and FFA levels. Elevated FFAs contribute to hepatocyte damage. Increased TG levels further aggravate ER stress. *P<0.05, **P<0.01
Discussion
CES2 is a serine esterase responsible for hydrolysis of ester and amide bonds present in prodrugs and xenobiotics (28). So far, the role of CES2 in lipid or glucose metabolism is completely unknown. In this report, we demonstrate that hepatic CES2 is markedly reduced under common conditions of metabolic stress (obesity, diabetes and NASH) likely as a result of reduced HNF4α expression, and that CES2 is sufficient and required for maintaining hepatic TG homeostasis by modulating lipolysis, ER stress and lipogenesis (Figure 7F). Since NAFLD starts with simple steatosis, our data suggest that hepatic CES2 plays a protective role in the development of NAFLD.
SREBP-1c and SREBP-2 are the master regulators of fatty acid and cholesterol biosynthesis, respectively. Recent data have shown that SREBP activities and ER stress are reciprocally regulated. Palmitate or lipid droplet accumulation in the ER is known to cause ER stress (29, 30). Activation of the ER stress pathways results in the increased proteolytic cleavage of the SREBP-1c and SREBP-2 isoforms and subsequent lipogenesis (26, 31). Conversely, inhibition of ER stress is shown to inhibit lipogenesis (27). As an ER-localized TGH, CES2 is a legitimate lipase for preventing TG accumulation in ER. As a result, over-expression of CES2 reduces hepatic ER stress and SREBP processing whereas loss of CES2 has opposite effects.
Defective lipolysis has now been recognized as one of the most important mechanisms contributing to the development of NAFLD (4, 6, 7). Our compelling in vitro and in vivo data have demonstrated that CES2 has TG lipase activity. TG hydrolysis in the liver is known to release FFAs, leading to PPARα activation and induction of FAO (21, 32). FFAs released from TG hydrolysis may also inhibit SREBP-1c expression by antagonizing LXR (23, 24). Indeed, overexpression of ATGL in db/db mice decreases hepatic TG levels through attenuating lipogenesis and inducing FAO (33). Our data indicate that CES2 over-expression has similar effects. Moreover, we show that CES2 over-expression alleviates ER stress, which may also account in part for the reduced lipogenesis. Thus, gain of hepatic CES2 function attenuates hepatic TG accumulation likely through both enhancing FAO and inhibition of lipogenesis via reducing SREBP-1c transcription and processing (Figure 7F, left panel).
Conversely, knockdown of hepatic CES2 induces ER stress and SREBP-1 processing, leading to increased lipogenesis and the development of fatty liver (Figure 7F, right panel). The importance of SREBP-1 is validated by the observation that loss of hepatic CES2 fails to induce liver steatosis in Srebp1−/− mice. Interestingly, transgenic expression of human PNPLA3(I148M) in mice also exhibits increased hepatic lipogenesis by inducing Srebp-1 processing and its target genes (10). These data suggest that ER stress-induced SREBP-1 processing may be one of the common mechanisms of liver steatosis triggered by defective lipolysis.
In HFD-fed mice, hepatic over-expression of CES2 increases whole body energy expenditure by inducing thermogenic genes in BAT. It has been shown that proteins secreted from the liver, such as FGF21 (34), can regulate genes involved in energy metabolism in BAT and thermogenesis. Nonetheless, CES2 over-expression does not affect FGF21 expression (data not shown). Therefore, it is possible that CES2 over-expression in HFD-fed mice results in secretion of other protein(s) which in turn affects thermogenesis in BAT. However, neither over-expression of Ces2 in db/db mice nor knockdown of Ces2 in wild-type mice affects whole body energy metabolism. Since Ces2 over-expression or knockdown alters hepatic TG level by >2 fold in these latter mice, we speculate that CES2 modulates hepatic TG metabolism likely independent of its regulation of whole body energy expenditure.
HNF4α controls the basal expression of many genes in the liver. Our recent studies have demonstrated that hepatic HNF4α expression is markedly reduced in NASH patients, diabetic mice and HFD-fed mice as a result of increased miR-34a expression (14). Since a reduction in hepatic HNF4α expression decreases CES2 expression significantly, our data suggest that the reduced CES2 expression in NASH, diabetes and obesity results, at least in part, from decreased HNF4α expression. Furihata et al. performed gel mobility shift assays and promoter-luciferase assays to show that HNF4α increases mouse Ces2 gene promoter activity via binding to a DR-1 (direct repeat separated by one base pair) element in the proximal promoter (35). However, Furihata et al. did not investigate whether HNF4α is able to regulate CES2 gene expression in vitro or in vivo (35). Nonetheless, their in vitro data on the Ces2 promoter activity, together with our ex vivo and in vivo data, demonstrate that CES2 is a direct downstream target of HNF4α.
Taken together, our current studies demonstrate that CES2 is a novel TG hydrolase that plays an essential role in controlling hepatic TG homeostasis by regulating lipolysis, FAO, ER stress and lipogenesis. In addition to alleviating liver steatosis, recapitulation of hepatic CES2 expression in db/db mice or HFD-fed mice also improves strikingly glucose tolerance and energy expenditure. Since hepatic CES2 is markedly reduced in diabetes, obesity and NASH, recapitulation of hepatic CES2 expression may be useful for prevention and treatment of NAFLD associated with diabetes and obesity.
Supplementary Material
Acknowledgments
Financial support: This work was supported by NIH grants R01HL103227, R01DK095895 and R01DK102619 to Y.Z.
Normal or pathologic human liver tissues were obtained through the Liver Tissue Cell Distribution System, Minneapolis, Minnesota, which was funded by NIH Contract # HHSN276201200017C.
Abbreviations
- CES2
carboxylesterase 2
- DNL
de novo lipogenesis
- FAO
fatty acid oxidation
- FFA
free fatty acid
- HNF4α
hepatocyte nuclear factor 4α
- HFD
high fat diet
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- PPARα
peroxisome proliferator-activated receptor α
- SREBP-1
sterol regulatory element-binding protein 1
- TG
triglyceride
- TGH
triglyceride hydrolase
References
- 1.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moon YA, Liang G, Xie X, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Brown MS, et al. The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metab. 2012;15:240–246. doi: 10.1016/j.cmet.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang JJ, Li JG, Qi W, Qiu WW, Li PS, Li BL, Song BL. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell Metab. 2011;13:44–56. doi: 10.1016/j.cmet.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs CD, Claudel T, Trauner M. Role of metabolic lipases and lipolytic metabolites in the pathogenesis of NAFLD. Trends Endocrinol Metab. 2014;25:576–585. doi: 10.1016/j.tem.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Kato M, Higuchi N, Enjoji M. Reduced hepatic expression of adipose tissue triglyceride lipase and CGI-58 may contribute to the development of non-alcoholic fatty liver disease in patients with insulin resistance. Scand J Gastroenterol. 2008;43:1018–1019. doi: 10.1080/00365520802008140. [DOI] [PubMed] [Google Scholar]
- 6.Schweiger M, Lass A, Zimmermann R, Eichmann TO, Zechner R. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am J Physiol Endocrinol Metab. 2009;297:E289–296. doi: 10.1152/ajpendo.00099.2009. [DOI] [PubMed] [Google Scholar]
- 7.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Speliotes EK, Butler JL, Palmer CD, Voight BF, Nash CRN, et al. Consortium G, Consortium MI. PNPLA3 variants specifically confer increased risk for histologic nonalcoholic fatty liver disease but not metabolic disease. Hepatology. 2010;52:904–912. doi: 10.1002/hep.23768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santoro N, Kursawe R, D’Adamo E, Dykas DJ, Zhang CK, Bale AE, Cali AM, et al. A common variant in the patatin-like phospholipase 3 gene (PNPLA3) is associated with fatty liver disease in obese children and adolescents. Hepatology. 2010;52:1281–1290. doi: 10.1002/hep.23832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li JZ, Huang Y, Karaman R, Ivanova PT, Brown HA, Roddy T, Castro-Perez J, et al. Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis. J Clin Invest. 2012;122:4130–4144. doi: 10.1172/JCI65179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53:116–126. doi: 10.1002/hep.24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu JW, Wang SP, Alvarez F, Casavant S, Gauthier N, Abed L, Soni KG, et al. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology. 2011;54:122–132. doi: 10.1002/hep.24338. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Li Y, Chen W, Xu Y, Yin L, Ge X, Jadhav K, et al. Hepatic carboxylesterase 1 is essential for both normal and farnesoid X-receptor-controlled lipid homeostasis. Hepatology. 2014;59:1761–1771. doi: 10.1002/hep.26714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Zalzala M, Xu J, Li Y, Yin L, Zhang Y. A metabolic stress-inducible miR-34a-HNF4alpha pathway regulates lipid and lipoprotein metabolism. Nat Commun. 2015;6:7466. doi: 10.1038/ncomms8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Yin L, Hillgartner FB. SREBP-1 integrates the actions of thyroid hormone, insulin, cAMP, and medium-chain fatty acids on ACCalpha transcription in hepatocytes. J Lipid Res. 2003;44:356–368. doi: 10.1194/jlr.M200283-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 18.Yin L, Ma H, Ge X, Edwards PA, Zhang Y. Hepatic hepatocyte nuclear factor 4alpha is essential for maintaining triglyceride and cholesterol homeostasis. Arterioscler Thromb Vasc Biol. 2011;31:328–336. doi: 10.1161/ATVBAHA.110.217828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge X, Yin L, Ma H, Li T, Chiang JY, Zhang Y. Aldo-keto reductase 1B7 is a target gene of FXR and regulates lipid and glucose homeostasis. J Lipid Res. 2011;52:1561–1568. doi: 10.1194/jlr.M015859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rune A, Osler ME, Fritz T, Zierath JR. Regulation of skeletal muscle sucrose, non-fermenting 1/AMP-activated protein kinase-related kinase (SNARK) by metabolic stress and diabetes. Diabetologia. 2009;52:2182–2189. doi: 10.1007/s00125-009-1465-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J, Li Y, Chen WD, Xu Y, Yin L, Ge X, Jadhav K, et al. Hepatic carboxylesterase 1 is essential for both normal and farnesoid X receptor-controlled lipid homeostasis. Hepatology. 2014;59:1761–1771. doi: 10.1002/hep.26714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Ge X, Heemstra LA, Chen WD, Xu J, Smith JL, Ma H, et al. Loss of FXR protects against diet-induced obesity and accelerates liver carcinogenesis in ob/ob mice. Mol Endocrinol. 2012;26:272–280. doi: 10.1210/me.2011-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ou J, Tu H, Shan B, Luk A, DeBose-Boyd RA, Bashmakov Y, Goldstein JL, et al. Unsaturated fatty acids inhibit transcription of the sterol regulatory element-binding protein-1c (SREBP-1c) gene by antagonizing ligand-dependent activation of the LXR. Proc Natl Acad Sci U S A. 2001;98:6027–6032. doi: 10.1073/pnas.111138698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshikawa T, Shimano H, Yahagi N, Ide T, Amemiya-Kudo M, Matsuzaka T, Nakakuki M, et al. Polyunsaturated fatty acids suppress sterol regulatory element–binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements. J Biol Chem. 2002;277:1705–1711. doi: 10.1074/jbc.M105711200. [DOI] [PubMed] [Google Scholar]
- 25.Lee JN, Ye J. Proteolytic activation of sterol regulatory element-binding protein induced by cellular stress through depletion of Insig-1. J Biol Chem. 2004;279:45257–45265. doi: 10.1074/jbc.M408235200. [DOI] [PubMed] [Google Scholar]
- 26.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bobrovnikova-Marjon E, Hatzivassiliou G, Grigoriadou C, Romero M, Cavener DR, Thompson CB, Diehl JA. PERK-dependent regulation of lipogenesis during mouse mammary gland development and adipocyte differentiation. Proc Natl Acad Sci U S A. 2008;105:16314–16319. doi: 10.1073/pnas.0808517105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merali Z, Ross S, Pare G. The pharmacogenetics of carboxylesterases: CES1 and CES2 genetic variants and their clinical effect. Drug Metabol Drug Interact. 2014;29:143–151. doi: 10.1515/dmdi-2014-0009. [DOI] [PubMed] [Google Scholar]
- 29.Fu S, Watkins SM, Hotamisligil GS. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15:623–634. doi: 10.1016/j.cmet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Ferre P, Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab. 2010;12(Suppl 2):83–92. doi: 10.1111/j.1463-1326.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- 31.Colgan SM, Tang D, Werstuck GH, Austin RC. Endoplasmic reticulum stress causes the activation of sterol regulatory element binding protein-2. Int J Biochem Cell Biol. 2007;39:1843–1851. doi: 10.1016/j.biocel.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Sapiro JM, Mashek MT, Greenberg AS, Mashek DG. Hepatic triacylglycerol hydrolysis regulates peroxisome proliferator-activated receptor alpha activity. J Lipid Res. 2009;50:1621–1629. doi: 10.1194/jlr.M800614-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turpin SM, Hoy AJ, Brown RD, Rudaz CG, Honeyman J, Matzaris M, Watt MJ. Adipose triacylglycerol lipase is a major regulator of hepatic lipid metabolism but not insulin sensitivity in mice. Diabetologia. 2011;54:146–156. doi: 10.1007/s00125-010-1895-5. [DOI] [PubMed] [Google Scholar]
- 34.Hondares E, Rosell M, Gonzalez FJ, Giralt M, Iglesias R, Villarroya F. Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 2010;11:206–212. doi: 10.1016/j.cmet.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furihata T, Hosokawa M, Masuda M, Satoh T, Chiba K. Hepatocyte nuclear factor-4alpha plays pivotal roles in the regulation of mouse carboxylesterase 2 gene transcription in mouse liver. Arch Biochem Biophys. 2006;447:107–117. doi: 10.1016/j.abb.2006.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.