Abstract
C-reactive Protein (CRP) as an indicator of cardiovascular disease (CVD) has shown limited sensitivity. We demonstrate that two isoforms of CRP (pentameric, pCRP and monomeric, mCRP) present in soluble form or on microparticles (MPs) have different biological effects and are not all measured by clinical CRP assays. The hsCRP assay did not measure pCRP or mCRP on MPs, whereas flow cytometry did. MPs derived from endothelial cells, particularly those bearing mCRP, were elevated in PAD patients compared to controls. The numbers of mCRP+ endothelial MPs did not correlate with hsCRP measurements of soluble pCRP, indicating their independent modulation. In controls, statins lowered mCRP+ endothelial MPs. In a model of vascular inflammation, mCRP induced endothelial shedding of MPs and was proinflammatory, while pCRP was anti-inflammatory. mCRP on endothelial MPs may be both an unmeasured indicator of, and an amplifier of, vascular disease, and its detection might improve risk sensitivity.
Keywords: CRP, peripheral artery disease, monomeric CRP, pentameric CRP, hsCRP, inflammation, microparticle
Introduction
C-reactive protein (CRP) is a prototypical acute phase reactant that plays an important role in innate immune defense and inflammation [1]. Although it was described historically as a single molecular entity, CRP exists in multiple forms. The soluble pentamer (pCRP) can be dissociated into monomers (mCRP) after binding to activated platelets, apoptotic/necrotic cells, or microparticles (MP) [2,3]. The existence of three forms, soluble pCRP, membrane-bound pCRP, and membrane-bound mCRP, may have contributed to the contradictory results showing that “CRP” has both pro- and anti-inflammatory activity and can either protect against or promote CVD in transgenic mouse models of atherosclerosis [4–7].
MPs are small, membrane-derived anucleoid vesicles that contain cytoplasmic material and cell surface proteins that are shed by parental cells in response to activation, stress or apoptosis [8]. MPs play a physiological role in processes such as thrombosis and coagulation [9] but also have been associated with vascular dysfunction and diseases such as atherosclerosis [10]. pCRP has been reported to exhibit anti-inflammatory properties [11–13] but may also serve as a precursor of mCRP that binds to lipid rafts to exert local proinflammatory responses in endothelial cells and neutrophils [14–16] and promote monocyte activation [2]. Free mCRP has not been detected in the circulation (likely because of its relative aqueous insolubility), but mCRP has been found in atherosclerosis plaques [2], within infarctions [17], and detected bound to MPs in the circulation of patients following myocardial infarction (MI) [3]. mCRP presence on MPs has been reported to be due to dissociation of pCRP in the lipid environment of the membrane, specifically that the altered lysophospholipids therein overcome electrostatic forces holding together the monomeric units so that they dissociate [18]. Similar to apoptotic and activated cells, MPs contain phospholipids on their surface not typically exposed in the outer leaflet of cell membranes, including lysophosphatidylcholine (LPC). The membrane-bound pCRP to mCRP conversion process has been found to be dependent on LPC, and MPs capable of mCRP conversion have elevated levels of LPC [2,3]. LPC generation is dependent on phospholipase A2 (PLA2) and blocking PLA2 in vivo inhibits localized mCRP formation in inflamed tissue [19]. Taken together, this suggests a mechanism by which MPs expressing LPC can convert pCRP to mCRP.
The experiments described in this paper investigated the hypothesis that mCRP is a relevant marker for chronic inflammatory diseases and could play a role in their pathophysiology. We tested our hypothesis by evaluating the different forms of CRP in patients that have peripheral (lower extremity) arterial disease (PAD), a malignant form of atherosclerosis with high morbidity and mortality [20]. We determined that an image-based cytometric method was effective in measuring the circulating mCRP and pCRP forms that were bound to microparticles of various origins, in addition to the soluble pCRP that was measurable by hsCRP. Our subsequent study demonstrated a significant increase in endothelial MPs bearing mCRP in PAD patients. To investigate possible mechanisms of this specificity in relation to a proinflammatory state, we used a model of transendothelial migration of mononuclear cells developed in our laboratory and investigated subsequent T cell and macrophage polarization in transmigrated cells. The study confirmed roles for both pCRP and mCRP in the modulation of T lymphocytes and monocyte responses. mCRP induced Th1/M1 activation and pCRP induced Th2/M2 responses.
For the first time, these studies show 1) the hsCRP assay does not measure pCRP or mCRP on MPs, 2) there are increased numbers of MPs of endothelial origin in PAD, 3) there are increased numbers of endothelial MPs bearing mCRP in PAD, 4) mCRP is pro-inflammatory and pCRP is anti-inflammatory in a model of leukocyte transendothelial migration and maturation, and 5) mCRP may institute a proinflammatory amplification loop via its induction of endothelial MP generation.
Methods
Plasma collection, MP and CRP depletion of plasma, and MP isolation
Sodium-citrated blood was collected with written informed consent from asymptomatic volunteers or PAD patients. Procedures for storage and manipulation as well as the criteria for donor selection are detailed in the supplementary material.
Preparation of mCRP
mCRP was prepared as described previously [21] using urea-EDTA denaturation as detailed in the supplementary material.
CRP Antibodies specific to isoform
Antibodies specific to pCRP (mIgG2a, clone 1D6) and mCRP (mIgG1, clones 8C10 and 3H12) were a kind gift from Dr. Larry Potempa (Roosevelt University, IL, USA)[22]. Clones 8C10 and 1D6 were purified using the Mouse TCS Purification System (Abcam, Cambridge, MA) and directly conjugated with a Lightning-link Rapid Dylight 488 kit (Novus Biologicals, Littleton, CO).
ELISA
ELISA was performed as described previously [23] using phosphocholine conjugated to Keyhole Limpet Hemocyanin as the capture particle for pCRP and detailed in the supplementary material.
hsCRP assay
Quantitative measurements of CRP by immunoturbidimetry were performed using an AU480 Chemistry Analyzer (Beckman Coulter, Brea CA) with CRP Ultra Wide Range Reagent (Sekisui Diagnostics, Lexington, MA) as detailed in the supplementary material.
Western Blots
MPs were purified from 50 ml of blood as detailed in the supplementary material. The MPs and 100 ng/ml pCRP control (R&D Systems) were mixed with native (nonreducing) sample buffer with 0.1% SDS as described previously [24], except here reduced levels of SDS were only added to the sample buffer and not the running buffer. Further details are in the supplementary material.
Flow cytometry
Directly conjugated lactadherin (Haematologic Tech Inc., Essex Junction, VT) was prepared using a Lightning-link PE labeling kit (Novus Biologicals). Cell marker antibodies are listed in the supplementary material.
For image based cytometry, we employed an ImagestreamX MkII (Amnis, Seattle, WA) which has previously been used to analyze MPs [25]. To measure MPs, we gated for small particles in the size range of 0.1–1 μm that also stained positive for lactadherin, which binds to the obligatorily-exposed phosphatidylserine on MPs. Details and the advantages for using imaging cytometry to measure MPs are in the supplementary material along with the gating strategy as shown in Fig. S1.
Lipid Profile
Lipid profile analysis for plasma was carried out on a Beckman Coulter AU480 automated chemistry analyzer utilizing appropriate standards and calibrators as per the manufacturer’s protocol and detailed in the supplementary material.
Transendothelial migration (TEM)
Blood mononuclear cells (MNLs) were fractioned from the blood by density centrifugation. As detailed previously [26] and in the supplementary material, human cardiac microvascular endothelial cells (HCMEC) were seeded onto inserts. MNLs were applied to the top and allowed to migrate through the HCMECs in response to MCP-1.
Immunofluorescence
Cells that transmigrated in the TEM assay onto poly D-lysine coated coverslips (NeuVitro, Vancouver, WA) were stained and imaged as described previously [26] using anti-CD86 (clone IT2.2, Biolegend), CD206 (clone EPR6828(B), Abcam), or appropriate isotype controls.
Protein arrays
TEM assays were performed as above for 96 hours, the transmigrated cells were expanded, and protein extracted as described previously [26]. Protein was loaded and analyzed on a standard human cytokine array membrane (ARY005, R&D Systems) as per the manufacturer’s protocol.
Statistical Analysis
Results are presented as mean ± SE. Since data were not always normally distributed, the nonparametric Mann-Whitney U-test was used for comparison of two data sets, whereas the Kruskal-Wallis test with Dunn’s multiple comparison posttest was used for more than two. A p value <0.05 was considered statistically significant.
Results
The relationship between circulating CRP in its soluble or MP-bound forms and atherosclerosis is unclear. mCRP is associated with MPs after acute cardiac injury, and is the only form detectable within atherosclerotic plaques [2], but soluble pCRP is the only isoform routinely measured clinically. We therefore studied mCRP and pCRP, soluble and on MPs, in PAD, a chronic disease with extensive atherosclerosis.
The hsCRP Assay Tested on Solubilized mCRP or Microparticle-bound CRP
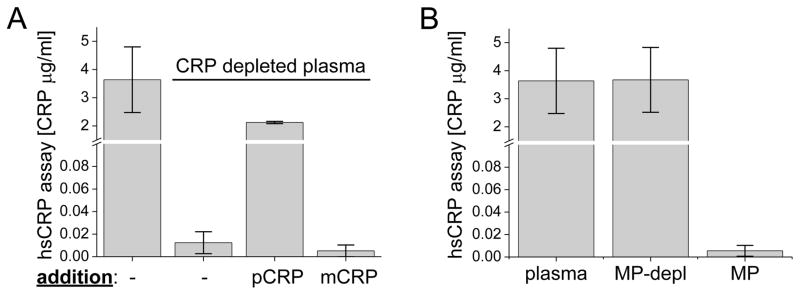
The hsCRP assay is currently used to gauge systemic inflammation and for cardiac risk assessment [27]. We asked which of the CRP species the assay would measure (soluble pCRP, solubilized mCRP, and pCRP or mCRP bound to MPs). Plasma gave a measurable value in the hsCRP assay (Fig. 1a), and that value decreased when CRP was depleted by passage of the plasma through phosphocholine-bound beads (phosphocholine is a ligand for CRP). Because the hsCRP assay is standardized for the use of plasma, we then used the CRP-depleted plasma to add back the two CRP forms. Soluble pCRP was measurable by the hsCRP assay in the depleted plasma (Fig. 1a). mCRP was prepared in a solubilized form and was also added to CRP-depleted plasma. Although the mCRP was detectable via our own ELISA, indicating that it was intact antigenically (Fig. S2 in the supplementary material), the Sekisui automated hsCRP assay failed to measure it (Fig. 1a). In another commercial assay, an ELISA (R&D Systems), the measurement of 2 μg/ml mCRP gave an optical density reading below that of the standards (range 0.78–500 ng/ml). In this assay, 1 μg/ml of pCRP added to the pCRP-depleted AB serum was measured as 0.825 ± 0.17 μg/ml. Therefore, this assay also failed to measure mCRP.
Fig. 1.
Detection of CRP isoforms and MPs by the hsCRP assay. a CRP detection in unmanipulated plasma (first column) or after passage over phosphocholine-coupled beads (second column, CRP depletion). The third column was 2 μg/ml pCRP added to CRP-depleted plasma and the fourth column was 2 μg/ml urea-solubilized mCRP added to the CRP-depleted plasma. b Levels of CRP in plasma with MPs removed (second column), or in MPs purified from the plasma (third column). (n=3)
We then tested MPs in the hsCRP assay. When MPs were depleted from plasma, there was no change in hsCRP measurements, and purified MPs from plasma had undetectable levels of CRP by that assay (Fig. 1b). To verify further that CRP bound to MPs was not detectable, we isolated MPs from plasma and loaded additional pCRP or mCRP to the MPs (Fig. S3 in the supplementary material). The hsCRP assay did not detect the CRP-loaded MPs, indicating that the assay failed to measure either pCRP or mCRP when bound to MPs (Fig. S3). The data indicate that the hsCRP assay is specific for soluble pCRP and is unable to detect other species of CRP.
Detection of Microparticles bearing CRP in the Plasma of PAD Patients Using Imaging Flow Cytometry
With the recognition of the limitations of the hsCRP assay, the detection of mCRP-bound MPs via immunoblot in the plasma of controls and the finding that MPs from adult human primary endothelial cells could convert pCRP to mCRP (Fig. S4 in supplementary material), we used image based flow cytometry to measure MPs bound with pCRP or mCRP in PAD patients versus asymptomatic controls with no prior history of cardiovascular disease (subjects characterized in Table 1). There was no significant difference in the total number of MPs found in controls compared to PAD patients (0.5–5 × 108 particles/ml, Table 2) or in the number of MPs bearing mCRP or pCRP in PAD patients compared to controls (Table 2), These data contrasted with the CRP levels measured by hsCRP, which were elevated in patients versus controls (Table 1), although there was a high degree of variability in the patient group.
Table 1.
Demographics and Clinical Characteristics of Donorsa
| Controls | PAD patients | |
|---|---|---|
| Demographics | ||
| Age (years) | 54.4 ± 5.3 | 65.3 ± 2.2 |
| Sex (male) % | 66.7 | 100 |
| hsCRP (μg/ml) | 1.7 ± 0.5 | 13.2 ± 4.3** |
| Risk factors % | ||
| Current smoker | 0 | 42.1 |
| Former smoker | - | 57.9 |
| Hypertension | - | 84.1 |
| Diabetes | - | 47.4 |
| History of CAD | 0 | 57.9 |
| Medication % | ||
| Aspirin | 16.7 | 68.4 |
| Statin | 50 | 73.7 |
| Clopidogrel | - | 31.6 |
| ACE inhibitor | - | 78.9 |
Data not collected for controls were indicated with a dash.
Data were reported as mean ± SE where applicable. (n=12 for controls, n=18 for PAD patients; **p ≤ 0.005) CAD coronary artery disease
Table 2.
MP characterization
| a Total MPs and those bearing CRP isoforms
| |||
|---|---|---|---|
| Controls | PAD patients | P value | |
| Total | 2.6×108 ± 0.6 | 2.4×108 ± 0.3 | 0.89 |
| mCRP+ | 1.1×107 ± 0.3 | 0.5×107 ± 0.1 | 0.28 |
| pCRP+ | 1.8×107 ± 0.5 | 1.6×107 ± 0.4 | 0.58 |
| b MPs of Specific Cellular Origins (x 105) | ||||
|---|---|---|---|---|
| Endothelial: | ||||
|
| ||||
| Controls | PAD patients | P value | % Increase | |
| Total | 2.7 ± 0.5 | 17.8 ± 4.5 | 0.003 | 559 |
| mCRP+ | 0.3 ± 0.1 | 1.2 ± 0.3 | 0.013 | 300 |
| pCRP+ | 1.2 ± 0.3 | 1.4 ± 0.5 | 0.36 | 17 |
| Platelet: | ||||
|
| ||||
| Controls | PAD patients | P Value | % Increase | |
|
| ||||
| Total | 117 ± 21 | 217 ± 52 | 0.36 | 85 |
| mCRP+ | 4.9 ± 1.2 | 4.4 ± 1.5 | 0.51 | −10 |
| pCRP+ | 10 ± 4 | 15 ± 5 | 0.94 | 50 |
|
| ||||
| Monocyte: | ||||
|
| ||||
| Controls | PAD patients | P Value | % Increase | |
|
| ||||
| Total | 14 ± 5 | 8 ± 3 | 0.42 | −43 |
| mCRP+ | 1.3 ± 0.6 | 0.5 ± 0.2 | 0.11 | −61 |
| pCRP+ | 4.6 ± 1.9 | 1.2 ± 0.6 | 0.51 | −74 |
|
| ||||
| B cell: | ||||
|
| ||||
| Controls | PAD patients | P Value | % Increase | |
|
| ||||
| Total | 1.4 ± 0.7 | 4.1 ± 1.3 | 0.08 | 193 |
| mCRP+ | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.76 | 50 |
| pCRP+ | 1.7 ± 1.0 | 1.9 ± 1.1 | 0.65 | 12 |
MPs were measured by flow cytometry as the number of lactadherin+ events per ml in the 0.1–1 μm size range. MPs were also measured for bound CRP isoforms and their cellular origin using markers for endothelial cells (CD144), platelets (CD41), monocytes (CD14), or B cells (CD19). Data were reported as mean ± SE. (n=11 for controls, n=18 for PAD patients)
PAD Patients Have Elevated Plasma Levels of Endothelial Microparticles Bearing mCRP Compared to Controls
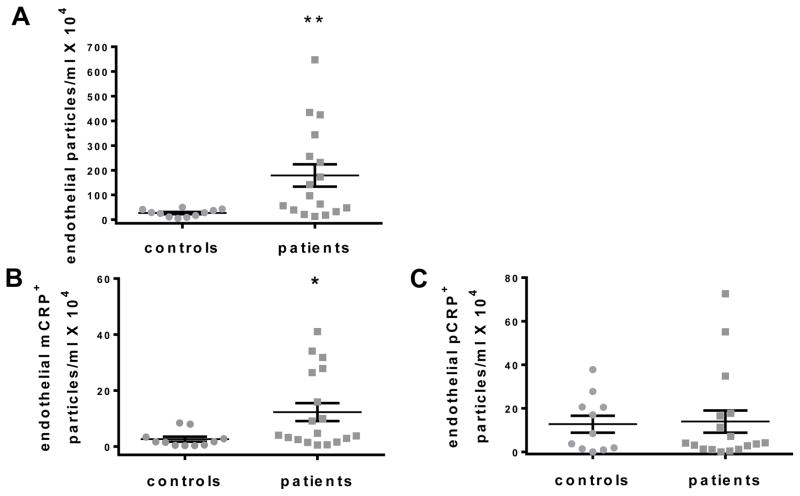
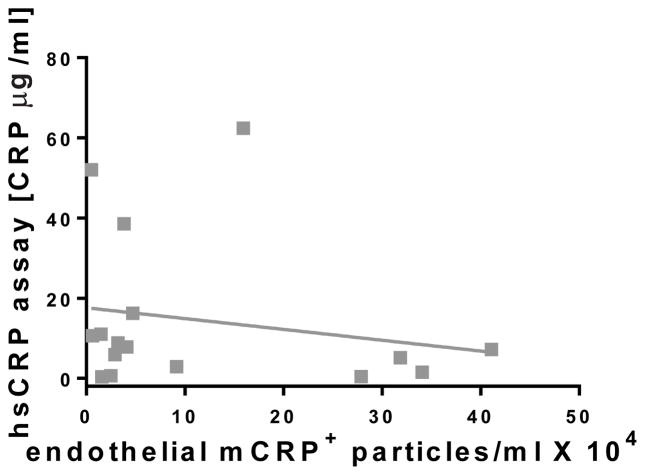
To investigate the possibility that the levels of a subpopulation of MPs might be altered in PAD patients, we examined the origin of the MPs using cellular markers for endothelial cells (CD144), platelets (CD41a), monocytes (CD14), neutrophils (CD66b), B cells (CD19), and T cells (CD3) in addition to the detection of lactadherin and CRP isoforms within our predetermined size range. A significant increase in endothelial-derived MPs (CD144+) was observed for PAD patients compared to controls (Fig. 2a and Table 2). Further, PAD patients had an elevated number of endothelial, mCRP-bearing MPs compared to controls (Fig. 2b), but not pCRP-bearing MPs (Fig. 2c). Although hsCRP measurements were increased in patients compared to controls (Table 1), there was no correlation between CRP levels measured by hsCRP and the number of endothelial MPs bearing mCRP in patients (Fig. 3).
Fig. 2.
Endothelial-derived MPs (and those bearing mCRP) measured by flow cytometry. (a) The number of lactadherin+ CD144+ particles was increased in PAD patients. The number of endothelial MPs bound with (b) mCRP, but not (c) pCRP, was increased for PAD patients. (n=11 for controls, n=18 for PAD patients, *p ≤ 0.05)
Fig. 3.
Correlation of hsCRP measurements with the number of endothelial MPs. There was no correlation (R=0.196 linear) between the elevated pCRP plasma levels measured by hsCRP in PAD patients compared to the number of endothelial derived MPs bearing mCRP (n=18)
mCRP-bearing MPs From Non-endothelial Origins in PAD Patients and Controls
We did not observe any significant difference in the number of platelet-derived MPs compared to controls, even when delineated by CRP isoform, although an increase was observed in the patients (Table 2). T cell or neutrophil-derived MPs were rarely detected and so are not included in the data. Although differences in monocyte and B cell derived MPs were observed, there were no significant differences between patients and controls (Table 2).
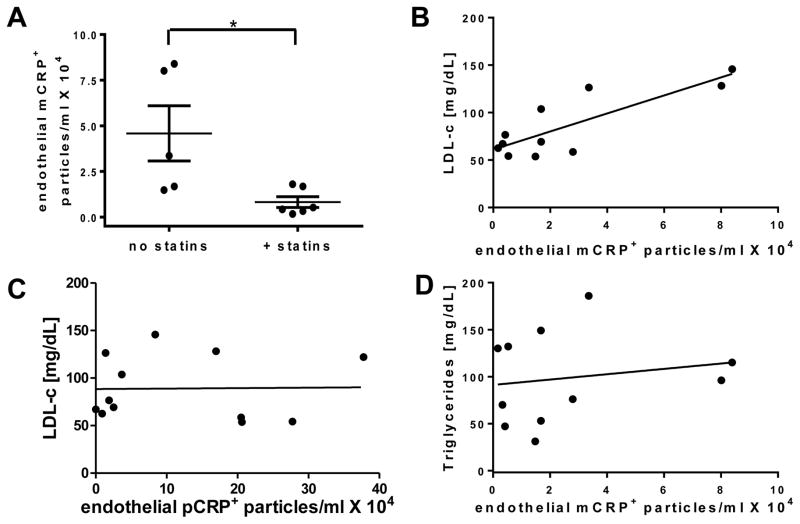
mCRP Levels Related to Statin Treatment
Almost all PAD patients are on statins, and so could not be evaluated in separate groups for any effects of statins. However, we found that our controls fell into two groups, those on statins, and those not. Because statins are known to decrease endothelial MPs [28], we evaluated the number of MPs bearing CRP isoforms in the controls based on statin treatment. The controls on statins had significantly reduced levels of endothelial mCRP+ MPs compared to controls without statins (Fig. 4a). By contrast, there was no statistically significant difference for MPs bearing pCRP (data not shown). It should be noted that the levels of endothelial mCRP+ MPs shown in Fig. 4a were significantly lower than those in PAD patients (17.8 ± 4.5 × 105, Table 2) but are shown using a scale to best discriminate the data from the two groups of controls. We then performed direct measurements of LDL-c and triglycerides on the same control samples. We found that increased numbers of mCRP+ endothelial MPs were seen in those controls with increased low density lipoprotein cholesterol (LDL-c) (R=0.85, Fig. 4b). However, there was no correlation for endothelial MPs bearing pCRP (Fig. 4c) or mCRP (Fig. 4d) and the levels of triglycerides.
Fig. 4.
Separation of normal controls by statin treatment. (a) The number of endothelial mCRP+ MPs in controls on or off statins. (b) The correlation (R=0.85) between the number of endothelial mCRP+ MPs and LDL-c in the plasma. No correlations were found for (c) endothelial pCRP + MPs and LDL-c (R=0.02) or (d) endothelial mCRP + MPs and triglycerides (R=0.17). (n=5 for controls, n=6 for controls + statin treatment, *p ≤ 0.05)
Transendothelial Migration Assay
We then ascertained if the mCRP or pCRP had any biological activity and if endothelial MPs could be involved. We examined the effects of soluble CRP isoforms in a model system of T cell/macrophage transendothelial migration (TEM) and polarization that mimics vascular inflammation. Monocytes and T cells transmigrate across an insert lined with human cardiac microvascular endothelial cells in response to monocyte chemoattractant protein-1 (MCP-1) and then differentiate into M1 and M2 macrophages, accompanied by a similar T cell polarization into Th1 and Th2 cells [26].
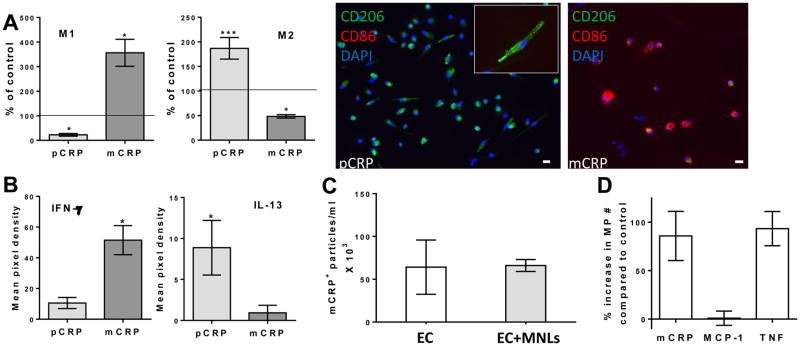
Soluble pCRP reduced the number of M1 (CD86+) macrophages significantly below background numbers, consistent with an anti-inflammatory effect, whereas solubilized mCRP treatment caused a significant increase in the number of M1s (Fig. 5a). Conversely, the number of M2 (CD206+) anti-inflammatory macrophages was increased by pCRP and decreased by mCRP. Furthermore, the relevant data from a protein array was that pCRP treatment promoted a Th2 response indicated by the production of interleukin-13 (IL-13), while mCRP treatment induced the production of interferon-γ (IFN-γ) representing a pro-inflammatory Th1 response (Fig. 5b).
Fig. 5.
Characterization of cells and MPs after transendothelial migration (TEM). a The number of M1 or M2 macrophages counted four days post-migration with the indicated treatment above the endothelial monolayer (pCRP and mCRP). (n=5; *p ≤ 0.05 and ***p ≤ 0.001). Migrated macrophages treated with pCRP were CD206 positive, whereas those treated with mCRP were CD86 positive. b The average signal (mean pixel density) was measured for cytokines using protein arrays. Protein levels of IFN-γ were higher after mCRP than after pCRP treatment, whereas levels of IL-13 were higher after pCRP treatment (n=4; p=0.02). c and d The number of MPs from endothelial cells (EC), with the presence or absence of mononuclear cells (MNLs). c Solubilized mCRP bound to MPs generated in the TEM assay. d mCRP, but not MCP-1 alone, induced the generation of MPs in the TEM assay similar to TNF stimulation. (n=3)
We used urea solubilized mCRP for the TEM experiments described above, and then tested the hypothesis that, because of its hydrophobicity, mCRP would bind to MPs from the microvascular endothelial cells used in the TEM assay. We found that added mCRP bound to MPs from those endothelial cells, whether they were grown in isolation or in the presence of MNLs (Fig. 5c). Therefore, the effects of added solubilized mCRP may have been mediated by mCRP bound to MPs produced during the TEM assay.
We then asked what the stimulus might be for production of MPs by endothelial cells in the TEM assay. We tested several possibilities, including the MCP-1 stimulus always present during the TEM, mCRP, and TNF as a possible positive control. TNF induces MP production in human umbilical vein endothelial cultures [29], and CRP has been reported to induce MP shedding by aortic endothelial cells [30], but neither has been tested in microvascular endothelium. Treatment with mCRP almost doubled the number of MPs produced from microvascular endothelial cells, while MCP-1 alone had no effect, and TNF was as active as mCRP (Fig. 5d). This indicates that mCRP can both induce and bind to MPs generated from cardiac microvascular endothelium.
Discussion
CRP has been proposed as a biomarker for risk of atherosclerotic diseases and other inflammatory states for several decades. Even using the most sensitive clinical assay available (hsCRP), reports of risk association have been mixed [27]. One of the reasons we and others suggest for these contradictory results is that they may be due to the measurement of only one isoform, pCRP, in its soluble form, by the hsCRP assay. We demonstrated that two forms of CRP, the pentamer and the monomer, circulate in the blood on microparticles, and that these are not measurable by two CRP assays. This indicates that a compartment of circulating CRP is currently invisible to clinical analysis. We therefore evaluated the two isoforms of CRP on microparticles in plasma samples by flow cytometry. For identification of MPs, we used lactadherin, which has the benefit of binding in a calcium independent and more sensitive manner than the commonly used annexin V [31]. Additionally, most of the PAD patients were treated with statins, which are known to decrease the expression of tissue factor, another marker for MPs, while not affecting phosphatidylserine exposure, to which lactadherin binds [32]. We thus maximized our likelihood of detecting as many MPs as possible.
CRP and MPs in PAD
PAD reflects endothelial dysfunction, so it is unsurprising that elevated levels of endothelial MPs were detected in PAD patients. However, this is the first time that an association has been found between mCRP and endothelial MPs in PAD patients. Specifically, an elevated number of endothelial MPs bearing mCRP was found in PAD patients compared to controls. Intriguingly, there was no correlation with hsCRP levels in PAD patients with elevated endothelial MPs or endothelial MPs bearing mCRP. The hsCRP assay fails to identify these forms of CRP and would therefore miss any possible consequences of the data observed here. The higher number of endothelial MPs with mCRP may indicate a more efficient conversion of pCRP to mCRP on patient MPs, similar to what has been found with MPs from activated platelets, due to increased amounts of lysophospholipids [3]. That the biological activity of mCRP is highly relevant to the PAD disease process may indicate that a subset of patients with poorly controlled disease have activated endothelial cells that produce high numbers of MPs and that those MPs tend to convert their burden of pCRP to mCRP with greater likelihood. Further, the proinflammatory activity of mCRP and its ability to promote endothelial shedding may be bases for amplification of inflammation and progression of the disease.
CRP Isoforms and Chronic Inflammation
mCRP+ endothelial MPs could be not only a marker for PAD, but also could be a means for mCRP to exert pro-inflammatory signaling throughout the body. MPs are able to convert the anti-inflammatory pCRP to the proinflammatory mCRP and thereby act as carriers for the CRP isoforms [3]. The MPs can mediate signaling of cells through ligand engagement (primarily FcγRI and II for pCRP [33] and FcγRIII and lipid rafts for mCRP [14]) or through transferring their components through cell membrane fusion or phagocytosis [8]. Thus, mCRP, a hydrophobic isoform not soluble in plasma, can be transported to cells where it will integrate into the lipid raft domain through a putative cholesterol binding domain that is hidden in the pentameric isoform [34]. mCRP can then induce pro-inflammatory effects on cells such as monocytes and endothelial cells, including the production of more MPs. More specifically, mCRP has been shown to bind predominantly to the apical surface of endothelial cells that are enriched in lipid raft domains, promoting the release of proinflammatory cytokines, the generation of reactive oxygen species, and adhesion molecule expression [34,35].
M1 proinflammatory and M2 anti-inflammatory macrophages [36] and activated T cells [37] are found in atherosclerotic plaques, but the mechanism by which macrophage polarization is controlled beyond the reported role of phagocytosis of lipid components is not well understood. In this work, we studied the role of pCRP versus mCRP in T cell and macrophage polarization after TEM through microvascular endothelium. While this in vitro model may not replicate the chronic inflammation associated with atherosclerosis, it does provide certain insights with regard to the potential mechanism by which CRP isoforms modulate that response. Our studies suggest that specific isoforms of CRP mediate chronic inflammation, in part, by modulating the polarization of monocytes and T lymphocytes migrating across the endothelial layer. Recently, it has been reported that CRP (untested for isoform content) directly stimulated Th2 polarization of the human Jurkat T cell line [38], and here we report that pCRP promoted a Th2 response with M2 macrophage differentiation, whereas mCRP promoted a Th1 response with M1 macrophage differentiation. By logical extension, the effect of CRP isoforms on T lymphocytes in the progression of atherosclerotic plaques presents a promising potential area of study.
Statins and Endothelial Microparticles
The lower numbers of mCRP+ MPs in controls taking statins could be the result of reduced exposure of circulating soluble pCRP to phosphatidylcholine, present on the surface of activated cells and MPs and necessary for the conversion of pentamer to monomer [2,3,19]. Statins are known to reduce the synthesis of phosphatidylcholine [39] as well as the release of MPs from endothelial cells, platelets, and monocytes [28]. Statins have also been reported to reduce the amount of circulating soluble pCRP [40], which could be a source for binding to MPs, even though there was no correlation between hsCRP measurements of pCRP and MPs bearing either isoform of CRP in patients. Thus, other variables also associated with atherosclerosis may supersede the effects of statin treatment, including the levels of tissue factor on MPs. Additionally, we do not know if the elevation of endothelial MPs pre-dated statin treatment and were decreased but not to control levels. Our study is not intended or powered to further examine this inference.
Both endothelial MPs [28] and soluble pCRP levels [41] have been found to be decreased by statins independent of the ability of statins to lower LDL cholesterol. However, here we found a strong correlation between LDL-c levels and the number of endothelial MPs bearing mCRP, suggesting LDL-c could be a modulator of the MP association with mCRP. The mechanism by which this occurs is unknown, but increased LDL-c is associated with chronic inflammation in vessels and generally associated with increased lipid deposition in the vascular wall, and statins decrease this phenomenon [42]. In addition to their effects on lipids, however, statins also suppress the inflammatory reaction through inhibition of isoprenylation in cytokines and chemokines [43]. In either case, the association of endothelial MPs and mCRP bound to them suggests a direct interaction with the endothelium (and by inference the LDL receptor). It is to be noted, however, that these inferences arise from the observations in our control population in which there is no history of PAD.
Limitations and future studies
Our study of mCRP in PAD patients was a blind pilot study that has inherent limitations. We were limited to samples from 18 PAD patients that were not collected longitudinally, and we did not have access to the patient’s disease progression or treatment outcomes. We were also limited in the number of controls that we could find with ages relatively close to the patient population. Nevertheless, our findings are notable in demonstrating that two CRP assays failed to detect all CRP isoforms and the measured levels did not correlate with elevated levels of mCRP+ endothelial MPs found in PAD patients. An expanded study could follow mCRP+ endothelial MP levels across both PAD progression and treatments to see if it is predictive of positive or negative outcomes and potentially improve risk assessment sensitivity.
Supplementary Material
Clinical Relevance.
The clinical significance of these findings is that signaling species may be sequestered in MPs and not be accessible to nor measured by standard plasma assays. We have no reason to believe that the findings will be limited to CRP. Furthermore the partition of these species from plasma to MPs appears to be dynamic, regulated, and dependent upon the MP source. In these data statins modified the partition as did the presence of PAD. Critically, the data suggest that our standard plasma assays of such species may be inaccurate, are at least underestimates, and may provide misleading information to the practitioner who happens to be assessing the plasma pool. Signaling species that are packaged with MPs are likely to have profound effects on the cell that ingests them and therefore be active.
The increased number of endothelial MPs in the patients, despite the fact that they are almost all on statins, may be due to the influence of health or lifestyle factors, such as smoking, diabetes, or aspects of cardiovascular disease other than PAD. Our patient sampling was too small to investigate the contributions of these other influences on the major findings in this report, but our intention is to promote the idea that further studies are warranted.
Acknowledgments
We are grateful for the CRP antibody donation of Dr. Larry Potempa (Roosevelt University, Chicago, Illinois). We would to thank all subjects who participated in the study, Tenille Epperson and Joe L. Raya for technical assistance, and the MD Anderson Cancer Center Flow Cytometry Core for assistance with the Amnis cytometer.
Sources of Funding
This work was supported by The National Institutes of Health [T32AI053831-10 to J.R.C., RO1 HL-089792 to M.L.E.], Bethesda, Maryland; the Hankamer Foundation, Houston, Texas; the Vivian L. Smith Foundation, Houston, Texas; and The Medallion Foundation, Houston, Texas.
Abbreviations
- CRP
C-reactive protein
- hsCRP
high sensitivity CRP assay
- mCRP
monomeric CRP
- MNL
mononuclear leukocyte
- MP
microparticle
- PAD
peripheral artery disease
- pCRP
pentameric CRP
- TEM
transendothelial migration
Footnotes
Disclosures
V.N. and R.C.H. are co-investigators on a provisional patent filed by Roche for use of biomarkers in heart failure prediction. The other authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Ethics Statement
The study was conducted in accordance with the ethical standards of the Helsinki declaration and its later amendments and with the ethical standards and approval of the Institutional Review Board of Baylor College of Medicine. Informed consent was obtained from all individual participants included in the study. No animal studies were carried out by the authors for this article.
References
- 1.Ansar W, Ghosh S. C-reactive protein and the biology of disease. Immunologic research. 2013;56(1):131–142. doi: 10.1007/s12026-013-8384-0. [DOI] [PubMed] [Google Scholar]
- 2.Eisenhardt SU, Habersberger J, Murphy A, Chen YC, Woollard KJ, Bassler N, et al. Dissociation of pentameric to monomeric C-reactive protein on activated platelets localizes inflammation to atherosclerotic plaques. Circulation research. 2009;105(2):128–137. doi: 10.1161/CIRCRESAHA.108.190611. [DOI] [PubMed] [Google Scholar]
- 3.Habersberger J, Strang F, Scheichl A, Htun N, Bassler N, Merivirta RM, et al. Circulating microparticles generate and transport monomeric C-reactive protein in patients with myocardial infarction. Cardiovascular research. 2012;96(1):64–72. doi: 10.1093/cvr/cvs237. [DOI] [PubMed] [Google Scholar]
- 4.Paul A, Ko KW, Li L, Yechoor V, McCrory MA, Szalai AJ, et al. C-reactive protein accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109(5):647–655. doi: 10.1161/01.CIR.0000114526.50618.24. [DOI] [PubMed] [Google Scholar]
- 5.Hirschfield GM, Gallimore JR, Kahan MC, Hutchinson WL, Sabin CA, Benson GM, et al. Transgenic human C-reactive protein is not proatherogenic in apolipoprotein E-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(23):8309–8314. doi: 10.1073/pnas.0503202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovacs A, Tornvall P, Nilsson R, Tegner J, Hamsten A, Bjorkegren J. Human C-reactive protein slows atherosclerosis development in a mouse model with human-like hypercholesterolemia. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(34):13768–13773. doi: 10.1073/pnas.0706027104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teupser D, Weber O, Rao TN, Sass K, Thiery J, Fehling HJ. No reduction of atherosclerosis in C-reactive protein (CRP)-deficient mice. The Journal of biological chemistry. 2011;286(8):6272–6279. doi: 10.1074/jbc.M110.161414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circulation research. 2010;107(9):1047–1057. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 9.Suades R, Padro T, Vilahur G, Badimon L. Circulating and platelet-derived microparticles in human blood enhance thrombosis on atherosclerotic plaques. Thrombosis and haemostasis. 2012;108(6):1208–1219. doi: 10.1160/TH12-07-0486. [DOI] [PubMed] [Google Scholar]
- 10.Leroyer AS, Tedgui A, Boulanger CM. Role of microparticles in atherothrombosis. Journal of internal medicine. 2008;263(5):528–537. doi: 10.1111/j.1365-2796.2008.01957.x. [DOI] [PubMed] [Google Scholar]
- 11.Sternik L, Samee S, Schaff HV, Zehr KJ, Lerman LO, Holmes DR, et al. C-reactive protein relaxes human vessels in vitro. Arteriosclerosis, thrombosis, and vascular biology. 2002;22(11):1865–1868. doi: 10.1161/01.atv.0000033821.96354.90. [DOI] [PubMed] [Google Scholar]
- 12.Khreiss T, Jozsef L, Potempa LA, Filep JG. Opposing effects of C-reactive protein isoforms on shear-induced neutrophil-platelet adhesion and neutrophil aggregation in whole blood. Circulation. 2004;110(17):2713–2720. doi: 10.1161/01.CIR.0000146846.00816.DD. [DOI] [PubMed] [Google Scholar]
- 13.Molins B, Pena E, Vilahur G, Mendieta C, Slevin M, Badimon L. C-reactive protein isoforms differ in their effects on thrombus growth. Arteriosclerosis, thrombosis, and vascular biology. 2008;28(12):2239–2246. doi: 10.1161/ATVBAHA.108.174359. [DOI] [PubMed] [Google Scholar]
- 14.Khreiss T, Jozsef L, Potempa LA, Filep JG. Conformational rearrangement in C-reactive protein is required for proinflammatory actions on human endothelial cells. Circulation. 2004;109(16):2016–2022. doi: 10.1161/01.CIR.0000125527.41598.68. [DOI] [PubMed] [Google Scholar]
- 15.Khreiss T, Jozsef L, Potempa LA, Filep JG. Loss of pentameric symmetry in C-reactive protein induces interleukin-8 secretion through peroxynitrite signaling in human neutrophils. Circulation research. 2005;97(7):690–697. doi: 10.1161/01.RES.0000183881.11739.CB. [DOI] [PubMed] [Google Scholar]
- 16.Zouki C, Haas B, Chan JS, Potempa LA, Filep JG. Loss of pentameric symmetry of C-reactive protein is associated with promotion of neutrophil-endothelial cell adhesion. Journal of immunology. 2001;167(9):5355–5361. doi: 10.4049/jimmunol.167.9.5355. [DOI] [PubMed] [Google Scholar]
- 17.Mok CC, Ying SK, Ma KM, Wong CK. Effect of raloxifene on disease activity and vascular biomarkers in patients with systemic lupus erythematosus: subgroup analysis of a double-blind randomized controlled trial. Lupus. 2013;22(14):1470–1478. doi: 10.1177/0961203313507987. [DOI] [PubMed] [Google Scholar]
- 18.Kiefer CR, Stock RE, Flanagan SS, Darling CE, Smith CS, Snyder LM. Early verification of myocardial ischemia with a novel biomarker of acute tissue damage: C-reactive protein fractional forms. Clinica chimica acta; international journal of clinical chemistry. 2012;413(19–20):1536–1541. doi: 10.1016/j.cca.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 19.Thiele JR, Habersberger J, Braig D, Schmidt Y, Goerendt K, Maurer V, et al. Dissociation of pentameric to monomeric C-reactive protein localizes and aggravates inflammation: in vivo proof of a powerful proinflammatory mechanism and a new anti-inflammatory strategy. Circulation. 2014;130(1):35–50. doi: 10.1161/CIRCULATIONAHA.113.007124. [DOI] [PubMed] [Google Scholar]
- 20.Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation. 2006;114(7):688–699. doi: 10.1161/CIRCULATIONAHA.105.593442. [DOI] [PubMed] [Google Scholar]
- 21.Kresl JJ, Potempa LA, Anderson BE. Conversion of native oligomeric to a modified monomeric form of human C-reactive protein. The international journal of biochemistry & cell biology. 1998;30(12):1415–1426. doi: 10.1016/s1357-2725(98)00078-8. [DOI] [PubMed] [Google Scholar]
- 22.Ying SC, Gewurz H, Kinoshita CM, Potempa LA, Siegel JN. Identification and partial characterization of multiple native and neoantigenic epitopes of human C-reactive protein by using monoclonal antibodies. Journal of immunology. 1989;143(1):221–228. [PubMed] [Google Scholar]
- 23.Potempa LA, Siegel JN, Fiedel BA, Potempa RT, Gewurz H. Expression, detection and assay of a neoantigen (Neo-CRP) associated with a free, human C-reactive protein subunit. Molecular immunology. 1987;24(5):531–541. doi: 10.1016/0161-5890(87)90028-9. [DOI] [PubMed] [Google Scholar]
- 24.Taylor KE, van den Berg CW. Structural and functional comparison of native pentameric, denatured monomeric and biotinylated C-reactive protein. Immunology. 2007;120(3):404–411. doi: 10.1111/j.1365-2567.2006.02516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Headland SE, Jones HR, D’Sa AS, Perretti M, Norling LV. Cutting-edge analysis of extracellular microparticles using ImageStream(X) imaging flow cytometry. Scientific reports. 2014;4:5237. doi: 10.1038/srep05237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trial J, Cieslik KA, Haudek SB, Duerrschmid C, Entman ML. Th1/M1 conversion to th2/m2 responses in models of inflammation lacking cell death stimulates maturation of monocyte precursors to fibroblasts. Frontiers in immunology. 2013;4:287. doi: 10.3389/fimmu.2013.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yousuf O, Mohanty BD, Martin SS, Joshi PH, Blaha MJ, Nasir K, et al. High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? Journal of the American College of Cardiology. 2013;62(5):397–408. doi: 10.1016/j.jacc.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Suades R, Padro T, Alonso R, Mata P, Badimon L. Lipid-lowering therapy with statins reduces microparticle shedding from endothelium, platelets and inflammatory cells. Thrombosis and haemostasis. 2013;110(2):366–377. doi: 10.1160/TH13-03-0238. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Tao J, Yang Z, Tu C, Xu MG, Wang JM, et al. Tumor necrosis factor-alpha induces release of endothelial microparticles from human endothelial cells. Zhonghua xin xue guan bing za zhi [Chinese journal of cardiovascular diseases] 2005;33(12):1137–1140. [PubMed] [Google Scholar]
- 30.Devaraj S, Kumaresan PR, Jialal I. C-reactive protein induces release of both endothelial microparticles and circulating endothelial cells in vitro and in vivo: further evidence of endothelial dysfunction. Clinical chemistry. 2011;57(12):1757–1761. doi: 10.1373/clinchem.2011.169839. [DOI] [PubMed] [Google Scholar]
- 31.Dasgupta SK, Abdel-Monem H, Niravath P, Le A, Bellera RV, Langlois K, et al. Lactadherin and clearance of platelet-derived microvesicles. Blood. 2009;113(6):1332–1339. doi: 10.1182/blood-2008-07-167148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mobarrez F, He S, Broijersen A, Wiklund B, Antovic A, Antovic J, et al. Atorvastatin reduces thrombin generation and expression of tissue factor, P-selectin and GPIIIa on platelet-derived microparticles in patients with peripheral arterial occlusive disease. Thrombosis and haemostasis. 2011;106(2):344–352. doi: 10.1160/TH10-12-0810. [DOI] [PubMed] [Google Scholar]
- 33.Devaraj S, Du Clos TW, Jialal I. Binding and internalization of C-reactive protein by Fcgamma receptors on human aortic endothelial cells mediates biological effects. Arteriosclerosis, thrombosis, and vascular biology. 2005;25(7):1359–1363. doi: 10.1161/01.ATV.0000168573.10844.ae. [DOI] [PubMed] [Google Scholar]
- 34.Ji SR, Ma L, Bai CJ, Shi JM, Li HY, Potempa LA, et al. Monomeric C-reactive protein activates endothelial cells via interaction with lipid raft microdomains. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2009;23(6):1806–1816. doi: 10.1096/fj.08-116962. [DOI] [PubMed] [Google Scholar]
- 35.Li HY, Wang J, Wu YX, Zhang L, Liu ZP, Filep JG, et al. Topological localization of monomeric C-reactive protein determines proinflammatory endothelial cell responses. The Journal of biological chemistry. 2014;289(20):14283–14290. doi: 10.1074/jbc.M114.555318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stoger JL, Gijbels MJ, van der Velden S, Manca M, van der Loos CM, Biessen EA, et al. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012;225(2):461–468. doi: 10.1016/j.atherosclerosis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Oliveira RT, Silva RM, Teo FH, Mineiro MF, Ferreira MC, Altemani A, et al. Detection of TCD4+ subsets in human carotid atheroma. Cytokine. 2013;62(1):131–140. doi: 10.1016/j.cyto.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, Liu SH, Wright TT, Shen ZY, Li HY, Zhu W, et al. C-reactive protein directly suppresses Th1 cell differentiation and alleviates experimental autoimmune encephalomyelitis. Journal of immunology. 2015;194(11):5243–5252. doi: 10.4049/jimmunol.1402909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanagita T, Yamamoto K, Ishida S, Sonda K, Morito F, Saku K, et al. Effects of simvastatin, a cholesterol synthesis inhibitor, on phosphatidylcholine synthesis in HepG2 cells. Clinical therapeutics. 1994;16(2):200–208. [PubMed] [Google Scholar]
- 40.Prasad K. C-reactive protein (CRP)-lowering agents. Cardiovascular drug reviews. 2006;24(1):33–50. doi: 10.1111/j.1527-3466.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 41.Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA: the journal of the American Medical Association. 2001;286(1):64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 42.Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends in molecular medicine. 2008;14(1):37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antonopoulos AS, Margaritis M, Lee R, Channon K, Antoniades C. Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Current pharmaceutical design. 2012;18(11):1519–1530. doi: 10.2174/138161212799504803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.