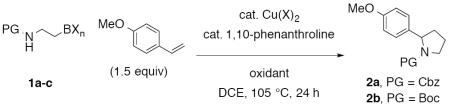
Abstract
2-Arylpyrrolidines occur frequently in bioactive compounds and thus methods to access them from readily available reagents are valuable. We report a copper-catalyzed intermolecular carboamination of vinylarenes with potassium N-carbamoyl-β-aminoethyltrifluoroborates. The reaction occurs with terminal, 1,2-disubstituted and 1,1-disubstituted vinylarenes bearing a number of functional groups. 1,3-Dienes are also good substrates and their reactions give 2-vinylpyrrolidines. Radical clock mechanistic experiments are consistent with the presence of carbon radical intermediates and do not support participation of carbocations.
Functionalized pyrrolidines are important nitrogen heterocycles found in numerous bioactive compounds of both natural and synthetic origin. 2-Arylpyrrolidines in particular are ubiquitous.1-3 The need to access these important moieties and related heterocycles has inspired the development of a number of methods.4-26 Many of these methods, however, utilize strong bases5 and water sensitive organometallic reagents.7,8 Additional intermolecular metal-catalyzed couplings18-20 and intramolecular metal- and Bronsted acid catalyzed cyclizations21-25 have been developed, providing various routes to 2-arylpyrrolidines and related products. These latter methods, however, are mainly limited to sulfonamides.
More recently, readily available vinyl arenes have been used to directly access 2-aryl pyrrolidines and related saturated heterocycles via intermolecular coupling with bi-functional heteroatom-substituted reagents that can undergo polar/radical [3+2]-type bond-forming reaction sequences under mild reaction conditions.26-31 The products of these reactions, by virtue of the required substrates, often contain additional functional groups (Schemes 1a and 1b) that may not be desired in all applications and primarily N-sulfonyl pyrrolidine synthesis has been reported.
Scheme 1.
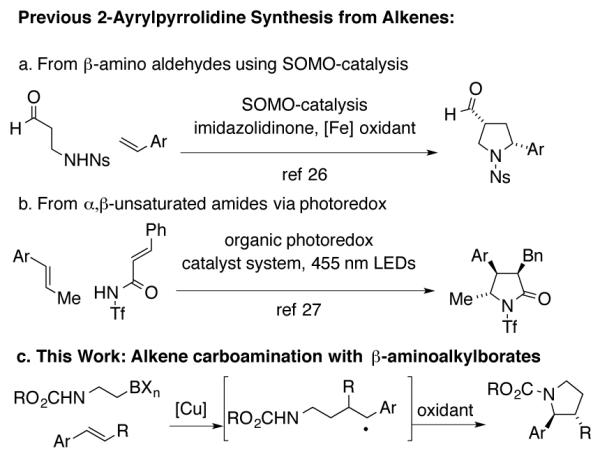
Polar/radical pyrrolidine syntheses
To address existing limitations as well as to explore an orthogonal reactivity mode, we envisaged that a β-aminoethyl carbon radical could serve as a bifunctional three-atom unit to affect a net intermolecular carboamination in the presence of an oxidant (Scheme 1c). The resulting products of the coupling with vinyl arenes are simple 2-arylpyrrolidines, whose applications in medicinal chemistry endeavors can be readily envisioned. Herein is presented our development of this method and its extension to 1,3-dienes. The method is ideal for the synthesis of N-carbamoyl pyrrolidines. Carbamates are generally considered to be more attractive than sulfonamides as the latter are higher molecular weight and often require more strenuous conditions to reveal the parent amine. The utility of carbamates in medicinal chemistry has been noted.32
We recently disclosed an oxidative alkyl Heck-type reaction between alkylboron reagents and vinyl arenes.33 In this transformation, alkyl radicals, generated in situ from [Cu(II)] oxidation of the alkylboron reagent, add to vinyl arenes. Oxidation of the resulting benzyl radical then provides the observed higher substituted vinyl arene products. We hypothesized that under the reaction conditions, the benzylic radical, or carbocation derived thereof, could be intercepted with an amine, resulting in 2-aryl pyrrolidine formation (Scheme 1c). The ready availability of N-carbamoyl-β-aminoethylboron reagents, due to the respective contributions of Overman and Molander,34,35 presented an excellent opportunity to test this hypothesis.
Copper(II) 2-ethylhexanoate [Cu(eh)2] is a readily available copper salt that has previously been shown to activate potassium alkyltrifluoroborates in coupling reactions with radical acceptors.33,36 However, an attempt at the copper(II) 2-ethylhexanoate-catalyzed coupling of potassium N-Cbz-β-aminoethyltrifluoroborate 1a with 4-methoxystyrene in the presence of MnO2 (2.55 equiv) as stoichiometric oxidant did not result in pyrrolidine formation (Table 1, entry 1). Upon changing the catalyst to [Cu(1,10-phenanthroline)](OTf)2,33 oxidative coupling readily occurred to give 82% yield of 2-arylpyrrolidine 2a (Table 1, entry 2). The Boc analog 1b also provided the coupling product 2b, but in lower yield (49%, entry 3). The boronic acid analog 1c underwent the coupling reaction, but also less efficiently (Table 1, entry 4, 52% yield).37 Lowering the copper loading from 20 mol % to 15 mol % led to a less efficient reaction with N-Cbz-β-aminoethyltrifluoroborate 1a (Table 1, entry 5, 67% yield). Ag2CO3 (2 equiv) could also serve as stoichiometric oxidant in place of MnO2 (Table 1, entry 6).
Table 1. Effect of Alkylborane Structure, Catalyst Loading and Oxidant on Reaction Efficiencya.
| entry | PG | BXn | oxidant | CuX2 (mol %) |
yield (%)c |
|---|---|---|---|---|---|
| 1b | Cbz | BF3K 1a |
MnO2 (2.55 equiv) |
Cu(eh)2 (20 mol %) |
NR |
| 2 | Cbz | BF3K 1a |
MnO2 (2.55 equiv) |
Cu(OTf)2 (20 mol %) |
82 |
| 3 | Boc | BF3K 1b |
MnO2 (2.55 equiv) |
Cu(OTf)2 (20 mol %) |
46 |
| 4 | Cbz | B(OH)2 1c |
MnO2 (2.55 equiv) |
Cu(OTf)2 (20 mol %) |
52 |
| 5d | Cbz | BF3K 1b |
MnO2 (2.55 equiv) |
Cu(OTf)2 (15 mol %) |
67 |
| 6 | Cbz | BF3K 1b |
Ag2CO3 (2 equiv) |
Cu(OTf)2 (20 mol %) |
63 |
25 mol % 1,1-phenanthroline was used unless otherwise noted.
1,10-phenanthroline was not used.
Isolated yield.
20 mol % 1,10-phenanthroline was used. MnO2 (85% by weight) was used in these reactions.
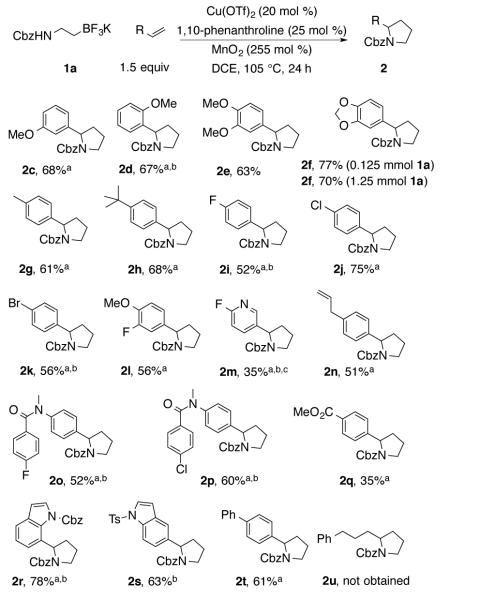
A number of styrenes underwent this reaction (Chart 1). Styrenes with electron-donating substituents were most reactive. While most reactions were performed at the 0.125 mmol scale of 1a (Chart 1), a 1.25 mmol scale for the efficient (70% yield) production of 2f was also performed. Ethers, alkyl substitutents, sulfonamides and amides were tolerated. Halide-substituted styrenes underwent the reaction, but longer reaction times were required. Substrates bearing two potentially reactive alkenes (terminal styrene, indole, terminal alkyl-substituted alkene) favored reaction at the terminal styrene. A styrene bearing a methyl ester also underwent the reaction but with lower efficiency. A 2-fluoropyridyl in place of a substituted phenyl was also tolerated albeit the reaction was less efficient. 1-Phenyl-4-pentene, lacking conjugation, did not react.
Chart 1. Annulation with Terminal Styrenes.
aTwo equiv of styrene was used. bReaction run for 48 h. cReaction run at 95 °C.
Disubstituted styrenes were next evaluated (Chart 2). 1-Alkyl and 1-aryl styrenes provided 3° amines and spirocycles 3a-3e. Coupling with indene gave cis-fused bicyclic pyrrolidine 3f. Both trans and cis-stilbene gave the same trans-pyrrolidine adduct 3g. 1-Phenyl-1-cyclohexene, a trisubstituted alkene, was unreactive (not shown).
Chart 2. Annulation with Di-substituted Styrenes.
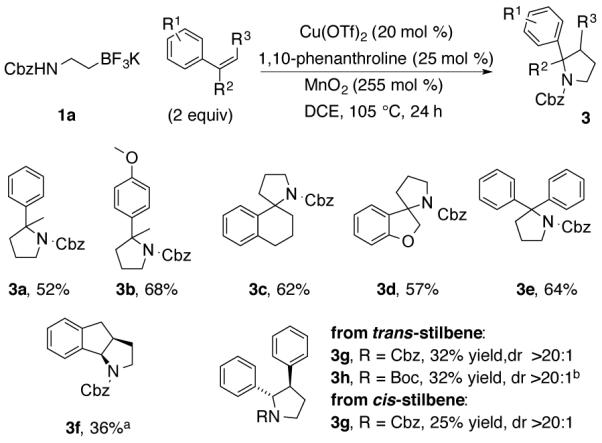
aReaction run in anhydrous 1,4-dioxane at 120 °C. bReaction run for 36 h.
Dienes and an ene-yne also underwent the coupling reaction to give the corresponding 2-vinyl and 2-propargyl pyrrolidines 4 and 5 (Scheme 2). trans-Ethyl cinnamate and an α,β-unsaturated diene provided β-amino acid esters 6 and 7 with good diastereoselectivity for the 2,3-trans pyrrolidines (Scheme 2).
Scheme 2.
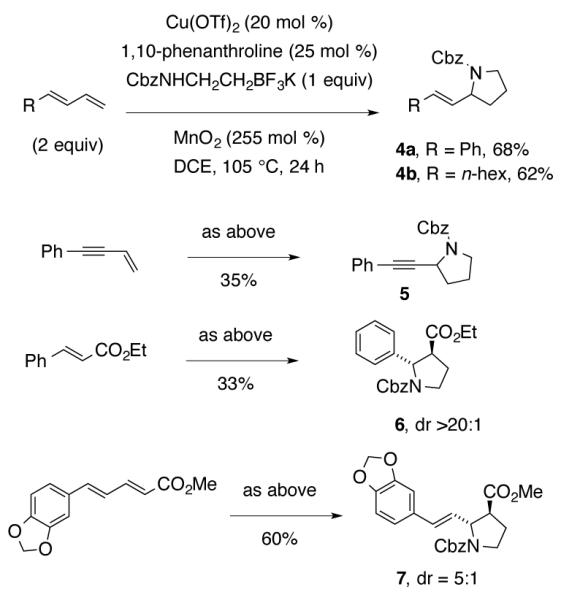
Reactions of Dienes and a Dienoate
Bexarotene methyl ester, the methyl ester of a retinoid anticancer agent that has recently shown promise in the treatment and prevention of Alzheimer’s disease,38 provided pyrrolidine 8 from the coupling reaction along with two alkyl Heck-type diastereomers 9 (Scheme 3).
Scheme 3.
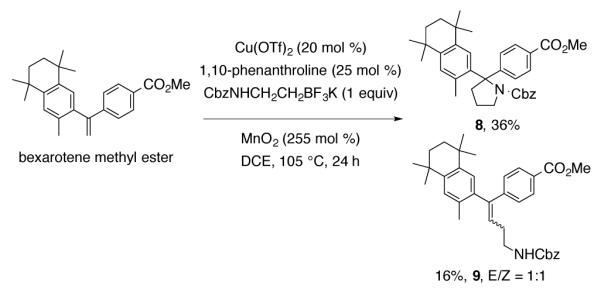
Reaction of Bexarotene Methyl Ester
These intermolecular coupling reactions appear to occur through copper-catalyzed/MnO2 mediated stepwise oxidative coupling sequence (Scheme 4). Copper(II)-catalyzed oxidation of the alkylborane to its corresponding alkyl radical initiates the process.33,36,39 The alkyl radical then adds to the styrene to produce a stabilized benzylic radical intermediate. This intermediate can combine with [Cu(II)] to form an alkylcopper(III) intermediate capable of undergoing C-N bond formation via reductive elimination (path I).40 Alternatively, the benzylic radical could be further oxidized by MnO2 to provide a benzylic carbocation that is then trapped by the pendant amine (path II).31 Oxidation of [Cu(I)] back to [Cu(II)] with MnO2 then closes the catalytic cycle. At the onset of our mechanistic investigation, we could not differentiate between path I and path II because, based on oxidation potentials, MnO2 is capable of oxidizing both [Cu(I)] and a benzylic radical, although [Cu(I)] is the more easily oxidized species.41
Scheme 4.
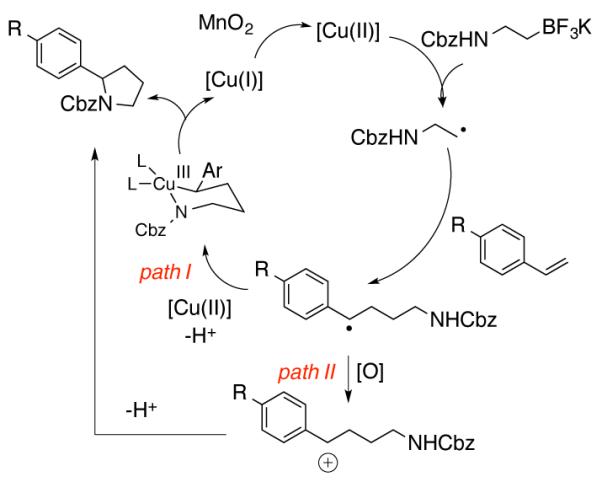
Proposed Reaction Mechanism
To investigate the mechanism further, a series of vinylcyclopropanes were submitted to the coupling reaction with N-Cbz-β-aminoethyltrifluoroborate 1a (Scheme 5). Reaction with 2-(buta-1,3-dienyl)cyclopropylbenzene provided a mixture of pyrrolidine diastereomers 10, and no cyclopropane ring-opened products were detected. Reaction with 2-((phenylcyclopropyl)vinyl)benzene provided both pyrrolidine diastereomers 11 and dihydronaphthalene 12.33 The regiochemistry of 12 indicates that cyclopropane ring opening occurred at the phenyl-bearing carbon. This supports a radical cyclopropane ring-opening mechanism over a metal-mediated ring opening where the less substituted organometallic would have been preferred.42 To differentiate between a carbocation and a radical cyclopropane opening, the 2-(tert-butoxy)-3-(1-phenylvinyl)cyclopropyl)benzene radical clock was applied. Newcomb has demonstrated this kind of radical clock will open at the oxygen-bearing carbon in carbocationic mechanisms, and at the phenyl-bearing carbon in radical mechanisms.43 In the event, a mixture of 4-phenylnaphthylene 13 and dihydronaphthalene 14 were obtained in this reaction. Naphthalene 13 is likely formed by elimination of t-BuOH from 14. The regioselectivity in these reactions support involvement of radical intermediates and do not provide evidence for carbocation intermediates. The lack of ring-opened product from reaction with the cyclopropyl diene probe could indicate that C-N bond formation is favored over ring-opening and/or radical addition to the arene when the carbon is less hindered. With increased steric hindrance at carbon, ring-opening and/or radical addition to the arene becomes competitive. While pyrrolidine formation without ring-opening is feasible, it is also possible the ring could open and subsequently close prior to pyrrolidine formation.44
Scheme 5.
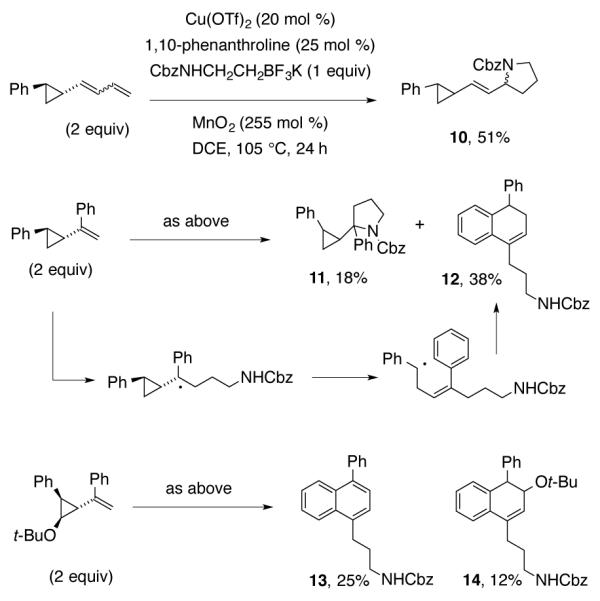
Radical Clock Mechanism Probes
In summary, we have developed new conditions for the synthesis of simple 2-arylpyrrolidines from vinyl arenes and dienes. The scope of the alkene partner is broad. Radical clock experiments support a purely radical mechanism, likely involving C-N bond formation through a copper(III) intermediate. Our future efforts involve reaction refinement and scope expansion.
Supplementary Material
ACKNOWLEDGMENT
We thank the National Institute of General Medical Science for support of this work (R01 078383) and the Bureau of Educational and Cultural Affairs of the U.S. Department of States for a Fullbright fellowship to C. U. We thank Mr. Shuklendu Karyakarte (UB chemistry) for assistance with some NMR spectra.
Footnotes
Experimental procedures, characterization of new compounds, and copies of NMR spectra. The Supporting Information is available free of charge on the ACS Publications website at DOI:
Author Contributions
The manuscript was written through contributions of all authors. / All authors have given approval to the final version of the manuscript.
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Klapars A, Campos KR, Waldman JH, Zewge D, Domer PG, Chen C-Y. J. Org. Chem. 2008;73:4986. doi: 10.1021/jo8006804. [DOI] [PubMed] [Google Scholar]
- (2).Cho-Schultz S, Patten MJ, Huang B, Elleraas J, Gajiwala KS, Hickey MJ, Wang J, Mehta PP, Kang P, Gehring MR, Kung P-P, Sutton SC. J. Comb. Chem. 2009;11:860. doi: 10.1021/cc900056d. [DOI] [PubMed] [Google Scholar]
- (3).Elliott RL, Ryther KB, Anderson DJ, Raszkiewicz JL, Campbell JE, Sullivan JP, Garvey DS. Bioorg. Med. Chem. Lett. 1995;5:991. [Google Scholar]
- (4).Wagner FF, Comins DL. Tetrahedron. 2007;63:8065. [Google Scholar]
- (5).Campos KR, Klapars A, Waldman JH, Dormer PG, Chen CY. J. Am. Chem. Soc. 2006;128:3538. doi: 10.1021/ja0605265. [DOI] [PubMed] [Google Scholar]
- (6).Wu S, Lee S, Beak P. J. Am. Chem. Soc. 1996;118:715. [Google Scholar]
- (7).Brinner KM, Ellman JA. Org. Biomol. Chem. 2005;3:2109. doi: 10.1039/b502080h. [DOI] [PubMed] [Google Scholar]
- (8).Reddy LR, Das SG, Liu Y, Prashad M. J. Org. Chem. 2010;75:2236. doi: 10.1021/jo902710s. [DOI] [PubMed] [Google Scholar]
- (9).Leemans E, Mangelinckx S, De Kimpe N. Chem. Commun. 2010;46:3122. doi: 10.1039/b925209f. [DOI] [PubMed] [Google Scholar]
- (10).Zuo Z, Ahneman DT, Chu L, Terrett JA, Doyle AG, MacMillan DWC. Science. 2014;345:437. doi: 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Broere DLJ, de Bruin B, Reek JNH, Lutz M, Dechert S, van der Vlugt JI. J. Am. Chem. Soc. 2014;136:11574. doi: 10.1021/ja502164f. [DOI] [PubMed] [Google Scholar]
- (12).Yus M, Soler T, Foubelo F. J. Org. Chem. 2001;66:6207. doi: 10.1021/jo010419n. [DOI] [PubMed] [Google Scholar]
- (13).Coldham I, Robinson SP, Baxter CA. Synlett. 2012;23:2405. [Google Scholar]
- (14).Bunrit A, Dahlstrand C, Olsson SK, Srifa P, Huang G, Orthaber A, Sjoberg PJR, Biswas S, Himo F, Samec JSM. J. Am. Chem. Soc. 2015;137:4646. doi: 10.1021/jacs.5b02013. [DOI] [PubMed] [Google Scholar]
- (15).Cui Z, Yu H-J, Yang R-F, Gao W-Y, Feng C-G, Lin G-Q. J. Am. Chem. Soc. 2011;133:12394. doi: 10.1021/ja2046217. [DOI] [PubMed] [Google Scholar]
- (16).Gribkov DV, Pastine SJ, Schnurch M, Sames D. J. Am. Chem. Soc. 2007;129:11750. doi: 10.1021/ja072577n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Henry CE, Xu Q, Fan YC, Martin TJ, Belding L, Dudding T, Kwon O. J. Am. Chem. Soc. 2014;136:11890. doi: 10.1021/ja505592h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Scarborough CC, Stahl SS. Org. Lett. 2006;8:3251. doi: 10.1021/ol061057e. [DOI] [PubMed] [Google Scholar]
- (19).Shi M, Liu L-P, Tang J. Org. Lett. 2006;8:4043. doi: 10.1021/ol0614830. [DOI] [PubMed] [Google Scholar]
- (20).Rao W, Chan PWH. Chem. Eur. J. 2008;14:10486. doi: 10.1002/chem.200801242. [DOI] [PubMed] [Google Scholar]
- (21).Schlummer B, Hartwig JF. Org. Lett. 2002;4:1471. doi: 10.1021/ol025659j. [DOI] [PubMed] [Google Scholar]
- (22).Chen J, Zhou L, Yeung Y-Y. Org. Biomol. Chem. 2012;10:3808. doi: 10.1039/c2ob25327e. [DOI] [PubMed] [Google Scholar]
- (23).Xu T, Qiu S, Liu G. J. Organomet. Chem. 2011;695:46. [Google Scholar]
- (24).O’Broin CQ, Fernandez P, Martinez C, Muniz K. Org. Lett. 2016;18:436. doi: 10.1021/acs.orglett.5b03476. [DOI] [PubMed] [Google Scholar]
- (25).Hennessy ET, Bentley TA. Science. 2013;340:591. doi: 10.1126/science.1233701. [DOI] [PubMed] [Google Scholar]
- (26).Jui NT, Garber JAO, Finelli FG, MacMillan DWC. J. Am. Chem. Soc. 2012;134:11400. doi: 10.1021/ja305076b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Gesmundo NJ, Grandjean J-MM, Nicewicz DA. Org. Lett. 2015;17:1316. doi: 10.1021/acs.orglett.5b00316. [DOI] [PubMed] [Google Scholar]
- (28).Grandjean J-MM, Nicewicz DA. Angew. Chem. Int. Ed. 2013;52:3967. doi: 10.1002/anie.201210111. [DOI] [PubMed] [Google Scholar]
- (29).Michaelis DJ, Shaffer CJ, Yoon TP. J. Am. Chem. Soc. 2007;129:1866. doi: 10.1021/ja067894t. [DOI] [PubMed] [Google Scholar]
- (30).Zhao B, Peng X, Zhu Y, Ramirez TA, Cornwall RG, Shi Y. J. Am. Chem. Soc. 2011;133:20890. doi: 10.1021/ja207691a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Lu D-F, Zhu C-L, Jia Z-X, Xu H. J. Am. Chem. Soc. 2014;136:13186. doi: 10.1021/ja508057u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Ghosh AK, Brindisi J. Med. Chem. 2015;58:2895. doi: 10.1021/jm501371s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Liwosz TW, Chemler SR. Org. Lett. 2013;15:3034. doi: 10.1021/ol401220b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Kamatani A, Overman LE. J. Org. Chem. 1999;64:8743. [Google Scholar]
- (35).Molander GA, Jean-Gerard L. J. Org. Chem. 2007;72:8422. doi: 10.1021/jo7015955. [DOI] [PubMed] [Google Scholar]
- (36).Sorin G, Mallorquin RM, Contie Y, Baralle A, Malacria M, Goddard JP, Fensterbank L. Angew. Chem. Int. Ed. 2010;49:8721. doi: 10.1002/anie.201004513. [DOI] [PubMed] [Google Scholar]
- (37).Attempted couplings with other aminoalkyltrifluoroborates were not successful (see Supporting Information).
- (38) (a).Qu L, Tang X. Cancer Chemotherapy and Pharmacology. 2010;65:201. doi: 10.1007/s00280-009-1140-4. [DOI] [PubMed] [Google Scholar]; (b) Habchi J, Arosio P, Perni M, Costa AR, Yagi-Utsumi M, Joshi P, Chia S, Cohen SIA, Muller MBD, Linse S, Nollen EAA, Dobson CM, Knowles TPJ, Vendruscolo M. Sci. Adv. 2016;2:e1501244. doi: 10.1126/sciadv.1501244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39) (a).Tang S, Liu C, Lei A. Chem. Soc. Rev. 2015;44:1070. doi: 10.1039/c4cs00347k. [DOI] [PubMed] [Google Scholar]; (b) Zhao W, Montgomery J. Angew. Chem. Int. Ed. 2015;54:12683. doi: 10.1002/anie.201507303. [DOI] [PubMed] [Google Scholar]
- (40).Clark SJ, Roche C. Chem. Commun. 2005:5175. doi: 10.1039/b509678b. [DOI] [PubMed] [Google Scholar]
- (41) (a).Milazzo G, Caroli S, Sharma VK. Tables of Standard Electrode Potentials. Wiley; Chichester: 1978. [Google Scholar]; (b) Bard AJ, Parsons R, Jordan J. Standard Potentials in Aqueous Solutions. Marcel Dekker; New York: 1985. [Google Scholar]; (c) Connelly NG, Geiger WE. Chem. Rev. 1996;96:877. doi: 10.1021/cr940053x. [DOI] [PubMed] [Google Scholar]; (d) Wayner DDM, McPhee DJ, Griller D. J. Am. Chem. Soc. 1988;110:132. [Google Scholar]
- (42).Wender PA, Dyckman AJ, Husfeld CO, Kadereit D, Love JA, Rieck H. J. Am. Chem. Soc. 1999;121:10442. [Google Scholar]
- (43) (a).Newcomb M, Chestney DL. J. Am. Chem. Soc. 1994;116:9753. [Google Scholar]; (b) Le Tadic-Biadatti M-H, Newcomb M. J. Chem. Soc., Perkin Trans. 1996;2:1467. [Google Scholar]; (c) Faulknew A, Race NJ, Scott JS, Bower JF. Chem. Sci. 2014;5:2416. [Google Scholar]
- (44).Benkovics T, Du J, Guzei IA, Yoon TP. J. Org. Chem. 2009;74:5545. doi: 10.1021/jo900902k. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.