Abstract
Background
Previous studies have suggested an association between particulate air pollution and cardiovascular disease, but the mechanism is still unclear.
Objective
We examined the association between workplace exposure to vehicle-related particles and cardiovascular disease related systemic inflammatory markers, C-reactive protein (hs-CRP), soluble intercellular adhesion molecule-1 (sICAM-1), and interleukin-6 (IL-6) in 137 trucking terminal workers (non-drivers) in the U.S. trucking industry.
Methods
We visited two large trucking terminals in 2009 and measured vehicle-related elemental carbon (EC), organic carbon (OC), and particulate matter with aerodynamic diameter ≤2.5μm (PM2.5), for 5 days consecutively at the main work areas. Each participant provided a blood sample and completed a health questionnaire during the sampling period. Individual workplace exposure level was calculated by 12-hr time weighted moving averages based on work shift. The association between each blood marker and exposure to each pollutant during 0-12, 12-24, 24-36, and 36-48 hours before the blood draw was examined by multivariable regression analyses.
Results
In general, OC and EC had a positive association with sICAM-1, especially for exposure periods 12-24 (lag12-24) and 24-36 (lag24-36) hrs prior to blood draw [β=54.9 (95%CI: 12.3-97.5) for lag12-24 and β=46.5 (95%CI: 21.2-71.8) for lag12-24; change in sICAM-1 (in ng/mL) corresponding to an IQR increase in OC]. A similar pattern was found for EC and PM2.5. We did not find an association between measured pollutants up to 48 hours before blood draw and hs-CRP or IL-6.
Conclusion
In this group of healthy workers, short-term exposure to vehicle-related air pollutants may be associated with sICAM-1. Our findings may be dependent on the exposure period studied.
Keywords: cardiovascular disease, inflammatory marker, occupational exposure, traffic air pollution, trucking industry
1. Introduction
Workers in the trucking industry are regularly exposed to exhaust from diesel trucks, light duty gasoline vehicles, and propane fork lift trucks, depending on job duties. Previous studies have suggested that exposure to particulate matter (PM) in general and from vehicle-related exhaust in particular is associated with elevated risks of cardiovascular disease (CVD) (Dockery et al. 1993; Hoek et al. 2013; Laden et al. 2006; Peters et al. 2004; Pope et al. 2004a; Wellenius et al. 2012). Occupational studies suggested that workers in the transportation industry, specifically drivers, have increased risk of CVD (Bigert et al. 2004; Robinson and Burnett 2005; Shin et al. 2013; Tuchsen et al. 2006), but limited studies have focused on terminal-based workers or non-drivers. In previous work by our research team, we observed elevated standardized mortality ratios for ischemic heart disease in both drivers and non-drivers (Hart et al. 2013; Laden et al. 2007).
It has been hypothesized that exposure to PM might induce a low-grade systemic inflammatory response that leads to an increased risk of CVD, and previous studies have suggested that exposure to PM is associated with elevated levels of systemic inflammatory markers (Chen et al. 2015; Dai et al. 2016; Delfino et al. 2009; Riediker et al. 2004; Ruckerl et al. 2007; Schwartz 2001), the potential predictors of susceptibility to CVD (Blake and Ridker 2002; Saadeddin et al. 2002; Tousoulis et al. 2007). However, the results have been inconsistent, and recently a growing number of studies have reported null associations (Brauner et al. 2008; Forbes et al. 2009; Mirowsky et al. 2015; Rudez et al. 2009), especially for C-reactive protein (CRP), a widely known acute phase protein produced by the liver in response to proinflammatory cytokines. It is possible that associations between air pollutants and CRP may be stronger in susceptible populations, and may not be as evident among healthy individuals. Positive associations between particulate air pollution and inflammatory markers have been observed among more susceptible populations such as myocardial infarction (MI) survivors (Ruckerl et al. 2007), patients with coronary or ischemic heart disease (Delfino et al. 2009; Siponen et al. 2015), individuals with type 2 diabetes (O'Neill et al. 2007; Ruckerl et al. 2014), and the elderly (Delfino et al. 2009; Pope et al. 2004b; Zeka et al. 2006). On the other hand, a few studies of healthy individuals did not find similar associations (Brauner et al. 2008; Mirowsky et al. 2015; Rudez et al. 2009).
Previous studies have also suggested that the association between air pollution and cardiopulmonary health outcomes might be modified by gene polymorphisms. Glutathione S-trans-ferase M1 (GSTM1), a class member of glutathione S-transferases (GSTs) gene family which is known for their ability to produce enzymes that are involved in detoxification and is part of the antioxidant defense system (Armstrong 1997), has been reported to modify the association between secondhand smoke exposure and inflammatory markers (Miller et al. 2003). It is also suggested that GSTM1 may modify the association between air pollution and heart rate variability, respiratory illness, as well as pro-inflammatory expression and cell adhesion molecule (Chahine et al. 2007; Madrigano et al. 2010; Ruckerl et al. 2014; Wu et al. 2012; Yang et al. 2008).
The purpose of this study was to examine the short-term relationship between occupational exposure to particulate air pollution and inflammatory markers, including high-sensitivity C-reactive protein (hs-CRP), soluble intercellular adhesion molecule-1 (sICAM-1), and interleukin-6 (IL-6), among trucking terminal workers (non-drivers) in the U.S. trucking industry. Additionally, we assessed gene-environment interaction by GSTM1 gene deletion to identify potential susceptible subpopulations in this relatively healthy working population.
2. Materials and Methods
2.1. Study subjects
We visited two large trucking terminals in Carlisle, PA, and Chicago, IL in March and June 2009, respectively. A total of 178 terminal-based workers (non-drivers) who worked at these two terminals during the sampling periods were recruited to participate in the study. The participants were asked to provide a blood sample and complete a health questionnaire. The detailed job categories in the trucking industry and the corresponding job duties are described elsewhere (Smith et al. 2006). In brief, the job titles of the participants included in the study were mainly dock worker (moving freight within the terminal), hostler (moving trucks in the terminal yard), and clerk (office worker). The protocol was approved by the Human Subjects Committees at the Brigham and Women's Hospital, the Harvard School of Public Health, and VA Boston Healthcare System, and each participant provided informed consent before participating.
2.2. Blood sample and health questionnaire
Phlebotomy was offered 24 hours per day during the field sampling period in each trucking terminal. At their convenience, participants provided approximately 30 mL of blood either before, during, or after one of their work shifts during the sampling week. Blood samples were stored in a refrigerator in the field and sent back to our laboratory with a coolant by overnight shipping every day. Upon arrival, the blood samples were centrifuged in a refrigerated unit and blood components aliquoted in cryotubes, which were stored in the vapor phase of liquid nitrogen freezers at lower than -130 °C. All samples were processed and stored within 24 hours of collection. The analyses of inflammatory markers were performed at the Clinical & Epidemiologic Research Laboratory, Department of Laboratory Medicine at Children's Hospital in Boston. Hs-CRP levels were determined by an immunoturbidimetric assay on a Hitachi 917 analyzer (Roche Diagnostics; Indianapolis, IN), using reagents and calibrators from Denka Seiken (Niigata, Japan). Concentrations of sICAM-1 and IL-6 were measured by an enzyme-linked immunosorbent assay (ELISA), which employs the quantitative sandwich enzyme immunoassay technique (R&D Systems, Minneapolis, MN). CRP is a well established non-specific systemic inflammatory marker related to CVD, and IL-6 has been linked to increased risk of stroke and MI, whereas sICAM-1 is directly involved in atherogenesis and has been considered as a good predictor of CVD in healthy individuals (Tousoulis et al. 2007). For the three inflammatory markers analyzed, all the samples were above the laboratory thresholds of detection. In addition, we randomly selected 100 samples from non-smokers to analyze the polymorphisms of an oxidative stress gene, GSTM1, to evaluate the potential for gene-environmental interaction. GSTM1 genotyping was performed by the assay consists of amplification of exons 4 and 5 of the GSTM1 allele by polymerase chain reaction (PCR) to differentiate between the null polymorphism and the presence of one or more copies of the gene (Schwartz et al. 2005).
A detailed health and exposure questionnaire was completed by each participant at the time of the blood draw. We asked about recent and past smoking habits, recent secondhand smoke (SHS) exposure, job title and job history, date and time of last work shift, specific duties on the day of blood draw, work schedule over the previous week, recent acute and chronic illnesses, medication use, physical activity, alcohol use, weight and height, and waist size.
2.3. Exposure assessment
We measured PM2.5 (particulate matter with aerodynamic diameter ≤2.5μm) as an overall measure of air pollution from multiple sources, elemental carbon (EC) in PM1 (particle matter with a diameter of ≤1.0 μm) as a marker of exposure to vehicle related combustion particles, and organic carbon (OC) in PM1. EC and OC were measured in PM1 to focus on exposures to freshly generated exhaust. In this work setting, EC has been shown to originate primarily from diesel exhaust (Davis et al. 2006). Area samples of PM2.5, EC, and OC were collected consecutively using 12-hr integrated sampling for 5 days and nights, changing the collectors at 7 a.m. and 7 p.m. every day. The particle collectors and their pumps were mounted in a box sampler connected to an external battery. Area samples were collected at the loading dock (2 box samplers in separate locations where loading was on-going), terminal yard (1 box sampler placed based on wind direction to reflect terminal background level from off-site sources), and the terminal office (1 box sampler), to represent exposures to airborne particles at work for dock workers, hostlers, and office workers, respectively. Mean concentration was calculated for the areas with more than 1 box sampler. The detailed sampling methods for each pollutant are described elsewhere (Smith et al. 2006). In brief, EC and OC in PM1 were measured by collecting PM1 on a 22-mm quartz tissue filter, which was then analyzed by thermal-optical carbon analyzer based on the NIOSH 5040 method (NIOSH 1998). PM2.5 was measured by a method consistent with the EPA PQ200 Federal Reference Method (Tainio et al. 2005). Particles were collected on a 37-mm Teflon filter (with a pore diameter of 0.2 μm) after passing through a precision-machined cyclone pre-selector (BGI, Inc., Waltham, MA) to remove particles greater than 2.5 μm aerodynamic diameter. In summary, for each on-site location, 10 consecutive integrated 12-hr samples of PM2.5, EC and OC were available over the 5 day sampling period. The overall correlations between averaged PM2.5, EC, and OC were low to moderate (Pearson's r=−0.18 for PM2.5 and EC; r=0.54 for PM2.5 and OC; r=0.09 for OC and EC).
Personal exposure to EC, OC, and PM2.5 for each participant was estimated from the area samples based on the participant's job title and work shift. We used the time of blood draw, the time the worker started working that day, and whether he or she worked the day before to estimate a time weighted average of occupational exposures to each pollutant for the 12 hours immediately preceding the blood draw (Lag0-12), and 12-24 hours, 24-36 hours, and 36-48 hours (Lag12-24, Lag24-36, and Lag36-48, respectively) before the blood draw (Figure 1). The workers on average worked 8-10 hour work shifts, and we assumed that occupational exposure levels were equal to zero when off work. In other words, the workers are occupationally exposed to the pollutants only during their work shifts and the rest of the time is a ‘wash-out’ period in terms of occupational exposures, and our approach was to account for both exposure at work and the wash-out period. In addition, we calculated the mean of Lag0-12 and Lag12-24 (Lag0-24), the mean of Lag24-36 and Lag36-48 (Lag24-48), and the mean of exposure levels during 0-48 hrs (Lag0-48) before the blood draw.
Figure 1. Relationship between area sampling level and estimated personal workplace exposure.
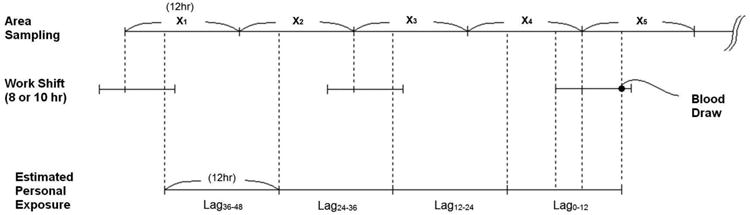
X1, X2…X5: Exposure level measured by area sampling.
2.4. Statistical Analysis
Workers with sufficient aliquots of blood for laboratory analysis and job information (job title and work shift) were included in the statistical analysis. We used multivariable linear regression analysis to assess the association between particulate air pollutants (EC, OC and PM2.5) and each marker of inflammation in separate models. Because the residuals of these regression models were non-normal, we used the robust variance for statistical inference (White 1980).
We considered several exposure metrics, including 12-hr exposure (Lag0-12, Lag12-24, Lag24-36, Lag36-48), 24-hr exposure (Lag0-24, Lag24-48), and 48-hr exposure (Lag0-48) before the time of blood draw. We also conducted multiple-lag models with an inclusion of all four lag periods, Lag0-12, Lag12-24, Lag24-36, and Lag36-48, in the same model. Regression coefficients and 95% confidence intervals (95%CIs) for each inflammatory marker were calculated in relation to an interquartile range (IQR) increase of each air pollutant for the corresponding exposure period. Age, gender, race, body mass index (BMI; weight divided by height squared, kg/m2), smoking status, secondhand smoke exposure in the last 24 hours, educational level, alcohol use, medical conditions (COPD, chronic rhinitis, high blood pressure, chronic heart problem, diabetes), and medication use were explored as potential confounders. We considered variables that were either known confounders or statistically significant in univariate analyses. We also examined effect modification by age, obesity (BMI≥30 kg/m2), smoking status, and city by fitting interaction terms and performing stratified analyses. In addition, we explored effect modification by GSTM1 polymorphisms (null vs. non-null) in the subset of non-smokers for whom genotype was successfully ascertained (93 of 100 samples randomly selected).
In sensitivity analyses, we performed the analyses restricted to the workers who provided their blood draw after the 2nd day of field sampling, since their exposure profile for every lag period was available. We also examined the association restricted to workers without any history of coronary heart disease. In addition, as CRP is a non-specific marker of inflammation which might be affected by recent acute illness (Tousoulis et al. 2007), we also performed sensitivity analysis additionally adjusted for self-reported recent illness (had a cold or flu in the past 30 days). Sensitivity analysis additionally adjusting for time of blood draw (i.e., morning, afternoon, evening, overnight) was also conducted to account for potential circadian variation of the inflammatory markers. Additionally, we ran the models additionally adjusted for duration at work before the blood draw, as well as including duration at work × cumulative exposure interaction term, to explore whether different timing of blood draw may influence the results observed. We also conducted sensitivity analysis only considering the un-weighted exposure level measured from the integrated area sample(s) collected during a worker's work shift before the blood draw (and if a worker's work shift spanned two area sampling sessions, the average level of the two sessions was taken), and adjusted for duration at work before blood draw; while this approach only focuses on exposure during the work shift but not the off-work wash-out period, it provides information on more acute effects due to workplace exposure and also allows us to ascertain whether there was a potential for ‘down-weighting’ the exposure effects when using our main approach which also accounted for the off-work period. Further, we performed logistic regression using a cut-point of 3 mg/L for hs-CRP, a level associated with greater risk of clinical coronary disease (Pearson et al. 2003) as well as other cut-points used in previous studies, such as the 90th percentile of hs-CRP (Peters et al. 2001). All analyses were performed in the SAS statistical package (v.9.3, SAS Institute Inc., Cary, NC).
3. Results
3.1. Participant characteristics
A total of 137 workers with complete work shift information for estimating personal exposure during all four lag periods (of Lag0-12, Lag12-24, Lag24-36, and Lag36-48) were eligible for statistical analysis. Participant characteristics, as well as levels of inflammatory markers, are presented in Table 1. The participants were predominantly male and Caucasian, with a median BMI of 28.3 kg/m2.
Table 1.
Levels of Inflammatory markers and characteristics of terminal workers in the U.S. trucking industry. a
| Continuous Variables | Median (IQR) | |
|---|---|---|
| CRP (mg/L) | 0.99 (0.38-1.98) | |
| sICAM-1 (ng/mL) | 248.1 (208.1-296.2) | |
| IL-6 (pg/mL) | 1.31 (0.86-2.22) | |
| Age (years) | 48 (42-54) | |
| BMI (kg/m2) | 28.4 (26.2-31.0) | |
| Categorical Variables | n | (%) |
|
| ||
| Male | 126 | 92.0 |
| Caucasian | 123 | 89.8 |
| Education >12yrs | 56 | 40.9 |
| Current smoker | 33 | 24.1 |
| Exposed to SHS in last 24hrs | 67 | 48.9 |
| Ever drink alcohol | 113 | 82.5 |
| Alcohol use in last 24 hrs | 28 | 20.4 |
| Recent illness | 30 | 21.9 |
| Cholesterol drug use | 22 | 16.1 |
| Exercise >2hrs/week | 66 | 48.2 |
| Job title | ||
| Dock worker | 68 | 49.6 |
| Hostler | 31 | 22.6 |
| Clerk | 38 | 27.7 |
| Work shift | ||
| Day | 63 | 46.0 |
| Evening | 34 | 24.8 |
| Night | 20 | 14.6 |
| Other/Rotating | 20 | 14.6 |
Variables with missing values: Exposed to SHS in last 24hrs (n=2), recent illness (n=1), ever drink alcohol (n=1), alcohol use in last 24 hrs (n=1).
3.2. Air pollutants
A total of 80 area samples were collected from 10 sampling sessions in the two trucking terminals. The overall median (IQR) level was 0.36 (0.22-0.54) μg/m3 for EC, 6.87 (5.13-10.5) for OC, and 14.7 (8.96-20.0) μg/m3 for PM2.5. Figure 2 presents the distribution of these pollutants by terminal and work area. In general, EC levels were similar across the work areas (dock, office, and yard), although levels in Chicago tended to be higher than the levels in Carlisle. Overall, the pattern of particulate levels at the two terminals was similar. The OC levels were proportionally higher than EC levels in the office area, possibly partially because office area is less ventilated comparing to other work areas; thus, cigarette smoke (which consists of high OC and low EC) are more likely to persist in the environment in the offices.
Figure 2. Distribution of area measurements of each particulate pollutant, by work area and terminal.
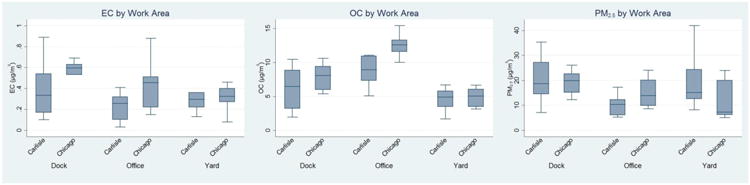
Levels of pollutants were measured at the field for 5 days/nights consecutively at each work area at the terminal (loading dock, office area, and terminal yard).
The levels of 12-hr lagged periods for estimated personal workplace exposure are summarized in Table 2. A majority of 112 participants (82%) provided their blood samples after or during their work shift, whereas the rest of the participants provided blood samples before their work shift. Thus, Lag0-12 generally represents work exposures for the day of blood draw and Lag24-36 represents work exposures the day before for the majority of the workers.
Table 2.
Levels of estimated personal occupational particulate exposure (μg/m3) of terminal-based workers during the sampling period.
| All | Dock Worker | Hostler | Clerk | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||
| Pollutant | Exposure period a | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD |
| EC | |||||||||||||
| Lag0-12 | 137 | 0.13 | 0.15 | 68 | 0.16 | 0.19 | 31 | 0.12 | 0.08 | 38 | 0.09 | 0.09 | |
| Lag12-24 | 120 | 0.13 | 0.14 | 59 | 0.15 | 0.16 | 26 | 0.10 | 0.08 | 35 | 0.11 | 0.14 | |
| Lag24-36 | 97 | 0.13 | 0.20 | 50 | 0.15 | 0.27 | 22 | 0.10 | 0.06 | 25 | 0.10 | 0.08 | |
| Lag36-48 | 73 | 0.13 | 0.20 | 38 | 0.17 | 0.24 | 16 | 0.12 | 0.16 | 19 | 0.06 | 0.07 | |
| OC | |||||||||||||
| Lag0-12 | 137 | 2.65 | 2.31 | 68 | 2.61 | 2.50 | 31 | 1.76 | 1.07 | 38 | 3.44 | 2.47 | |
| Lag12-24 | 120 | 2.32 | 2.17 | 59 | 2.13 | 2.21 | 26 | 1.52 | 1.04 | 35 | 3.24 | 2.43 | |
| Lag24-36 | 111 | 2.24 | 2.08 | 57 | 1.93 | 2.15 | 25 | 1.76 | 0.96 | 29 | 3.25 | 2.36 | |
| Lag36-48 | 73 | 2.15 | 2.19 | 38 | 2.02 | 2.48 | 16 | 1.84 | 1.41 | 19 | 2.65 | 2.10 | |
| PM2.5 | |||||||||||||
| Lag0-12 | 137 | 6.15 | 6.54 | 68 | 7.52 | 7.51 | 31 | 6.17 | 6.79 | 38 | 3.69 | 2.89 | |
| Lag12-24 | 120 | 4.68 | 5.00 | 59 | 5.64 | 5.64 | 26 | 4.12 | 5.49 | 35 | 3.46 | 2.76 | |
| Lag24-36 | 111 | 4.66 | 4.88 | 57 | 5.31 | 5.50 | 25 | 4.50 | 5.36 | 29 | 3.51 | 2.49 | |
| Lag36-48 | 73 | 4.48 | 5.99 | 38 | 6.04 | 7.38 | 16 | 3.07 | 4.37 | 19 | 2.54 | 2.20 | |
Lag0-12: workplace exposure during 0-12 hours before the time of blood draw. Lag12-24: workplace exposure during 12-24 hours before the time of blood draw. Lag24-36: workplace exposure during 24-36 hours before the time of blood draw. Lag36-48: workplace exposure during 36-48 hours before the time of blood draw
3.3. Association between particulate exposure at work and inflammatory markers
The results from the multivariable-adjusted linear regression model assessing the associations of inflammatory markers with the corresponding IQR increase of each particulate pollutant are presented in Table 3 for multiple-lag models. For sICAM-1, in general we observed a positive relationship with EC and OC, especially for exposure periods of Lag12-24 and Lag24-36. We also observed a similar pattern for PM2.5, although the results were less significant. The net effect of OC exposure throughout 48 hours before the blood draw was significantly associated with sICAM-1. Overall, air pollutants were positively associated with IL-6 levels at the exposure period immediately before the blood draw (Lag0-12), whereas no other exposure periods appeared to be associated, although all the results did not reach statistical significance. We did not find a significant association between air pollutants and hs-CRP. The results from models only included one 12-hr lagged period at a time (Supplemental Material, Table S-1) as well as models for 24-hr and 48 hr moving averages (Supplemental Material, Table S-2) also showed similar patterns.
Table 3.
Results from multiple-lag models examining association of inflammatory markers [change (95%CI)] with an interquartile range increase of air pollutants in the U.S. trucking industry a
| Inflam. | Exposure | ECb | occ | PM2.5d | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Marker | Period | Change | (95%CI) | Change | (95%CI) | Change | (95% CI) |
| CRP | Lag0-12 | -0.40 | (-1.44, 0.64) | 1.18 | (-2.43, 4.79) | 1.12 | (-1.31, 3.54) |
| (mg/L) | Lag12-24 | -1.04 | (-3.59, 1.50) | -1.77 | (-4.72, 1.19) | -0.84 | (-3.05, 1.36) |
| Lag24-36 | -0.34 | (-1.13, 0.45) | -2.37 | (-6.72, 1.99) | -1.19 | (-3.85, 1.46) | |
| Lag36-48 | 0.08 | (-0.85, 1.00) | 0.06 | (-1.86, 1.97) | 0.28 | (-0.99, 1.55) | |
| Net effect | -1.71 | (-5.07, 1.66) | -2.90 | (-7.36, 1.57) | -0.64 | (-3.19, 1.90) | |
| IL-6 | Lag0-12 | 0.06 | (-0.48, 0.61) | 0.47 | (-1.19, 2.14) | 0.30 | (-0.79, 1.39) |
| (pg/mL) | Lag12-24 | -0.15 | (-1.52, 1.22) | 1.19 | (-1.16, 3.53) | 0.66 | (-0.98, 2.31) |
| Lag24-36 | 0.00 | (-0.36, 0.37) | -0.22 | (-2.09, 1.65) | 0.48 | (-0.82, 1.78) | |
| Lag36-48 | -0.22 | (-0.95, 0.51) | -1.69 | (-4.29, 0.91) | -0.61 | (-1.74, 0.53) | |
| Net effect | -0.30 | (-1.88, 1.28) | -0.25 | (-2.76, 2.26) | 0.84 | (-0.88, 2.56) | |
| slCAM-1 | Lag0-12 | 0.94 | (-9.88, 11.8) | 0.52 | (-27.2, 28.3) | -10.8 | (-28.9, 7.15) |
| (ng/mL) | Lag12-24 | 24.9 | (2.95, 46.8) | 54.9 | (12.3, 97.5) | 24.5 | (-6.15, 55.1) |
| Lag24-36 | 12.1 | (2.10, 22.1) | 46.5 | (21.2, 71.8) | 29.2 | (4.36, 54.1) | |
| Lag36-48 | -13.2 | (-28.3, 1.91) | -16.9 | (-64.5, 30.8) | -15.5 | (-39.4, 8.48) | |
| Net effect | 24.7 | (-2.37, 51.8) | 85.0 | (28.8, 141.3) | 27.4 | (-20.4, 75.2) | |
Linear regression model using robust variance; adjusted for age (quartile), race, sex, obesity (BMI≥30 kg/m2), exercise (>2hrs/wk), smoking status, secondhand smoke exposure in the past 24 hrs, and cholesterol drug use.
Corresponds to an IQR increase of Lag0-12 EC: 0.16 μg/m3
Corresponds to an IQR increase of Lag0-12 OC: 3.47 μg/m3
Corresponds to an IQR increase of Lag0-2 PM2.5: 6.85 μg/m3
Net effect represents the summed up effect estimates of Lag0-12, Lag12-24, Lag24-36, and Lag36-48. Standard error of the net effect was calculated by square-root of the sum of variance and covariance of the four effect estimates.
3.4. Sensitivity Analyses
Sensitivity analysis additionally including recent and chronic illness as well as time of blood draw in the models did not materially changed the results (Supplemental Material, Table S-1). Further, additionally including duration at work before the blood draw in the multiple-lag models did not alter our main findings (Supplemental Material, Table S-3), and duration at work before the blood draw was not significantly related to the three inflammatory markers studied. We did not find any significant positive interactions between duration at work and cumulative exposure levels in the interaction models (all p-values for interaction>0.2). We also did not find significant associations in the analysis only considering un-weighted area exposure levels during a worker's work shift on the day of blood draw (Supplemental Material, Table S-4); this is similar to our findings in the main analysis when using weighted exposure estimates taking the off-work period into account (Table 3), where we also did not find associations between Lag0-12 exposures and inflammatory markers. The results from the analyses restricted to the 73 (53%) workers who provided their blood draw after the 2nd day of field sampling were similar to the analyses performed for all participants. Restricting to 123 (90%) workers without any coronary heart disease history also did not alter our conclusions. The results were materially unchanged when we performed multivariable logistic regression using 3 mg/L or the upper 90th percentile as a cut point for elevated hs-CRP (data not shown).
3.5. Effect modification
We did not find any significant evidence of effect modification by age, obesity, smoking status, coronary heart disease history, or city (data not shown). For the subset of the study population with GSTM1 data (n=93), overall we observed a nonsignificant elevation in inflammatory markers among those with the GSTM1-null (deletion) polymorphism (n=48), compared to those with the non-null polymorphism (n=45). However, no statistical evidence of a gene-environmental interaction between particulate exposure and inflammatory markers was found (p-value for interaction >0.3).
4. Discussion
We examined the associations between workplace particulate air pollutants and three blood markers, hs-CRP, sICAM-1, and IL-6, as indicators of inflammatory responses, among 137 employees in the U.S. trucking industry. In this population of truck terminal workers, we observed suggestive positive associations of occupational exposures to EC and OC 12-36 hours before the blood draw with elevated sICAM-1. A similar pattern was observed for PM2.5, but the results were less consistently statistically significant. We did not find any statistically significant relationships of the three measured air pollutants with hs-CRP or IL-6. In addition, no statistically significant interaction between GSTM1 and air pollutants was found.
Previous literature has suggested that sICAM-1 is a good predictor of cardiovascular risk in healthy individuals (Tousoulis et al. 2007). sICAM-1 is expressed mainly on endothelial cells under the stimulation of proinflammatory cytokines (Saadeddin et al. 2002; Tousoulis et al. 2007), and studies suggest that a greater level of peripheral blood sICAM-1 is associated with the development of symptomatic peripheral arterial disease and MI in healthy individuals (Blake and Ridker 2002; Ridker et al. 1998). However, studies have found inconsistent results on the association between air pollution exposure and sICAM-1. To date, little information on sICAM-1 is available to make an inference of the potential exposure-response timeline. Similar to our findings, significant associations of short-term (within 3 days) PM2.5 exposure and sICAM-1 has been found in other populations such as patients with coronary heart disease (Ruckerl et al. 2006), and the elderly who were free of known chronic medical conditions at enrollment (Dai et al. 2016). Seven-day exposure to PM2.5 (Wilker et al. 2011) as well as median-term (4-12 weeks) exposure to black carbon (BC) (Alexeeff et al. 2011), another marker of vehicular-related pollution, have also been associated with elevated sICAM-1 levels. However, other studies did not find an association between short-term air pollution and sICAM-1, including studies of the elderly and welders (Fang et al. 2010; Madrigano et al. 2010). Further studies are needed to identify the potential susceptible populations and exposure-response timing of the relationship between air pollution and sICAM-1 levels.
CRP is an acute phase protein synthesized by the liver in response to proinflammatory cytokines, such as IL-6 (Blake and Ridker 2002; Saadeddin et al. 2002). It is the most well established inflammatory marker to date (Tousoulis et al. 2007) because of its association with increased cardiac risk (Blake and Ridker 2002; Pearson et al. 2003; Saito et al. 2003) and possibly atherosclerotic plaque vulnerability (Blake and Ridker 2002). Both CRP and sICAM-1 have been shown to remain independently predictive of coronary heart disease after adjustment for metabolic syndrome among men (Pischon et al. 2007). Elevated IL-6 has been found to be associated with CVD and MI (Bennet et al. 2003; Cesari et al. 2003), and also to be negatively correlated with carotid plaque echogenicity, suggesting increased plaque vulnerability (Yamagami et al. 2004). However, IL-6 has a large daily variability and relatively short half-life (2-6 hrs) (Riches et al. 1992; Tousoulis et al. 2007), and thus it is possible that our sampling method might not be able to capture its rapid variation. On the other hand, it is suggested that CRP may have a relatively longer half-life (19 hrs) and requires an induction time of 1-2 days (Koenig et al. 2003; Ruckerl et al. 2007). Thus, it is possible that the association between CRP and particulate pollutants might have been missed in our study because we did not have sufficient information about workers' exposure at work >48 hours prior to blood draw. In addition, CRP is a non-specific marker of inflammation, which can be affected by recent acute illness (Tousoulis et al. 2007); however, our results were not altered after controlling for recent illness. On the other hand, it is possible that we did not find an association with CRP because our participants are generally healthier workers, as some of the previous studies conducted in healthy populations also did not observe a significant association between air pollution and CRP (Brauner et al. 2008; Mirowsky et al. 2015; Rudez et al. 2009).
One of the primary work-related exposures for workers in the trucking industry is diesel exhaust, which is a complex mixture of fine particles and combustion gases (HEI 1995). Environmental sampling of diesel exhaust is challenging because there is no single specific marker. Numerous previous epidemiological studies have used job title/job history information, census data, and self-reports to assess exposure to diesel exhaust (Garshick et al. 2003). For studies using markers of exposure, particulate matter, EC, OC, black carbon, oxides of nitrogen (NOx), and polycyclic aromatic hydrocarbons (PAHs) are most commonly used as exposure indicators (Garshick et al. 2003). PM2.5 and PM10 have been widely used in air pollution studies because particulate matter is one of the six “criteria pollutants” regulated by the U.S. Environmental Protection Agency. We measured PM2.5 in our study as an overall exposure marker of occupational air pollution in this work setting. Previous measurements conducted by our research team indicate that diesel exhaust is the dominant source of EC in the terminal work locations (representing all engine operating conditions), and exposure to EC in gasoline car emissions and propane emissions are relatively small (Davis et al. 2006). EC accounts for a significant fraction of diesel particles because historically these particles have EC cores with OC, hydrocarbons, and sulfate (HEI 1995). Furthermore, EC is not a significant component of cigarette smoke (0.49%) (HEI 1995), and workers in the trucking industry are less likely to be exposed to other sources of EC such as coal or wood smoke.
We examined the association between the exposure of particulate pollutants and inflammatory markers in an occupational setting, focusing upon a healthy working population with daily exposure to vehicle-related exhaust at work. Our exposure monitoring is not directly comparable to that of other studies in the literature. Most previous epidemiological studies have utilized air pollution data collected by local or national air quality monitors to represent ambient exposure levels either by close-to-point estimation or spatial modeling. In our study, we measured particle levels at each work area in the trucking terminals consecutively for five work days, and took into consideration how long each worker was on shift at the specific work area. A limitation of our exposure assessment is that we had to make assumptions about the relative contribution of the area measurements to an individual's personal occupational exposure. Nevertheless, our previous work on exposure assessment at the trucking terminals throughout the U.S. showed that the correlation between the average personal exposures and the matched area sampling by work area was fairly high, especially for EC (Smith et al. 2006). In addition, our data do not allow us to examine the potential effects of peak exposures (for example, 2-hour of very high exposures before the blood draw) as we only had integrated exposure data from the area samplings for every 12 hours. Another limitation is that, repeated measurements of the studied exposure and outcome biomarkers were not available. It is also possible that we did not completely account for the circadian variation of the inflammatory markers, but the results of our analyses were unchanged when adding the time of blood draw (i.e., morning, afternoon, evening, overnight) into our regression models. The estimated Lag0-12 exposures for workers who gave blood in the beginning or early in their work shift may not capture their entire exposure profile throughout the work shift on the day of the blood draw, but it is a true reflection of averaged occupational exposure during 0-12 hours before the blood draw for these workers. Although the participants were volunteers, selection bias is not likely to be an issue since the workers should not be aware of their levels of inflammatory markers. Finally, the sample size was small and thus the association between occupational particulate exposure and inflammatory markers might have been missed because of insufficient power. It is also possible that the small sample size may be one of the reasons that we did not find significant effect modification by GSTM1 polymorphism on these associations.
In summary, we observed a suggestive positive relationship of short-term vehicle-related air pollutants at work with sICAM-1 among workers in the trucking industry for EC, OC, and PM2.5 especially at the exposure lag period of 12-36 hours before the blood draw. No significant evidence of the relationships of particulate air pollutants with IL-6 and hs-CRP was found. Our findings suggest that the short-term vehicular exposure may be associated with sICAM-1 in a relatively healthy working population, and the findings may be affected by the induction time and half-life of the biomarkers studied.
Supplementary Material
Highlights.
Associations between vehicular air pollutants and inflammatory markers were examined.
We focused on non-drivers of truck terminal workers as literature is mainly in drivers.
Short-term exposure to OC and EC may be associated with increased sICAM-1.
Acknowledgments
This study is supported by the NIH/NIEHS R21 ES013726 (Garshick E), P30 ES000002 (Dockery D) and American Heart Association (AHA) Award 0815689D (Chiu YHM).
The study protocol was approved by the Human Subjects Committees at the Brigham and Women's Hospital, the Harvard T.H. Chan School of Public Health, and VA Boston Healthcare System, and each participant provided informed consent before participating.
Abbreviations
- BMI
body mass index
- EC
elemental carbon
- GSTM1
gluthatione S-transferase M1
- hs-CRP
high-sensitivity C-reactive protein
- IL-6
interleukin-6
- OC
organic carbon
- PM2.5
particulate matter with aerodynamic diameter ≤2.5μm
- PM1
particle matter with a diameter of ≤1.0 μm
- SHS
secondhand smoke
- sICAM-1
soluble intercellular adhesion molecule-1
Footnotes
The authors have no actual or potential competing financial interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexeeff SE, Coull BA, Gryparis A, Suh H, Sparrow D, Vokonas PS, et al. Medium-term exposure to traffic-related air pollution and markers of inflammation and endothelial function. Environ Health Perspect. 2011;119:481–486. doi: 10.1289/ehp.1002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RN. Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem Res Toxicol. 1997;10:2–18. doi: 10.1021/tx960072x. [DOI] [PubMed] [Google Scholar]
- Bennet AM, Prince JA, Fei GZ, Lyrenas L, Huang Y, Wiman B, et al. Interleukin-6 serum levels and genotypes influence the risk for myocardial infarction. Atherosclerosis. 2003;171:359–367. doi: 10.1016/j.atherosclerosis.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Bigert C, Klerdal K, Hammar N, Hallqvist J, Gustavsson P. Time trends in the incidence of myocardial infarction among professional drivers in Stockholm 1977-96. Occup Environ Med. 2004;61:987–991. doi: 10.1136/oem.2004.012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med. 2002;252:283–294. doi: 10.1046/j.1365-2796.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- Brauner EV, Moller P, Barregard L, Dragsted LO, Glasius M, Wahlin P, et al. Exposure to ambient concentrations of particulate air pollution does not influence vascular function or inflammatory pathways in young healthy individuals. Part Fibre Toxicol. 2008;5:13. doi: 10.1186/1743-8977-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, et al. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study) Am J Cardiol. 2003;92:522–528. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- Chahine T, Baccarelli A, Litonjua A, Wright RO, Suh H, Gold DR, et al. Particulate air pollution, oxidative stress genes, and heart rate variability in an elderly cohort. Environ Health Perspect. 2007;115:1617–1622. doi: 10.1289/ehp.10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Zhao Z, Sun Q, Lin Z, Zhao A, Wang C, et al. Size-fractionated particulate air pollution and circulating biomarkers of inflammation, coagulation, and vasoconstriction in a panel of young adults. Epidemiology. 2015;26:328–336. doi: 10.1097/EDE.0000000000000273. [DOI] [PubMed] [Google Scholar]
- Dai L, Bind MA, Koutrakis P, Coull BA, Sparrow D, Vokonas PS, et al. Fine particles, genetic pathways, and markers of inflammation and endothelial dysfunction: Analysis on particulate species and sources. J Expo Sci Environ Epidemiol. 2016 doi: 10.1038/jes.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ME, Smith TJ, Laden F, Hart JE, Ryan LM, Garshick E. Modeling particle exposure in U.S. trucking terminals. Environ Sci Technol. 2006;40:4226–4232. doi: 10.1021/es052477m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Gillen DL, Polidori A, Arhami M, et al. Air pollution exposures and circulating biomarkers of effect in a susceptible population: clues to potential causal component mixtures and mechanisms. Environ Health Perspect. 2009;117:1232–1238. doi: 10.1289/ehp.0800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, 3rd, Xu X, Spengler JD, Ware JH, Fay ME, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Fang SC, Eisen EA, Cavallari JM, Mittleman MA, Christiani DC. Circulating adhesion molecules after short-term exposure to particulate matter among welders. Occup Environ Med. 2010;67:11–16. doi: 10.1136/oem.2008.043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes LJ, Patel MD, Rudnicka AR, Cook DG, Bush T, Stedman JR, et al. Chronic exposure to outdoor air pollution and markers of systemic inflammation. Epidemiology. 2009;20:245–253. doi: 10.1097/EDE.0b013e318190ea3f. [DOI] [PubMed] [Google Scholar]
- Garshick E, Laden F, Hart JE, Smith TJ. Health Effects Institute, Communication 10. 2003. Exposure assessment issues in epidemiology studies on chronic health effects of diesel exhaust; pp. 17–26. [Google Scholar]
- Hart JE, Garshick E, Smith TJ, Davis ME, Laden F. Ischaemic heart disease mortality and years of work in trucking industry workers. Occup Environ Med. 2013;70:523–528. doi: 10.1136/oemed-2011-100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEI. Special Report. Cambridge, MA: Diesel Working Group, Health Effects Institute; 1995. Diesel exhaust: A critical analysis of emissions, exposure, and health effects. [Google Scholar]
- Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, et al. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ Health. 2013;12:43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig W, Sund M, Frohlich M, Lowel H, Hutchinson WL, Pepys MB. Refinement of the association of serum C-reactive protein concentration and coronary heart disease risk by correction for within-subject variation over time: the MONICA Augsburg studies, 1984 and 1987. Am J Epidemiol. 2003;158:357–364. doi: 10.1093/aje/kwg135. [DOI] [PubMed] [Google Scholar]
- Laden F, Hart JE, Smith TJ, Davis ME, Garshick E. Cause-specific mortality in the unionized U.S trucking industry. Environ Health Perspect. 2007;115:1192–1196. doi: 10.1289/ehp.10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: Extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med. 2006;173:667–672. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigano J, Baccarelli A, Wright RO, Suh H, Sparrow D, Vokonas PS, et al. Air pollution, obesity, genes and cellular adhesion molecules. Occup Environ Med. 2010;67:312–317. doi: 10.1136/oem.2009.046193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EA, Pankow JS, Millikan RC, Bray MS, Ballantyne CM, Bell DA, et al. Glutathione-S-transferase genotypes, smoking, and their association with markers of inflammation, hemostasis, and endothelial function: the atherosclerosis risk in communities (ARIC) study. Atherosclerosis. 2003;171:265–272. doi: 10.1016/j.atherosclerosis.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Mirowsky JE, Peltier RE, Lippmann M, Thurston G, Chen LC, Neas L, et al. Repeated measures of inflammation, blood pressure, and heart rate variability associated with traffic exposures in healthy adults. Environ Health. 2015;14:66. doi: 10.1186/s12940-015-0049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIOSH. Elemental Carbon (Diesel Particulate) 5040. In: Cassinelli ME, O'Connor PF, editors. NIOSH Manual of Analytical Methods. Fourth. National Institute for Occupational and Safety and Health; 1998. [Google Scholar]
- O'Neill MS, Veves A, Sarnat JA, Zanobetti A, Gold DR, Economides PA, et al. Air pollution and inflammation in type 2 diabetes: a mechanism for susceptibility. Occup Environ Med. 2007;64:373–379. doi: 10.1136/oem.2006.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Peters A, Frohlich M, Doring A, Immervoll T, Wichmann HE, Hutchinson WL, et al. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg Study. Eur Heart J. 2001;22:1198–1204. doi: 10.1053/euhj.2000.2483. [DOI] [PubMed] [Google Scholar]
- Peters A, von Klot S, Heier M, Trentinaglia I, Hormann A, Wichmann HE, et al. Exposure to traffic and the onset of myocardial infarction. N Engl J Med. 2004;351:1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- Pischon T, Hu FB, Rexrode KM, Girman CJ, Manson JE, Rimm EB. Inflammation, the metabolic syndrome, and risk of coronary heart disease in women and men. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004a;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Hansen ML, Long RW, Nielsen KR, Eatough NL, Wilson WE, et al. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect. 2004b;112:339–345. doi: 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riches P, Gooding R, Millar BC, Rowbottom AW. Influence of collection and separation of blood samples on plasma IL-1, IL-6 and TNF-alpha concentrations. J Immunol Methods. 1992;153:125–131. doi: 10.1016/0022-1759(92)90314-j. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351:88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- Riediker M, Cascio WE, Griggs TR, Herbst MC, Bromberg PA, Neas L, et al. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med. 2004;169:934–940. doi: 10.1164/rccm.200310-1463OC. [DOI] [PubMed] [Google Scholar]
- Robinson CF, Burnett CA. Truck drivers and heart disease in the United States, 1979-1990. Am J Ind Med. 2005;47:113–119. doi: 10.1002/ajim.20126. [DOI] [PubMed] [Google Scholar]
- Ruckerl R, Greven S, Ljungman P, Aalto P, Antoniades C, Bellander T, et al. Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors. Environ Health Perspect. 2007;115:1072–1080. doi: 10.1289/ehp.10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckerl R, Hampel R, Breitner S, Cyrys J, Kraus U, Carter J, et al. Associations between ambient air pollution and blood markers of inflammation and coagulation/fibrinolysis in susceptible populations. Environ Int. 2014;70:32–49. doi: 10.1016/j.envint.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Ruckerl R, Ibald-Mulli A, Koenig W, Schneider A, Woelke G, Cyrys J, et al. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med. 2006;173:432–441. doi: 10.1164/rccm.200507-1123OC. [DOI] [PubMed] [Google Scholar]
- Rudez G, Janssen NA, Kilinc E, Leebeek FW, Gerlofs-Nijland ME, Spronk HM, et al. Effects of ambient air pollution on hemostasis and inflammation. Environ Health Perspect. 2009;117:995–1001. doi: 10.1289/ehp.0800437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadeddin SM, Habbab MA, Ferns GA. Markers of inflammation and coronary artery disease. Med Sci Monit. 2002;8:RA5–12. [PubMed] [Google Scholar]
- Saito M, Ishimitsu T, Minami J, Ono H, Ohrui M, Matsuoka H. Relations of plasma high-sensitivity C-reactive protein to traditional cardiovascular risk factors. Atherosclerosis. 2003;167:73–79. doi: 10.1016/s0021-9150(02)00380-5. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Air pollution and blood markers of cardiovascular risk. Environ Health Perspect. 2001;109(3):405–409. doi: 10.1289/ehp.01109s3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Park SK, O'Neill MS, Vokonas PS, Sparrow D, Weiss S, et al. Glutathione-S-transferase M1, obesity, statins, and autonomic effects of particles: gene-by-drug-by-environment interaction. Am J Respir Crit Care Med. 2005;172:1529–1533. doi: 10.1164/rccm.200412-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SY, Lee CG, Song HS, Kim SH, Lee HS, Jung MS, et al. Cardiovascular disease risk of bus drivers in a city of Korea. Ann Occup Environ Med. 2013;25:34. doi: 10.1186/2052-4374-25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siponen T, Yli-Tuomi T, Aurela M, Dufva H, Hillamo R, Hirvonen MR, et al. Source-specific fine particulate air pollution and systemic inflammation in ischaemic heart disease patients. Occup Environ Med. 2015;72:277–283. doi: 10.1136/oemed-2014-102240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TJ, Davis ME, Reaser P, Natkin J, Hart JE, Laden F, et al. Overview of particulate exposures in the US trucking industry. J Environ Monit. 2006;8:711–720. doi: 10.1039/b601809b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tainio M, Tuomisto JT, Hanninen O, Aarnio P, Koistinen KJ, Jantunen MJ, et al. Health effects caused by primary fine particulate matter (PM2.5) emitted from buses in the Helsinki metropolitan area, Finland. Risk Anal. 2005;25:151–160. doi: 10.1111/j.0272-4332.2005.00574.x. [DOI] [PubMed] [Google Scholar]
- Tousoulis D, Antoniades C, Stefanadis C. Assessing inflammatory status in cardiovascular disease. Heart. 2007;93:1001–1007. doi: 10.1136/hrt.2006.088211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchsen F, Hannerz H, Roepstorff C, Krause N. Stroke among male professional drivers in Denmark, 1994-2003. Occup Environ Med. 2006;63:456–460. doi: 10.1136/oem.2005.025718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenius GA, Burger MR, Coull BA, Schwartz J, Suh HH, Koutrakis P, et al. Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med. 2012;172:229–234. doi: 10.1001/archinternmed.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. A Heteroskedasticity-Consistent Covariance Matrix Estimator and a Direct Test for Heteroskedasticity. Econometrica. 1980;48:817–838. [Google Scholar]
- Wilker EH, Alexeeff SE, Suh H, Vokonas PS, Baccarelli A, Schwartz J. Ambient pollutants, polymorphisms associated with microRNA processing and adhesion molecules: the Normative Aging Study. Environ Health. 2011;10:45. doi: 10.1186/1476-069X-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Peden DB, McConnell R, Fruin S, Diaz-Sanchez D. Glutathione-S-transferase M1 regulation of diesel exhaust particle-induced pro-inflammatory mediator expression in normal human bronchial epithelial cells. Part Fibre Toxicol. 2012;9:31. doi: 10.1186/1743-8977-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagami H, Kitagawa K, Nagai Y, Hougaku H, Sakaguchi M, Kuwabara K, et al. Higher levels of interleukin-6 are associated with lower echogenicity of carotid artery plaques. Stroke. 2004;35:677–681. doi: 10.1161/01.STR.0000116876.96334.82. [DOI] [PubMed] [Google Scholar]
- Yang IA, Fong KM, Zimmerman PV, Holgate ST, Holloway JW. Genetic susceptibility to the respiratory effects of air pollution. Thorax. 2008;63:555–563. doi: 10.1136/thx.2007.079426. [DOI] [PubMed] [Google Scholar]
- Zeka A, Sullivan JR, Vokonas PS, Sparrow D, Schwartz J. Inflammatory markers and particulate air pollution: characterizing the pathway to disease. Int J Epidemiol. 2006;35:1347–1354. doi: 10.1093/ije/dyl132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


