Abstract
Background
Inorganic arsenic is a lung, bladder, and skin carcinogen. One of the major sources of exposure to arsenic is through naturally contaminated drinking water. While positive associations have been observed between arsenic in drinking water and prostate cancer, few studies have explored this association in the United States.
Objectives
To evaluate the association between inorganic arsenic concentrations in community water systems and prostate cancer incidence in Illinois using an ecologic study design.
Methods
Illinois Environmental Protection Agency data on arsenic concentrations in drinking water from community water systems throughout the state were linked with county-level prostate cancer incidence data from 2007 to 2011 from the Illinois State Cancer Registry. Incidence rates were indirectly standardized by age to calculate standardized incidence ratios (SIRs) for each county. A Poisson regression model was used to model the association between county-level SIRs and mean arsenic tertile (0.33 to 0.72, 0.73 to 1.60, and 1.61 to 16.23 ppb), adjusting for potential confounders.
Results
For counties with mean arsenic levels in the second tertile, the SIR was 1.05 (95% CI: 0.96–1.16). For counties with mean arsenic levels in the third tertile, the SIR was 1.10 (95% CI: 1.03–1.19). There was a significant linear dose-response relationship observed between mean arsenic levels and prostate cancer incidence (p for trend = 0.003).
Conclusions
In this ecologic study, counties with higher mean arsenic levels in community water systems had significantly higher prostate cancer incidence. Individual-level studies of prostate cancer incidence and low-level arsenic exposure are needed.
Keywords: Arsenic, prostate cancer, water, Poisson, spatial autocorrelation
1. Introduction
Arsenic is ubiquitous in nature, and is the 20th most common element in the earth’s crust.1 Arsenic is emitted from volcanic activity and industrial activities, in addition to being historically used as a pesticide. For humans, the major source of exposure is through food and drinking water.2 In the United States, arsenic is distributed in surface and groundwater at varying concentrations, but arsenic in public/community water supplies is not to exceed 10 parts per billion (ppb) based on the current standard from the Environmental Protection Agency (EPA).3 However, concerns remain regarding the carcinogenicity of arsenic in drinking water at levels at or below the current guideline.4 In Illinois, the majority of community water supplies have arsenic levels below 10 ppb, while private wells are not regulated for arsenic concentrations in groundwater.5
The International Agency for Research on Cancer (IARC) has categorized arsenic as a “Group 1 Carcinogen,” meaning there is sufficient evidence of carcinogenicity in humans. However, the majority of epidemiologic studies focused on the carcinogenicity of arsenic have been limited to skin, urinary bladder, and lung cancers.6 There is some evidence of an association between arsenic exposure and prostate cancer, the second leading cause of cancer death in males in the United States, but this association is not well established for low-level arsenic exposure. The majority of existing epidemiologic studies evaluating arsenic in relation to prostate cancer have been conducted outside of the United States where exposure levels were in excess of 10 ppb.7–14 To date, only two studies on arsenic and prostate cancer have been conducted in the United States (Table 1).15,16 Garcia-Esquinas et al. (2013) found a 4-fold increase in the hazard of prostate cancer mortality (hazard ratio: 4.58, 95% CI: 1.31–16.6) when comparing those in the highest tertile of total urinary arsenic (>13.32 µg/g creatinine) to those in the lowest tertile (<6.91 µg/g creatinine) among American Indians in Arizona, Oklahoma, North Dakota, and South Dakota, in what is to date the only prospective cohort study of low-dose arsenic exposure in the United States. Lewis et al. (1999) found elevated mortality from prostate cancer among men exposed to medium (1,000–4,999 ppb-years) and high levels (≥5,000 ppb-years) of cumulative arsenic exposure based on ecologic measurements of arsenic in community water supplies in Utah. It has been suggested that arsenic can impact prostate cancer cell progression through androgen-independence, which is often associated with advanced and lethal prostate cancers that are difficult to treat.17, 18 Other research has suggested that arsenic exposure through drinking water inhibits DNA repair processes as part of its carcinogenic mechanism of action.19
Table 1.
Epidemiologic Studies of Arsenic Exposure and Prostate Cancer
| Study | Country | Study design |
Sample size | Exposure | Result Summary |
|---|---|---|---|---|---|
| Chen and Wang (1990)8 |
Taiwan | Ecologic | 314 precincts and townships |
Arsenic in drinking water (range = 50 ppb to ≥350 ppb) |
Increased prostate cancer mortality |
| Chen et al. (1988)12 | Taiwan | Ecologic | 313 townships | Arsenic in drinking water (range = ≤300 ppb to ≥600 ppb) |
Increased prostate cancer mortality |
| Garcia-Esquinas et al. (2013)15 |
U.S. | Prospective | 18 deaths, 3,932 total cohort |
Inorganic arsenic levels in urine (interquartile range = 5.8 to 15.6 µg/g creatinine) |
Increased prostate cancer mortality |
| Hinwood et al. (1999)11 |
Australia | Ecologic | 22 geographic areas |
Arsenic in surface soil and drinking water (<10 to >200 ppb) |
Increased prostate cancer incidence |
| Hsu et al. (2013)10 | Taiwan | Prospective | 9 cases, 1,231 total cohort |
Arsenic skin lesions (present vs. absent) |
Increased prostate cancer incidence |
| Lewis et al. (1999)16 | U.S. | Ecologic | 7 communities | Arsenic in drinking water (range = 3.5 to 620 ppb) |
Increased prostate cancer mortality |
| Rivara et al. (1997)14 | Chile | Ecologic | 2 regions | Arsenic in drinking water (range = 10 ppb to 860 ppb) |
No association with prostate cancer mortality |
| Tsai et al. (1999)13 | Taiwan | Ecologic | 4 townships | Arsenic in drinking water (range = 250 ppb to 1,140 ppb) |
Increased prostate cancer mortality |
| Wu et al. (1989)9 | Taiwan | Ecologic | 42 villages | Arsenic in drinking water (range = 10 ppb to 1,752 ppb) |
Increased prostate cancer mortality |
| Yang et al. (2008)7 | Taiwan | Ecologic time series |
4 townships | Arsenic in drinking water (range = <10 to 2,500 ppb) |
Reduced prostate cancer mortality associated with elimination of arsenic from well water |
Given the limited existing epidemiologic studies examining the association between low-level arsenic exposure and prostate cancer, we sought to examine the association between inorganic arsenic concentration in community water supplies and prostate cancer incidence in Illinois using an ecologic study design.
2. Methods
The county-level concentration of arsenic in finished drinking water (water that has been treated and is ready for distribution and consumption by the public), provided by community water systems (CWSs) between 2000 and 2006, was the main exposure of interest. Prostate cancer incidence data from the Illinois State Cancer Registry for 2007–2011 aggregated at the county-level were merged with county-level population and demographic data from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program. As such, an ecological analysis was conducted at the county-level. This study was approved by the University of Illinois at Chicago Institutional Review Board.
2.1 Water Data
Arsenic levels in finished drinking water provided by community water systems (CWSs) were obtained from Illinois Safe Drinking Water Information System (SDWIS) for the period January 1, 2000 to December 31, 2006. CWSs are public water systems that supply water for human consumption to the same population-year round through at least 15 service connections or to at least 25 people.20 The Arsenic and Clarifications to Compliance and New Source Monitoring Rule 66 FR 6976, which was finalized in January 2001, required CWSs using groundwater to take one sample between 1999–2001, 2002–2004, and 2005–2006; annual measurements were required for CWSs using surface water.
The most frequently reported limits of detection (for samples identified below the detection limit) were 0.5 ppb (n=1,509 samples) and 1 ppb (n=1,401 samples), and ranged from 0–50 ppb. For samples below the limit of detection, the value imputed was ½ the limit of detection. If the limit of detection was reported as zero, then 0.25 ppb was imputed (n=6 samples). Overall, 50.9% of samples were indicated to be below the limit of detection.
CWSs were linked to counties based on the CWS address. County-level monthly average arsenic levels were calculated by averaging the arsenic levels in finished water for all CWSs in each county. The exposure metric was the county-level average arsenic level over the period 2000 through 2006, which was the average of the county-level monthly average arsenic levels. No arsenic data were available for 2 of the 102 counties in Illinois and were excluded from the analysis. Data may not have been available for these counties because they were served by CWSs with addresses in other counties, or lack of arsenic measurement.
Since some households in counties may be served by private wells, we accounted for the proportion of residents in a county who reported domestic private well use to the United States Geological Survey in 2000 rather than use of CWSs, which was included as a covariate in our analyses.21 While arsenic may also be present in private well water, arsenic concentrations were not available for these water sources since there is no systematic monitoring of arsenic in private wells in Illinois.
2.2 Cancer Data
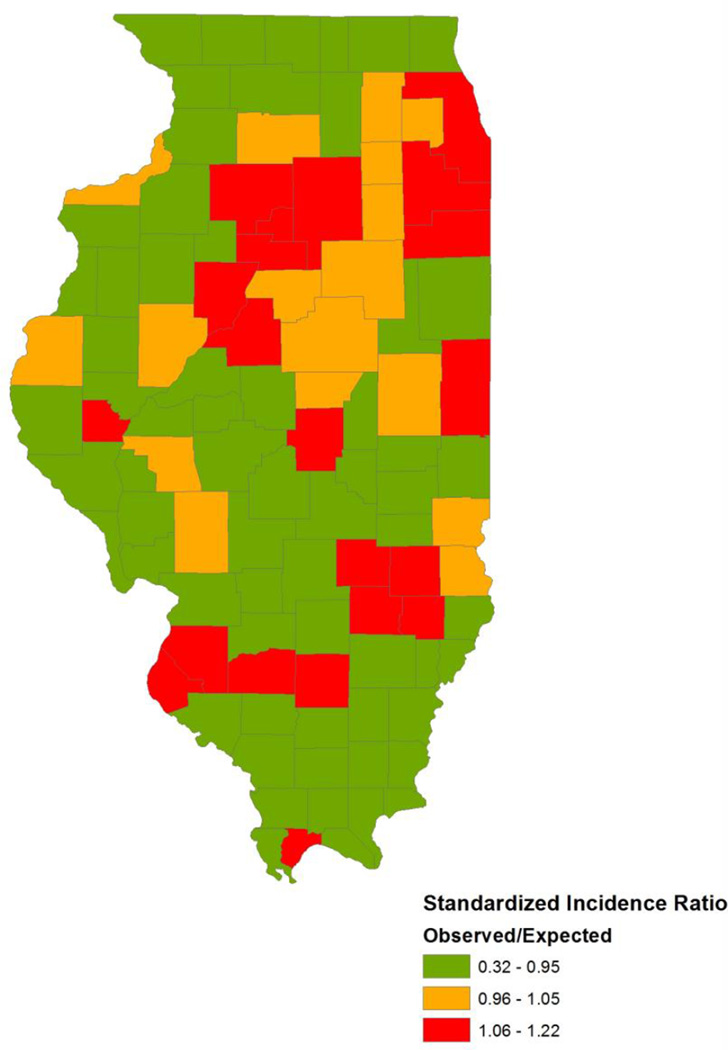
The Illinois State Cancer Registry (ISCR) provided data for all incident cases of prostate cancer between 2007 and 2011 among adults (aged ≥ 15 years) residing in Illinois at the time of diagnosis. Age-specific (crude) prostate cancer incidence rates from 2007–2011 for the whole state of Illinois were calculated in order to indirectly standardize the county-level incidence rates by age. Standardized incidence ratios (SIRs) were calculated for each county by dividing the number of observed cases by expected cases. As such, a value of greater than 1 indicates that more cancer cases were observed in that county than expected based on the age demographics of males in that county, and a value of less than 1 indicates fewer cancer cases were observed in that county than expected.
2.3 County Population and Demographic Data
Population and demographic data for 102 Illinois counties were obtained from SEER, courtesy of the Illinois State Cancer Registry. Population estimates incorporated intercensal years (for 2007 to 2009) and Vintage 2012 (for 2010 to 2011). More information on the population estimates and associated methodology can be found elsewhere.22 Supplementary data on the percent of individuals in the county living under the federal poverty level, used as a metric for socioeconomic status, were obtained from the 2010 Small Area Income and Poverty Estimates (SAIPE) program at the U.S. Census Bureau.23
2.4 Geographic Data
Choropleth maps to depict mean arsenic level by county and standardized incidence ratios by county were created using ArcGIS 10 (ESRI, Redlands, CA). County shapefiles were obtained from the U.S. Census Bureau’s 2010 TIGER/Line files.24
2.5 Statistical Analysis
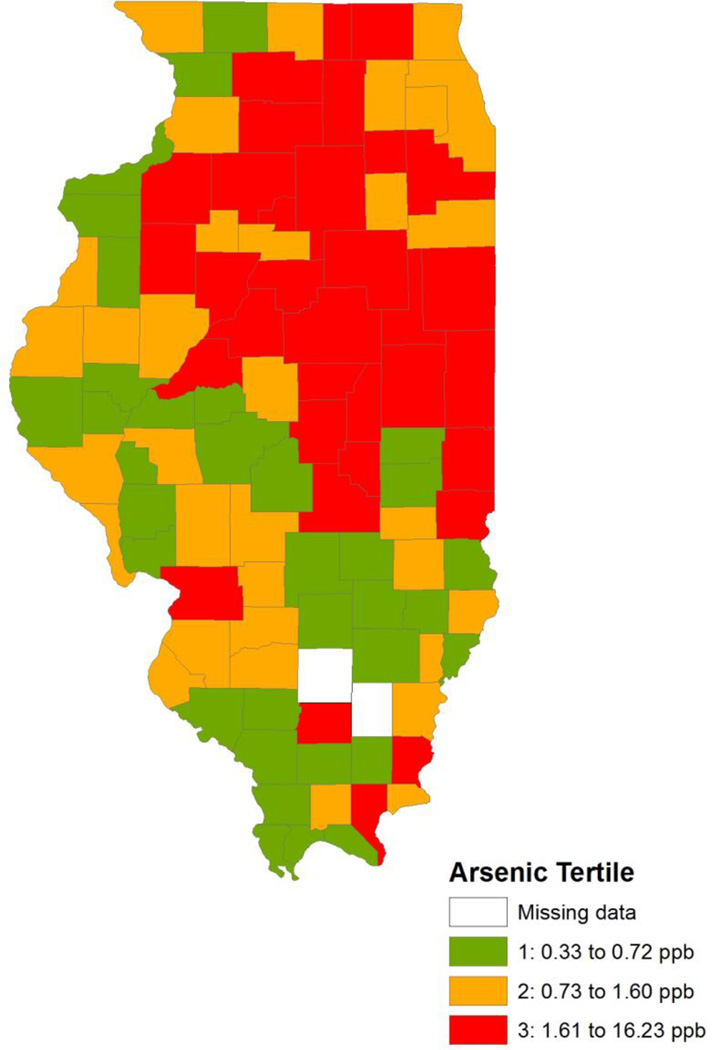
A Poisson regression model with robust standard errors was constructed under the assumption that the number of observed incident cancer cases for each county had a Poisson distribution, where the expected number of cases for that county was based on the county’s age demographics. The natural log of the expected number of cases was included in the model as an offset term. The explanatory variables were arsenic tertiles, with the lowest tertile serving as the reference category. The average level of arsenic in CWSs for each county from 2000 to 2006 was categorized into tertiles, with the first tertile representing a mean arsenic level between 0.33 and 0.72 parts per billion (ppb), the second tertile representing a mean arsenic level between 0.73 and 1.60 ppb, and the third tertile representing a mean arsenic level between 1.61 and 16.23 ppb. Arsenic tertiles were also modeled as an ordinal variable to calculate the p-value for trend. Since there was evidence of linear trend, models were also run using arsenic as a continuous exposure variable as an average per 10 ppb increase. In addition to crude regression models, adjusted model 1 included the percentage of black male residents and the percentage of other race male residents in the county. Adjusted model 2 included the covariates in adjusted model 1, with the addition of the percentage of the county population living below the federal poverty line. Adjusted model 3 included all covariates in adjusted model 2, in addition to the percentage of private well users in the county. Covariates were all modeled as continuous variables.
Robust standard errors for parameter estimates were used to control for mild violations of the Poisson distribution assumption that the mean equals the variance.25 The model residuals were tested for spatial autocorrelation by calculating a Global Moran’s I statistic. Poisson regression models that included a random effect for each county with a spatial covariance structure based on the latitude and longitude of each county’s centroid were also performed to control for spatial autocorrelation. Since prostate cancer is rare among younger males and may be related to misclassification or different etiology, we performed a sensitivity analysis excluding males <45 years old. All statistical analyses were performed using SAS version 9.3 (Cary, NC).
3. Results
Between 2007 and 2011, there were 45,595 incident prostate cancer cases among 4,936,634 males ≥15 years old in the state of Illinois (Table 2). The majority of cases occurred among men between the ages of 55 and 74 years old. Mean arsenic levels and prostate cancer SIRs by county are presented in Figures 1 and 2. The distributions of county-level covariates across arsenic tertiles are provided in Table 3. Both the crude and adjusted Poisson regression results suggested prostate cancer incidence greater than expected among counties with elevated arsenic levels (Table 4). For counties with mean arsenic levels in the second tertile, the SIR was 1.05 (95% CI: 0.96–1.16) when adjusted for private well use, racial demographics, and socioeconomic status. For counties with mean arsenic levels in the third tertile, the SIR was 1.10 (95% CI: 1.03–1.19) accounting for covariates. There was a significant linear dose-response relationship observed between mean arsenic levels and prostate cancer incidence (p for trend = 0.003). Results when modeling arsenic as a continuous variable showed that a 10 ppb increase in mean arsenic levels was associated with a 12% increase in the standardized incidence ratio (95% CI: 1.04–1.20) for prostate cancer when adjusting for confounders.
Table 2.
Prostate Cancer Cases Diagnosed between 2007–2011 and Population Age Demographics
| Prostate Cancer Cases N=45,595 |
Illinois Male Population N = 4,936,634* |
|
|---|---|---|
| Age, N (%) | ||
| 15 to 34 years | 4 (0.01) | 1,804,545 (36.55) |
| 35 to 44 years | 285 (0.63) | 869,273 (17.61) |
| 45 to 54 years | 4,753 (10.42) | 910,508 (18.44) |
| 55 to 64 years | 15,027 (32.96) | 688,680 (13.95) |
| 65 to 74 years | 16,250 (35.64) | 378,657 (7.67) |
| 75 years and older | 9,276 (20.34) | 284,971 (5.77) |
Population data were averaged across the 5-year period from 2007–2011 based on intercensal data17
Figure 1.
Mean Arsenic Values (ppb) by County from 2000 to 2006
Figure 2.
Prostate Cancer Standardized Incidence Ratios by County for 2007 to 2011
Table 3.
County-Level Demographics by Arsenic Tertile
| Arsenic Tertile 1 (0.33–0.72 ppb) N=34 Counties |
Arsenic Tertile 2 (0.73–1.60 ppb) N=33 Counties |
Arsenic Tertile 3 (1.61–16.23 ppb) N=33 Counties |
|
|---|---|---|---|
| Percent of White Males | 91.10 | 76.23 | 89.50 |
| Percent of Black Males | 7.43 | 16.93 | 7.22 |
| Percent of Other Race Males | 1.47 | 6.84 | 3.28 |
| Percent of Individuals Living in Poverty | 14.62 | 14.43 | 11.75 |
| Percent of Private Well Users | 31.90 | 39.77 | 31.07 |
Table 4.
Standard Poisson Regression Results (Crude and Adjusted)
| Model | Exposure Variable | SIR (95% CI) | p-value | p for trend |
|---|---|---|---|---|
| Crude | Arsenic tertiles | 0.181 | ||
| 0.33 to 0.72 ppb | 1.00 (reference) | |||
| 0.73 to 1.60 ppb | 1.17 (1.08–1.26) | <0.001 | ||
| 1.61 to 16.23 ppb | 1.13 (1.05–1.21) | 0.001 | ||
| Adjusted 1 | Arsenic tertiles | 0.001 | ||
| 0.33 to 0.72 ppb | 1.00 (reference) | |||
| 0.73 to 1.60 ppb | 1.07 (0.98–1.17) | 0.146 | ||
| 1.61 to 16.23 ppb | 1.12 (1.05–1.20) | 0.001 | ||
| Adjusted 2 | Arsenic tertiles | 0.004 | ||
| 0.33 to 0.72 ppb | 1.00 (reference) | |||
| 0.73 to 1.60 ppb | 1.05 (0.96–1.15) | 0.299 | ||
| 1.61 to 16.23 ppb | 1.10 (1.02–1.18) | 0.011 | ||
| Adjusted 3 | Arsenic tertiles | 0.003 | ||
| 0.33 to 0.72 ppb | 1.00 (reference) | |||
| 0.73 to 1.60 ppb | 1.05 (0.96–1.16) | 0.264 | ||
| 1.61 to 16.23 ppb | 1.10 (1.03–1.19) | 0.008 | ||
| Crude | Arsenic (per 10 ppb) | 1.01 (0.93–1.10) | 0.794 | |
| Adjusted 1 | Arsenic (per 10 ppb) | 1.06 (0.96–1.17) | 0.233 | |
| Adjusted 2 | Arsenic (per 10 ppb) | 1.10 (1.02–1.19) | 0.013 | |
| Adjusted 3 | Arsenic (per 10 ppb) | 1.12 (1.04–1.20) | 0.004 |
Adjusted for the percentage of black male residents, and the percentage of other race male residents
Adjusted for the percentage of black male residents, the percentage of other race male residents, and the percentage of residents living below the federal poverty line
Adjusted for the percentage of black male residents, the percentage of other race male residents, the percentage of residents living below the federal poverty line, and the percentage of residents reporting private well use
Residuals from the standard Poisson regression model were significantly positively spatially autocorrelated (Moran’s I statistic: 0.19, p-value: <0.001). The results from the spatial autocorrelation model were similar to the standard Poisson regression model (Table 5). For counties with mean arsenic levels in the second tertile, the SIR was 1.05 (95% CI: 0.98–1.13) when adjusted for private well use, racial demographics, socioeconomic status, and spatial autocorrelation. For counties with mean arsenic levels in the third tertile, the SIR was 1.08 (95% CI: 1.00–1.15) accounting for covariates and spatial autocorrelation. Again, there was a significant linear dose-response relationship observed (p for trend = 0.039). When analyzed continuously, an average 10 ppb increase in arsenic levels was associated with an 8% increase in the standardized incidence ratio (95% CI: 1.01–1.16) of prostate cancer after adjusting for confounders and controlling for spatial autocorrelation. We found no appreciable differences in model estimates when restricting the analyses to males older than 45 years (data not shown).
Table 5.
Spatial Autocorrelation Poisson Regression Results (Crude and Adjusted)
| Model | Exposure Variable | SIR (95% CI) | p-value | p for trend |
|---|---|---|---|---|
| Crude | Arsenic tertiles | 0.013 | ||
| 0.33 to 0.72 ppb | 1.00 (reference) | |||
| 0.73 to 1.60 ppb | 1.08 (1.00–1.16) | 0.036 | ||
| 1.61 to 16.23 ppb | 1.09 (1.02–1.17) | 0.011 | ||
| Adjusted 1 | Arsenic tertiles | 0.021 | ||
| 0.33 to 0.72 ppb | 1.00 (reference) | |||
| 0.73 to 1.60 ppb | 1.07 (1.00–1.15) | 0.061 | ||
| 1.61 to 16.23 ppb | 1.10 (1.02–1.17) | 0.010 | ||
| Adjusted 2 | Arsenic tertiles | 0.037 | ||
| 0.33 to 0.72 ppb | 1.00 (reference) | |||
| 0.73 to 1.60 ppb | 1.05 (0.97–1.13) | 0.216 | ||
| 1.61 to 16.23 ppb | 1.08 (1.01–1.15) | 0.037 | ||
| Adjusted 3 | Arsenic tertiles | 0.039 | ||
| 0.33 to 0.72 ppb | 1.00 (reference) | |||
| 0.73 to 1.60 ppb | 1.05 (0.98–1.13) | 0.173 | ||
| 1.61 to 16.23 ppb | 1.08 (1.00–1.15) | 0.037 | ||
| Crude | Arsenic (per 10 ppb) | 1.07 (1.00–1.15) | 0.066 | |
| Adjusted 1 | Arsenic (per 10 ppb) | 1.07 (0.99–1.15) | 0.070 | |
| Adjusted 2 | Arsenic (per 10 ppb) | 1.08 (1.01–1.16) | 0.032 | |
| Adjusted 3 | Arsenic (per 10 ppb) | 1.08 (1.01–1.16) | 0.024 |
Adjusted for the percentage of black male residents, and the percentage of other race male residents
Adjusted for the percentage of black male residents, the percentage of other race male residents, and the percentage of residents living below the federal poverty line
Adjusted for the percentage of black male residents, the percentage of other race male residents, the percentage of residents living below the federal poverty line, and the percentage of residents reporting private well use
4. Discussion
The majority of counties in Illinois had mean arsenic levels in the CWSs below the current U.S. EPA standard of 10 ppb, and all counties had mean arsenic levels in CWSs below the prior EPA standard of 50 ppb which was in place until 2006.3 Prostate cancer incidence was significantly higher in counties with higher mean CWS arsenic levels, even after controlling for known confounding factors and spatial autocorrelation.
While skin, lung, and bladder cancers are well-established arsenic-related cancers, the link with prostate cancer is less known. Biologically, it has been suggested that arsenic exposure increases the risk of prostate cancer through epigenetic mechanisms that increase cell growth and cell survival while decreasing apoptosis. In the prostate specifically, studies of human prostate epithelial cells in culture have demonstrated that low level exposure to inorganic arsenic induces malignant transformations that involve increases in matrix metalloproteinase-9 secretion,26 inhibition of apoptosis,27 aberrant genomic DNA methylation,28 and K-ras oncogene activation and overexpression29, 30 among others.17 Additionally, inorganic arsenic exposure stimulates androgen independence, which is often associated with advanced stages of prostate cancer and a poor prognosis due to resistance to certain types of treatment.18,31, 32 Recent research suggests that arsenic exposure can transform human prostate epithelial stem/progenitor cells into cancer stem-like cells that result in highly pleomorphic and aggressive tumors, and that these arsenic-transformed malignant prostate epithelial cells can then recruit nearby non-contiguous normal stem cells into a cancer phenotype.33, 34
Since this study was performed on county-level data, individual-level inferences are limited. It is unknown whether prostate cancer cases in these Illinois counties were exposed to higher concentrations of arsenic than non-cases. Therefore, individual-level studies of prostate cancer incidence and arsenic exposure are needed to confirm the associations observed in this analysis.
As an ecologic study, it is possible that confounding may have biased our results. We adjusted for age by standardizing, and further controlled for county-level covariates including race/ethnicity, socioeconomic status, and private well use in the regression models. Family history, an established risk factor of prostate cancer, was not accounted for due to absence of an appropriate data source for this information. It is possible that observed associations may be confounded by family history of prostate cancer if family history is related to reduced family mobility and thus ingestion of arsenic through the CWS. The findings of the current study require replication in other observational studies.
Another limitation of this analysis is the representativeness of CWS data. Community water systems are one of three types of public water systems.20 Other types of public water systems include non-transient non-community water systems, which regularly provide water to at least 25 of the same people for at least 6 months per year, and transient non-community water systems, which provide water to places where people do not remain for long periods of time.20 While CWS data are likely to reflect the major source of residential exposure to arsenic through drinking water, non-transient water sources like schools, hospitals, office buildings, and transient sources like campgrounds were not included in this analysis. The absence of arsenic exposure through private household well water sources is also a limitation. Private wells in Illinois are not regulated and therefore data on arsenic concentrations in these wells is not available. We included the percent of private well users as a covariate in our regression models, but this may not adequately address the lack of well arsenic data. Other limitations include prostate cancer latency and exposure misclassification. With arsenic exposure data from 2000 to 2006 and prostate cancer incidence data from 2007 to 2011, our data allow for a latency period up to 11 years. The average estimated latency period for prostate cancer is approximately 7 to 12 years, so some cases associated with arsenic may have been missed.35 Furthermore, as an ecologic study we did not have individual data on residential history, which may have resulted in misclassification bias of arsenic exposure through the CWSs.
5. Conclusions
This is one of few studies to analyze low-level arsenic exposure through drinking water and prostate cancer. The significant association observed between counties with higher arsenic levels in community water systems and prostate cancer incidence greater than expected warrants further research. Future studies should examine this association using individual-level data including individual arsenic exposure assessments.
Highlights.
Arsenic is a known carcinogen, but it is unclear if it causes prostate cancer.
We used data on arsenic levels in community water systems in Illinois.
County-level mean arsenic was associated with increased prostate cancer incidence.
There was a significant linear dose-response.
Acknowledgments
Funding
Catherine Bulka was supported in part by NIOSH Grant T42 OH008672 and NHLBI Grant T32 HL125294.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Approval
This study was approved by the University of Illinois at Chicago Institutional Review Board (#2010-0907).
Contributor Information
Catherine M. Bulka, Email: cbulka2@uic.edu.
Rachael M. Jones, Email: rjones25@uic.edu.
Mary E. Turyk, Email: mturyk1@uic.edu.
Leslie T. Stayner, Email: lstayner@uic.edu.
Maria Argos, Email: argos@uic.edu.
References
- 1.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Arsenic, Metals, Fibres and Dusts. 100C. Lyon, France: IARC; 2012. pp. 41–93. Arsenic and Arsenic Compounds. [PMC free article] [PubMed] [Google Scholar]
- 2.Toxicological Profile for Arsenic. Atlanta, GA: US Department of Health and Human Services, Public Health Service; 2007. [Accessed April 1, 2015]. Agency for Toxic Substances Control and Disease Registry, Centers for Disease Control and Prevention, US Department of Health and Human Services, Public Health Service. ( http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=22&tid=3) [Google Scholar]
- 3.United States Environmental Protection Agency. Basic Information about the Arsenic Rule. < http://water.epa.gov/lawsregs/rulesregs/sdwa/arsenic/Basic-Information.cfm>.
- 4.Smith AH. Arsenic Health Effects Research Program. Is the proposed new arsenic water standard of 10 ug/L sufficiently protective of public health? < http://wwwbrr.cr.usgs.gov/projects/GWC_chemtherm/FinalAbsPDF/smith.pdf>.
- 5.Warner KL, Marin A, Arnold TL. Arsenic in Illinois Ground Water – Community and Private Supplies. United States Geological Survey Water-Resources Investigations Report 03-4103. 2003 < http://solon.er.usgs.gov/pubs/wrir03_4103.pdf>.
- 6.Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan HM, Wood R, Kosnett MJ, Smith MT. Cancer risks from arsenic in drinking water. Environmental Health Perspectives. 1992;97:259–267. doi: 10.1289/ehp.9297259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang CY, Chang CC, Chiu HF. Does arsenic exposure increase the risk for prostate cancer? Journal of Toxicology and Environmental Health. 2008;71(23):1559–1563. doi: 10.1080/15287390802392065. [DOI] [PubMed] [Google Scholar]
- 8.Chen CJ, Wang CJ. Ecological correlation between arsenic level in well water and age-adjusted mortality from malignant neoplasms. Cancer Research. 1990;50(17):5470–5474. [PubMed] [Google Scholar]
- 9.Wu MM, Kuo TL, Hwang YH, Chen CJ. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. American Journal of Epidemiology. 1989;130(6):1123–1132. doi: 10.1093/oxfordjournals.aje.a115439. [DOI] [PubMed] [Google Scholar]
- 10.Hsu LI, Chen GS, Lee CH, Yang TY, Chen YH, Wang YH, Hsueh YM, Chiou HY, Wu MM, Chen CJ. Use of arsenic-induced palmoplantar hyperkeratosis and skin cancers to predict risk of subsequent internal malignancy. American Journal of Epidemiology. 2013;177(3):202–212. doi: 10.1093/aje/kws369. [DOI] [PubMed] [Google Scholar]
- 11.Hinwood AL, Jolley DJ, Sim MR. Cancer incidence and high environmental arsenic concentrations in rural populations: results of an ecological study. International Journal of Environmental Health Research. 1999;9:131–141. [Google Scholar]
- 12.Chen CJ, Kuo TL, Wu MM. Arsenic and Cancers. The Lancet. 1988;1(8582):414–415. doi: 10.1016/s0140-6736(88)91207-x. [DOI] [PubMed] [Google Scholar]
- 13.Tsai SM, Wang TN, Ko YC. Mortality for certain diseases in areas with high levels of arsenic in drinking water. Archives of Environmental Health. 1999;54(3):186–193. doi: 10.1080/00039899909602258. [DOI] [PubMed] [Google Scholar]
- 14.Rivara MI, Cebrián M, Corey G, Hernández M, Romieu I. Cancer risk in an arsenic-contaminated area of Chile. Toxicology and Industrial Health. 1997;13(2–3):321–338. doi: 10.1177/074823379701300217. [DOI] [PubMed] [Google Scholar]
- 15.García-Esquinas E, Pollán M, Umans JG, Francesconi KA, Goessler W, Guallar E, Howard B, Farley J, Best LG, Navas-Acien A. Arsenic exposure and cancer mortality in a US-based prospective cohort: the strong heart study. Cancer Epidemiology, Biomarkers & Prevention. 2013;22(11):1944–1953. doi: 10.1158/1055-9965.EPI-13-0234-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis DR, Southwick JW, Ouellet-Hellstrom R, Rench J, Calderon RL. Drinking water arsenic in Utah: A cohort mortality study. Environmental Health Perspectives. 1999;107(5):359–365. doi: 10.1289/ehp.99107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benbrahim-Tallaa L, Waalkes MP. Inorganic arsenic and human prostate cancer. Environmental Health Perspectives. 2008;116(2):158–164. doi: 10.1289/ehp.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tibetts J. Arsenic and Prostate Cancer: Acquiring Androgen Independence. Environmental Health Perspectives. 2005;113(9):A614–A615. doi: 10.1289/ehp.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrew AS, Burgess JL, Meza MM, Demidenko E, Waugh MG, Hamilton JW, Karagas MR. Arsenic exposure is associated with decreased DNA repair in vitro in individuals exposed to drinking water arsenic. Environmental Health Perspectives. 2006;114(8):1193–1198. doi: 10.1289/ehp.9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.United States Environmental Protection Agency. Information about Public Water Systems. < https://www.epa.gov/dwreginfo/information-about-public-water-systems>.
- 21.United States Geological Survey. Estimated Use of Water in the United States County-Level Data for 2000. < http://water.usgs.gov/watuse/data/2000/>.
- 22.National Vital Statistics System. U.S. Census Populations With Bridged Race Categories. < http://www.cdc.gov/nchs/nvss/bridged_race.htm>.
- 23.United States Census Bureau. Small Area Income and Poverty Estimates. < http://www.census.gov/did/www/saipe/data/statecounty/data/2010.html>.
- 24.United States Census Bureau. TIGER/Line ® Shapefiles and TIGER/Line ® Files. https://www.census.gov/geo/maps-data/data/tiger-line.html.
- 25.Cameron AC, Trivedi PK. Microeconometrics Using Stata. College Station, TX: Stata Press; 2009. [Google Scholar]
- 26.Achanzar WE, Brambila EM, Diwan BA, Webber MM, Waalkes MP. Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. Journal of the National Cancer Institute. 2002;94(24):1888–1891. doi: 10.1093/jnci/94.24.1888. [DOI] [PubMed] [Google Scholar]
- 27.El-Atta HM, El-Bakary AA, Attia AM, Lotfy A, Khater SS, Elsamanoudy AZ, Abdalla HA. DNA fragmentation, caspase 3 and prostate-specific antigen genes expression induced by arsenic, cadmium, and chromium on nontumorigenic human prostate cells. Biological Trace Element Research. 2014;162(1–3):95–105. doi: 10.1007/s12011-014-0100-y. [DOI] [PubMed] [Google Scholar]
- 28.Pelch KE, Tokar EJ, Merrick BA, Waalkes MP. Differential DNA methylation profile of key genes in malignant prostate epithelial cells transformed by inorganic arsenic or cadmium. Toxicology and Applied Pharmacology. 2015;286(3):159–167. doi: 10.1016/j.taap.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ngalame NN, Tokar EJ, Person RJ, Waalkes MP. Silencing KRAS overexpression in arsenic-transformed prostate epithelial and stem cells partially mitigates malignant phenotype. Toxicological Sciences. 2014;142(2):489–496. doi: 10.1093/toxsci/kfu201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benbrahim-Tallaa L, Waterland RA, Styblo M, Achanzar WE, Webber MM, Waalkes MP. Molecular events associated with arsenic-induced malignant transformation of human prostatic epithelial cells: aberrant genomic DNA methylation and K-ras oncogene activation. Toxicology and Applied Pharmacology. 2005;206:28–29. doi: 10.1016/j.taap.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 31.Benbrahim-Tallaa L, Webber MM, Waalkes MP. Acquisition of androgen independence by human prostate epithelial cells during arsenic-induced malignant transformation. Environmental Health Perspectives. 2005;113(9):1134–1139. doi: 10.1289/ehp.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benbrahim-Tallaa L, Webber MM, Waalkes MP. Mechanisms of Acquired Androgen Independence during Arsenic-Induced Malignant Transformation of Human Prostate Epithelial Cells. Environmental Health Perspectives. 2007;115(2):243–247. doi: 10.1289/ehp.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tokar EJ, Diwan BA, Waalkes MP. Arsenic exposure transforms human epithelial stem/progenitor cells into a cancer stem-like phenotype. Environmental Health Perspectives. Environmental Health Perspectives. 2010;118(1):108–115. doi: 10.1289/ehp.0901059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y, Tokar EJ, Sun Y, Waalkes MP. Arsenic-transformed malignant prostate epithelia can convert noncontiguous normal stem cells into an oncogenic phenotype. Environmental Health Perspectives. 2012;120(6):865–871. doi: 10.1289/ehp.1204987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Etzioni R, Cha R, Feuer EJ, Davidov O. Asymptomatic incidence and duration of prostate cancer. American Journal Epidemiology. 1998;148(8):775–785. doi: 10.1093/oxfordjournals.aje.a009698. [DOI] [PubMed] [Google Scholar]