Abstract
Background & Aims
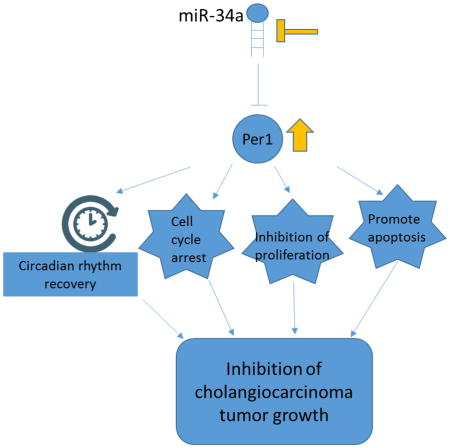
Disruption of circadian rhythm is associated with cancer development and progression. MicroRNAs (miRNAs) are a class of small non-coding RNAs that trigger mRNA translation inhibition. We aimed to evaluate the role of Per1 and related miRNAs in cholangiocarcinoma growth.
Methods
The expression of clock genes was evaluated in human cholangiocarcinoma tissue arrays and cholangiocarcinoma lines. The rhythmic expression of clock genes was evaluated in cholangiocarcinoma cells and H69 (non-malignant cholangiocytes) by q-PCR. We measured cell proliferation, cell cycle and apoptosis in Mz-ChA-1 cells after Per1 overexpression. We examined tumor growth in vivo after injection of Per1 overexpressing cells. We verified miRNAs that targets Per1. The circadian rhythm of miR-34a was evaluated in cholangiocarcinoma and H69 cells. We evaluated cell proliferation, apoptosis and invasion after inhibition of miR-34a in vitro, and the potential molecular mechanisms by mRNA profiling after overexpression of Per1.
Results
Expression of Per1 was decreased in cholangiocarcinoma. The circadian rhythm of Per1 expression was lost in cholangiocarcinoma cells. Decreased cell proliferation, lower G2/M arrest, and enhanced apoptosis were shown in Per1 overexpressing cells. An in vivo study revealed decreased tumor growth, decreased proliferation, angiogenesis and metastasis after overexpressing Per1. Per1 was verified as a target of miR-34a. miR-34a was rhythmically expressed in cholangiocarcinoma cells and H69. The inhibition of miR-34a decreased proliferation, migration and invasion in cholangiocarcinoma cells. mRNA profiling has shown that overexpression of Per1 inhibits cell growth through regulation of multiple cancer related pathway, such as cell cycle, cell growth and apoptosis pathway.
Conclusions
Disruption of circadian rhythms of clock genes contributes to the malignant phenotypes of human cholangiocarcinoma.
Keywords: biliary epithelium, circadian rhythm, clock genes, cholangiocarcinoma, microRNAs
Graphical Abstract
INTRODUCTION
Cholangiocarcinoma (CCA) is a malignancy of the biliary epithelium with non-specific symptoms, making early diagnosis difficult [1]. Surgical resection remains the only treatment to prolong survival. Circadian rhythms are endogenous and autonomous oscillations generated by a set of clock genes. In addition to the central nervous system, circadian rhythms are present in peripheral tissues and single cells [2]. For example, dysregulated circadian clock gene expression and disrupted circadian rhythm is associated with tumor development and progression in experimental models [3]. In humans, epidemiologic studies have demonstrated a correlation between the disruption of circadian rhythms and tumor development [4]. The mechanisms underlying the maintenance of the autonomous circadian rhythm involve two multi-gene feedback loops: the negative feedback and the positive loop. The CLOCK (Circadian Locomotor Output Cycles Kaput) and BMAL1 (ARNTL, Aryl hydrocarbon receptor nuclear translocator-like) proteins form heterodimers that bind to the E-box in the promoter region upstream of circadian transcription repressor genes Period (Per) and Cryptochrome (Cry). The Per and Cry proteins inhibit the transcription of CLOCK and BMAL1, acting as the negative loop in the autonomous oscillation [5].
A number of genes, including core components of the clock, metabolic genes, and other tissue-specific transcripts are rhythmically expressed in the liver [6]. Decreased Per1 expression is observed in prostate and lung cancer [7, 8]. However, the circadian rhythm in biliary cancer remains unexplored.
microRNAs (miRNAs) are small non-coding RNAs that bind to mRNA in a complementary sequence manner, leading to the silencing or inhibition of the translation of target mRNAs. A single miRNA can regulate the expression of multiple genes and has multiple effects on cellular functions. During tumorigenesis, a group of critical miRNAs, including miR21, miR-141 and miR-29 are aberrantly expressed and regulate CCA development [9]. However, the interaction of circadian rhythms with miRNAs in CCA is unknown. We aimed to evaluate the (i) role of clock genes in CCA and the effect of overexpressing Per1 on CCA growth; (ii) role of miRNAs that target Per1 and the effect of inhibiting of miRNA in CCA; and (iii) mechanisms by which Per1 regulates the progression of CCA.
MATERIALS AND METHODS
Materials
Reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. The rabbit polyclonal antibody against fibronectin (ab2413) was purchased from Abcam (Cambridge, MA). The rabbit polyclonal antibody against VEGF was purchased from Abcam, Cambridge, MA). The following antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX): BMAL1 (N-20); CLOCK (H-276); Cry1 (A-20); Per1 (N-20); PCNA (FL-261). mirVana™ miR-34a inhibitors and negative controls were purchased from Ambion Inc., (Austin, Texas). pCMV6-Per1 and controls were purchased from OriGene (Rockville, MD). siPer1 and controls were purchased from Santa Cruz Biotechnology.
Cell lines
Six human CCA cell lines (Mz-ChA-1, TFK-1, CCLP-1, HuCC-T1, SG231 and HuH-28) with different biliary origin were used. Mz-ChA-1 cells (from gallbladder) were a gift from Dr. G. Fitz (University of Texas Southwestern Medical Center, Dallas, TX) [10]; HuH-28 cells (from intrahepatic bile ducts) [11] and TFK-1 cells (from extrahepatic bile ducts) [12] were obtained from Cancer Cell Repository, Tohoku University, Japan. HuCC-T1 [13], and SG231 [14] (from intrahepatic bile ducts) cells were obtained from Dr. A.J. Demetris (University of Pittsburgh, PA) and cultured as described [13–15]. The human immortalized, non-malignant cholangiocyte cell line, H69, (from Dr. G.J. Gores, Mayo Clinic, MN) was cultured as described [16]. The human immortalized cholangiocyte line, MMNK-1 [17] was a gift of the laboratory of Dr. Ramille Shah, Northwestern University, Chicago, IL.
Expression of clock genes in non-malignant human cholangiocytes, CCA cells and human biopsies
The expression of the clock genes, BMAL1, CLOCK, Per1 and Cry1 was evaluated by q-PCR [18] in H69 and CCA cells. We also evaluated the immunoreactivity of clock genes in commercially available Accumax tissue arrays (ISU ABXIS Co., Seoul, Korea) by semi-quantitative immunohistochemistry (IHC). These tissue arrays contain 48 well-characterized CCA biopsy samples from different tumor differentiation grades as well as 4 non-malignant liver biopsy samples. Light microscopy and IHC observations were taken with a BX-40 light microscope (Olympus, Tokyo, Japan) with a video-cam (Spot Insight, Diagnostic Instrument, Inc.).
To further demonstrate that Per1 is downregulated in CCA we analyzed publically available datasets [19] of gene expression in CCA compared to normal bile ducts. We have evaluated Per1 expression from microarray databases from this profiling data set via NextBio software (www.nextbio.com).
Circadian expression of core clock genes in CCA and non-malignant cells
An established in vitro model of circadian expression of clock genes [2] was exploited to determine the 24-hr circadian expression of clock genes in non-malignant (H69 and MMNK-1) [20] and CCA lines. The cells were deprived of serum for 48 hr, transferred to a medium containing 50% serum for 2 hr, and returned to serum-free medium. Cells were harvested every 4 hr after serum stimulation. Total RNA was isolated with a mirVana miRNA isolation kit (Ambion Inc.) and evaluated for the expression of clock genes by q-PCR. Cells that were harvested just before adding 50% serum were considered as 0 hr. The mRNA level of core clock genes in H69 for time point 0 is denoted as 1.
Evaluation of the functional role of suppression of miR-34a and regulation of circadian expression of Per1 in human CCA cells
Next we evaluated the functional effect of miR-34a in CCA cells. miR-34a precursor antisense inhibitors and controls (Ambion) were transfected into Mz-ChA-1, TFK-1 and H69 cells with Dharma reagent (Thermo Scientific, Waltham, MA) using a standard protocol. Cell proliferation, apoptosis and invasion were evaluated 48 hr after transfection. Cell proliferation after silencing of miR-34a in the selected cells was examined via MTS assay, while cell invasion was evaluated using a cell invasion assay kit (Chemicon, Temecula, CA). We measured the 24-hr circadian expression of Per1 after inhibiting the expression of miR-34a in Mz-ChA-1 and TFK-1 cells. To determine that knockdown of Per1 reverses the anti-proliferative and anti-invasive effects of the miR-34a inhibitor, we co-transfected Per1 siRNA with miR-34a inhibitor in Mz-ChA-1 cells before measuring cell proliferation.
In vivo studies
Animal procedures were performed under the guidelines of the Baylor Scott & White IACUC Committee. Male BALB/c nude (nu/nu) mice (n = 8 per group) were kept in a temperature-controlled (20–22°C) environment with 12-hr light-dark cycles and with free access to drinking water and standard mouse chow. Mz-ChA-1 cells (5×106) stably transfected with control vector or Per1 were suspended in 0.25 mL of extracellular matrix gel and injected subcutaneously in both the right and left back flank of nude mice. Tumor growth was measured three times per week, and volume was determined as follows: tumor volume (mm3) = π/6 * length (mm) * width (mm) * height (mm). Tumors were allowed to grow until maximum allowable tumor burden was reached as set forth by the Baylor Scott & White IACUC tumor burden policy. After 43 days, mice were euthanized with sodium pentobarbital (50 mg/kg BW i.p.).
Tumor sections (4–5 μm) were prepared and stained for: (i) H&E, (ii) PCNA; (iii) fibronectin; and iv) VEGF by IHC [21]. We evaluated the mRNA expression of Per1, PCNA, VEGF-A, NGF, TIMP2, TIMP3, MMP-3, MMP-9 and S100A4 in tumor tissue by q-PCR.
OneArray DNA Microarrays and Filtering Genes
The gene expressing profiling was performed in empty vector (EV) and Per1 overexpressing Mz-ChA-1 cells by Phalanx Biotech Group (Belmont, CA) using the Human whole genome OneArray DNA microarray HOA 7.1. The raw profiling data has been submitted to the ArrayExpress database (www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-4156. The profiling data were input into Ingenuity Pathway Analysis (IPA) to identify the pathways in which they are involved. The details are described in supplementary information.
Statistics
A double-sided Student t-test was performed to compare two groups (p <0.05 was considered significant) unless otherwise indicated.
Please see Supplementary data for more detailed information of this section.
RESULTS
Core clock genes are aberrantly expressed in CCA
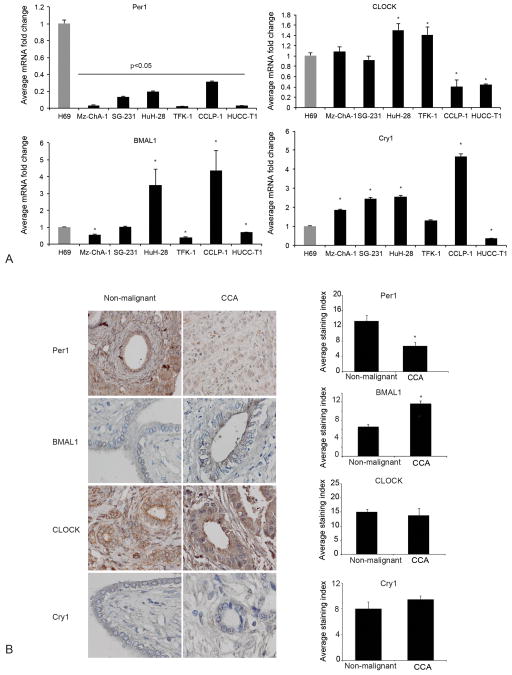
We have previously demonstrated that: (i) melatonin inhibits CCA growth in vitro and in vivo; and (ii) melatonin deregulation is associated with disruption of circadian rhythm [22]. Therefore, we evaluated the expression of core clock genes and circadian rhythm in CCA cells compared to non-malignant cholangiocytes. Per1 mRNA expression decreased in all CCA lines compared to H69 cells (Figure 1A). Similarly, there was decreased expression of Per1 in human CCA biopsies compared to non-malignant samples (Figure 1B). The mRNA expression of BMAL1 increased in HuH-28 and CCLP-1 compared to H69, but decreased in other CCA cells (Figure 1A). The expression of BMAL1 increased in human CCA biopsies compared to non-malignant controls (Figure 1B). The expression of CLOCK increased in HuH-28 and TFK-1 cells (Figure 1A). Cry1 expression increased in HuCC-T1 and decreased in Mz-ChA-1, SG-231, HuH-28 and CCLP-1 cells. (Figure 1A). The expression of Cry1 and CLOCK did not show significant changes in human CCA biopsies compared to non-malignant samples (Figure 1B). By analysis of published microarray data [19], we found Per1 expression decreased in CCA when compared to normal intrahepatic bile ducts (Fold Change: 0.032, p-Value: 2.2 × 10−9) or non-cancerous surrounding tissue (Fold Change 0.165, P-Value: 4.2 × 10−10) [19].
Figure 1.
[A–B] Expression of core clock genes, BMAL1, CLOCK, Per1 and cry1 in CCA cell lines and tissue array of human CCA biopsies. [A] Per1 mRNA expression decreased in all CCA cells compared with H69 cells, while other clock genes displayed altered expression in the different CCA cell lines. Data are expressed as mean ± SEM of 4 experiments. *p<0.05 compared to H69. [B] Decreased expression of Per1 and enhanced expression of BMAL1 in human CCA biopsies (n=48) as compared with non-malignant control tissue (n=4). Data are expressed as mean ± SEM.*p <0.05 vs. non-malignant. Original magnification X40.
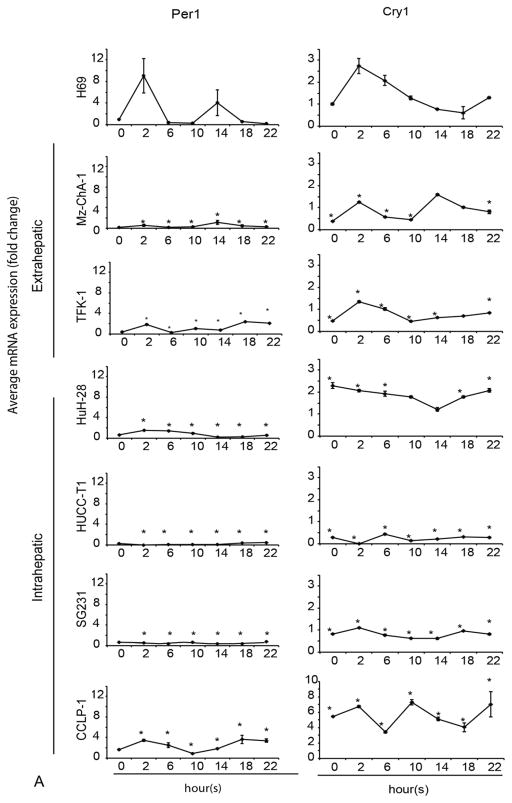
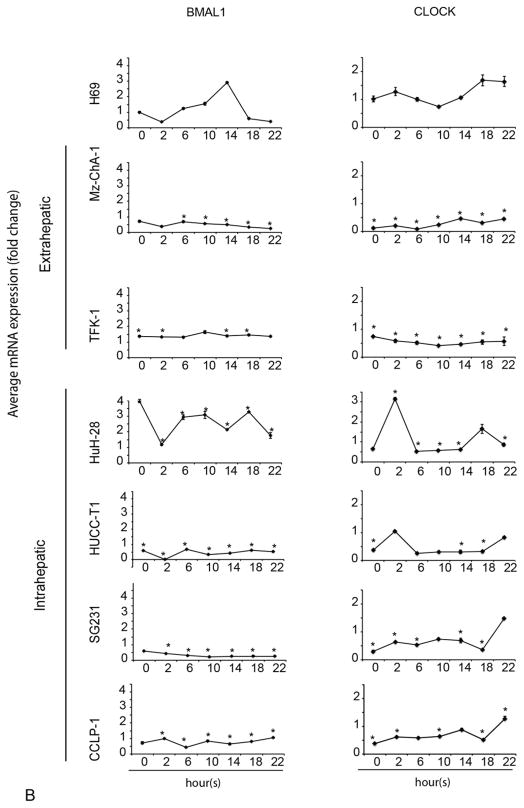
Loss of 24-hr rhythmic expression of clock genes in CCA
Since the 24-hour circadian rhythm has been exclusively found in animals, tissues and single cells [2], we evaluated the presence of the 24-hour circadian rhythm in non-malignant cholangiocytes and CCA cells. The rhythmic expression of Per1 was present in H69 (Figure 2A) and MMNK-1 (Suppl. Figure 1), but was lost in all CCA cells. BMAL1 showed a loss of circadian rhythm in Mz-ChA-1, TFK-1, SG231, CCLP-1 and HuCC-T1 cell lines. BMAL1 showed rhythmic expression with phase shift in HuH-28 compared to H69 cells (Figure 2B). Cry1 lost circadian rhythm in HuCC-T1 and SG231 cell lines (Figure 2A). CLOCK lost circadian rhythm in two extrahepatic cell lines (Mz-ChA-1 and TFK-1) and two intrahepatic cell lines (SG231 and CCLP-1), but still displayed circadian rhythm in HuH-28 and HUCC-T1 cells. These findings suggest that Per1 (whose circadian rhythm was lost in all the CCA lines used) may be key in the regulation of CCA growth (Figure 2A).
Figure 2.
A–B The 24-hr circadian rhythm of BMAL1, CLOCK, Per1 and Cry1 mRNA expression levels in CCA and H69. The cells were stimulated with 50% serum for 2 hr after being serum starved for 48 hr. Samples were taken every 4 hr until 24 hr. The points represent the mean ± SEM for 3 to 6 experiments. *p < 0.05 vs. H69 at corresponding time points.
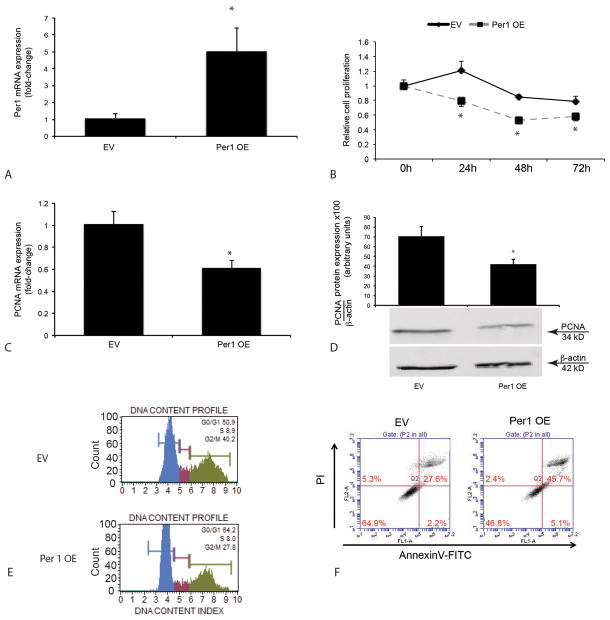
Effect of restoration of Per1 expression on CCA proliferation, apoptosis and invasiveness in CCA cell lines
Since Per1 expression and its circadian rhythm was decreased in all CCAs used, we aimed to demonstrate that restoration of Per1 expression decreases the growth of Mz-ChA-1, HuCC-T1 cells. Overexpression of Per1 was confirmed by q-PCR (Figure 3A). There was decreased proliferation of Mz-ChA-1 cells after overexpression (OE) of Per1 compared to control cells (receiving empty vector, EV), which was confirmed by q-PCR and immunoblots for PCNA (Figure 3 B–D). There were fewer G2/M phase cells in Per1 OE cells compared to EV cells, whereas the number of quiescent cells in G0/G1 phase increased in Per1 OE cells compared to EV cells (Figure 3E). There was enhanced apoptosis in Mz-ChA-1 Per1 OE cells compared to EV cells (Figure 3F). Similarly, overexpression of Per1 decreased proliferation, invasion and cell cycle in HuCC-T1 cells (Suppl. Figure 2A–C). We also demonstrated that silencing of Per1 expression in H69 cells increased proliferation, but did not affect the invasiveness of these cells (Suppl. Figure 2D–E).
Figure 3.
Decreased proliferation, enhanced apoptosis and decreased number of cells in S and G2/M phase in Mz-ChA-1 cells that overexpress Per1. [A] The mRNA expression of Per1 was confirmed by q-PCR. The points represent the mean ± SEM for 4 experiments. *p < 0.05 vs. EV. EV=empty vector; Per1 OE = Per1 overexpressing cells; [B] Decreased proliferation was shown by MTS assay at 24, 48 and 72 hr. Experiments for MTS are mean ± SEM of 3 repeated experiments. Decreased expression of PCNA was measured by q-PCR [C] and blots [D]. [E] Decreased number of cells in S phase and G2/M phase in Per1 OE Mz-ChA-1 cells. [F] Enhanced apoptosis in Per1 OE Mz-ChA-1 cells as measured by AnnexinV-FITC apoptosis kit. The gate was set using three controls: unstained, and staining with FITC only or PI only. The data are from double staining of PI and AnnexinV-FITC.
miR-34a was predicted and verified as the target of Per1
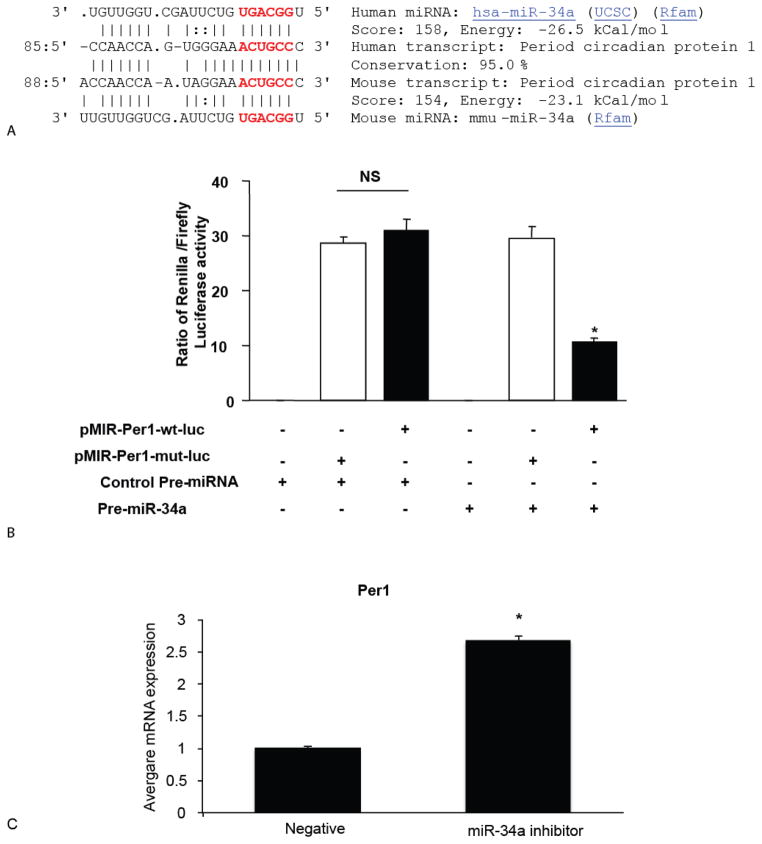
The molecular mechanisms by which Per1 decreased CCA growth are unknown. One possible mechanism is that Per1 might be regulated by some specific miRNAs that acts as oncomiRs. Therefore, we determined the specific miRNAs that target Per1. Per1 was identified by all three databases (DIANA-MicroT, Miranda and RNAhybrid) as the target of miR-34a. As shown in Figure 4A, there is one conserved binding site for miR-34a in the Per1 3′UTR. miR-34a expression was upregulated in CCA cells as determined by miRNA microarray [23]. Based on these findings, miR-34a was selected for study in subsequent experiments.
Figure 4.
Per1 is a novel target of miR-34a. [A] Conserved target site of hsa-miR-34a on Per1 gene. The potential binding sites are labeled in red. [B] Mz-ChA-1 cells were transfected with a Renilla luciferase expression construct pRL-TK and the pMIR-PER1-wt-luc or pMIR-PER1-mut-luc firefly luciferase expression construct, along with either miR-34a precursor or control precursor. Luciferase assays were performed after 48 hr. The expression of Renilla luciferase activity was normalized to that of firefly luciferase activity for each sample. [C] The mRNA expression of Per1 was increased when the miR-34a expression was inhibited. Data represent mean ± SEM from 8 separate experiments. *p <0.05 relative to control.
To substantiate that miR-34a directly targets Per1, we cloned the 3′UTR containing potential miR-34a binding sites for further validation by luciferase assay (Figure 4A). miR-34a significantly suppressed the expression of pMIR-Per1-WT but not pMIR-Per1-mut, indicating this suppression effect is specific to 3′-UTR regions (Figure 4B). Per1 expression increased after inhibition of miR-34a expression (Figure 4C).
Aberrant expression of miR-34a in CCA cell lines
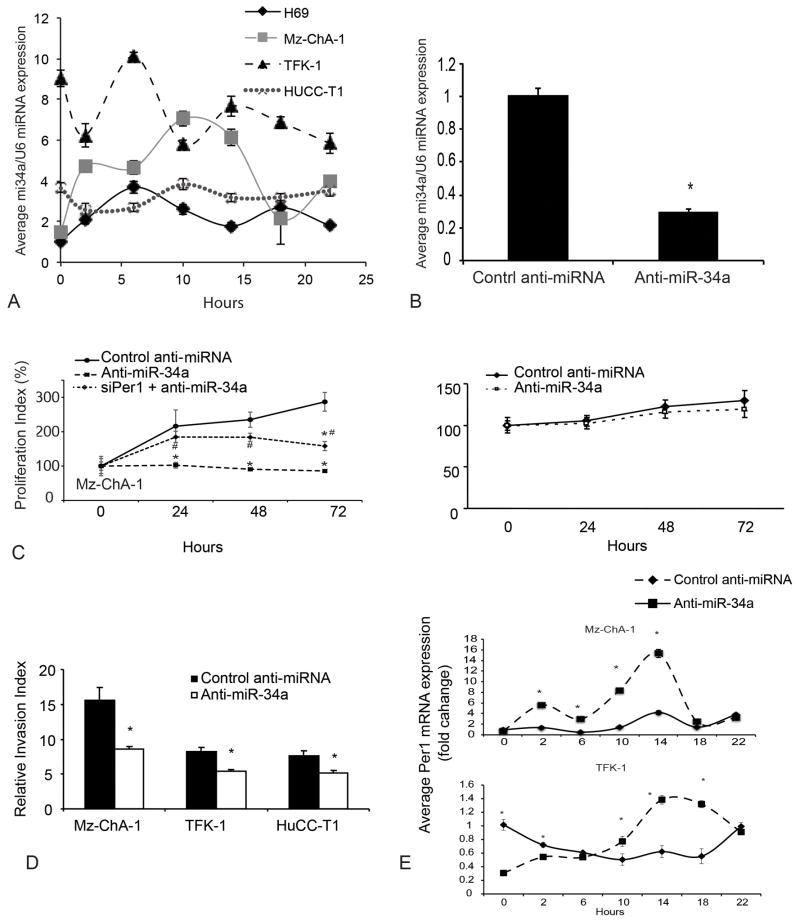
It is interesting to point out that not only the clock genes show circadian rhythm, but also specific miRNAs are regulated by clock genes or target circadian genes and also display a circadian rhythm, such as miR-122 [24]. Since Per1 is a primary output rhythmical gene, we determined whether the 24-hr expression of miR-34a showed circadian rhythm (Figure 5A). In Mz-ChA-1 cells, the expression of miR-34a displayed a shifted phase, higher amplitude and higher expression at all time points compared with H69 cells. In TFK-1, the overall amplitude was not altered significantly; however, the period was significantly shortened and the expression level was higher compared to H69 cells. For HuCC-T1, the amplitude diminished and the circadian rhythm was lost compared to H69 cells (Figure 5A).
Figure 5.
Functional role of miR-34a in CCA cells. [A] 24-hr expression pattern of miR-34a is shown in CCA cells and H69 cells. Data represent mean ± SEM of 4 experiments. [B] Decreased expression of miR-34a was confirmed by Taqman miRNA real-time PCR array. *p<0.05 vs. control anti-miRNA. [C] Decreased proliferation was shown in anti-miR-34a transfected cell line at 24, 48 and 72 hr in Mz-ChA-1 and this inhibitory effect was diminished by silencing of Per1. *p<0.05 vs. control anti-miRNA, #p<0.05 vs. anti-miR-34a. [D] Decreased invasive ability in Mz-ChA-1, TFK-1 and HuCC-T1 cells that were transfected with anti-miR-34a. *p<0.05 vs. control anti-miRNA. [E] Inhibition of miR-34a expression restored or shifted the phase of Per1 24-hr circadian rhythm. Expression of Per1 was evaluated by q-PCR at different time points after inhibition of miR-34a. All time points are compared to control anti-miRNA 0 hr. Data are mean ± SEM for 3–4 experiments. *p<0.05 vs. control anti-miRNA.
Inhibition of miR-34a expression restored Per1 24-hr circadian rhythm and diminished proliferation and invasion in vitro
Since miR-34a acts as an oncomiR in CCA, we next evaluated whether inhibition of miR-34a affects Per1 expression and circadian rhythm and tumor cell growth or invasion. Decreased expression of miR-34a was confirmed in miR-34a inhibitor-transfected cells (Figure 5B). After inhibition of miR-34a expression, the proliferation of Mz-ChA-1 cells was inhibited at 24, 48 and 72 hr, whereas the proliferation of H69 did not show significant change after inhibition of miR-34a expression (Figure 5C). Furthermore, after inhibition of miR-34a expression, cell invasiveness was decreased in CCA cell lines compared to vector-transfected cells (Figure 5D). Significant in vitro oscillation of Per1 was noted in both Mz-ChA-1 and TFK-1 cell lines (Figure 5E). Interestingly, the increased amplitude and phase shift of Per1 circadian rhythm was observed as compared to control in Mz-ChA-1 and TFK-1 respectively. However, the period or the peak of Per1 expression did not change (Figure 5E). Furthermore, silencing of Per1 expression in miR-34a-transfected Mz-ChA-1 cells diminished the inhibition of proliferation in CCA cells (Figure 5C).
Overexpression of Per1 inhibits CCA xenograft tumor growth in vivo
We next evaluated the tumor suppressor role of Per1 overexpression in vivo. We found significant inhibition of tumor growth from 20 days until 43 days in xenograft models that were injected with Mz-ChA-1 cells stably overexpressing Per1 compared to control cells (Figure 6A). We demonstrated that the expression of PCNA, VEGF-A, NGF, epithelial-mesenchymal transition (EMT) markers (TIMP2, TIMP3, MMP-3 and MMP9) and the metastasis marker S100A4 were inhibited in the Per1 OE group (Figure 6B). We found decreased expression of PCNA, fibronectin and VEGF by IHC in Per1 OE tumors, indicating that overexpressing Per1 may inhibit proliferation, EMT and angiogenesis (Figure 6C).
Figure 6.
Overexpression of Per1 inhibits tumor growth in xenograft model. [A] There was a significant inhibition in tumor growth in nude mice injected with Per1 overexpressing cells. Three representative tumors from both the EV and Per1 OE group and a summary graph of data time points are shown. Data are mean ± SEM of tumor size evaluations from 8 mice per each group of animals. *p<0.05 vs. the corresponding values of tumor volume in the EV group. [B] Overexpression of Per1, decreased proliferation (by PCNA), inhibition of angiogenesis factors (VEGF-A, NGF), EMT-related genes (TIMPs and MMPs) and a metastasis markers (S100A4) are shown by q-PCR in tumor tissue of the Per1 OE group. Data are mean ± SEM for 3 different tissue samples. [C] Decreased PCNA, fibronectin and VEGF expression are shown by IHC in the Per1 OE group. Original magnification: 20X.
Overexpression of Per1 inhibits xenograft tumor growth through regulation of cell cycle, cell growth and apoptosis pathway
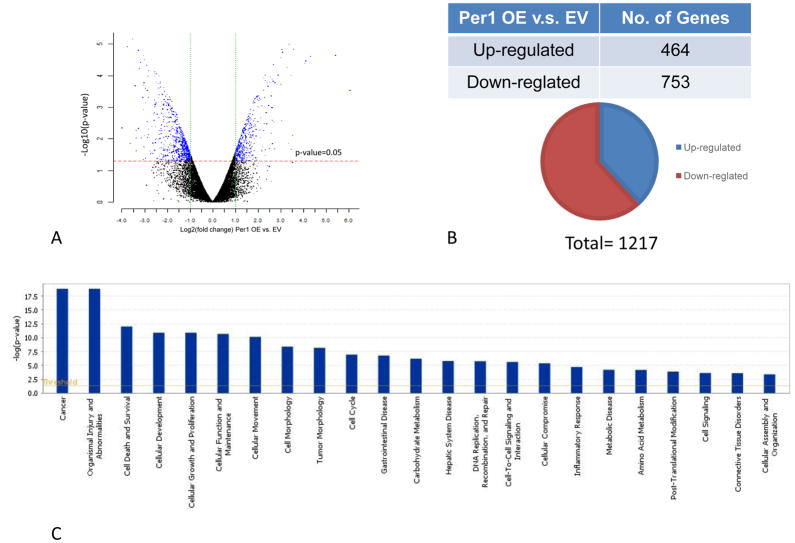
We performed microarray to determine which pathway and biological process is most affected by Per1 deregulation in CCA. The gene expression profile showed that more than 1000 genes were modulated by overexpressing Per1, among which 484 genes are up-regulated and 793 genes are down-regulated (Figure 7A–B). As shown in Suppl. Table 1 and Figure 7C, more than 800 genes modulated by overexpressing Per1 are related to cancer, which stands for the most regulated diseases. Organismal injury and abnormalities, cell death and survival, cellular development, growth and proliferation and cellular movement stand for the top 10 affected cellular functions (Figure 7C). More than 100 genes in these functions showed differential expression between EV and Per1 OE cells, indicating overexpressing Per1 gene regulates tumorigenesis in aspects of proliferation, apoptosis and cell invasiveness which is consistent with functional study of Per1 OE (Suppl. Table 1 and Figure 3). Overexpression of Per1 inhibits several canonical pathway that are related with tumorigenesis, such as IGF-1 signaling, p53 signaling, cAMP-medicated signaling and growth hormone signaling, which is based on the z-score prediction provided by IPA® (Suppl. Table 2).
Figure 7.
mRNA profiles are significantly altered by overexpression of Per1 in Mz-ChA-1 cells. mRNA expression was evaluated by the Human whole genome OneArray DNA microarray HOA 7.1, which is designed by Phalanx Biotech Group. [A] Volcano plot shows the distribution of differentially expressed probes according to fold-change (x-axis) and significance (negative logarithm of the P-value on the y-axis). The red dotted line is the P-value cut-off (0.05), and the green dotted line is the fold change cut-off (log2 |fold change|≧ 1). Expression data are plotted for all probes excluding control and flagged probes. [B] A total of 1217 genes were modulated in Per1 OE cells compared with EV, among which 464 genes were upregulated and 753 genes were downregulated. [C] The most significant canonical pathways across the ingenuity database were shown in bar chart. The significant canonical pathways for the gene list are displayed along the x-axis. The y-axis displayed the –log of p value, which indicates the probability of association of molecules from our gene list with the canonical pathway by random chance alone. Orange line sets the significant threshold as 0.05.
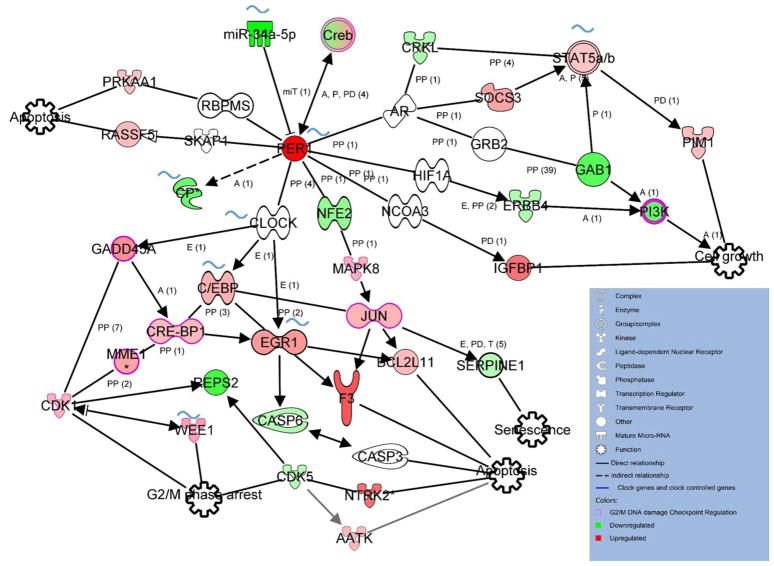
Through IPA® pathway analysis we analyzed how modulation of Per1 can affect those genes, how those genes interact with each other and how many circadian genes are involved. As shown in Figure 8, overexpressing Per1 mostly promotes apoptosis through direct or indirect interaction of Per1. At least seven molecules that increased are shown to facilitate apoptosis. We also found nine molecules, including Wee1, CRE-BP1, CDK1 and GADD45A, that relate to cell cycle regulation are upregulated in per1 overexpressing group. There are eight genes in this network that core clock genes or clock-controlled genes, which eventually leads to different functional regulation of tumorigenesis (marked with curved blue line). In addition, expression of Serpine1, which is involved in senescence, is decreased after overexpressing Per1 (Figure 8).
Figure 8.
Putative networks predicted by IPA and based on microarray profiling after overexpressing Per1. Icons filled with green indicate the genes that are downregulated after overexpressing Per1 in microarray profiling while icons filled with red indicate the genes that are upregulated in microarray profiling;. Outlined with purple are the genes involved in the G2/M DNA damage checkpoint regulation. Blue curved mark highlights those reported to be circadian expressed and clock controlled by at least one of the microarray studies in other publications [40–43]. Black arrows indicate activation, dead-end lines inhibition. The solid line that connected between two genes shows direct protein-protein interaction as provided in the literature. Detailed IPA legend refer to http://ingenuity.force.com/ipa/IPATutorials?id=kA250000000TN2wCAG.
DISCUSSION
We demonstrated that the rhythmic expression of core clock genes was disrupted in CCA cells compared to non-malignant cholangiocytes. The expression of Per1 was decreased in all CCA cell lines evaluated. Restoration of Per1 inhibited proliferation and increased apoptosis of CCA cells in vitro and reduced tumor growth in vivo. Per1 was verified as a novel target of miR-34a in CCA cells. Inhibition of miR-34a reduced proliferation and invasiveness of CCA cells.
In humans, circadian disruption increases the susceptibility to cancer development including hepatocellular carcinoma, lung, pancreatic, prostate and colorectal cancer [25–27]. Parallel to our findings, decreased Per1 expression is found in colon cancer, which correlated with estrogen receptor-beta expression via epigenetic regulation [28]. By analyzing published microarray data, we found that Per1 expression decreased in CCA when compared with intrahepatic bile ducts or with non-cancerous surrounding. Similarly, deregulation of Per1 was found in pancreatic and hepatocellular cancer [29]. In our study, decrease of Per1 expression in non-malignant cholangiocytes promotes cellular proliferation and shows an increased trend of invasion indicating Per1 as a critical tumor suppressor in the progression of CCA.
Per1 regulates cell growth by modulating cell cycle [30]. When Per1 expression reached a high level, the percentage of cells that enter into the M phase is reduced. Herein, we show that overexpressing Per1 increased G2/M phase and S phase while G1 phase decreased. The potential mechanisms could be explained by the finding that Per1 protein interacts with the checkpoint protein ataxia telangiectasia mutated (ATM) and the checkpoint kinase 2 (CHK2), leading to DNA repair and cell cycle arrest and/or apoptosis [31]. We also have found eight molecules (13.5%) ATM signaling was modulated in microarray profiling, which supports the concept that Per1 plays an important role in DNA damage and cell growth control in CCA. Wee1, an important regulator checkpoint of G2 expression and a primary output clock controlled gene [32], is upregulated in Per1 OE cell line. Indeed, Wee1 is downregulated in hepatocellular carcinoma [33]. The overexpression of Per1 partly restored the expression of Wee1. However, whether the circadian rhythm of Wee1 is affected in Per1 OE cells needs to be further evaluated. miR-34a acts as an oncogenic miRNA in hepatocellular [23] and CCA cells [9]. miR-34a regulates invasion and migration [29], apoptosis [34] cell cycle progression and proliferation [35] by targeting different genes. miR-34a is rhythmically expressed in our study, which has been predicted by a computational model for the interaction of core clock genes with miRNAs [36]. Modulation of miR-34a enlarged the amplitude of the 24-hr circadian rhythm of Per1 in Mz-ChA-1 cells and shifted the phase in TFK-1 but did not change the period in our study. Previous studies demonstrate that modulation of miRNA alters the phase and amplitude of circadian rhythm of clock genes in many organisms.
Overexpression of Per1 also affects other clock-controlled genes, indicating the coordination of circadian rhythm of different core clock genes and clock controlled genes plays a role in tumorigenesis of cholangiocarcinoma (Figure 8). Per1 is modulated by CREB [37]. The study demonstrated cAMP-responsive elements (CREs) in mPer1 promoter region that binds CRE-binding protein (CREB), which is responsive to synergistic activation of the cAMP and mitogen-activated protein kinase pathways [37]. Here we also found that modulation of Per1 may regulate the CREB complex indicating a bi-directional regulation between CREB and Per1. Through IPA analysis, we found that overexpression of Per1 affects tumorigenesis of CCA through regulation of multiple functional pathways (Figure 8). Specifically, cell death and apoptosis related genes seem affected. Overexpression of Per1 also inhibited the expression of Serpine1, one of the markers of senescence. Actually, the expression of Serpine1 is upregulated in primary sclerosing cholangitis [38], which is an important risk factor for the development of CCA [39].
In summary, our study defines the role of the circadian rhythm of clock genes in the modulation of CCA growth, the correlation of miRNA to clock genes and CCA and the therapeutic effect of Per1 and miR-34a in CCA growth. We evaluated the possible molecular mechanism(s) of how Per1 act as a tumor suppressor inhibits the malignant transformation of cholangiocytes. However, how overexpressing Per1 interacts with genes leading to the control of cell cycle, cell growth, senescence and apoptosis needs to be further elucidated.
Supplementary Material
Acknowledgments
Statement of financial support: This work was supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Baylor Scott & White, the Texas Chapter of the Ladies Auxiliary to the Veterans of Foreign Wars to Dr. Alpini and Dr. Francis, a VA Research Career Scientist Award, a VA Merit award to Drs. Alpini (5I01BX000574), Meng (1I01BX001724) and Glaser (5I01BX002192) and NIH DK076898, DK054811 and DK062975 grants to Drs. Alpini, Meng and Glaser, and a VA CDA-2 Award to Dr. Francis (IK2 BX001760).
This manuscript is the result of work supported by resources at the Central Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Abbreviations
- BMAL1
brain and muscle-Arnt-like1
- CCA
cholangiocarcinoma
- CDK1
Cyclin-dependent kinase 1
- CLOCK
circadian locomotor output cycles kaput
- CREB
cAMP-responsive element binding protein
- Cry1
cryptochrome 1
- EV
empty vector
- GADD45A
Growth arrest and DNA-damage-inducible protein 45 alpha
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- MMP
metalloproteinase
- NGF
nerve growth factor
- OE
overexpressing
- PCNA
proliferating cellular nuclear antigen
- PCR
polymerase chain reaction
- Per1
Period 1
- TIMP
tissue inhibitor of metalloproteinase
- 3′UTR
3′ untranslated region
- VEGF
vascular endothelial growth factor
Footnotes
Author names in bold designate shared co-first authorship
Conflict of interest: No conflict of interest to report.
Individual contributions: Yuyan Han assisted in the design of study, performed experiments, analyzed/interpreted data, and drafted the manuscript; Gianfranco Alpini developed the study and study design, interpreted data, provided funding and helped draft the manuscript; Fanyin Meng helped design and interpret the microRNA study, provided technical support and helped to revise the manuscript; Julie Venter, Nan Wu, Ying Wan, Holly Standeford, Laurent Ehrlich helped performed some of the experiments; Cynthia Meininger and John Greene Jr. contributed to study design and revision of the manuscript; Heather Francis and Shannon Glaser contributed to study design, interpreted data, and helped with manuscript revision and provided funding.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cardinale V, Bragazzi MC, Carpino G, Torrice A, Fraveto A, Gentile R, et al. Cholangiocarcinoma: increasing burden of classifications. Hepatobiliary Surg Nutr. 2013;2:272–280. doi: 10.3978/j.issn.2304-3881.2013.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 3.Filipski E, King VM, Li X, Granda TG, Mormont MC, Liu X, et al. Host circadian clock as a control point in tumor progression. J Natl Cancer Inst. 2002;94:690–697. doi: 10.1093/jnci/94.9.690. [DOI] [PubMed] [Google Scholar]
- 4.Viswanathan AN, Schernhammer ES. Circulating melatonin and the risk of breast and endometrial cancer in women. Cancer Lett. 2009;281:1–7. doi: 10.1016/j.canlet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cermakian N, Sassone-Corsi P. Multilevel regulation of the circadian clock. Nat Rev Mol Cell Biol. 2000;1:59–67. doi: 10.1038/35036078. [DOI] [PubMed] [Google Scholar]
- 6.Bozek K, Rosahl AL, Gaub S, Lorenzen S, Herzel H. Circadian transcription in liver. Biosystems. 2010;102:61–69. doi: 10.1016/j.biosystems.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Cao Q, Gery S, Dashti A, Yin D, Zhou Y, Gu J, et al. A role for the clock gene per1 in prostate cancer. Cancer Res. 2009;69:7619–7625. doi: 10.1158/0008-5472.CAN-08-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gery S, Komatsu N, Kawamata N, Miller CW, Desmond J, Virk RK, et al. Epigenetic silencing of the candidate tumor suppressor gene Per1 in non-small cell lung cancer. Clin Cancer Res. 2007;13:1399–1404. doi: 10.1158/1078-0432.CCR-06-1730. [DOI] [PubMed] [Google Scholar]
- 9.Meng F, Henson R, Lang M, Wehbe H, Maheshwari S, Mendell JT, et al. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology. 2006;130:2113–2129. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 10.Knuth A, Gabbert H, Dippold W, Klein O, Sachsse W, Bitter-Suermann D, et al. Biliary adenocarcinoma. Characterisation of three new human tumor cell lines. J Hepatol. 1985;1:579–596. doi: 10.1016/s0168-8278(85)80002-7. [DOI] [PubMed] [Google Scholar]
- 11.Kusaka Y, Muraoka A, Tokiwa T, Sato J. Establishment and characterization of a human cholangiocellular carcinoma cell line. Hum Cell. 1988;1:92–94. do not know. [PubMed] [Google Scholar]
- 12.Saijyo S, Kudo T, Suzuki M, Katayose Y, Shinoda M, Muto T, et al. Establishment of a new extrahepatic bile duct carcinoma cell line, TFK-1. Tohoku J Exp Med. 1995;177:61–71. doi: 10.1620/tjem.177.61. [DOI] [PubMed] [Google Scholar]
- 13.Miyagiwa M, Ichida T, Tokiwa T, Sato J, Sasaki H. A new human cholangiocellular carcinoma cell line (HuCC-T1) producing carbohydrate antigen 19/9 in serum-free medium. In Vitro Cell Dev Biol. 1989;25:503–510. doi: 10.1007/BF02623562. [DOI] [PubMed] [Google Scholar]
- 14.Storto PD, Saidman SL, Demetris AJ, Letessier E, Whiteside TL, Gollin SM. Chromosomal breakpoints in cholangiocarcinoma cell lines. Genes Chromosomes Cancer. 1990;2:300–310. doi: 10.1002/gcc.2870020408. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu Y, Demetris AJ, Gollin SM, Storto PD, Bedford HM, Altarac S, et al. Two new human cholangiocarcinoma cell lines and their cytogenetics and responses to growth factors, hormones, cytokines or immunologic effector cells. Int J Cancer. 1992;52:252–260. doi: 10.1002/ijc.2910520217. [DOI] [PubMed] [Google Scholar]
- 16.Grubman SA, Perrone RD, Lee DW, Murray SL, Rogers LC, Wolkoff LI, et al. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am J Physiol Gastrointest Liver Physiol. 1994;266:G1060–1070. doi: 10.1152/ajpgi.1994.266.6.G1060. [DOI] [PubMed] [Google Scholar]
- 17.Maruyama M, Kobayashi N, Westerman KA, Sakaguchi M, Allain JE, Totsugawa T, et al. Establishment of a highly differentiated immortalized human cholangiocyte cell line with SV40T and hTERT. Transplantation. 2004;77:446–451. doi: 10.1097/01.TP.0000110292.73873.25. [DOI] [PubMed] [Google Scholar]
- 18.Tazawa H, Tsuchiya N, Izumiya M, Nakagama H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci U S A. 2007;104:15472–15477. doi: 10.1073/pnas.0707351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021–1031. e1015. doi: 10.1053/j.gastro.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francis H, DeMorrow S, Venter J, Onori P, White M, Gaudio E, et al. Inhibition of histidine decarboxylase ablates the autocrine tumorigenic effects of histamine in human cholangiocarcinoma. Gut. 2012;61:753–764. doi: 10.1136/gutjnl-2011-300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francis H, Onori P, Gaudio E, Franchitto A, DeMorrow S, Venter J, et al. H3 histamine receptor-mediated activation of protein kinase Calpha inhibits the growth of cholangiocarcinoma in vitro and in vivo. Mol Cancer Res. 2009;7:1704–1713. doi: 10.1158/1541-7786.MCR-09-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han Y, Demorrow S, Invernizzi P, Jing Q, Glaser S, Renzi A, et al. Melatonin exerts by an autocrine loop antiproliferative effects in cholangiocarcinoma: its synthesis is reduced favoring cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol. 2011;301:G623–633. doi: 10.1152/ajpgi.00118.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gatfield D, Le Martelot G, Vejnar CE, Gerlach D, Schaad O, Fleury-Olela F, et al. Integration of microRNA miR-122 in hepatic circadian gene expression. Genes Dev. 2009;23:1313–1326. doi: 10.1101/gad.1781009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, et al. Night-shift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst. 2003;95:825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 26.Kloog I, Haim A, Stevens RG, Portnov BA. Global co-distribution of light at night (LAN) and cancers of prostate, colon, and lung in men. Chronobiol Int. 2009;26:108–125. doi: 10.1080/07420520802694020. [DOI] [PubMed] [Google Scholar]
- 27.Rich T, Innominato PF, Boerner J, Mormont MC, Iacobelli S, Baron B, et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11:1757–1764. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- 28.Mostafaie N, Kallay E, Sauerzapf E, Bonner E, Kriwanek S, Cross HS, et al. Correlated downregulation of estrogen receptor beta and the circadian clock gene Per1 in human colorectal cancer. Mol Carcinog. 2009;48:642–647. doi: 10.1002/mc.20510. [DOI] [PubMed] [Google Scholar]
- 29.Sato F, Nagata C, Liu Y, Suzuki T, Kondo J, Morohashi S, et al. PERIOD1 is an anti-apoptotic factor in human pancreatic and hepatic cancer cells. J Biochem. 2009;146:833–838. doi: 10.1093/jb/mvp126. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006;22:375–382. doi: 10.1016/j.molcel.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 32.Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, et al. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci U S A. 2007;104:3342–3347. doi: 10.1073/pnas.0611724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin YM, Chang JH, Yeh KT, Yang MY, Liu TC, Lin SF, et al. Disturbance of circadian gene expression in hepatocellular carcinoma. Mol Carcinog. 2008;47:925–933. doi: 10.1002/mc.20446. [DOI] [PubMed] [Google Scholar]
- 34.Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A. 2008;105:13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun F, Fu H, Liu Q, Tie Y, Zhu J, Xing R, et al. Downregulation of CCND1 and CDK6 by miR-34a induces cell cycle arrest. FEBS Lett. 2008;582:1564–1568. doi: 10.1016/j.febslet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 36.Liu K, Wang R. MicroRNA-mediated regulation in the mammalian circadian rhythm. J Theor Biol. 2012;304:103–110. doi: 10.1016/j.jtbi.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 37.Travnickova-Bendova Z, Cermakian N, Reppert SM, Sassone-Corsi P. Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc Natl Acad Sci U S A. 2002;99:7728–7733. doi: 10.1073/pnas.102075599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tabibian JH, Trussoni CE, O’Hara SP, Splinter PL, Heimbach JK, LaRusso NF. Characterization of cultured cholangiocytes isolated from livers of patients with primary sclerosing cholangitis. Lab Invest. 2014;94:1126–1133. doi: 10.1038/labinvest.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004;99:523–526. doi: 10.1111/j.1572-0241.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 40.Bozek K, Relogio A, Kielbasa SM, Heine M, Dame C, Kramer A, et al. Regulation of clock-controlled genes in mammals. PLoS One. 2009;4:e4882. doi: 10.1371/journal.pone.0004882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brewer M, Lange D, Baler R, Anzulovich A. SREBP-1 as a transcriptional integrator of circadian and nutritional cues in the liver. J Biol Rhythms. 2005;20:195–205. doi: 10.1177/0748730405275952. [DOI] [PubMed] [Google Scholar]
- 42.Nicolas M, Noe V, Ciudad CJ. Transcriptional regulation of the human Sp1 gene promoter by the specificity protein (Sp) family members nuclear factor Y (NF-Y) and E2F. Biochem J. 2003;371:265–275. doi: 10.1042/BJ20021166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duffield GE, Best JD, Meurers BH, Bittner A, Loros JJ, Dunlap JC. Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr Biol. 2002;12:551–557. doi: 10.1016/s0960-9822(02)00765-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.