Abstract
Sustained JNK activation has been implicated in many models of cell death and tissue injury. P-JNK interacts with the mitochondrial outer membrane protein, Sab (SH3BP5). Using knockdown or liver specific deletion of Sab we aimed to elucidate the consequences of this interaction on mitochondrial function in isolated mitochondria and liver injury models in vivo. Respiration in isolated mitochondria was directly inhibited by P-JNK+ATP. Knockdown or liver specific knockout of Sab abrogated this effect and markedly inhibited sustained JNK activation and liver injury from acetaminophen (APAP) or TNF/galactosamine. We then elucidated an intramitochondrial pathway in which interaction of JNK and Sab on the outside of the mitochondria released SHP1 (PTPN6) from Sab in the inside of the mitochondrial outer membrane leading to its activation and transfer to the inner membrane where it dephosphorylates P-Y419Src (active) which required a platform protein, DOK4, on the inner membrane. Knockdown of mitochondrial DOK4 or SHP1 inhibited the inactivation of mitochondrial P-Src and the effect of P-JNK on mitochondria. Conclusions; the binding to and phosphorylation of Sab by P-JNK on the outer mitochondrial membrane leads to SHP1 and DOK4 dependent inactivation of P-Src on the inner membrane. Inactivation of mitochondrial Src inhibits electron transport and increases ROS release, which sustains JNK activation and promotes cell death and organ injury.
Keywords: acetaminophen, TNF, galactosamine, phospho-Src, oxidative phosphorylation, SHP1 (PTPN6), DOK4, apoptosis, necrosis
Sustained JNK activation has been implicated in many models of cell death and tissue injury.1–7 We have previously identified translocation of JNK to mitochondria in several models of liver cell death and organ injury, including acetaminophen (APAP), TNF-galactosamine (GalN), chemical-induced ER stress and palmitic acid lipotoxicity.8–12 In these circumstances, P-JNK binds to a protein found exclusively on the outer membrane of mitochondria, namely SH3BP5 or Sab.10 This interaction is associated with disturbed mitochondrial function in intact cells and increased mitochondrial ROS production.9 The oxidative stress then contributes to the activation of the MAPK cascade, sustaining JNK activation.13–17 Knockdown of Sab in vivo using adenoviral shSab mitigated this self-sustaining pathway and blocked liver cell death in all these models.8–10 Others have shown similar effects in brain and cardiac injury.18,19
The mechanism of how the interaction of JNK with Sab on the cytoplasmic surface of the mitochondria leads to impairment of mitochondria function is unknown. Therefore, our objectives were to assess the Sab dependent direct effects of P-JNK on isolated mitochondria and determine the intramitochondrial signaling pathway that mediates these effects both in vitro and in vivo, the latter using two models of acute liver injury: APAP- induced necrosis and TNF/GalN induced apoptosis. The current studies employed liver specific conditional knockout of Sab for the first time as well as adenoviral knockdown to assess Sab dependency. Our findings have identified a link between the interaction of P-JNK and Sab and inhibition intramitochondrial Src leading to inhibition of mitochondrial respiration in the promotion of cell death and organ injury.
Materials and Methods
Animals
All animal experiments followed procedures approved by the IACUC of the University of Southern California. Male C57BL/6NHsd mice (6–8 weeks of age) were obtained from Harlan Bioproducts for Science Inc. (Indianapolis, IN). loxP flanked Sab mutant mice (Sh3bp5tm1a(KOMP)Wtsi) were generated in C57BL/6N background by KOMP, UC Davis. Exon 5, 6 and 7 were flanked by loxP. Primers for genotyping of Sab mutant mice are: for floxed mice GAGATGGCGCAACGCAATTAATG and TGTGTAGCAGAGTAGACCCTCATGC, for post-Cre mice GCTACCATTACCAGTTGGTCTGGTGTC and TGTGTAGCAGAGTAGA CCCTCATGC. To generate tamoxifen-induced hepatocyte specific Sab KO mice (Tam-SabΔHep), Sab floxed mice were crossbred with Alb-CreERT2 mice (Albtm1(cre/ERT2)Mtz)20,21 kindly provided by Dr. Pierre CHAMBON, Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC), France. Sabf/f;Alb-CreERT2+/− were crossbred with Sabf/f floxed mice at least 10 times, and then 6 weeks old male mice were fed with tamoxifen (Tam) 1g/kg of diet pellet for 7 days and experiment was performed 5–7 days later after last day of Tam-diet.22
In-vitro kinase effect on mitochondria using Seahorse XF24 analyzer or by western blot
For Seahorse XF24 analyzer: Livers from 6–8 weeks old mice were homogenized in mitochondrial assay solution (MAS) (70mM sucrose, 220mM mannitol,10mM KH2PO4, 5mM MgCl2, 2mM HEPES, 1mM EGTA, pH7.2 at 37°C) supplemented with pyruvate and malate or succinate and rotenone without protease and phosphatase inhibitors. Mitochondria were isolated by differential centrifugation and resuspended in MAS with substrates.23,24 To 20μg of mitochondria in 50μl MAS supplemented with substrate with or without ATP 6μM, 50ng of ~160U/mg kinase or chemical inhibitors were added on ice. Pre-mixed mitochondria 20μg (50μl) was loaded into each well of XF24 cell culture microplate on ice and was spun at 3000rpm for 20min at 8°C, and then incubated at 37°C in CO2 free incubator for 15min. After incubation, each well was fed with 450μl of 37°C prewarmed MAS supplemented with substrate, and OCR was measured. The program was set to equilibrate at 37°C for 5min before measurement of basal OCR (State 2 respiration). ADP (4mM final), oligomycin (2.5μg/ml final), CCCP (4μM final) or antimycin A (4μM final) was injected from port-A, port-B, port-C or port-D respectively and OCR was measured sequentially. ADP induced mitochondrial oxidative-phosphorylation (State 3 respiration) was determined after ADP injection. OCR after oligomycin injection was defined as State 4 respiration. CCCP induced mitochondrial oxidative respiration was defined as maximal respiratory capacity of mitochondria. When required, measurements were normalized to average of measurement points of the basal (starting) level of OCR of each well. Error bars in one representative experiment represent S.D of 3–5 wells. Experiments were repeated 3–5 times with different mitochondria preps. Data was analyzed in groups of wells of each sample prep and statistical analysis was done by t-test.
For western blot analysis: Mitochondria after incubation with various kinases and inhibitors were spun at 20,000 ×g for 5 min at 4°C and protein was extracted in RIPA lysis buffer with protease and phosphatase inhibitors.
Submitochondrial fractionation
Liver mitochondria were isolated from 6 weeks old mice using homogenizing buffer with protease and phosphatase inhibitors as described. To avoid the changes of mitochondrial inner membrane integrity with the mitochondrial swelling method, submitochondrial fractionation was performed by digitonin permeabilization followed by mechanical disruption of outer membrane. Resuspended mitochondria were incubated with digitonin dissolved in homogenizing buffer (0.5 mg digitonin/mg mitochondria/0.2ml buffer) for 20 min at 25°C. After incubation, the mitochondrial outer membrane was stripped by vigorous pipetting up and down X60 times with 1ml pipette in digitonin buffer, and then spun 9000 Xg for 10min at 8°C to separate outer membrane in supernatant and mitoplast in pellet. Mitoplasts were washed by pipetting up and down X30 times in 1ml homogenizing buffer, and then spun 9000 Xg for 10min at 8°C. After 3 washes, mitoplasts were resuspended in homogenizing buffer and mechanically disrupted several times by vacuum created in 30G needle attached insulin syringe, and then spun at 20,000 Xg for 10min to separate matrix as supernatant and inner membrane as pellet. Mitoplast, inner membrane, matrix and outer membrane fractions were solubilized and diluted in RIPA buffer up to equal volume of submitochondrial fractions. Equal volume of fractions was used to examine the distribution of proteins in submitochondrial fractions. To confirm the separations, fraction marker proteins were immunoblotted with specific antibodies.
In-vitro model system for assessing the interaction of Src, DOK4 and SHP1
The in-vitro phosphatase reaction system25 was modified. 1μg of Src recombinant protein (Origene) was diluted in 150 μl of reaction buffer (50mM Tris-HCl, pH7.5, 0.1mM Na2EDTA, 2mM MnCl2, 5mM DTT, 0.01% Brij35 (nonionic detergent), and 600μM ATP) and kept at 30°C for 30 min to activate Src by auto-phosphorylation. 1μg of DOK4 recombinant protein (Origene) was added into the reactions as indicated, and incubated for 30 min. 1μg of protein phosphatase SHP1(PTPN6) (Origene) was added to the reaction, and incubated for 30min. Reaction was stopped by addition of ice cold 150μl of buffer with protease and phosphatase inhibitor cocktails. Each reaction was aliquoted for Western blot and immunoprecipitation. Src in the reaction was immunoprecipitated with c-Src antibody (Cell Signaling Technology) in 1% BSA TBS 500μl for 60 min and protein A agarose for 60 min, and washed with TBS-Tween 0.05% 3 times, and eluted in 2XLB with 2βME and denatured. Src associated proteins in elutes were determined by Western blot.
Results
Effect of conditional knockout of Sab in APAP and TNF/galactosamine toxicity
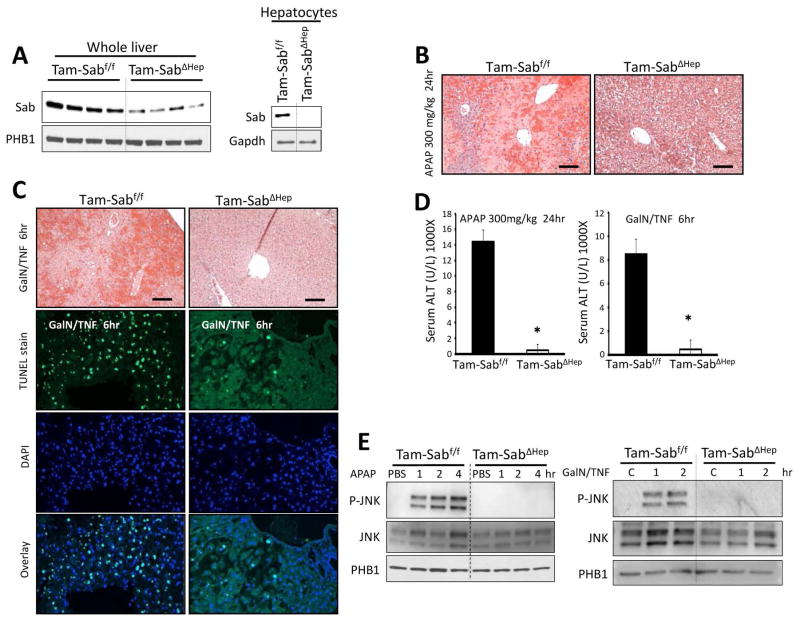
We generated homozygous floxed Sab mice (Supporting Fig. 1A) and administered adenoviral-Cre (either AAV8-TBG-Cre or adenoviral-Cre) to adult mice and performed studies 10 days later. After AAV8 delivery of liver specific TBG-Cre, Sab was undetectable in hepatocytes and hepatocyte Sab deletion abrogated APAP liver injury (Supporting Fig. 1B). Using adenoviral Cre delivery, we examined the early phase (up to 4 hours) of APAP toxicity and observed no association of P-JNK with mitochondria in the absence of Sab (Supporting Fig. 1C). We also crossed homozygous floxed Sab mice with heterozygous tamoxifen inducible albumin Cre mice. This approach obviated potential concerns about the use of adenovirus to knockdown Sab or to deliver Cre (inflammation or nonparenchymal knockdown) and allowed comparison with tamoxifen treated littermates (Tam-Sabf/f versus Tam-SabΔHep). There was no histological abnormality or change in baseline ALT in littermate controls after tamoxifen feeding followed by 5–7 days washout. The remaining Sab in whole liver homogenates was absent in hepatocytes isolated from these mice (Fig. 1A). The Tam-SabΔHep mice were markedly protected from APAP and TNF/GalN induced liver injury as reflected in histological injury and serum ALT (Fig. 1B–D). Furthermore, TUNEL positive apoptosis was markedly reduced in the Tam-SabΔHep mice after TNF/GalN (Fig. 1C). In the homozygous floxed littermates the expected sustained JNK activation was observed after APAP and TNF/GalN administration, while in the tamoxifen inducible Sab-deleted mice, JNK activation was not sustained and there was no association of P-JNK with mitochondria (Fig. 1E). Deletion of Sab did not affect APAP adduct formation or GSH depletion and had no effect on basal Cyp2e1 expression (Supporting Fig. 2).
Fig. 1. Effect of conditional knockout of Sab in liver injury models.
Mice were fed with tamoxifen diet (Tam-diet) for 7 days. (A) Depletion of Sab in mitochondria isolated from whole liver homogenate with residual Sab presumably in nonparenchymal cells and undetectable Sab in freshly isolated mouse hepatocytes. One week later tamoxifen treated littermate control (Tam-Sabf/f Control) and tamoxifen treated Sabf/f;Alb-CreERT2+/− Sab KO (Tam-SabΔHep KO) were treated with APAP 300mg/kg i.p or TNF 12μg/kg/GalN 800mg/kg i.p. (B) Comparison of H and E liver histology 24 hours after APAP. Scale bars represent 100μm. (C) Comparison of H and E and TUNEL stain of liver 6 hours after TNF/GalN. Scale bars represent 100μm. (D) Serum ALT 24 hours after APAP or 6 hours after TNF/GalN. Error bars represent S.D of 3 separate experiments. N = 5; (*) represent p <0.05 (t-test), Tam-Sabf/f Control vs Tam-SabΔHep KO (E) Comparison of P-JNK translocation to mitochondria in early hours after APAP or GalN/TNF in Tam-Sabf/f versus Tam-SabΔHep mice.
Direct effects of JNK on mitochondria and dependence on Sab
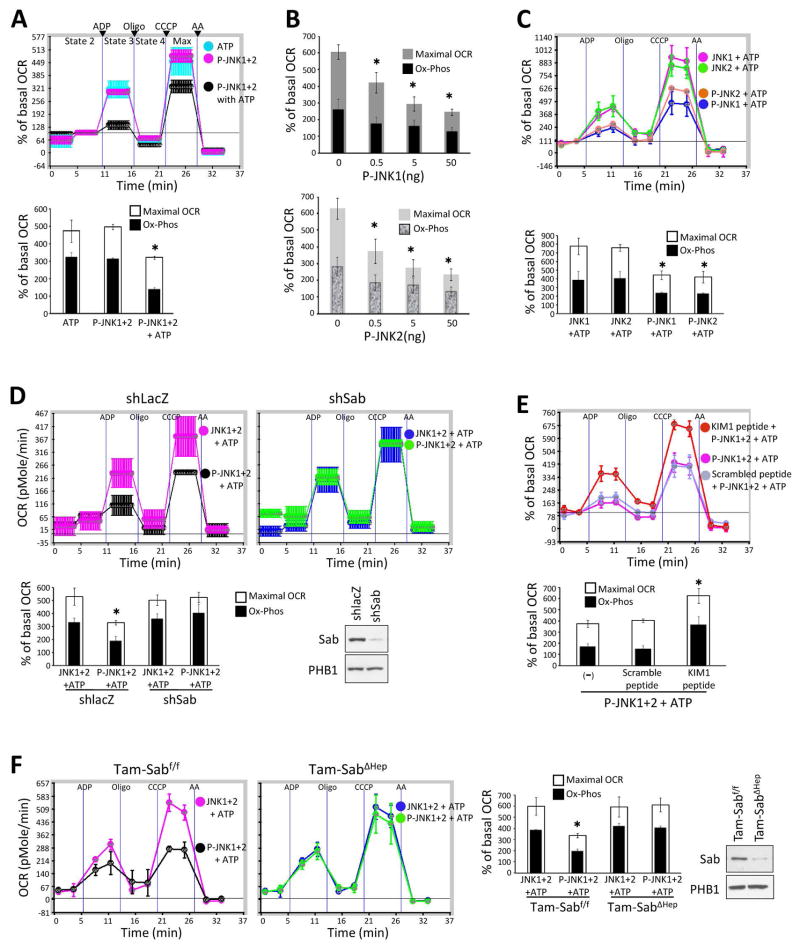
To determine the direct effects of JNK on mitochondria, we first compared western blot densitometric intensity of P-JNK standards with aliquots of cytoplasmic extracts prepared 2 hours after APAP. This provided a densitometric intensity of P-JNK which could be compared with that of cytoplasmic P-JNK available to mitochondria observed in vivo after APAP, allowing us to approximate the P-JNK levels to which mitochondria are exposed (Supporting Fig. 3). Based on these findings we used a range 0.5 – 50ng P-JNK per 20μg mitochondrial protein. Incubation of normal liver mitochondria with P-JNK 1 and 2, individually or combined, rapidly and dose-dependently inhibited ADP-inducible respiration (state 3) and CCCP-inducible maximum respiration in the presence of ATP whereas no effect of P-JNK was seen without ATP (Fig. 2A and B). The inhibition of respiration was seen in the presence of either complex I or II substrates (Supporting Fig. 4A). ATP alone had no effect. Since P-JNK is known to phosphorylate Sab,26,27 this finding strongly suggests that the effect of JNK on mitochondria requires phosphorylation of Sab. We then examined JNK1 and 2 individually in activated and non-activated form (both with ATP). Non-activated JNK 1 or 2 with ATP did not inhibit mitochondrial respiration (Fig. 2C). In contrast, activated JNK1 or 2 individually exerted comparable inhibition of mitochondrial respiration (Fig. 2B and C) and as noted above, did so in a dose dependent fashion within the range of exposure we estimated to occur in vivo (Fig. 2B).
Fig. 2. Sab dependent effect of P-JNK on OCR of isolated mitochondria.
(A) Mitochondrial Oxygen consumption rate (OCR) of isolated mitochondria (20μg) from wild type mouse liver treated with activated JNK (P-JNK1+2 50ng each) with or without ATP (6μM final) in 50μl mitochondrial assay solution supplemented with pyruvate/malate. Data represents real-time continuous measurement of OCR using in Seahorse XF24 analyzer. (*) represent p <0.05 (t-test), vs P-JNK or ATP alone. (B) OCR of isolated mitochondria treated with serially diluted P-JNK1 or P-JNK2 in the presence of ATP and pyruvate/malate. (*) represent p <0.05 (paired t-test) for both mitochondrial maximal oxidative capacity and oxidative phosphorylation, vs without P-JNK. (C) Mitochondria (20μg) were treated with 50ng of recombinant unactivated JNK (JNK1 or JNK2) or activated JNK (P-JNK1 or P-JNK2) in the presence of ATP and pyruvate/malate. (*) represent p <0.05, JNK vs P-JNK. (D) Adenoviral shSab or adenoviral shlacZ was given to mice intravenously and 10 days later the mitochondria were isolated. Efficiency of Sab knockdown was confirmed by western blot of isolated mitochondria. 20μg of mitochondria were treated with unactivated JNK1+2 or P-JNK1+2 (50ng each) in the presence of ATP and pyruvate/malate. (*) represents p <0.05, shlacZ vs shSab treated with P-JNK1+2 + ATP. (E) Mitochondria (20μg) isolated from wild type mice were treated with P-JNK1+2 with scrambled peptide or KIM1 peptide in the presence of ATP. (F) Mitochondria (20μg) isolated from Tam-Sabf/f *ATP and pyruvate/malate. Efficiency of Sab knockout was confirmed by western blot of isolated mitochondria. (*) represents p <0.05, Tam-Sabf/f Control vs Tam-SabΔHep KO treated with P-JNK+ATP. The OCR was measured in following order: after acquisition of basal (State 2) OCR, state 3 respiration after ADP injection, state 4 respiration after oligomycin injection, maximal respiration after CCCP injection, and non-mitochondrial OCR after antimycinA (AA) were measured. Error bars represent the S.D of triplicate or pentaplicate samples in one representative experiment and bar graphs compare maximum respiration and oxidative phosphorylation (OCR after ADP minus oligomycin) for three experiments. In bar graph, (*) indicates both max OCR and oxidative phosphorylation were significant.
Next we assessed the Sab dependence of the effect of JNK on mitochondria using three approaches: mitochondria from mouse liver after in vivo treatment with adenoviral-shSab, the effect of KIM1 Sab blocking peptide, and liver specific conditional knockout (Tam-SabΔHep). KIM1 peptide corresponding to the JNK docking site on Sab has been shown to inhibit the binding of P-JNK to Sab but did not inhibit JNK activity (eg. with c-Jun as substrate).8,9,28,29 As shown in Fig. 2D, after efficient in vivo Sab knockdown, mitochondria were completely protected from the inhibition of respiration by P-JNK 1+2 with ATP. Similarly, the KIM1 blocking peptide inhibited the effect of P-JNK 1+2 with ATP on normal mitochondria (Fig. 2E) whereas mitochondria from liver specific inducible Sab knockout were also resistant to P-JNK 1/2 plus ATP (Fig. 2F). In addition, we performed similar studies with purified active p38 kinase and MKK4. P-MKK4 also translocates to mitochondria after APAP.10 Neither kinase alone with or without ATP exerted an effect on mitochondrial respiration (Supporting Fig. 4B). Of-note, mitochondria from inducible knockout mice did not exhibit any changes in mitochondrial respiration (not shown).
Role of Src in liver mitochondria
The interaction and phosphorylation of Sab by P-JNK is known to occur on the cytoplasmic face of the mitochondria at the C-terminus of Sab. The topology of Sab was verified with N- and C-terminal antibodies after proteinase K digestion of the cytoplasmic facing portion of intact mitochondria (Supporting Fig. 5). Since P-JNK binds to the C-terminus of Sab on the outside of mitochondria and does not enter mitochondria, how does the binding of P-JNK and its phosphorylation of Sab on the outside surface of mitochondria lead to impaired respiration? We focused our attention on the known role of intramitochondrial activated Src kinases30–32 in regulating mitochondrial function by being required for various steps in electron transport.33,34
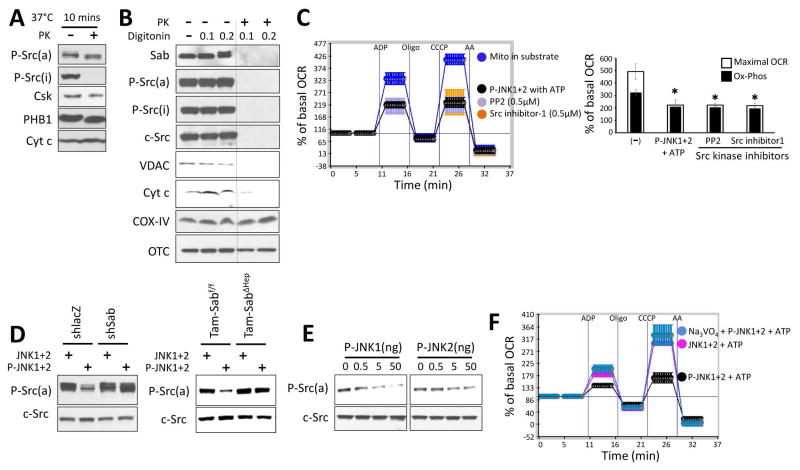
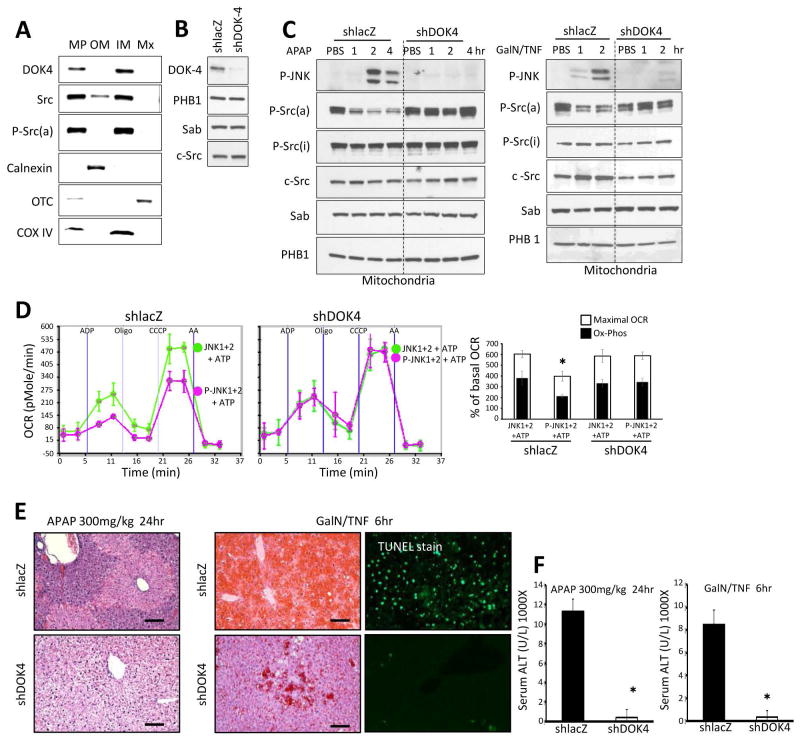
We first examined the localization of total c-Src, P-Y527Src (inactive), P-Y419Src (active), and the inactivating kinase, Csk, in isolated normal mouse liver mitochondria with or without proteinase K digestion. Csk, c-Src and P-Y419Src were present in mitochondria after proteinase K whereas P-Y527Src was barely detectable inside the mitochondria (Fig. 3A). Permeabilization of the outer membrane allowed proteinase K to destroy these intramitochondrial proteins (including P-Y419Src) along with VDAC (outer membrane) and cytochrome C (bound to intermembrane face of the inner membrane) while sparing matrix, OTC and inner membrane cytochrome oxidase IV (Fig. 3B). Thus, activated Src was exposed to the intermembrane space. Further localization of Src in the mitochondria is discussed below.
Fig. 3. Localization of Src in mitochondria and effect of P-JNK on P-Src.
(A) Isolated mitochondria from wild type mice were incubated with or without proteinase K (PK) (1mg/ml) at 37°C for 10 min. (B) Isolated mitochondria were incubated with digitonin 0.1 mg or 0.2 mg per mg of mitochondria protein in ice for 30min followed by PK (1mg/ml) (or no PK) for 10 minutes at 37°C. Western blot analysis in A and B was performed using antisera against Sab, P-Src(a) (active form, Y419), P-Src(i) (inactive form, Y527), c-Src, VDAC, cytochrome c (Cyt c), cytochrome oxidase (COX) IV and ornithine carbamoyl-transferase (OTC). (C) Mitochondria were incubated with substrate (pyruvate/malate) alone or substrate with P-JNK plus ATP, or PP2 (Src inhibitor), or Src inhibitor 1. Error bars represent the S.D of pentaplicate samples in one representative experiment of 3 different preps summarized in bar graph. (*) represent p <0.05, vs substrate alone. (D) Effect of JNK on Src activity in isolated liver mitochondria. Mitochondria from shlacZ or shSab treated mice or mitochondria from Tam-Sabf/f Control or Tam-SabΔHep KO mice were incubated as in (C) and western blot was performed for P-Src(a) and c-Src. Western blots are representative of 3 different preps. (E) Immunoblots of isolated mitochondria treated with serially diluted P-JNK1 or P-JNK2 in the presence of ATP and pyruvate/malate. (F) Isolated liver mitochondria from wild type mice were incubated in pyruvate/malate with unactivated JNK+ATP or P-JNK+ATP with or without sodium thiovanadate 2mM. Error bars represent the S.D of pentaplicate samples in one representative experiment of 3 different preps.
In other contexts, decreased mitochondrial Src expression or Src inhibitors have been shown to directly inhibit mitochondrial respiration.30,31 We confirmed this in isolated normal liver mitochondria. Two Src inhibitors impaired mitochondrial respiration to a comparable degree as P-JNK1+2 with ATP (Fig. 3C). In this experimental setting the addition of P-JNK1+2 with ATP led to a rapid dephosphorylation of active Src without affecting total mitochondrial c-Src (Fig. 3D). Liver mitochondria isolated after in vivo Sab knockdown or Sab knockout (Tam-SabΔHep) were resistant to dephosphorylation of P-Y419Src (Fig. 3D). The P-JNK dose dependent inhibition of isolated mitochondrial respiration, as shown in Fig. 2B, directly correlated with the extent of dephosphorylation of P-Y419 Src (Fig. 3E). Not surprisingly, the rapid dephosphorylation of active Src was inhibited by the tyrosine phosphatase inhibitor, sodium orthovanadate (Na3VO4), which also prevented the inhibition of respiration induced by P-JNK 1+2 with ATP (Fig. 3F). Na3VO4 alone had no effect on respiration and the serine/threonine phosphatase inhibitor, sodium fluoride, did not ameliorate the effect of P-JNK on mitochondria (not shown). These data strongly suggest that the effect of JNK on mitochondria is mediated by a Sab dependent effect on intramitochondrial activated Src leading to its inactivation, supporting the conclusion that this effect on Src can account for Sab dependent effect of JNK on mitochondrial function.
Sab-dependent inactivation of mitochondrial Src in liver injury models
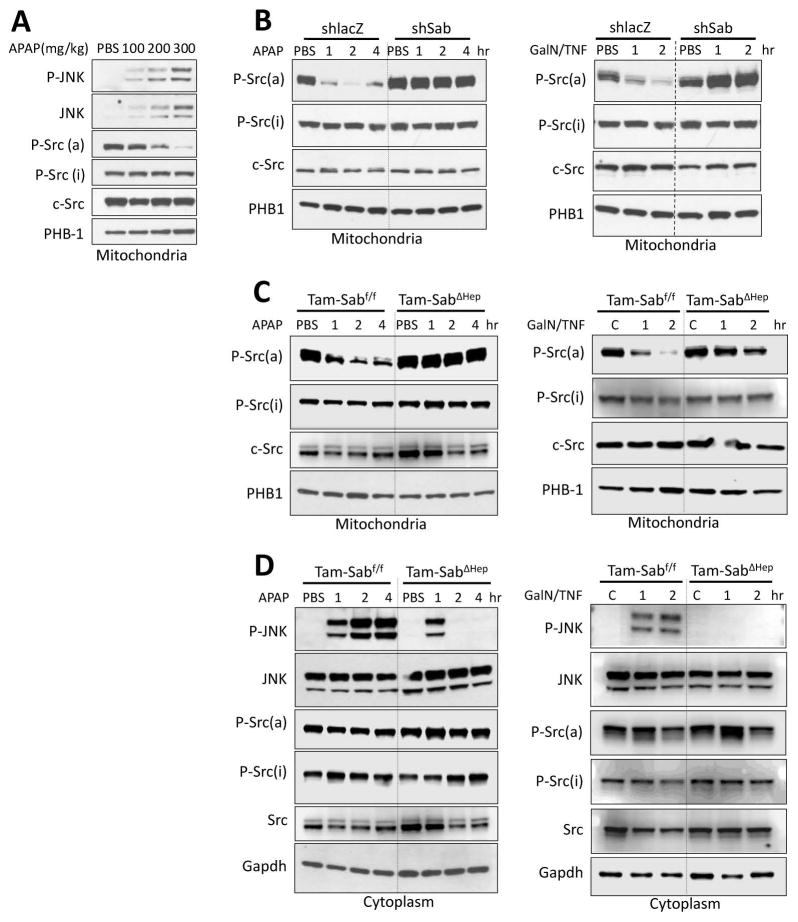
We then assessed the status of mitochondrial Src in two in vivo models of massive liver injury: APAP-induced necrosis and TNF/galactosamine–induced apoptosis. As shown on Fig. 4A, there was an inverse relationship with P-JNK and P-Src(a) in mitochondrial extracts as the dose of APAP was increased from nontoxic (100mg/kg), borderline (200mg/kg) and toxic APAP (300mg/kg). In lacZ controls, APAP-induced sustained cytoplasmic JNK activation and translocation to mitochondria. This was accompanied by markedly decreased mitochondrial P-Y419Src with no change in total Src or cytoplasmic P-Src forms (Fig. 4B). In contrast, efficient knockdown of Sab prevented the dephosphorylation of Src and sustained JNK activation. Similarly, TNF/GalN lead to a rapid dephosphorylation of active Src in mitochondria. Knockdown of Sab prevented this change (Fig. 4B). Importantly, decreased mitochondrial P-Y419Src, seen in littermate controls, was abrogated in the Tam-SabΔHep mice in both models of toxicity (Fig. 4C). Cytoplasmic P-Y419Src was unaffected (Fig. 4D). Thus, the findings in two Sab-dependent in vivo liver injury models mirror the findings in isolated mitochondria.
Fig. 4. Sab dependence of mitochondrial Src inactivation in liver injury models in vivo.
(A) Wild type mice were treated with PBS or APAP 100, 200, 300 mg/kg i.p and mitochondria were isolated at 2 hours later for western blot analysis. (B) Ad-shlacZ or Ad-shSab was given to wild type mice intravenously and 10 days later treatments with APAP (300mg/kg i.p) in warm PBS or D-GalN (800mg/kg i.p)/TNF-α (12μg/kg i.p) in pyrogen-free PBS. D-GalN was given 30 min prior to TNF-α. (C, D) Mice were fed with Tam-diet for 7 days. One week later Tam-Sabf/f Control and Tam-SabΔHep KO were treated with APAP or TNF/GalN. Mitochondria (C) and cytoplasm (post mitochondria) (D) were isolated from liver at indicated time points by differential centrifugation. Western blot analysis was performed using antisera against P-Src(a) (Y419), P-Src(i) (Y527), c-Src, P-JNK, JNK, PHB1 and Gapdh. Western blots are representative of 3 different experiments.
Role of intramitochondrial DOK4 on JNK activation and mitochondrial dysfunction
DOK4 is a member of the DOK platform protein family.35 DOK4 has been reported to interact with Src in mitochondria of endothelial cells.36 Using confocal microscopy and subcellular fractionation, DOK4 was found only in mitochondria in the liver (Supporting Fig. 6A and B). Using liver mitochondria, we confirmed that DOK4 is an intramitochondrial protein (Supporting Fig. 6C) and localized to the inner membrane (Fig. 5A) with a cleavable peptide when digitonin permeabilized mitochondria were treated with proteinase K, indicating that DOK4 was partially exposed to the intermembrane space (Supporting Fig. 6D). Fractionation of mitochondria revealed that both DOK4 and P-Y419Src were localized to the inner membrane (Fig. 5A). Src is known to be lipidated and associated with membranes.37 Neither P-Src nor DOK4 was associated with the outer membrane. We then knocked down DOK4 expression in vivo with adenoviral shDOK4 (Fig. 5B). Sustained P-JNK activation was prevented in DOK4 depleted mice in both APAP and TNF/GalN liver injury models (Fig. 5C). DOK4 knockdown prevented dephosphorylation of P-Src(a). Levels of mitochondrial c-Src and Sab were unchanged after DOK4 knockdown. The basal function (OCR) of liver mitochondria after DOK4 knockdown was not changed (not shown) but exhibited resistance to the direct effect of P-JNK1+2 with ATP on mitochondrial respiration (Fig. 5D). Thus, the protective effect on mitochondria after knockdown of DOK4 was identical to the knockdown of Sab in mitochondria, suggesting that the role of DOK4 is downstream of Sab in mitochondria.
Fig. 5. DOK4 dependence of JNK effect on isolated mitochondria in vivo and in vitro.
(A) Isolated mitochondria were fractionated into mitoplasts (MP), outer membrane (OM), inner membrane (IM) and matrix (MX). Western blot was then performed using marker enzymes to verify fractionation. Note, DOK4 and P-Src(a) were localized to the inner membrane. (B) Ad-shlacZ or Ad-shDOK4 was given to wild type mice intravenously and 10 days later efficiency of DOK4 knockdown was confirmed by western blot of isolated mitochondria. (C) Mice were treated with APAP (300mg/kg i.p) in warm PBS or D-GalN (800mg/kg i.p)/TNF-α (12μg/kg i.p) in pyrogen-free PBS. D-GalN was given 30min prior to TNF-α. Mitochondria were isolated at indicated times for western blot analysis using DOK4, P-JNK, P-Src(a), P-Src(i), c-Src, Sab and PHB1. Western blots are representative of 3 different preps. (D) Mitochondria (20μg) isolated from shlacZ or shDOK4 treated mice were incubated with pyruvate/malate and treated with JNK1+2 or P-JNK1+2 in the presence of ATP. Error bars in E represent the S.D of pentaplicate of samples in one representative experiment and bar graph summarizes 3 experiments. (*) represents p <0.05 t-test, shlacZ vs shDOK4 treated with P-JNK+ATP. N = 5 mice per group. (E) H & E staining of liver tissue section 24 hours after APAP treatment or 6 hours after TNF/GalN treatment and TUNEL staining of liver tissue section 6 hours after TNF/GalN treatment. Scale bars represent 100μm. (F) serum ALT was measured at 24 hours after APAP treatment or 6 hours after TNF/GalN treatment. Error bars represent S.D for N = 5 mice per group. (*) represent p <0.05, shlacZ vs shDOK4.
DOK4 dependent inactivation of mitochondrial Src in liver injury models
We effectively knocked down DOK4 using Ad-shDOK4 with Ad-shlacZ as control. As described above, the in vivo treatment with APAP or TNF/GalN lead to the dephosphorylation of active P-Src in the Ad-shlacZ controls. However, DOK4 knockdown abrogated this effect. The marked in vivo liver injury (histology and serum ALT) in both models was prevented in the DOK4 knockdown mice (Fig. 5E and F).
Role of intramitochondrial SHP-1 in JNK induced P-Src inactivation
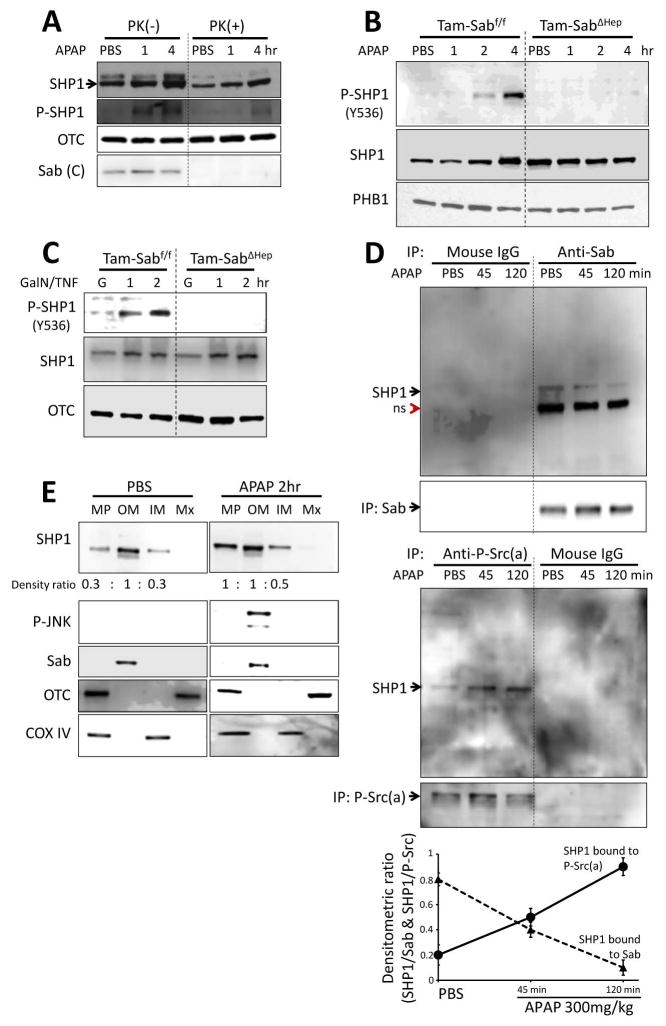
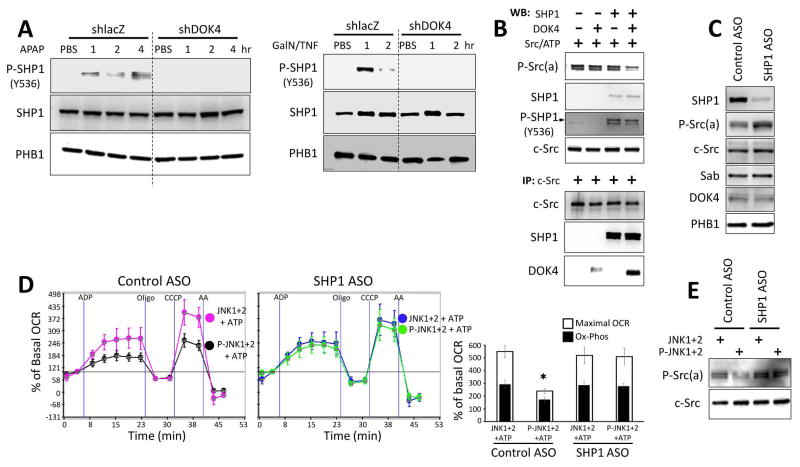
DOK family proteins are known to bind SHP1 or 2.38,39 We found that SHP1 (PTPN6) but not SHP2 (PTPN11) was present in liver mitochondria (Supporting Fig. 6B and E, and Fig. 6A). Under basal conditions, trace detectable activated SHP1 was found in mitochondria; however, after APAP (Fig. 6A and B) or TNF/GalN (Fig. 6C) in vivo, activated P-Y536SHP1 progressively increased over time in mitochondria from Tam-Sabf/f mice (Fig. 6A); this did not occur in Tam-SabΔHep mice (Fig. 6B and C). SHP1 was found to be associated with Sab after IP of Sab (Fig. 6D) and with outer membrane under basal conditions (Fig. 6E). After APAP treatment, IP of Sab revealed that the association of SHP1 with Sab decreased; in contrast, after APAP, IP of P-Src(a) revealed increased association with SHP1 (Fig. 6D). Since P-Src was associated with the inner membrane, the results in figure 6D and E indicate that SHP1 transferred from Sab on the intermembrane face of the outer membrane to the intermembrane face of the inner membrane where P-Y419Src and DOK4 are located. In addition knockdown of DOK4 prevented SHP1 activation in the APAP and TNF/GalN models (Fig. 7A). Knockout of Sab or knockdown of DOK4 did not significantly change basal SHP1 levels in mitochondria (Fig. 6B and C, and Fig. 7A). As noted above, in the absence of inner membrane DOK4, P-Y419 Src was not dephosphorylated suggesting that DOK4 provides a platform for the interaction of SHP1 and P-Src.
Fig. 6. SHP1 activation mediates mitochondrial Src inactivation in liver injury models in vivo.
(A) Mitochondria isolated from PBS or APAP injected mice were treated with proteinase K (PK) to determine intramitochondrial localization of SHP1. Mitochondrial SHP1 is inside of mitochondrial outer membrane and resistant to PK digestion. Activated P-SHP1 after APAP also resisted PK. PK digestion of C-terminus of Sab is shown as positive control. (B, C) Mitochondrial SHP1 was phosphorylated (activated) at Y536 in APAP or D-Gal/TNF treated TAM-Sabff mouse liver but not in APAP or D-Gal/TNF treated TAM-SabΔHep mouse liver. (D) SHP1 co-immunoprecipitated with Sab and with P-Y419Src. Association of SHP1 with Sab decreased significantly and association with P-Src increased after APAP treatment as summarized in the plot shown below the blots (n=3); p <0.05, all data points at 45 and 120 minutes versus PBS control. (E) SHP1 is predominantly localized at outer membrane but relocated to inner membrane after APAP treatment. The densitometric ratio of MP/OM and IM/OM is shown below the SHP1 blots and is representative of 2 experiments.
Fig. 7. Effect of JNK on mitochondria after knockdown of DOK4 or SHP1.
(A) SHP1 activation by APAP or D-Gal/TNF treatment was prevented in DOK4 knockdown mouse liver. (B) In a cell free system with recombinant proteins dephosphorylation of P-Src (Y419) required association with SHP1, DOK4 and P-Src (Y419). (C) SHP1 ASO effectively depleted mitochondrial SHP1 and increased mitochondrial P-Src(a). (D, E) SHP1 knockdown prevented P-JNK+ATP mediated inhibition of isolated mitochondria respiration and dephosphorylation of Src. (*) represents p <0.05, Control ASO vs SHP1 ASO treated with P-JNK+ATP. Error bars represent the S.D of triplicate or pentaplicate samples in one representative experiment and bar graphs compare maximum respiration and oxidative phosphorylation (OCR after ADP minus oligomycin) for three experiments.
To confirm these observations, we used a cell free model system (see methods) with recombinant c-Src, SHP1 and DOK4 (Fig. 7B). We incubated recombinant Src and ATP and as expected c-Src underwent self-activation to P-Y419Src. Activated Src then activated SHP1. However, dephosphorylation of P-Src by activated recombinant SHP1 occurred in the presence of recombinant DOK4 but not in its absence. IP of c-Src from these incubations revealed association of Src with DOK4 and SHP1. Thus, P-Src activated SHP1 and the efficient phosphatase mediated dephosphorylation of P-Src was facilitated by the platform function of DOK4.
Finally, we knocked down SHP1 with ASO treatment in vivo and documented a marked decrease in mitochondrial SHP1 without an effect on expression of Sab, c-Src, or DOK4 (Fig. 7C). Interestingly, the basal level of P-Src (a) increased after SHP1 knockdown but total c-Src levels did not change. This suggests that the low level of basal active SHP1 in mitochondria modulates or dampens P-Src(a) level. Exposure of the liver mitochondria from scrambled control ASO treated mice to P-JNK and ATP impaired OCR but the mitochondria from ASO-induced depletion of SHP1 were resistant to the inhibition of respiration induced by P-JNK and ATP (Fig. 7D) as well as the dephosphorylation of Src (Fig. 7E).
Discussion
In the current study for the first time we have demonstrated a direct, unequivocal effect of pure P-JNK on mitochondrial respiration. This effect depends on the ATP substrate of the kinase. Either activated JNK1, 2, or both exert this effect to rapidly impair state 3 respiration and respiratory capacity. This direct effect of P-JNK plus ATP is Sab dependent as it was abrogated in liver mitochondria after Sab knockdown or knockout or by the addition of the Sab blocking peptide.
We have previously shown that P-JNK directly increases mitochondrial ROS production in isolated mitochondria which is also prevented by the KIM1 Sab peptide.9 The present studies indicate that the presumed phosphorylation of Sab by JNK leads to a block in electron transport, promoting oxidative stress. The site(s) of the block is currently uncertain but likely beyond complex I and II as impaired respiration is seen with either complex I or II substrates. An important question is what mediates this effect within mitochondria. To begin to answer this question, we considered factors which regulate electron transport. One factor of recent interest is tyrosine phosphorylation of various intermediates of the electron transport chain, mediated by Src kinases.30–32 For example, the tyrosine phosphorylation of cytochrome oxidase regulates its activity.31,33,34 In osteoclasts, Hela and neuronal cells and cardiac ischemia model, c-Src is present in the mitochondria and its inhibition directly inhibits mitochondrial electron transport and promotes ROS production with pathological consequences.30–32 We confirmed that a similar effect occurs in liver mitochondria treated with Src inhibitors and the effect was similar to that of P-JNK in inhibiting respiratory activity, suggesting for the first time a link between JNK and Sab with intramitochondrial Src.
We then determined if Src is present in liver mitochondria and found that it resides both on the outer membrane and inside mitochondria, the latter in the intermembrane space associated with the inner membrane and not in the matrix. Interestingly, the intramitochondrial P-Src was mainly in the active phosphorylated form (P-Y419). Following exposure of mitochondria to P-JNK and ATP, rapid dephosphorylation of Src occurred which was accompanied by impaired respiration, and the tyrosine phosphatase inhibitor, Na3VO4 blocked the dephosphorylation of active Src and the inhibitory effect of P-JNK on respiration.
We further confirmed that in two models of liver injury, as we previously reported,10 knockdown of Sab and, as we describe for the first time, hepatocyte specific inducible knockout of Sab, prevented sustained JNK activation and liver injury. In the APAP model, necrotic cell death predominates, whereas in the TNF/GalN model, apoptotic cell death predominates. In both models, floxed littermate controls exhibit rapid dephosphorylation of the active phosphorylated form of Src in mitochondria indicating that, as we observed in direct studies in isolated mitochondria, P-JNK interaction with Sab leads to rapid dephosphorylation of Src and impaired mitochondrial function which preceded liver injury. Knockdown or knockout of Sab abrogated the inactivation of mitochondrial Src in vivo. Thus, the parallel between our in vitro and in vivo data support our conclusion that the effect of JNK on Sab leads to inactivation of Src which mediates impaired respiration, increased ROS, sustained JNK activation and, cell death.
It is noteworthy that the above mechanism leads to a self-sustaining cycle8–10,28 which then accounts for sustained JNK activation, presumably by continuous exposure to ROS and activation of upstream MAP3K such as ASK1 and MAP2K such as MKK4.13–17 Sustained JNK activation amplifies ROS production after APAP treatment which is believed to contribute to MPT mediated necrotic cell death.9,10,40–44 In the case of TNF/GalN-induced apoptosis sustained JNK activation also depends on the mitochondrial Sab mediated cycle but the apoptotic outcome is known to be mediated by the effects of JNK on the pro-apoptotic Bcl family members (eg. Bax)45,46 and anti-apoptotic members (eg. Bcl-XL and Mcl-1)47,48 which leads to mitochondrial outer membrane permeabilization, cytochrome c release, and caspase activation.5 Caspase activation does not occur in the APAP model as it is inhibited by GSH depletion and ROS.42
We also explored the role of DOK4, a platform protein, because it has been previously found to bind Src in mitochondria of endothelial cells.36 We confirmed that DOK4 is present in liver mitochondria and found that it localizes to the inner membrane. The knockdown of DOK4 resulted in a strikingly similar outcome as knockdown or knockout of Sab, namely abrogation of dephosphorylation of mitochondrial Src, resistance to the effects on P-JNK plus ATP on OCR in isolated mitochondria, prevention of sustained JNK activation in vivo, and marked protection against liver injury from APAP or TNF/GalN. Remarkably, two proteins found exclusively in mitochondria, Sab on the outer membrane and DOK4 on the inner membrane, are linked to inactivation of intramitochondrial Src.
An important issue is how does Sab on the outer membrane exert this effect on Src in a DOK4 dependent fashion on the inner membrane. We could find no evidence that Src directly binds to Sab. As noted above, Src was associated with the inner membrane. On the other hand, as expected, we found that vanadate-inhibitable tyrosine phosphatase activity mediates the inactivation of Src. The interplay between tyrosine kinase and phosphatase is favored by their proximity on platform proteins, eg. DOK4. Using a cell free system, we demonstrated that the dephosphorylation of P-Src(a) was facilitated in the presence of DOK4. Thus, we examined the possibility that the intermembrane domain of Sab sequesters a phosphatase which is released after JNK binding and phosphorylation of Sab. DOK family proteins are known to bind SHP1 or 2.38,39 We identified SHP1 but not SHP2 in liver mitochondria, associated with the outer membrane. Indeed, SHP1 co-immunoprecipitated with Sab in mitochondrial extracts under basal conditions. SHP1 associated with Sab was not in phospho active form. However, after APAP treatment in vivo, the SHP1 associated with Sab was decreased, suggesting its release from Sab. Under these conditions (APAP treatment) SHP1 became activated after redistributing to the inner membrane where it was immunoprecipitated with inner membrane associated P-Src(a). As reported by others, we demonstrated that P-Src(a) activates SHP149 but P-SHP1 inactivates P-Src only if DOK4 is present. Both P-Src(a) and DOK4 were at least partially degraded by proteinase K after permeabilization of the outer membrane indicating that they are accessible to the intermembrane space. Our data suggest that DOK4 facilitates the interaction of SHP1 and P-Src on the inner membrane, leading to dephosphorylation of P-Src. Inactive SHP1 appears to be sequestered by Sab and, following release from Sab, is activated by P-Src on inner membrane and then P-SHP1-mediated dephosphorylation of Src is facilitated by the DOK4 platform. The precise mechanism for the requirement of DOK4 for activated SHP1 to dephosphorylate Src will require more study but likely is related to stabilizing the complex to permit the phospho-site on Src to be accessible to SHP1 active site. Furthermore, in addition to inactivating Src, it is theoretically possible that SHP1 might also dephosphorylate respiratory chain phosphotyrosines after transfer to the mitochondrial innermembrane.49 Regardless of this uncertainty, our findings clearly show that sustained JNK activation in the cytoplasm is mediated by a self-sustaining loop in which the interaction and presumed phosphorylation of Sab by P-JNK leads to activation of a phospho-tyrosine phosphatase dependent dephosphorylation of intramitochondrial Src, the consequence of which is impaired electron transport and increased ROS release. ROS then continues to activate upstream MAPKinases. Sustained JNK activation then amplifies mitochondrial ROS in the development of APAP necrosis and mediates Bcl family induced apoptosis in the TNF/GalN model (Fig. 8).
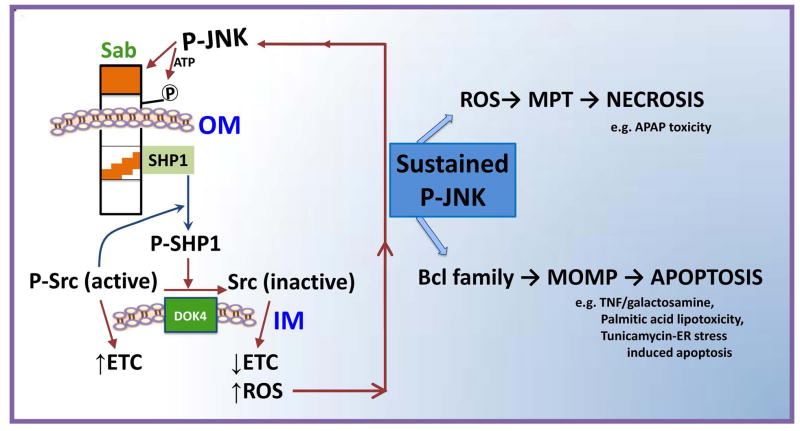
Fig. 8. Model of the pathway for the interplay of JNK and mitochondria in cell death.
JNK is initially activated by the MAPK cascade either extrinsically by receptor signaling or intrinsically by organelle stress emanating from mitochondria, ER, or nucleus. Activated JNK translocates to the mitochondria where it binds and phosphorylates Sab on the cytoplasmic side of the outer membrane (OM). This leads to release of inactive SHP1 sequestered by Sab which then is activated by P-Src and mediates inactivation of P-Y419Src, facilitated by the inner membrane (IM) DOK4 platform. Active Src maintains electron transport whereas inactivation leads to impaired electron transport which promotes increased ROS release. The ROS release continues to activate upstream MAPK leading to JNK activation in a self-sustaining loop which accounts for JNK activation being sustained. In APAP toxicity, the amplified mitochondrial ROS from damaged mitochondria due to this loop promotes mitochondrial permeability pore (MPT) opening and necrosis, whereas in TNF-induced apoptosis the sustained JNK activation is known to lead to enhanced activity of pro-apoptotic Bcl proteins and impairment of anti-apoptotic Bcl proteins; the result is mitochondrial outer membrane permeablization (MOMP) which permits release of cytochrome c and other mitochondrial proteins, followed by caspase activation, and apoptosis. The key feature is that the Sab-dependent effect of P-JNK on mitochondria via an intramitochondrial signaling pathway is the mechanism for sustained activation of P-JNK in the cytoplasm which is necessary for cell death.
An interesting point in our studies is that there is a graded response to different levels of exposure of mitochondria to P-JNK with respect to P-Src inactivation and impairment of electron transport. Our in vivo studies employed two models of severe acute liver failure in which high levels of JNK activation and marked dephosphorylation of Src are observed. In contrast sublethal dosing with APAP lead to lower levels of sustained JNK activation corresponding to less Src inactivation. A dose effect of P-JNK was also observed in isolated mitochondria. Thus, we hypothesize that lesser degrees of impairment of mitochondrial function may establish a steady state in which sustained JNK has nonlethal physiological or pathophysiological consequences, such as effects on insulin signaling, lipid metabolism, inflammatory signaling and even protective adaptations. Additional work is required to clarify this intriguing hypothesis which may account for the complex, sometimes contradictory reported roles of JNK.
Supplementary Material
Acknowledgments
Financial Support
This research was supported by R01DK067215 (N.K.) and the USC Research Center for Liver Disease’s Cell Culture, Cell and Tissue Imaging, Histology and Metabolic/Analytical/Instrumentation Cores (P30DK048522) (N.K).
Nonstandard Abbreviations
- APAP
acetaminophen
- CCCP
Carbonyl cyanide 3-chlorophenyl-hydrazone
- Csk
C-terminal Src kinase
- DOK4
Docking Protein 4
- Fyn
FYN Proto-Oncogene Src Family Tyrosine Kinase
- GalN
galactosamine
- JNK
c-Jun N-terminal kinases
- JNK1
mitogen-activated protein kinase 8 (MAPK8)
- JNK2
mitogen-activated protein kinase 9 (MAPK9)
- KIM1
kinase interacting motif-1
- Lyn
LYN Proto-Oncogene Src Family Tyrosine Kinase
- MAPK
Mitogen-activated protein kinases
- NFkB
nuclear factor kappa-light-chain-enhancer of activated B cells
- OTC
Ornithine Carbamoyltransferase
- PHB1
prohibitin
- PTPase
protein tyrosine phosphatase
- PK
Proteinase K
- Sab (SH3BP5)
SH3 homology associated BTK binding protein
- SHP1 (PTPN6)
Protein tyrosine phosphatase, non-receptor type 6
- SHP2 (PTPN11)
Tyrosine-protein phosphatase non-receptor type 11
- c-Src
SRC proto-oncogene (non-receptor tyrosine kinases)
- Tam-SabΔHep
liver-specific promoter tamoxifen-inducible chimeric Cre Sab Knockout
- TNFR
tumor necrosis factor receptor
- VDAC
Voltage-dependent anion channel
Footnotes
Conflict of interest statement: S.W, T.A.T, R.W.M.M and N.K have declared that no conflict of interest exists. M.A. is an employee and shareholder of Ionis Pharmaceuticals.
Author Contributions
Sanda Win and Tin Aung Than designed and executed all experiments and participated in writing the manuscript. Robert Win Maw Min assisted with experiments involving immunohistochemistry and western blotting. Mariam Aghajan participated in synthesis and application of ASO. Neil Kaplowitz provided oversight and design of all experiments and writing of the manuscript.
References
- 1.Seki E, Brenner DA, Karin M. A Liver Full of JNK: Signaling in Regulation of Cell Function and Disease Pathogenesis, and Clinical Approaches. Gastroenterology. 2012;143:307–320. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han D, Dara L, Win S, Than TA, Yuan L, Abbasi SQ, et al. Regulation of drug-induced liver injury by signal transduction pathways: critical role of mitochondria. Trends Pharmacol Sci. 2013;34:243–253. doi: 10.1016/j.tips.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ibrahim SH, Gores GJ. Who pulls the trigger: JNK activation in liver lipotoxicity? J Hepatol. 2012;56:17–19. doi: 10.1016/j.jhep.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czaja MJ. JNK regulation of hepatic manifestations of the metabolic syndrome. Trends Endocrinol Metab. 2010;21:707–713. doi: 10.1016/j.tem.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplowitz N, Than TA, Shinohara M, Ji C. Endoplasmic reticulum stress and liver injury. Semin Liver Dis. 2007;27:367–377. doi: 10.1055/s-2007-991513. [DOI] [PubMed] [Google Scholar]
- 7.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 8.Win S, Than TA, Le BH, García-Ruiz C, Fernandez-Checa JC, Kaplowitz N. Sab (Sh3bp5) dependence of JNK mediated inhibition of mitochondrial respiration in palmitic acid induced hepatocyte lipotoxicity. J Hepatol. 2015;62:1367–1374. doi: 10.1016/j.jhep.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Win S, Than TA, Fernandez-Checa JC, Kaplowitz N. JNK interaction with Sab mediates ER stress induced inhibition of mitochondrial respiration and cell death. Cell Death Dis. 2014;5:e989. doi: 10.1038/cddis.2013.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Win S, Than TA, Han D, Petrovic LM, Kaplowitz N. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 2011;286:35071–35078. doi: 10.1074/jbc.M111.276089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283(20):13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 13.Xie Y, Ramachandran A, Breckenridge DG, Liles JT, Lebofsky M, Farhood A, et al. Inhibitor of apoptosis signal-regulating kinase 1 protects against acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 2015;286:1–9. doi: 10.1016/j.taap.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma M, Gadang V, Jaeschke A. Critical role for mixed-lineage kinase 3 in acetaminophen-induced hepatotoxicity. Mol Pharmacol. 2012;82(5):1001–1007. doi: 10.1124/mol.112.079863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holzer RG, Park EJ, Li N, Tran H, Chen M, Choi C, et al. Saturated fatty acids induce c-Src clustering within membrane subdomains leading to JNK activation. Cell. 2011;147:173–184. doi: 10.1016/j.cell.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, et al. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Nishitoh H, Saitoh M, Mochida Y, Takeda K, Nakano H, Rothe M, et al. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol Cell. 1998;2:389–395. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- 18.Chambers JW, Howard S, LoGrasso PV. Blocking c-Jun N-terminal kinase (JNK) translocation to the mitochondria prevents 6-hydroxydopamine-induced toxicity in vitro and in vivo. J Biol Chem. 2013;288:1079–1087. doi: 10.1074/jbc.M112.421354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambers JW, Pachori A, Howard S, Iqbal S, LoGrasso PV. Inhibition of JNK mitochondrial localization and signaling is protective against ischemia/reperfusion injury in rats. J Biol Chem. 2013;288:4000–4011. doi: 10.1074/jbc.M112.406777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metzger D, Clifford J, Chiba H, Chambon P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc Natl Acad Sci U S A. 1995;92:6991–6995. doi: 10.1073/pnas.92.15.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P. Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci U S A. 1996;93:10887–10890. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonzo JA, Ferry CH, Matsubara T, Kim JH, Gonzalez FJ. Suppression of hepatocyte proliferation by hepatocyte nuclear factor 4α in adult mice. J Biol Chem. 2012;287:7345–7356. doi: 10.1074/jbc.M111.334599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers GW, Brand MD, Petrosyan S, Ashok D, Elorza AA, Ferrick DA, et al. High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLoS One. 2011;6:e21746. doi: 10.1371/journal.pone.0021746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Than TA, Win S, Kaplowitz N. In vitro assays of mitochondrial function/dysfunction. Clin Pharmacol Ther. 2014;96:665–668. doi: 10.1038/clpt.2014.178. [DOI] [PubMed] [Google Scholar]
- 25.Somani AK, Bignon JS, Mills GB, Siminovitch KA, Branch DR. Src kinase activity is regulated by the SHP-1 protein-tyrosine phosphatase. J Biol Chem. 1997;272:21113–21119. doi: 10.1074/jbc.272.34.21113. [DOI] [PubMed] [Google Scholar]
- 26.Wiltshire C, Matsushita M, Tsukada S, Gillespie DA, May GH. A new c-Jun N-terminal kinase (JNK)-interacting protein, Sab (SH3BP5), associates with mitochondria. Biochem J. 2002;367:577–585. doi: 10.1042/BJ20020553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Court NW, Kuo I, Quigley O, Bogoyevitch MA. Phosphorylation of the mitochondrial protein Sab by stress-activated protein kinase 3. Biochem Biophys Res Commun. 2004;319:130–137. doi: 10.1016/j.bbrc.2004.04.148. [DOI] [PubMed] [Google Scholar]
- 28.Chambers JW, LoGrasso PV. Mitochondrial c-Jun N-terminal kinase (JNK) signaling initiates physiological changes resulting in amplification of reactive oxygen species generation. J Biol Chem. 2011;286:16052–16062. doi: 10.1074/jbc.M111.223602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chambers JW, Cherry L, Laughlin JD, Figuera-Losada M, Lograsso PV. Selective inhibition of mitochondrial JNK signaling achieved using peptide mimicry of the Sab kinase interacting motif-1 (KIM1) ACS Chem Biol. 2011;6:808–818. doi: 10.1021/cb200062a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogura M, Yamaki J, Homma MK, Homma Y. Mitochondrial c-Src regulates cell survival through phosphorylation of respiratory chain components. Biochem J. 2012;447:281–289. doi: 10.1042/BJ20120509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyazaki T, Neff L, Tanaka S, Horne WC, Baron R. Regulation of cytochrome c oxidase activity by c-Src in osteoclasts. J Cell Biol. 2003;160:709–718. doi: 10.1083/jcb.200209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salvi M, Brunati AM, Bordin L, La Rocca N, Clari G, Toninello A. Characterization and location of Src-dependent tyrosine phosphorylation in rat brain mitochondria. Biochim Biophys Acta. 2002;1589:181–195. doi: 10.1016/s0167-4889(02)00174-x. [DOI] [PubMed] [Google Scholar]
- 33.Tibaldi E, Brunati AM, Massimino ML, Stringaro A, Colone M, Agostinelli E, et al. Src-Tyrosine kinases are major agents in mitochondrial tyrosine phosphorylation. J Cell Biochem. 2008;104:840–849. doi: 10.1002/jcb.21670. [DOI] [PubMed] [Google Scholar]
- 34.Foster DB, Van Eyk JE, Marbán E, O’Rourke B. Redox signaling and protein phosphorylation in mitochondria: progress and prospects. J Bioenerg Biomembr. 2009;41:159–168. doi: 10.1007/s10863-009-9217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai D, Dhe-Paganon S, Melendez PA, Lee J, Shoelson SE. Two new substrates in insulin signaling, IRS5/DOK4 and IRS6/DOK5. J Biol Chem. 2003;278:25323–25330. doi: 10.1074/jbc.M212430200. [DOI] [PubMed] [Google Scholar]
- 36.Itoh S, Lemay S, Osawa M, Che W, Duan Y, Tompkins A, et al. Mitochondrial Dok-4 recruits Src kinase and regulates NF-kappaB activation in endothelial cells. J Biol Chem. 2005;280:26383–26396. doi: 10.1074/jbc.M410262200. [DOI] [PubMed] [Google Scholar]
- 37.Draper JM, Xia Z, Smith CD. Cellular palmitoylation and trafficking of lipidated peptides. J Lipid Res. 2007;48:1873–1884. doi: 10.1194/jlr.M700179-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berg KL, Siminovitch KA, Stanley ER. SHP-1 regulation of p62(DOK) tyrosine phosphorylation in macrophages. J Biol Chem. 1999;274:35855–35865. doi: 10.1074/jbc.274.50.35855. [DOI] [PubMed] [Google Scholar]
- 39.Celis-Gutierrez J, Boyron M, Walzer T, Pandolfi PP, Jonji S, Olive D, et al. Dok1 and Dok2 proteins regulate natural killer cell development and function. EMBO J. 2014;33:1928–1940. doi: 10.15252/embj.201387404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;246(1–2):8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 42.Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Inhibition of Fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol Appl Pharmacol. 1999;156:179–186. doi: 10.1006/taap.1999.8635. [DOI] [PubMed] [Google Scholar]
- 43.Kim JS, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun. 2003;304:463–470. doi: 10.1016/s0006-291x(03)00618-1. [DOI] [PubMed] [Google Scholar]
- 44.Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 2011;45:156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- 46.Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto Y, et al. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J. 2004;23:1889–1899. doi: 10.1038/sj.emboj.7600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basu A, Haldar S. Identification of a novel Bcl-xL phosphorylation site regulating the sensitivity of taxol- or 2-methoxyestradiol-induced apoptosis. FEBS Lett. 2003;538:41–47. doi: 10.1016/s0014-5793(03)00131-5. [DOI] [PubMed] [Google Scholar]
- 48.Morel C, Carlson SM, White FM, Davis RJ. Mcl-1 integrates the opposing actions of signaling pathways that mediate survival and apoptosis. Mol Cell Biol. 2009;29:3845–3852. doi: 10.1128/MCB.00279-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frank C, Burkhardt C, Imhof D, Ringel J, Zschörnig O, Wieligmann K, et al. Effective dephosphorylation of Src substrates by SHP-1. J Biol Chem. 2004;279:11375–11383. doi: 10.1074/jbc.M309096200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.