Abstract
Diundecyl phthalate (DUP) is a high production volume chemical used as a plasticizer in polyvinyl chloride and other plastics. Specific biomarkers of DUP would be useful for human exposure assessment. To identify such biomarkers, we investigated the in vitro metabolism of DUP with human liver microsomes using online solid phase extraction coupled to HPLC-mass spectrometry. Using high resolution mass spectrometry, we conclusively confirmed the structures of four DUP specific metabolites: mono-undecyl phthalate (MUP), mono-hydroxyundecyl phthalate (MHUP), mono-oxoundecyl phthalate (MOUP), and mono-carboxydecyl phthalate (MCDP). We also used high resolution mass spectrometry to isolate MCDP and MHUP from co-eluting isobaric metabolites of diisononyl phthalate (i.e., mono-carboxyisononyl phthalate) and diisododecyl phthalate (i.e., monohydroxyisododecyl phthalate), respectively, that could not be separated with low resolution tandem mass spectrometry. To evaluate the potential usefulness of the newly identified DUP metabolites as exposure biomarkers, we analyzed 36 human urine samples by high resolution mass spectrometry. We detected MHUP and MCDP in > 83% of the samples; median concentrations were 0.21 ng/mL and 0.36 ng/mL, respectively. MOUP was detected only in 14% of the samples analyzed, and MUP was not detected. All three metabolites eluted as peak clusters likely because of the presence of multiple oxidation sites and multiple isomers in DUP technical mixtures. Taken together, these findings suggest that with the appropriate mass spectrometry quantification techniques, MHUP and MCDP may serve as suitable biomarkers for assessing background exposure to DUP.
Keywords: Biomonitoring, In vitro, Mass spectrometry, Oxidative metabolites, DUP, diundecyl phthalate
1. Introduction
Diundecyl phthalate (DUP, Palatinol®111P-1, Jayflex™ L11P-E), a high molecular weight phthalate comprised of a pair of 11-carbon esters linked to a benzene-1,2-dicarboxylic moiety, is manufactured as a mixture of primarily branched chain isomers. DUP is a high production volume chemical (OECD, 2015) mainly used as a polyvinyl chloride (PVC) plasticizer in applications requiring low fog and low temperature flexibility such as wiring, cable jacketing and insulation, furniture and automobile upholstery, and flooring and wall covering (NICNAS, 2008; US Consumer Product Safety Commission, 2010). DUP can also be used in non-PVC polymers; in flame retardant nylon, rubbers, paints, and adhesives (NICNAS, 2008; US Consumer Product Safety Commission, 2010). US production of DUP and its proportion in the total phthalate production market have been slowly increasing since the implementation of chemical tracking in 1982 (US Consumer Product Safety Commission, 2010).
Toxicity data for DUP are relatively limited (Barber et al., 2000; David et al., 2001; Kwack et al., 2009, 2010; Saillenfait et al., 2013). Exposure to C7-11 phthalate monoester mixture did not show mutagenic potential in the L5178Y mouse lymphoma mammalian cell mutation assay, or genotoxicity in the Balb/3T3 cell transformation assay (Barber et al., 2000). In another in vitro study, cell-to-cell communication was inhibited by the C7-C11 phthalate monoester mixture with rat and mouse hepatocytes, but not with hamster, cynomolgus monkey, or human hepatocytes or in a human liver cell line; the same C7-11 monoester mixture did not markedly change peroxisomal beta-oxidation in hepatocytes from any species (Kamendulis et al., 2002). Exposure to DUP (500 mg/kg body weight/day) for 4 weeks in Sprague-Dawley male rats decreased sperm count and sperm mobility, although it did not significantly affect liver or testis weights (Kwack et al., 2009). In another study, male Sprague-Dawley rats administered 500 mg/kg body weight/day of DUP for two weeks showed significant increases in aspartate aminotransferase and alkaline phosphatase (Kwack et al., 2010). In Sprague-Dawley rats dosed with 0.5 and 1 g DUP/kg body weight/day by gavage on gestation days 6–20, maternal body weight and food consumption were not affected, but, compared to controls, treated fetuses showed small decreases in anogenital distance (males) as well as a higher incidence of supernumerary lumbar ribs (Saillenfait et al., 2013). Although health effects of DUP in humans are largely unknown, identifying biomarkers of DUP would facilitate human exposure assessment, particularly because exposures to phthalates and phthalate alternatives may be changing as a result, at least in part, of legislative activity, industrial practices, public concerns and consumers' demands (Goen et al., 2011; Health Canada 2013; Schutze et al., 2014; Silva et al., 2013; Wittassek et al., 2007; Zota et al., 2014).
In vitro metabolism has been used to identify potential bio-markers of exposure to select environmental chemicals (Choi et al., 2013; Roberts et al., 2012; Silva et al., 2015). For the current study, we used high resolution mass spectrometry to conclusively identify potential DUP specific exposure biomarkers after in vitro metabolism of DUP using human liver microsomes. We also quantified the urinary concentrations of these DUP metabolites in 36 samples collected in 2013–2015 from a convenience sample of adults with no known occupational exposure to DUP.
2. Materials and methods
2.1. Reagents and standards
DUP ( > 99%, CAS # 3648-20-2) and acetic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). 4-methyl-7-oxo-octyl phthalate (MONP), 4-methyl-7-hydroxyoctyl phthalate (MHNP), 4-methyl-carboxyheptyl phthalate (MCOP), d4-MONP, d4-MHNP and 13C6-MCOP were purchased from ADM (Teltow, Germany). β-glucuronidase (Escherichia coli-K12) was purchased from Roche Biomedical (Mannheim, Germany). Acetonitrile (HPLC grade) was purchased from Burdick and Jackson (VWR, Radnor, PA). All reagents, solvents and standard materials were used without further purification.
2.2. Urine collection
We used 36 spot urine specimens collected anonymously from U.S. adult volunteers in Atlanta, GA in 2013–2015, and stored at −70 °C until analysis. The volunteers had no documented occupational exposure to DUP. CDC's Subjects Institutional Review Board reviewed and approved the collection of the samples. A waiver of informed consent was requested under 45 CFR 46.116(d); no personal or demographic data were available.
We also used archived urine, stored at −70 °C until analysis, that had been collected from rats dosed with diisodecyl phthalate (DiDP) (500 mg/kg body weight) as presented elsewhere (Kato et al., 2007).
2.3. In vitro metabolism of DUP
We followed a procedure similar to the approach described previously (Choi et al., 2013; Roberts et al., 2012; Silva et al., 2015). In a 30 mL Qorpak™ Clear Wide Mouth French square bottle (Fisher Scientific, Pittsburg, PA, USA), DUP standard solution (1 mL, 1.3 mg/mL) was concentrated to approximately 0.5 mL under a stream of nitrogen. Then, we added pH 7.4 phosphate buffer (0.1 M, 8 mL), water (1 mL), NADPH solution (A) (500 μL, BD Gentest™, Woburn, MA, USA), NADPH solution (B) (100 μL, BD Gentest™) to generate NADPH in situ using an enzymatic reaction, and female pooled human liver microsomes (200 μL, BD Gentest™). The bottle was capped and the contents were gently mixed and placed in an incubator (Fisher Scientific, Hampton, NH, USA) at 37 °C for 8 h. Because of the limited water solubility of DUP and to produce in vitro metabolites in sufficient amounts for mass spectrometry identification, after 8 h, the solution was replenished with 500 μL NADPH solution (A), 100 μL NADPH solution (B), and 200 μL female human liver microsomes, gently mixed, and in-cubated for another 17 h. Aliquots of microsomal suspension (1 mL) were transferred into microcentrifuge tubes, vortex mixed, and centrifuged at 12,500 rpm for 20 min on an Avanti high performance centrifuge (Beckman Coulter Inc, Brea, CA, USA). The supernatant was transferred into autosampler vials for analysis (Silva et al., 2015). The above procedure was repeated without DUP, but with water (500 μL) for the preparation of the control samples.
2.4. Identification of in vivo and in vitro DUP metabolites
The HPLC gradient for separation of DUP metabolites and the on-line solid phase extraction (SPE) procedure were adapted from methods published elsewhere (Silva et al., 2007). Briefly, in vitro metabolites in the supernatant of the human liver microsomal homogenate (500 μL) obtained after incubating with DUP were extracted using on-line SPE on a Chromolith RP-18 pre-column (Merck KGaA, Darmstadt, Germany) and resolved on a Betasil phenyl HPLC column (3 μM, 2.1 mm × 25 mm, ThermoFisher Scientific, San Jose, CA, USA) using a water/acetonitrile gradient. The mass spectral analysis was initially performed on a TSQ Vantage AM triple quadrupole mass spectrometer (ThermoFisher Scientific, San Jose, CA, USA). All ions on Q1 were scanned from m/z = 150 to m/z = 480 in electrospray ionization (ESI)-negative ion mode. ESI Q1 full scan produced multiple peaks at m/z of 319, 333, 335 and 349. For in vivo metabolite identification, human and rat urine (1 mL) were treated with β-glucuronidase before separation and detection of the DUP biomarkers (Silva et al., 2007). The identity of the DUP metabolites was confirmed by high resolution mass spectrometry using parallel reaction monitoring (PRM) on a QEx-active Plus mass spectrometer (ThermoFisher Scientific, San Jose, CA, USA) in negative mode to obtain accurate m/z of precursors without applying collision energy [CE] and products of the DUP metabolites after applying CE (Table 1).
Table 1.
QExactive high resolution mass spectrometric parameters for measuring the metabolites of diundecyl phthalate.
| DUP metabolite |
m/z
|
NCE* | |
|---|---|---|---|
| Precursor | Product | ||
| Mono-oxoundecyl phthalate (MOUP) | 333.1708 | 121.030 | 29 |
| Mono-hydroxyundecyl phthalate (MHUP) | 335.1864 | 187.170 | 25 |
| Mono-carboxydecyl phthalate (MCDP) | 349.1657 | 201.150 | 12 |
NCE–normalized collision energy

The structure shown is for one isomer only.
2.5. Quantification of DUP metabolites in human urine
After vortex mixing and aliquoting, human urine (100 μL) was spiked with the internal standard solution containing d4-MHNP, d4-MONP, and 13C6-MCOP. The target metabolites were extracted after hydrolysis with β–glucuronidase, chromatographically resolved by HPLC, and detected by high resolution mass spectrometry in PRM mode after applying CE (Table 1). The mobile phases were 0.1% acetic acid in water and 0.1% acetic acid in acetonitrile. The DUP metabolites were quantified using the calibration curves derived from analogous metabolites of di-isononyl phthalate (DiNP), namely MHNP, MONP, and MCOP. The limits of detection (LOD) of all analytes were set at the lowest standard concentration (0.01 ng/mL).
3. Results and discussion
The metabolism of high molecular weight phthalates, such as DUP, generally involves the formation of hydrolytic monoesters, followed by oxidation (Phase I reaction) and/or conjugation (Phase II reaction) before urinary excretion. These metabolites, in turn, can serve as biomarkers of exposure to the parent phthalate (Silva et al., 2013). We undertook the current study to identify and characterize in vitro and in vivo exposure biomarkers of DUP.
Full scan mass spectra in negative ion mode from m/z = 150 to m/z = 480 of the supernatant of the human liver microsomes incubated with DUP revealed four unique peaks with m/z = 319, 333, 335 and 349. We did not observe these peaks in the microsomal suspension when incubated without DUP suggesting that the m/z = 319, 333, 335 and 349 peaks corresponded to in vitro metabolites of DUP. First, we analyzed fragmentation patterns through product ion scans and tentatively identified the m/z specific for three oxidative (m/z = 333, 335, 349) and one hydrolytic (m/z = 319) DUP metabolites. Then, we determined the accurate masses of these metabolites and their mass fragments by high resolution mass spectrometry and conclusively identified the metabolites as monoundecyl phthalate (MUP, m/z = 319.1915, Fig. 1S), mono-oxoundecyl phthalate (MOUP, m/z = 333.17075, Fig. 1), mono-hydroxyundecyl phthalate (MHUP, m/z = 335.18640, Fig. 2), and mono-carboxydecyl phthalate (MCDP, m/z = 349.16566, Fig. 3). Interestingly, for the in vitro study we used reagent grade DUP, and MOUP and MHUP eluted as peak clusters most likely because oxidation occurred at different carbons on the C11 side chain. By contrast, because MCDP can only result from ω-oxidation in the side chain, the fact that MCDP eluted as three distinct peaks suggests that the DUP used for the in vitro study also contained branched isomers (Fig. 4). Similarly, we observed three closely eluting MUP peaks (Fig. 4).
Fig. 1.
High resolution mass spectrometric fragmentation of mono-oxoundecyl phthalate identified in human liver microsomes (HLM) after in vitro metabolism of DUP (top) and in human urine (bottom).
Fig. 2.
High resolution mass spectrometric fragmentation of monohydroxyundecyl phthalate identified in human liver microsome (HLM) homogenate after in vitro metabolism of DUP (top) and in human urine (bottom). Different ratios of fragments resulted due to structural differences in isomers of DUP used in in vitro study (mainly linear) and in commercial formulations (mainly branched).
Fig. 3.
High resolution mass spectrometric fragmentation of monocarboxydecyl phthalate identified in human liver microsomes (HLM) after in vitro metabolism of DUP (top) and in human urine (bottom).
Fig. 4.
Chromatographic separation of DUP metabolites detected in human liver microsomal (HLM) suspension after incubation with DUP, in human urine, and in DiDP dosed rat urine.
We also detected these metabolites in human urine (Fig. 4). Both in vivo and in vitro metabolites produced similar mass fragments (Figs. 1–3, S1). However, in vitro metabolites resulting from incubation of DUP retained longer on the HPLC column than the in vivo metabolites present in the human urine (Fig. 4), perhaps reflecting differences in metabolite structures (e.g., fraction of branched vs. linear isomers). Using the same analytical method, we evaluated the elution of DUP metabolites in archived urine samples from rats dosed with a commercial DiDP, a product that also contained branched DUP isomers (European Chemicals Bureau, 2003). The DUP metabolites in the rat urine eluted at similar retention times and with similar peak shape as the DUP metabolites detected in human urine. Of interest, in both human and rat urine, MCDP eluted as a abroad peak cluster suggesting that the DUP used in commercial products contains mainly branched iso-mers with numerous potential ω-oxidation sites.
To study environmental exposure of DUP, we measured four DUP metabolites in 36 spot urine samples collected from a convenience sample of adults with no known occupational exposure to DUP using online SPE coupled with HPLC and high resolution mass spectrometry. We detected MHUP (86%) and MCDP (83%) in most samples, whereas MOUP was detected only in 14% of the samples tested (Table 2). MHUP and MCDP were also detected at higher concentrations than MOUP (Table 2). Because MUP is relatively hydrophobic and would be further metabolized before its excretion in urine, MUP was not detected. As expected, the urinary concentrations of MHUP correlated well with MCDP (p < 0.001). Because high resolution mass selection excludes most isobaric chemical interferences (Be et al., 2013), we achieved well defined chromatographic peak clusters even at concentrations below the LOD of 0.01 ng/mL.
Table 2.
Urinary concentrations of diundecyl phthalate metabolites in a convenience sample of US adultsa (N=36).
| Percentile | Urinary diundecyl phthalate metabolite concentrationsb, ng/mL |
|||
|---|---|---|---|---|
| MUP | MOUP | MHUP | MCDP | |
| 50th | <LODc | <LODc | 0.21 | 0.36 |
| 75th | <LODc | <LODc | 0.64 | 0.67 |
| 90th | <LODc | 0.20 | 13.7 | 11.5 |
| 95th | <LODc | 0.48 | 29.2 | 12.8 |
Spot urine samples were collected in 2013–2015 from a group of US adults with no known occupational exposure to DUP.
Estimated concentrations based on the calibration curves of DiNP metabolite analogs
LOD=0.01 ng/mL
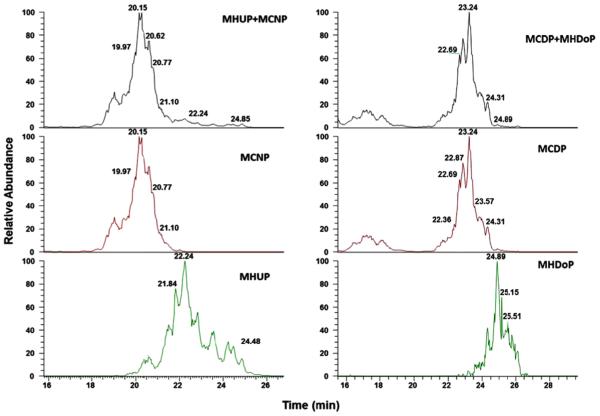
Simultaneous exposure to DiDP (C10 side chain), DUP, and diisododecyl phthalate (DiDoP, C12 side chain) in humans would produce urinary metabolites isobaric to some DUP metabolites [e.g., MHUP (m/z = 335.186) and monocarboxynonyl phthalate (MCNP, m/z = 335.150); and MCDP (m/z = 349.166) and mono-hydroxydodecyl phthalate (MHDoP, m/z = 349.202)] (Fig. 5). Because all of these metabolites have similar chemical characteristics and elute as peak clusters, full chromatographic separation is difficult to achieve (Fig. 5). However, high resolution mass spectrometry adequately separated DUP metabolites in the presence of isobaric DiDP and DiDoP metabolites. We observed a higher contribution from the isobaric DiDP metabolite (MCNP) to MHUP than of the isobaric DiDoP metabolite (MHDoP) to MCDP, perhaps because human exposure to DiDP is higher than the exposure to DiDoP. Because the relative abundance of the specific fragments is different in isobars, when using low resolution tandem mass spectrometry for quantifying MHUP and MCDP, isobaric interferences can be minimized by selecting appropriate fragment (s) for product ion scan.
Fig. 5.
Interfering isobars MCNP (m/z = 335) and MHDoP (m/z = 349), from the metabolites of DiDP and DDoP to DUP metabolites, MHUP (m/z = 335) and MCDP(m/z = 349).
In summary, we identified four DUP specific in vitro metabolites, MUP, MHUP, MOUP, and MCDP. Our data also suggest that MHUP and MCDP could serve as suitable biomarkers for assessing DUP exposure in humans. High resolution mass spectrometry would provide the best choice for quantification, due to low LOD and because these metabolites may partially co-elute with isobaric metabolites of DiDP or DiDoP. Therefore, if using the more common low resolution tandem mass spectrometry biomonitoring approach to detect DUP biomarkers, attention should be given to achieve adequate chromatographic separation to remove isobaric interferences or to select appropriate fragments to distinguish co-eluting isobaric metabolites from DiDP and DiDoP.
Supplementary Material
Acknowledgement
This work was supported in part by the appointment of Trevor W. Bontke to the Research Participation Summer Program at the Centers for Disease Control and Prevention (CDC), administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the CDC. We thank Dr. L. Earl Gray and Johnathan Furr from the U.S. Environmental Protection Agency for conducting the rat dosing experiments.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The authors declare they have no competing financial interests.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.envres.2016.03.037.
References
- Barber ED, Cifone M, Rundell J, Przygoda R, Astill BD, Moran E, et al. Results of the L5178Y mouse lymphoma assay and the Balb/3T3 cell in vitro transformation assay for eight phthalate esters. J. Appl. Toxicol. 2000;20:69–80. doi: 10.1002/(sici)1099-1263(200001/02)20:1<69::aid-jat630>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Be X, Moore ES, Zhao Z, Wells MC. LC-MS/MS bioanalytical method development for AMG 900: resolution of an isobaric interference in rodent in vivo studies. J. Pharm. Biomed. Anal. 2013;74:171–177. doi: 10.1016/j.jpba.2012.10.026. [DOI] [PubMed] [Google Scholar]
- Choi K, Joo H, Campbell JL, Andersen ME, Clewell HJ. in vitro intestinal and hepatic metabolism of Di(2-ethylhexyl) phthalate (DEHP) in human and rat. Toxicol. in vitro. 2013;27:1451–1457. doi: 10.1016/j.tiv.2013.03.012. [DOI] [PubMed] [Google Scholar]
- David RM, McKee RH, Butala JH, Barter RA, Kayser M. Esters of aromatic mono-, di-, and tricarboxylic acids, aromatic diacids, and di-, tri-, or polyalcohols. In: Bingham E, Cohrssen B, Powell CH, editors. Patty's Toxicology. 5th Vol. 6. John Wiley and Sons; New York: 2001. pp. 635–932. [Google Scholar]
- European Chemicals Bureau Risk Assessment Report on 1,2-Benezedicarboxylic Acid, Di-C9-11 Bracnched Alkyl Esters, C10-Rich, and Di-“Iso-decyl” Phthalate (DIDP) Institute for Health and Consumer Protection, European Union. 2003 ⟨http://echa.europa.eu/documents/10162/b66cca3a-5303-455b-8355-63bf741e263b⟩ (accessed 07.03.16)
- Goen T, Dobler L, Koschorreck J, Muller J, Wiesmuller GA, Drexler H, et al. Trends of the internal phthalate exposure of young adults in Germany-follow-up of a retrospective human biomonitoring study. Int. J. Hyg. Env. Health. 2011;215:36–45. doi: 10.1016/j.ijheh.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Health Canada Second report on human biomonitoring of environmental chemicals in Canada: Results of the Canadian Health Measures Survey Cycle 2 (2009–2011) Health Canada, Ottawa, Ontario, Canada. 2013 Available: ⟨http://www.hc-sc.gc.ca/ewh-semt/pubs/contaminants/chms-ecms-cycle2/index-eng.php⟩ (accessed 07.03.16)
- Kamendulis LM, Isenberg JS, Smith JH, Pugh G, Lington AW, Klaunig JE. Comparative effects of phthalate monoesters on gap junctional inter-cellular communication and peroxisome proliferation in rodent and primate hepatocytes. J. Toxicol. Environ. Health Part A. 2002;65:569–588. doi: 10.1080/152873902317349736. [DOI] [PubMed] [Google Scholar]
- Kato K, Silva MJ, Wolf C, Gray LE, Needham LL, Calafat AM. Urinary metabolites of diisodecyl phthalate in rats. Toxicoloy. 2007;236:114–122. doi: 10.1016/j.tox.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Kwack SJ, Han EY, Park JS, Bae JY, Ahn IY, Lim SK, et al. Comparison of the short term toxicity of phthalate diesters and monoesters in sprague-dawley male rats. Toxicol. Res. 2010;26:75–82. doi: 10.5487/TR.2010.26.1.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwack SJ, Kim KB, Kim HS, Lee BM. Comparative toxicological evaluation of phthalate diesters and metabolites in Sprague-Dawley male rats for risk assessment. J. Toxicol. Environ. Health A. 2009;72:1446–1454. doi: 10.1080/15287390903212923. [DOI] [PubMed] [Google Scholar]
- NICNAS, 6–1–2008 Existing Chemical Hazard Assessment Report: Diundecyl Phthalate. ⟨http://www.nicnas.gov.au/_data/assets/pdf_file/0006/4983/DUP-hazard-assessment.pdf⟩ (accessed 07.03.16)
- OECD OECD Existing chemicals database. 2015 ⟨http://webnet.oecd.org/HPV/UI/SIDS_Details.aspx?key = 8a22d820-e86f-4fde-afc1-db366ccdd2e0&idx = 0⟩ (accessed 01.03.16)
- Roberts SC, Macaulay LJ, Stapleton HM. in vitro metabolism of the brominated flame retardants 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB) and bis(2-ethylhexyl) 2,3,4,5-tetrabromophthalate (TBPH) in human and rat tissues. Chem. Res. Toxicol. 2012;25:1435–1441. doi: 10.1021/tx300086x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saillenfait AM, Gallissot F, Sabate JP, Remy A. Prenatal developmental toxicity studies on diundecyl and ditridecyl phthalates in Sprague-Dawley rats. Reprod. Toxicol. 2013;37:49–55. doi: 10.1016/j.reprotox.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Schutze A, Kolossa-Gehring M, Apel P, Bruning T, Koch HM. Entering markets and bodies: increasing levels of the novel plasticizer Hexamoll (R) DINCH (R) in 24 h urine samples from the German Environmental Specimen Bank. Int. J. Hyg. Environ. Health. 2014;217:421–426. doi: 10.1016/j.ijheh.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Jia T, Samandar E, Preau JL, Calafat AM. Environmental exposure to the plasticizer 1,2-cyclohexane dicarboxylic acid, diisononyl ester (DINCH) in US adults (2000–2012) Environ. Res. 2013;126:159–163. doi: 10.1016/j.envres.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Calafat AM, Ye X. Identification of di-2-ethylhexyl terephthalate (DEHTP) metabolites using human liver microsomes for biomonitoring applications. Toxicol. in vitro. 2015;29:716–721. doi: 10.1016/j.tiv.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL, Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. J. Chromatogr. B. 2007;860:106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- US Consumer Product Safety Commission Toxicity Review for Diundecyl phthalate. 2010 ⟨https://www.cpsc.gov/PageFiles/125795/dup.pdf⟩ (accessed 08.03.16)
- Wittassek M, Wiesmuller GA, Koch HM, Eckard R, Dobler L, Muller J, et al. Internal phthalate exposure over the last two decades – a retrospective human biomonitoring study. Int. J. Hyg. Environ. Health. 2007;210:319–333. doi: 10.1016/j.ijheh.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ. Health Perspect. 2014;122:235–241. doi: 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.