Abstract
Despite the clinical success and growth in the utilization of continuous flow ventricular assist devices (cfVADs) for the treatment of advanced heart failure, hemolysis and thrombosis remain major limitations. Inadequate and/or ineffective anticoagulation regimens, combined with high pump speed and non-physiological flow patterns can result in hemolysis which often is accompanied by pump thrombosis. An unexpected increased in cfVADs thrombosis was reported by multiple major VAD implanting centers in 2014, highlighting the association of hemolysis, and a rise in lactate dehydrogenase (LDH) presaging thrombotic events. It is well established that thrombotic complications arise from abnormal shear stresses generated by cfVADs. What remains unknown is the link between cfVAD-associated hemolysis and pump thrombosis. Can hemolysis of red blood cells (RBCs) contribute to platelet aggregation thereby facilitating prothrombotic complication in cfVADs? Herein, we examine the effect of RBC-hemolysate and selected major constituents, i.e. LDH and plasma free hemoglobin (pHb) on platelet aggregation utilizing electrical resistance aggregometry. Our hypothesis is that elements of RBCs, released as a result of shear-mediated hemolysis, will contribute to platelet aggregation. We show that RBC-hemolysate and pHb, but not LDH, are direct contributors to platelet aggregation, posing an additional risk mechanism for cfVAD thrombosis.
Keywords: Hemolysis, Thrombosis, Ventricular Assist Devices, Platelet Aggregation, Lactate Dehydrogenase (LDH), Multiplate Analyzer
Introduction
Continuous flow ventricular assist devices (cfVADs) have emerged as standard-of-care therapeutics, restoring hemodynamics for advanced heart failure patients, as either bridge-to-transplant or destination (long-term) therapy1, 2. Despite their clinical success, cfVADs remain plagued by thrombosis and hemolysis resulting in pump malfunction, recurrent heart failure, neurologic events, embolic complications and possible death. In an attempt to limit thrombosis, cfVAD patients require life-long antiplatelet and anticoagulation regimens. Inadequate or ineffective anti-thrombotic drug regimens, combined with high pump speed, perturbed flow, and high shear stress often result in red cell hemolysis and subsequent thrombosis. As such careful management of anti-thrombotic regimens and trending of specific thrombosis- or hemolysis-associated biomarkers may help minimize adverse prothrombotic events and post-implant complications.
Over the past year an increase in cfVAD thrombosis has been observed at multiple centers.3 These investigators report that the development of pump-related hemolysis and a rise in LDH were noted to presage thrombotic events. Similarly, the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) also reported an increase of pump exchange or death due to pump thrombosis during 2011 and 2012, where elevated LDH in the first month post-implant was noted to be a strong predictor of pump thrombosis4. In previous work, we and others have demonstrated and further defined the role of non-physiological flow and elevated shear stresses as to their contribution to platelet aggregation and activation5–7. Similarly, these perturbed flows may lead to hemolysis and release of RBC contents i.e. LDH and plasma free hemoglobin (pHb). What remains unknown is the link between cfVAD-mediated hemolysis and pump thrombosis. Are RBC released contents a risk factor or a risk maker for predicting adverse prothrombotic events in patients implanted with cfVADs? In the present study, we hypothesized that elements of RBCs, released as a result of shear-mediated hemolysis will contribute to platelet activation. As such, we specifically examined the effect of adding RBC-hemolysate (RBC-hemo), pHb, and LDH to platelets as to their subsequent impact on platelet aggregation using impedance aggregometry.
Methods
Sample Preparation and Material Collection
Fresh human blood was collected from healthy adult volunteers (n = 4) via venipuncture and mixed with 10% (v/v) Anticoagulant Citrated Dextrose – Formula A (ACD-A). Blood was collected only from those donors free of alcohol consumption the night before or caffeine the morning of blood donation, to maximize platelets count and prevent desensitization of platelets. All volunteers provided informed consent for the study, which had University of Arizona Institutional Review Board (IRB) approval)8. Blood samples were centrifuged to separate platelet rich plasma (PRP) from white and red blood cells (RBCs). RBCs were washed (x3) in PLT buffer (Hepes-modified Ca2+-free Tyrode’s buffer) via centrifugation and counted using a Z2 Particle Counter (Beckman Coulter, Miami, FL). Washed RBCs (5 ×106 cells/µl) were subjected to high frequency mechanical sonification (Branson Sonifier SLP, Atkinson, NH) at 60 W, 20 kHz for 5 minutes to generate RBC-hemolysate (RBC-hemo). Platelets were also counted using a Z2 Particle Counter (Beckman Coulter, Miami, FL) and normalized to 1.5 × 105 platelets/µl with PLT buffer for aggregation studies using a Multiplate Analyzer (Roche Diagnostics, Mannheim, Germany). To examine the effect of hemoglobin and LDH on platelet aggregation these compounds were added exogenously to PRP, as outlined below. Lactate Dehydrogenase 1 (LDH, Lee Biosolutions, St. Louis, MI) and human plasma free hemoglobin (pHb, MyBioSources, San Diego, CA) were purchased for the study. Plasma free hemoglobin (pHb) was solubilized in normal saline to achieve a stock of 6 mg/ml, and diluted for use, with concentrations verified using a NanoDrop 2000 C UV-Vis Spectrophotometer (Thermo Fisher Scientific Inc, MA, USA). pHb was studied at concentration range of 2 – 120 mg/dl. Stock LDH was obtained as a solution with an enzymatic activity of 740 IU/ml. LDH was studied at a concentration range of 200 – 1800 U/L.
Platelet Aggregation Study
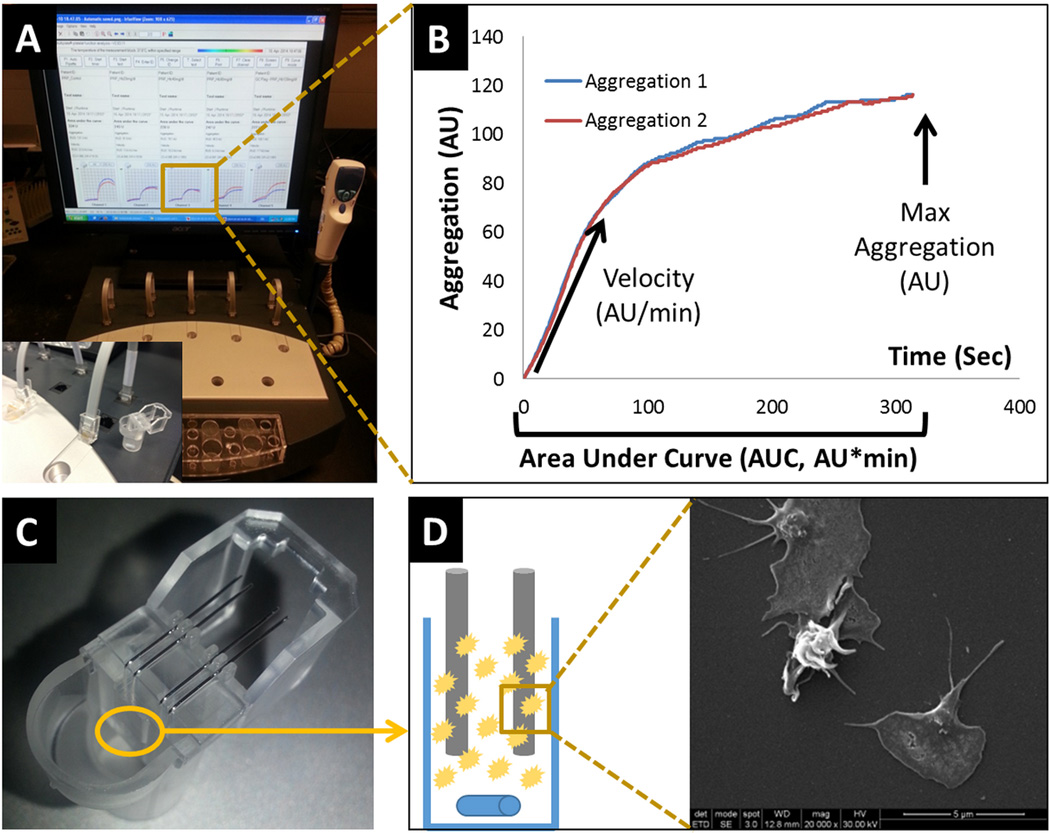
RBC-hemo, LDH and pHb as test reagents were added to platelets (PRP) at defined concentrations. PRP + test reagent mixtures were incubated at 37°C for 3 min. Platelet aggregation was then measured utilizing the Multiple Analyzer (MA) (Figure 1A) - an impedance aggregometer that measures the increase in electrical impedance across two metal wires in a test cell when platelets accumulate and aggregate in response to an agonist (Figure 1C, D). The MA has 5 channels, each one containing a single use test cell with two independent sensor units9 (Figure 1C). The change of impedance, or resistance, is proportional to the amount of platelets sticking to the electrodes and is continuously detected from each sensor unit separately, transformed to arbitrary aggregation units (AU) and displayed as two aggregation curves. Aggregation is plotted against time. The maximum height of the curve represents the aggregation level, while the curve slope (Figure 1B) represents the reaction velocity. The most important parameter is the area under the aggregation curve (AUC) or AU*min.10
Figure 1. Multiplate Analyzer Impedance Aggregometry.
A) Multiplate Analyzer console. B) Example of an aggregation measurement with 6 minute runtime. C) Multiplate test cells. D) Platelets aggregate to electrodes, thus increasing the electrical impedance.
Aggregation experiments were performed exposing PRP to different agonists. Each experiment was repeated four times using PRP (150,000 platelets/µl) obtained from different donors. In the first experiment, PRP was exposed to different dilutions of RBC hemolysate: 100%, 40% and 20%. After 12 minutes, 5 µM of adenosine diphosphate (ADP) was added to each channel and the test continued for another 18 minutes to validate and verify the viability and optimal aggregation of tested platelets. Two control samples were utilized: PRP without any agonist and intact RBCs (washed RBCs). Similarly, we tested pHb at 2, 20, 40 (cfVAD-induced threshold), 80 and 120 mg/dl and LDH at 200, 600 (cfVAD-induced hemolysis threshold), 1200 and 1800 IU/L.
Scanning Electron Microscopy Analysis
Aggregated or control (un-activated) platelets (150 µL) were added to 150 µL of 2% v/v glutaraldehyde in platelet buffer, pH 7.4 and simultaneously placed onto 12mm circular glass coverslips in 24-well plates for 15 minute. Excess solution was partially aspirated, leaving approximately 50 µL of mixture behind. Coverslips were washed with 25%, 50%, 75%, and 100% of double-distilled H2O in 1% glutaraldehyde. Coverslips were then dehydrated through a graded ethanol series of 0%, 25%, 50%, 75%, and 100% in double-distilled H2O. Samples were stored in 100% ethanol until critical point drying through a series of 25%, 50%, 75%, and 100% hexamethyldisiloxane (HMDS) in ethanol exposures. Each preparation stage required a 5 min immersion at room temperature. Coverslips were then mounted on double-sided carbon tape, allowed to air dry overnight, and then sputter-coated with islanded gold in an argon chamber. Samples were imaged at 15 kV with an aperture spot size of 3 by using an Inspect F50 scanning electron microscope (FEI, Hillsboro, OR) to identify platelet morphological changes, which represent platelet activation and platelet aggregation.
Statistical analysis
Statistical tests were performed using Microsoft Excel v2010. Single comparison t-tests were used in two-population comparisons. Differences were considered significant if p < 0.05.
Results
Effect of Addition of RBC Components on Platelet Aggregation
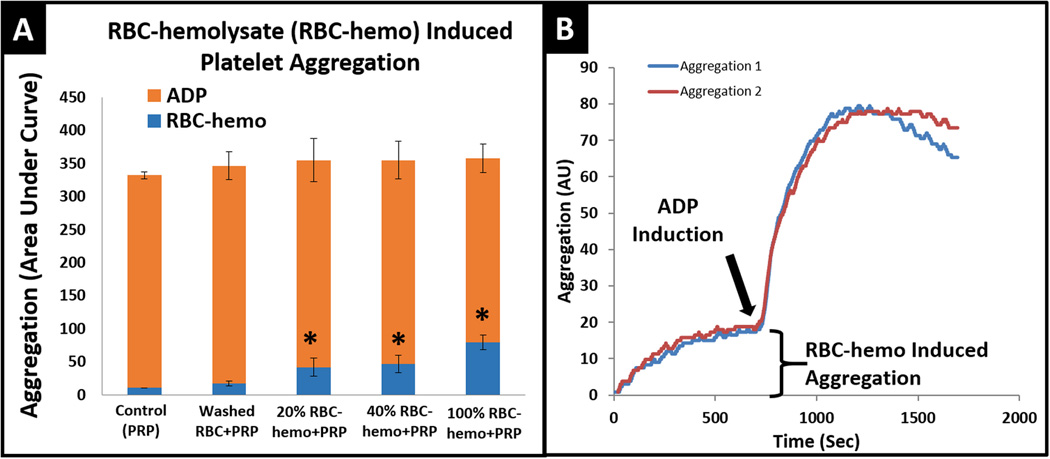
The addition of RBC hemolysate to PRP resulted in a definitive increase in platelet aggregation versus control (Figure 2). Further, when different dilutions of RBC hemolysate (100%, 40% and 20%) were added to PRP a concentration-dependent behavior, as to this augmentation of platelet aggregation was observed (Figure 2A-blue). No aggregation was measured for controls of PRP alone or addition of washed intact RBCs. After 12 minutes of measurement, ADP was added to drive aggregation to a functional maximum (Figure 2A-red, 2B) to validate and verify platelet viability and functionality.
Figure 2. Platelet aggregation by red blood cell hemolysate (RBC-hemo) and Adenosine diphosphate (ADP).
A) Partial platelet aggregation induced by RBC-hemo and maximal platelet aggregation mediated by the addition of ADP. All aggregation induced by RBC-hemo are significant from control, which has only clean PRP. * p-value < 0.05.B) Example of a single experiment showing spontaneous aggregation measured within 12 minutes then subsequently induced by ADP to demonstrate platelet’s viability and functionality. n-samples = 4.
The addition of LDH to PRP however did not increase platelet aggregation, even with concentrations three times higher than the typical clinical LDH hemolysis threshold level of 600 IU/L (Figure 3A). Plasma free hemoglobin showed little to no aggregation at a physiological concentration (2 mg/dL) but did increase aggregation at higher concentrations (normal serum threshold value of 40 mg/dL) with progressive increases to 120 mg/dL (Figure 3C).
Figure 3. Platelet Aggregation by lactate dehydrogenase (LDH) and Plasma Hemoglobin (pHb).
A) Platelet aggregation induced by various concentration of LDH showing no effect. B) Platelet aggregation mediated by red blood cell hemolysate (RBC-hemo) extrapolated from Figure 2 for comparison purposes. C) Platelet aggregation induced at various pHb concentrations showing a significant increase with 120 mg/dL when compared to the control sample, which has no pHb. *p-value <0.05. n-samples = 4.
Effect of Addition of RBC Components on Platelet Activation and Morphology
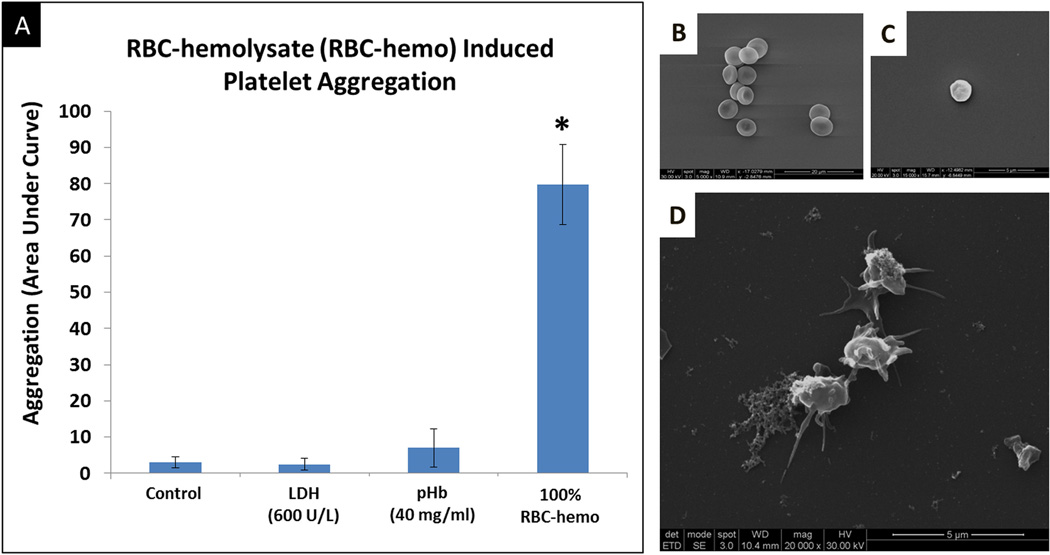
RBC-hemo generated from washed RBCs (5 ×106 cells/µl, Figure 4B) significantly aggregated platelets to an extent far greater than that observed with exposure to both LDH and pfHb, at concentrations typically associated with cfVADs in vivo in implanted patients (i.e. LDH (600 IU/L) and pHb (40mg/dL),(Figure 4A) as reported in the Interagency Registry for Mechanically Assisted Circulation Support (INTERMACS))4, 11, 12. SEM analysis of platelets aggregated by RBC-hemo showed significant morphological change with pseudopods connecting to each other (Figure 4D) when compared to control platelets from normal volunteers (Figure 4C).
Figure 4. Prothrombotic activity of RBC-hemolysate.
A) Platelet aggregation effectiveness of RBC-hemolysate in comparison to that of LDH and pHB (at levels observed in cfVAD patients), RBC-hemo demonstrated at least a 7-fold increase in aggregation when compared to pHb and 15-fold when compared to LDH and control (PRP alone). *p-value <0.05. B) Scanning electron microscope (SEM) of RBC. C) SEM of non-activated platelets. D) SEM of RBC-hemo-induced platelet aggregation. n-samples = 4. * p-value <0.05.
Discussion
In this study we examined the effect of exogenous addition of mechanically damaged RBCs and specific RBC constituents on platelet aggregation and activation. Our results clearly show that RBC hemolysate and pHB, added to platelets at the concentrations tested, led to enhanced platelet aggregation and activation. In contrast LDH, a clinical marker of hemolysis, did not contribute mechanistically to platelet activation or aggregation.
The studies presented here highlight a potential mechanistic link between cfVAD-induced RBC hemolysis and thrombosis. We found that RBC-hemolysate effectively and significantly activated platelets (Figure 2) in a concentration-dependent manner (Figure 2A-blue). This raises the possibility that the potential for pump thrombosis clinically might be closely associated with the level of hemolysis in vivo.
RBC hemolysate contains numerous constitutive elements that may induce platelet aggregation and activation. To consider mechanism, it is possible that RBC contents, membrane fragments, or a combination of both, act as agents stimulating platelet activation or provide a substrate for enhanced aggregation. RBC composition has been studied extensively over the years, with summary finding of protein being 49%, lipids 46% and carbohydrates, other metabolites and ions as the reminder of the constituents13, 14. Beyond the dominant RBC protein – i.e. Hb, which we herein, and others,12, 15, 16 have demonstrated to lead to enhanced platelet activation and aggregation, other RBC proteins as well may also drive aggregation. Recent studies of the RBC proteome have now found > 750 protein constituents in RBCs17, 18. Proteomic analysis has demonstrated many functional classes of proteins in RBCs including kinases, glycoproteins, adhesion proteins, transporters and proteases, all of which are recognized as having pro-thrombotic members within each class17. RBCs also contain many strong pro-thrombotic metabolic constituents including ADP19–21 and calcium22. Further, RBC membranes contain a range of lipids including a predominance of phospholipids and cholesterol, with small amounts of glycolipids. Lipids are asymmetrically distributed in RBC membranes with most choline containing phospholipids found in the outer monolayer, whereas most aminophospholipids are found in the inner membrane23, 24. Aminophospholipids such as phosphatidylserine and phosphatidylethanolamine, being negatively charged, have been demonstrated to be prothrombotic25. With hemolysis these inner membrane elements are effectively everted allowing direct exposure to platelets, posing prothrombotic risk. Further, phosphatidylserine within membrane fragments has been postulated to regulate thrombin release26. Therefore, to further narrow down mechanistic contributors individual hemolytic components should be further characterized and investigated as to their contribution to cfVAD-induced hemolysis and thrombosis.
Our observations of total RBC hemolysate-enhanced platelet aggregation, and our findings of the pro-activation and aggregation effects of hemoglobin, are consistent with and supported by the reports of both Iuliano et al27 and Villagra et al16. Iuliano examined the effect of adding either supernatants derived from “stirred erythrocytes,” or exogenous hemoglobin on platelet aggregation utilizing PRP and light aggregometry. They observed that stirred supernatant i.e. RBC contents derived from mild to moderate mechanical damage containing hemoglobin, as well as direct exogenous hemoglobin exposure, both led to enhanced platelet aggregation. Similarly, Villagra et al examined the effect of cell free hemoglobin on the activation of platelets in the form of PRP, with activation determined based upon gpIIb/IIIa and p-selectin expression. They similarly demonstrated a dose-dependent increase in platelet activation with Hgb exposure. Our studies go beyond these studies. Here we show that Hgb can activate platelets which are in a more pure form, i.e. as purified cells, namely as gel filtered platelets, free of association with plasma proteins, which may have additive effects on both activation and aggregation.
The recent work of Helms et al further supports our findings of the connection and role of hemolysis as a driver of platelet activation15. Their studies attempted to dissect which intra-RBC constituents induce or amplify platelet activation, observing, similar to our study, that whole RBC hemolysate, despite slight preparation differences, drives platelet activation when added to PRP. They further showed that hemoglobin alone activates platelets, consistent with our findings. Interestingly they observed that when apyrase, a nucleotide di- and tri-phosphatase, was added to dialysed hemoglobin, a diminution of the extent of activation of platelets was observed, compared to untreated control, arguing for a dominant role of hemoglobin-bound ADP as a major actor in hemolysis-mediated activation. Their observations and our finding remain congruent in that: 1. even with exposure of PRP to apyrase-treated dialyzed Hgb, platelet activation was still observed to increase over baseline, 2. our Hgb treatment involved exposure of PRP to highly purified reagent grade Hgb free of small molecules such as ADP with observed significant activation, 3. Iuliano as well demonstrated that exposure of PRP to Hgb led to enhanced platelet activation, with or without apyrase admisture, implying a direct role for Hgb, independent of concomitant ADP27.
Scanning electron microscopy analysis of platelets exposed to RBC-hemolysate showed high level of aggregation and activation as evidenced by extruding filopods, adhesion, and irregular morphology when compared to inactivated platelets (Figure 4). The finding is congruent with Kuwahara and colleagues findings where platelet changes shape28 and platelets interact with other platelets forming aggregates29.
On the clinical level in addition to pHb, the level of hemolysis can be identified by the use of LDH as a clinical biomarker16, 30, 31. In fact, INTERMACS has established two criteria for defining hemolysis as adverse events: 1) LDH above 600 IU/L or 2.5 times the upper limit of laboratory normal and 2) plasma free hemoglobin above 40 mg/dL with signs/symptoms4, 12. As was a starting goal of this study we have clarified the role of LDH, establishing that it does not aggregate platelets (Figure 3), suggesting that it is solely a risk marker. In contrast, as discussed above, while we found that pHb activated platelets, our findings show that it does so at a concentration three times higher than the hemolysis criteria defined by INTERMACS. This finding is also consistent with Helms et al15 and previous studies16, 27 as previously stated. As such, the level of LDH and pHb are therefore best utilized for trending cfVADs-induced hemolysis, rather than for firm pump thrombosis diagnosis. Such needs require clinical tests including analysis of pump power consumption, ramping studies, echocardiography and radiological imaging studies.
The time sequence and initiating events in cfVAD thrombosis remain a challenge and a paradox to a degree. It is a bit of a “chicken and the egg situation,” in that it remains unclear whether pump thrombosis leads to RBC hemolysis, further potentiating platelet activation and thrombus formation or if vice versa cfVADs hemolyze RBCs, thus amplifying platelet activation. still remains to be investigated. What we now know, in conjunction with previous studies15, 16, 20, 21, 32, 33, is that RBC-hemolysate mediated platelets aggregation, but not LDH, and marginal platelet activation occurs with high plasma free hemoglobin levels. Further understanding of the mechanism of platelet activation via RBC hemolytic elements may provide additional pathways to limit VAD (shear)-mediated platelet activation and reduce the thrombotic risk for VAD patients.
Supplementary Material
References
- 1.Yamakawa M, Kyo S, Yamakawa S, Ono M, Kinugawa K, Nishimura T. Destination therapy: The new gold standard treatment for heart failure patients with left ventricular assist devices. Gen. Thorac. Cardiovasc. Surg. 2013;61:111–117. doi: 10.1007/s11748-012-0181-5. [DOI] [PubMed] [Google Scholar]
- 2.Porepa LF, Starling RC. Destination Therapy With Left Ventricular Assist Devices: For Whom and When? Can J Cardiol. 2014;30:296–303. doi: 10.1016/j.cjca.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Starling RC, Moazami N, Silvestry SC, Ewald G, Rogers JG, Milano Ca, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370:33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 4.Kirklin JK, Naftel DC, Kormos RL, Pagani FD, Myers SL, Stevenson LW, et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the HeartMate II left ventricular assist device. J Hear Lung Transplant. 2014;33:12–22. doi: 10.1016/j.healun.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Xenos M, Girdhar G, Alemu Y, Jesty J, Slepian M, Einav S, et al. Device Thrombogenicity Emulator (DTE)--design optimization methodology for cardiovascular devices: a study in two bileaflet MHV designs. J Biomech. 2010;43(12):2400–2409. doi: 10.1016/j.jbiomech.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alemu Y, Bluestein D. Flow-induced platelet activation and damage accumulation in a mechanical heart valve: numerical studies. Artif Organs. 2007 S;31(9):677–688. doi: 10.1111/j.1525-1594.2007.00446.x. [DOI] [PubMed] [Google Scholar]
- 7.Bluestein D, Chandran KB, Manning KB. Towards non-thrombogenic performance of blood recirculating devices. Ann Biomed Eng. 2010;38(3):1236–1256. doi: 10.1007/s10439-010-9905-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin W, Alemu Y, Affeld K, Jesty J, Bluestein D. Flow-induced platelet activation in bileaflet and monoleaflet mechanical heart valves. Ann Biomed Eng. 2004;32(8):1058–1066. doi: 10.1114/b:abme.0000036642.21895.3f. [DOI] [PubMed] [Google Scholar]
- 9.Tóth O, Calatzis A, Penz S, Losonczy H, Siess W. Multiple electrode aggregometry: A new device to measure platelet aggregation in whole blood. Thromb Haemost. 2006;96:781–788. [PubMed] [Google Scholar]
- 10.Steinlechner B, Dworschak M, Birkenberg B, Duris M, Zeidler P, Fischer H, et al. Platelet dysfunction in outpatients with left ventricular assist devices. Ann Thorac Surg. 2009;87(1):131–137. doi: 10.1016/j.athoracsur.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, et al. The Fourth INTERMACS Annual Report: 4,000 implants and counting. J Heart Lung Transplant. 2012;31(2):117–126. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Cowger JA, Romano MA, Shah P, Shah N, Mehta V, Haft JW, et al. Hemolysis: a harbinger of adverse outcome after left ventricular assist device implant. J Heart Lung Transplant. 2014;33(1):35–43. doi: 10.1016/j.healun.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Pennel R. Composition of Normal Human Red Blood Cells. In: Surgenor M, editor. The Red Blood Cell. New York: Academic Press; 1974. pp. 93–146. [Google Scholar]
- 14.Beutler E. Composition of the Erythrocyte. In: Beutler E, Lichtman M, Coller B, Kipps T, Seligsohn U, editors. Hematology. New York: McGraw Hill; 2001. pp. 289–293. [Google Scholar]
- 15.Helms CC, Marvel M, Zhao W, Stahle M, Vest R, Kato GJ, et al. Mechanisms of hemolysis-associated platelet activation. J Thromb Haemost. 2013;11(12):2148–2154. doi: 10.1111/jth.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT, Kato GJ. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007;110:2166–2172. doi: 10.1182/blood-2006-12-061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kakhniashvili DG, Bulla LA, Goodman SR. The human erythrocyte proteome: analysis by ion trap mass spectrometry. Mol Cell Proteomics. 2004;3:501–509. doi: 10.1074/mcp.M300132-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Goodman SR, Kurdia A, Ammann L, Kakhniashvili D, Daescu O. The human red blood cell proteome and interactome. Exp Biol Med (Maywood) 2007;232:1391–1408. doi: 10.3181/0706-MR-156. [DOI] [PubMed] [Google Scholar]
- 19.Woulfe D, Yang J, Brass L. ADP and platelets: the end of the beginning. J Clin Invest. 107(12):1503–1505. doi: 10.1172/JCI13361. 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puri RN, Colman RW. ADP-induced platelet activation. Crit Rev Biochem Mol Biol. 1997;32:437–502. doi: 10.3109/10409239709082000. [DOI] [PubMed] [Google Scholar]
- 21.Polgár J, Eichler P, Greinacher A, Clemetson KJ. Adenosine diphosphate (ADP) and ADP receptor play a major role in platelet activation/aggregation induced by sera from heparin-induced thrombocytopenia patients. Blood. 1998;91:549–554. [PubMed] [Google Scholar]
- 22.Rao AK, Disa J, Yang X. Concomitant defect in internal release and influx of calcium in patients with congenital platelet dysfunction and impaired agonist-induced calcium mobilization. Thromboxane production is not required for internal release of calcium. J Lab Clin Med. 1993;121(1):52–63. [PubMed] [Google Scholar]
- 23.Verkleij AJ, Zwaal RF, Roelofsen B, Comfurius P, Kastelijn D, van Deenen LL. The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim Biophys Acta. 1973;323:178–193. doi: 10.1016/0005-2736(73)90143-0. [DOI] [PubMed] [Google Scholar]
- 24.Zwaal RF, Roelofsen B, Comfurius P, van Deenen LL. Organization of phospholipids in human red cell membranes as detected by the action of various purified phospholipases. Biochim Biophys Acta. 1975;406:83–96. doi: 10.1016/0005-2736(75)90044-9. [DOI] [PubMed] [Google Scholar]
- 25.Presseizen K, Friedman Z, Shapiro H, Radnay J, Ellis MH. Phosphatidylserine expression on the platelet membrane of patients with myeloproliferative disorders and its effect on platelet-dependent thrombin formation. Clin Appl Thromb Hemost. 2002;8(1):33–39. doi: 10.1177/107602960200800104. [DOI] [PubMed] [Google Scholar]
- 26.Schoenwaelder SM, Yuan Y, Josefsson EC, White MJ, Yao Y, Mason KD, et al. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood. 2009;114(3):663–666. doi: 10.1182/blood-2009-01-200345. [DOI] [PubMed] [Google Scholar]
- 27.Iuliano L, Violi F, Pedersen JZ, Praticò D, Rotilio G, Balsano F. Free radical-mediated platelet activation by hemoglobin released from red blood cells. Arch Biochem Biophys. 1992;299(2):220–224. doi: 10.1016/0003-9861(92)90267-z. [DOI] [PubMed] [Google Scholar]
- 28.Kuwahara M. Platelet Shape Changes and Adhesion Under High Shear Flow. Arterioscler Thromb Vasc Biol. 2002;22(2):329–334. doi: 10.1161/hq0202.104122. [DOI] [PubMed] [Google Scholar]
- 29.Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109(12):5087–5095. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- 30.Shah P, Mehta VM, Cowger JA, Romano MA, Haft JW, Aaronson KD, et al. Lactate Dehydrogenase Is Superior To Serum-Free Hemoglobin As A Marker Of Pump Thrombosis In Left Ventricular Assist Devices. J Am Coll Cardiol. 2013;61(10):E665. [Google Scholar]
- 31.Shah P, Mehta VM, Cowger JA, Aaronson KD, Pagani FD. Diagnosis of hemolysis and device thrombosis with lactate dehydrogenase during left ventricular assist device support. J Heart Lung Transplant. 2014;33(1):102–104. doi: 10.1016/j.healun.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Sill JC, Proper JA, Johnson ME, Uhl CB, Katusic ZS. Reactive oxygen species and human platelet GP IIb/IIIa receptor activation. Platelets. 2007;18:613–619. doi: 10.1080/09537100701481385. [DOI] [PubMed] [Google Scholar]
- 33.Hu W, Jin R, Zhang J, You T, Peng Z, Ge X, et al. The critical roles of platelet activation and reduced NO bioavailability in fatal pulmonary arterial hypertension in a murine hemolysis model. Blood. 2010;116(9):1613–1622. doi: 10.1182/blood-2010-01-267112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.