Abstract
Objectives
To assess associations between residences location, risky sexual behaviours and sexually transmitted diseases (STDs) among adults living in Guangzhou, China.
Methods
Data were obtained from 751 Chinese adults aged 18–59 years in Guangzhou, China, using stratified random sampling by using spatial epidemiological methods. Face-to-face household interviews were conducted to collect self-report data on risky sexual behaviours and diagnosed STDs. Kulldorff’s spatial scan statistic was implemented to identify and detect spatial distribution and clusters of risky sexual behaviours and STDs. The presence and location of statistically significant clusters were mapped in the study areas using ArcGIS software.
Results
The prevalence of self-reported risky sexual behaviours was between 5.1% and 50.0%. The self-reported lifetime prevalence of diagnosed STDs was 7.06%. Anal intercourse clustered in an area located along the border within the rural–urban continuum (p=0.001). High rate clusters for alcohol or other drugs using before sex (p=0.008) and migrants who lived in Guangzhou <1 year (p=0.007) overlapped this cluster. Excess cases for unprotected sex (p=0.031) overlapped the cluster for college students (p<0.001). Five of nine (55.6%) students who had sexual experience during the last 12 months located in the cluster of unprotected sex.
Conclusions
Short-term migrants and college students reported greater risky sexual behaviours. Programmes to increase safer sex within these communities to reduce the risk of STDs are warranted in Guangzhou. Spatial analysis identified geographical clusters of risky sexual behaviours, which is critical for optimising surveillance and targeting control measures for these locations in the future.
INTRODUCTION
Worldwide, more than one million people acquire sexually transmitted diseases (STDs) every day.1 The heavy burden of STDs has important public health significance, as it is associated with worse sexual health, lower health quality of life and higher risk of HIV acquisition.1,2
China’s reform and opening up appear to have rapidly laid the foundation for the re-emergence of STDs since 1980s.3 It was estimated that in 2014 the total number of new STD cases in adults was about 1.70 million,4 an increase of >10 times since 1989.3 Changes in sexual behaviours, relationships and norms among Chinese general population contributed to the epidemic.5 For example, in 2000, 16.9% of college students reported having sex and the number nearly doubled (32%) in 2006.6 The national prevalence of multiple sexual partnerships among young adults significantly increased from 16.8%6 in 2000 to 47.3%7 in 2010.
Massive population movements occurred in China during the last 30 years and this also facilitated the transmission dynamics of STDs. A complete hukou (residency management) system was built in China in 1958, which strictly limits the migration of farmers flowing into cities. Since the late 1980s, driven by the reform of the market-oriented economy, a multitude of farmers left their homelands and families for urban areas to make a living. By the end of 2014, there were 253 million internal migrants in China, accounting for 18.5% of the total population.8 The majority of rural-to-urban migrants are sexually active, less aware of sexual health and fall outside of social and family control measures.9 According to the social control theory10 and social isolation theory,11 migrants with social isolation and lax social control may lead to behaviour changes. A meta-analysis conducted by Zou et al12 demonstrated rural-to-urban migrants in China had higher risk of STDs and hepatitis C infection than the registered population.
Most studies on risky sexual behaviours and STDs mainly focused on key populations thought to be most at risk such as men who have sex with men, female sex workers and injection drug users. Much of our understanding of risky sexual behaviours and STD epidemiology within the general population (non-key population groups) comes from clinic-based screening at prenatal clinics and blood donation stations.13 These conventional surveillance measures used to understand STD risk fail to capture migrants and others who frequently have informal residence status and are unlikely to seek formal clinical services.14 Uncertainty about the prevalence and distribution of risky sexual behaviours and STDs among the general population introduces fundamental questions about optimal surveillance for understanding the spread of STDs. This has broad significance in optimising surveillance, targeting control measures within specific geographical locations and informing STD policy. Recent developments in spatial sampling have paved the way for population-representative sampling methods to examine the prevalence and geographical distribution of risky sexual behaviours and STDs in the general population.
The purpose of this study was to investigate a geospatial dimension of the distribution of self-report sexual behaviours and diagnosed STDs among a sample of adults aged 18–59 years in Guangzhou, China using a population-representative survey.
METHODS
Study sites
Data collection, between April and November 2014, occurred in two districts (Yuexiu and Tianhe) in the South China city of Guangzhou, Guangdong Province. Yuexiu and Tianhe are two representative districts in Guangzhou. Yuexiu district is the old centre of town and a traditional residential area. It covers a total area of 33.8 km2 and has a population of 117.5 thousand residents. Tianhe district covers a total area of 137.4 km2, which contains many non-residential areas like forests, unavailable low-hilly land and uninhabitable industrial land. Tianhe district has 54 km2 of comparable residential area and a residential population of 148.4 thousand people. The two districts have a similar population size and density based within comparable residential areas.
Population and study protocol
Stratified cluster sampling was used to select participants, aged 18–59 years, in study sites. We chose 700 households within each district using geographical information system (GIS). Random geographical coordinates were pinned and overlaid on Google Earth images to locate the nearest residential building, including informal or temporary settlements where migrants lived, for each point. Not more than one eligible participant was selected within each chosen building in order to minimise similarity and spatial autocorrelation. Participants and households were selected randomly by earliest birthday date method and a random number generator application, respectively. If a potential interviewee in a household refused to participate, or there was no eligible interviewee in that household, we moved to next randomised location for no more than three times in total within the same building. Selected households were visited in the evening during weekdays and in the daylight at the weekend. We visited the same household not more than three times if nobody responded, and marked this coordinate as a non-response.
Trained Chinese interviewers using mobile tablet devices conducted face-to-face interviews. All interviewers received extensive training in interview methods, research ethics and the study protocol before conducting interviews. Participants responded to sensitive items related to sexual health and STDs independently and privately, and interviewers provided assistance as needed. After the completion of the study, participants received a ¥50 (US$8) phone card as a token of appreciation.
Measurement
Sociodemographic characteristics included self-reported age, sex, education level, marital and employment status, migrants (defined as no household registration in Guangzhou), sexual orientation, student status and duration of living in Guangzhou.
Risky sexual behaviours in the last 12 months included, consumption of alcohol or other drugs before sex, anal intercourse, multiple sexual partnerships, casual sex partners (defined as any non-steady and non-cohabitating sex partner) and unprotected sex (defined as rarely or never using condom).
Diagnosed STDs in participants’ lifetime were collected by self-report data, including chlamydia, gonorrhoea, genital warts, syphilis, trichomonas, herpes, hepatitis B virus (HBV), pubic lice, non-specific urethritis (NSU), epididymitis, pelvic inflammatory disease (PID), vulvovaginal candidiasis and bacterial vaginosis.
Analysis
The spatial scan statistics (SaTScan)15 software (V.9.4, http://www.satscan.org) was used to detect the potential excess of measured variables noted above. The method tested geographical clusters by considering the rates of nearby clusters across multiple spatial scales, minimising the potential for error resulting from the small sample sizes within each cluster of households. A circular window of varying radius was centred at each location and moved across the map so that at any given position the window includes different sets of residences. The radius of each circular window varies repeatedly from zero up to a set maximum radius, which restricts the maximum size from exceeding 50% of the total study population. Clusters were detected by comparing the number of observed cases with expected cases generated by random simulation within the window: high rate clusters indicating the number of observed cases exceeded that of expected cases while low rate clusters indicating the opposite.
Primary or secondary clusters were identified according to the value of likelihood ratio (LLR) for variables. The cluster with largest LLR was called ‘primary cluster’ and multiple subordinate clusters ordered by LLR were called ‘secondary clusters’. The significance of the clusters was deduced by an LLR test based on Monte Carlo simulations: 9999 repetitions were performed for dichotomous variables and 999 repetitions for ordinal or nominal variables. In this paper, we reported primary clusters for all variables no matter whether they were statistical significance or not, and secondary clusters which do not overlap with the primary cluster and have p≤0.1.
A standard GIS program in the ArcGIS10.0 software was used to translate the outputs generated by SaTScan into maps that revealed the distribution of significant clusters.
RESULTS
Sociodemographic characteristics and risky sexual behaviours
In total, there were 1215 attempted surveys, with 14 partial completions, 368 refusals and 82 uninhabited locations, resulting in a sample of 751 complete surveys. This yields a total cooperation rate of 61.8%, which is favourable for household surveys conducted in Mainland China. Participants’ sociodemographic characteristics and risky sexual behaviours were shown in table 1. 42.7% (321/751) were between 18 and 28 years, 50.3% (378/751) were female. And 57.5% were migrants (432/751), 54.6% (410/751) of the participants reported being in a sexual relationship in the last 12 months. Among those individuals, risky sexual behaviours varied between 5.1% (21/410) and 50% (205/410).
Table 1.
Participant characteristics (n=751)
| Sociodemographic characteristics | n | % | Sociodemographic characteristics | n | % |
|---|---|---|---|---|---|
| Age group | Marital status | ||||
| >18 | 321 | 42.7 | Single | 277 | 36.9 |
| >29 | 199 | 26.5 | Married or cohabitating | 464 | 61.8 |
| >39 | 139 | 18.5 | Missing data | 10 | 1.3 |
| 49–59 | 92 | 12.3 | Migrants | ||
| Sex | Yes | 432 | 57.5 | ||
| Female | 378 | 50.3 | No | 319 | 42.5 |
| Male | 373 | 49.7 | Missing data | 0 | – |
| Education | Employment | ||||
| Primary or never attended school | 41 | 5.5 | Employed or self-employed | 617 | 82.1 |
| Any secondary | 287 | 38.2 | Unemployed | 99 | 13.2 |
| Postsecondary | 422 | 56.2 | Missing data | 35 | 4.7 |
| Missing data | 1 | 0.1 | College students | ||
| Location of living in Guangzhou | Yes | 70 | 9.3 | ||
| Yuexiu | 366 | 48.7 | No | 681 | 90.7 |
| Tianhe | 385 | 51.3 | Sexual orientation | ||
| Duration of living in Guangzhou | Heterosexual | 717 | 95.5 | ||
| ≤1 year | 91 | 12.1 | Not heterosexual | 31 | 4.1 |
| >1 year | 660 | 87.9 | Missing data | 3 | 0.4 |
|
| |||||
| Sexual behaviours in the last 12 months | n | % | Sexual behaviours in the last 12 months | n | % |
|
| |||||
| Sexual relationship | Multiple sexual partnerships | ||||
| Yes | 410 | 54.6 | Yes | 73 | 17.8 |
| No | 284 | 37.8 | No | 326 | 79.5 |
| Refuse to answer | 54 | 7.2 | Refuse to answer | 6 | 1.5 |
| Missing data | 3 | 0.4 | Missing data | 5 | 1.2 |
| Consumption of alcohol or other drug before sex | Casual sex partners | ||||
| Yes | 21 | 5.1 | Yes | 41 | 10.0 |
| No | 389 | 94.9 | No | 363 | 88.5 |
| Missing data | 0 | – | Missing data | 6 | 1.5 |
| Anal intercourse | Unprotected sex | ||||
| Yes | 28 | 6.8 | Yes | 205 | 50.0 |
| No | 345 | 84.2 | No | 61 | 14.9 |
| Refuse to answer | 33 | 8.0 | Refuse to answer | 129 | 31.5 |
| Missing data | 4 | 1.0 | Missing data | 15 | 3.6 |
Prevalence of STDs
Among all participants who have ever tested for STDs (n=386), 53 (13.7%) reported at least one STD (table 2), resulting in a self-reported STD prevalence of 7.06% (95% CI 5.23% to 8.89%). Vulvovaginal candidiasis was the most common STDs (5.82%, 95% CI 3.46% to 8.18%) followed by PID (3.97%, 95% CI 2% to 5.94%). Prevalence of other STDs was between 0% and 2.13%. More than one STD occurred in 22 participants (2.93%, 95% CI 1.72% to 4.14%) and coinfection was particularly common among those with vulvovaginal candidiasis, HBV, PID, bacterial vaginosis and chlamydia.
Table 2.
Results of self-report STD screening and infection among participants
| Screening (n) | Infection (n) | Self-report prevalence (%) | 95% CI | |
|---|---|---|---|---|
| STDs | 386 | 53 | 7.06 | 5.23 to 8.89 |
| Chlamydia | 189 | 4 | 0.53 | 0.02 to 1.04 |
| Gonorrhoea | 195 | 4 | 0.53 | 0.02 to 1.04 |
| Genital warts | 150 | 2 | 0.27 | 0.08 to 0.46 |
| Syphilis | 197 | 2 | 0.27 | 0.08 to 0.46 |
| Trichomonas vaginalis | 119 | 6 | 1.59 | 0.33 to 2.85 |
| HBV | 363 | 16 | 2.13 | 1.10 to 3.16 |
| NSU | 50 | 2 | 0.27 | 0.08 to 0.46 |
| PID | 131 | 15 | 3.97 | 2.0 to 5.94 |
| Vulvovaginal candidiasis | 134 | 22 | 5.82 | 3.46 to 8.18 |
| Bacterial vaginosis | 131 | 13 | 3.44 | 1.60 to 5.82 |
| Herpes | 137 | 0 | 0.00 | – |
| Pubic lice | 120 | 0 | 0.00 | – |
| Epididymitis | 42 | 0 | 0.00 | – |
| Others | 142 | 1 | 0.13 | 0.06 to 0.20 |
| More than one | – | 22 | 2.93 | 1.72 to 4.14 |
| STDs |
HBV, hepatitis B virus; NSU, non-specific urethritis; PID, pelvic inflammatory disease; STDs, sexually transmitted diseases.
Distribution of high and low rate spatial clusters
Spatial cluster analysis identified seven significantly high rate clusters in our study (table 3, figure 1 and see online supplementary table S1). The cluster for migrants was the largest (p<0.001), with 148 migrants and 101.24 expected cases, resulting in a cluster rate ratio (RR) of 1.46. Among all migrants in the cluster, 27.7% (41/148) had unprotected sex, 10.1% (15/148) had multiple sexual partnerships, 7.4% (11/148) had casual sex partners, 4.1% (6/148) had anal intercourse and 4.1% (6/148) used alcohol or other drugs before sex. A high rate cluster for college students (cluster radius=6.95 km, p<0.001) detected in Tianhe included 52 observed cases with 27.02 expected.
Table 3.
Results of cluster analysis: clusters with high and low rates
| Case definition | High rate clusters
|
Low rate clusters
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster type | No of observed cases | No of expected cases | LLR | Cluster RR | p Value | Cluster type | No of observed cases | No of expected cases | LLR | Cluster RR | p Value | |
| Sociodemographic characteristics | ||||||||||||
| Gender: male | Primary | 10 | 4.97 | 7.06 | 2.01 | 0.352 | Primary | 24 | 40.73 | 7.88 | 0.59 | 0.171 |
| Marital status: married/cohabitated | Primary | 201 | 166.56 | 15.36 | 1.21 | <0.001 | Primary | 167 | 202.88 | 15.08 | 0.82 | <0.001 |
| Employment: unemployed | Primary | 4 | 0.55 | 7.98 | 7.23 | 0.108 | Primary | 18 | 33.46 | 6.85 | 0.54 | 0.240 |
| Duration of living in Guangzhou: ≤1 year | Primary | 16 | 4.60 | 11.64 | 3.47 | 0.007 | Primary | 27 | 45.20 | 8.50 | 0.60 | 0.051 |
| Migrants: yes | Primary | 148 | 101.24 | 36.39 | 1.46 | <0.001 | Primary | 131 | 189.25 | 37.98 | 0.69 | <0.001 |
| Migrants: yes | Secondary | 49 | 34.51 | 8.55 | 1.42 | 0.10 | Secondary | 1 | 8.63 | 9.03 | 0.12 | 0.078 |
| College students: yes | Primary | 52 | 27.02 | 19.58 | 1.92 | <0.001 | Primary | 16 | 36.26 | 13.15 | 0.44 | <0.001 |
| Sexual orientation: not heterosexual | Primary | 5 | 0.41 | 9.57 | 12.06 | 0.051 | Primary | 0 | 4.02 | 4.40 | 0 | 0.610 |
| Risky sexual behaviours | ||||||||||||
| Consumption of alcohol/other drug before sex: yes | Primary | 4 | 0.26 | 9.82 | 15.62 | 0.010 | Primary | 0 | 5.99 | 7.28 | 0 | 0.038 |
| Anal intercourse: yes | Primary | 5 | 0.38 | 13.39 | 13.32 | <0.001 | Primary | 0 | 4.58 | 5.22 | 0 | 0.309 |
| Multiple sexual partnerships: yes | Primary | 3 | 0.55 | 5.15 | 5.47 | 0.680 | Primary | 5 | 17.93 | 9.29 | 0.28 | 0.025 |
| Lived with one of multiple sex partners: no | Primary | 9 | 5.77 | 4.41 | 1.56 | 0.269 | Primary | 7 | 13.45 | 6.37 | 0.52 | 0.084 |
| Casual sex partners: yes | Primary | 4 | 0.51 | 6.93 | 7.88 | 0.296 | Primary | 1 | 10.45 | 9.07 | 0.09 | 0.012 |
| Unprotected sex: yes | Primary | 30 | 23.12 | 8.37 | 1.30 | 0.036 | Primary | 0 | 3.08 | 5.99 | 0 | 0.249 |
| STDs | ||||||||||||
| Self-reported STDs: yes | Primary | 2 | 0.13 | 5.52 | 15.46 | 0.630 | Primary | 0 | 3.49 | 3.75 | 0 | 0.960 |
All dichotomous, ordinal and nominal variables were scanned for high and low rate clusters by Bernoulli model, ordinal model and multinomial model, respectively. LLR, likelihood ratio; STDs, sexually transmitted diseases; RR, rate ratio.
Figure 1.
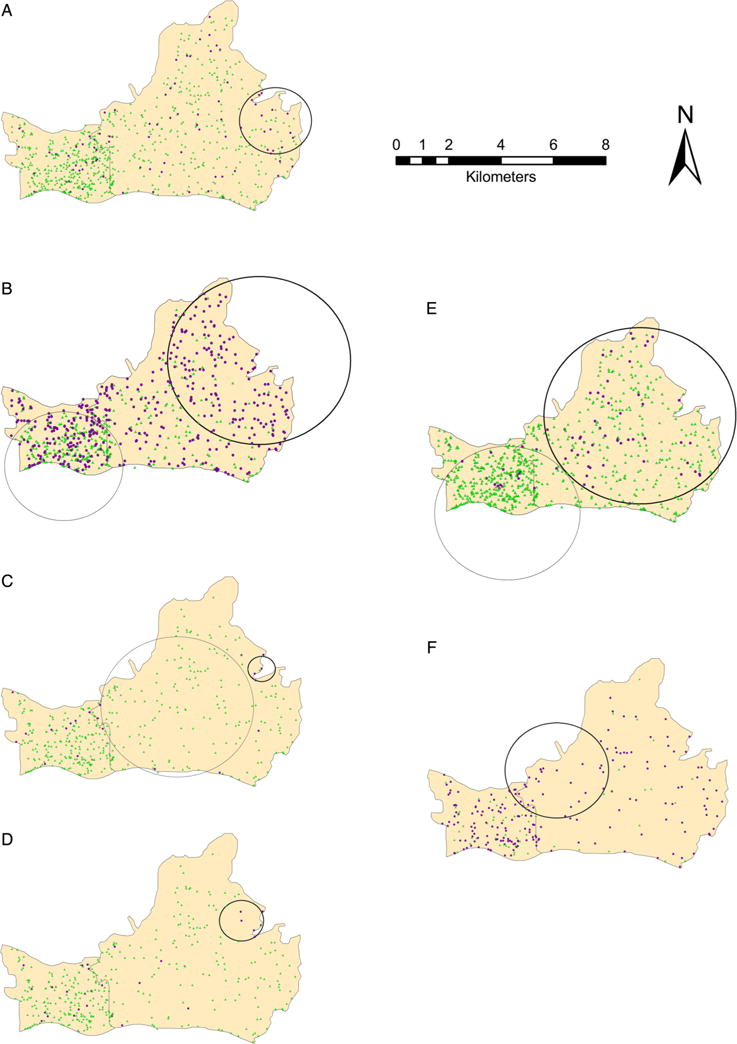
Results of cluster analysis. (A) The cluster for duration of living in Guangzhou (purple circular: ≤1 year, green triangle: >1 year). (B) The cluster for migrants (purple circular: yes, green triangle: no). (C) The cluster for consumption of alcohol or other drug before sex in the last 12 months (purple circular: yes, green triangle: no). (D) The anal intercourse cluster in the last 12 months (purple circular: yes, green triangle: no). (E) The cluster for college students (purple circular: yes, green triangle: no). (F) Results of cluster analysis for unprotected sex in the last 12 months (purple circular: yes, green triangle: no). Note: heavy line circles stand for high rate cluster and fine line circles stand for low rate cluster.
There were three significant clusters identified by risky sexual behaviours. The cluster for consumption of alcohol or other drugs before sex (p=0.01) included four participants and 0.26 expected (RR=15.62). The anal intercourse cluster (p<0.001) included five individuals compared with the expected number of 0.38 (RR=13.32). The migrants cluster overlapped both of the two clusters in Tianhe district. The cluster for unprotected sex (p=0.036) included 30 participants and 23.12 expected cases (RR=1.30), which was overlapped by the college students cluster. Five of nine (55.6%) students located in the cluster for unprotected sex had a sexual experience during the last 12 months.
Six significant low rate clusters were demonstrated in table 3, figure 1 and see online supplementary table S2. No statistically significant high rate clusters were identified for other sexual risk behaviours or for STDs.
DISCUSSION
The lifetime prevalence of any self-reported sexually transmitted infection (7.06%) in our study is greater than that in other population-representative studies in China containing self-report data (2.8%)7 or urine test-based data (5.8%).16 There are three main reasons that may explain the difference in prevalence of STDs between our study and others. First, Huang et al7 collected self-report data in the last 12 months. However, the current study assessed STDs in participants’ lifetime, which may include more cases occurring previous to the last year. Second, previous studies were conducted before 2010 or even before 2000, so there may be period and cohort effects. As we know, the prevalence of STDs in China has increased rapidly during the last decades, especially in the new millennium.3,4 Finally, the difference in participants’ age between our study and others may be a contributory factor. Previous studies were restricted to participants aged 18–39 years, however, the current study assessed STDs among adults aged 18–59 years. Although the physiology of sexual function declines with age, there is no significant decline in sexual interest. For example, Pearline et al17 found, among elders in China (>50 years), 46% acknowledged purchasing commercial sex, nearly 24% stated they had multiple sexual partners and <4% acknowledged condom use and STD prevalence in the elderly has been rising gradually. Risky sexual behaviours were also very common among our study population, with prevalence between 5.1% and 50%, which was similar to a meta-analysis focused on general population in China.18 Studies conducted around 2010 demonstrated that prevalence of multiple sexual partnerships was about 30% among migrants and 10% among college students, and prevalence of unprotected sex at the last intercourse was about 55% for the two groups. This trend suggests that the general population has the potential to drive prevalence of STDs in China and this need to be studied in the future. In addition, our data suggests an urgent need to enhance screening and testing of STDs among the general population. Rapid testing approaches are recommended for pilot testing in health facilities and communities due to high sensitivity and specificity.19 Further studies are required to evaluate the effectiveness and cost-effectiveness of such programmes in China.
This present study is consistent with social geographical network theory,20 which emphasises that geographical connection and migration network influences the geographical distribution of STDs/HIV and related high-risk behaviours. Our study displayed great convergence with only minor differences in migrants’ spatial distribution (especially short-term migrants) and risky sexual behaviours. Worldwide, epidemiological studies observed a high prevalence of risky sexual behaviours among immigrants.21 In China, rural-to-urban migrants may have different sexual norms, and limited knowledge about either contraception and reproductive health or protection against STDs/HIV, which suggests that migrants are particularly vulnerable to STDs.22 In addition, most migrants who move to urban regions without their families may cohabitate with rural companions, and an estimated 52% may have temporary husbands or wives to search for social support, which may increase risk of STD/HIV transmission.23 Implementing interventions and public policies are needed to reduce the risk of STDs for migrant communities. These initiatives can include the provision of sexual and reproductive health education and STD/HIV-related health services (screening and counselling).
In this study, excess cases for unprotected sex overlapped the college students’ cluster. Similarly, Ali et al24 found a high prevalence of unprotected sex among sexually active students in the Netherlands, of whom 58.9% boys and 75.8% girls reporting inconsistent condom use with their steady partner. In the USA, the percentage of students who used a condom during the last intercourse decreased from 2003 to 2013 (63%–59%).25 A possible explanation could be that sexually active young adults lacked sexual health knowledge and contraceptive use26 due to the absence of sexual health education. According to Chinese government reports, the STD/HIV epidemic among young students is clearly on the rise in recent years.27 These findings highlight college students, as they make the transition to adulthood, should be another key population targeted with enhanced efforts to increase the strength of health education and STD/HIV-related health services.
All the high rate clusters noted above were found in the middle and northeast parts of Tianhe district. These parts are considered rural–urban continuums and industrial parks, where the cost of living is relatively lower and more employment opportunities are available. Consequently, a greater number of migrants clustered there. Meanwhile, there are >60 colleges and universities locate in the middle of Tianhe district, which explains the large cluster for college students localised there.
In terms of STD cases, our study found that there was no spatial cluster for STDs, which seemed to be contradictory to studies done in Africa28 and China29 previously. This study was designed to obtain a population-representative sample in Guangzhou. Studies conducted in Africa mainly targeted key populations or areas, which may not represent the general population well. Another possible reason is thought to be the difference in data measurements. STD cases reported by official public clinics or clinical trial sites were analysed in previous studies, while self-report data were used in the current study. In China, sexual health, especially STDs/HIV, was and remains a taboo topic. Participants may worry about information disclosure and stigma; therefore under-report their history of STDs even though they used tablet devices in our study. Furthermore, official STD testing services are only available in authorised hospitals and specialised STD/dermatology clinics, which are mostly concentrated in the downtown area of Guangzhou. The centralisation of these institutions as well as low income and lack of health insurance among migrants limit their options for seeking and receiving services.30 Consequently, they often seek STD services at unofficial local clinics that do not routinely report STD cases. In addition, many STDs are asymptomatic so that migrants are less likely to seek medical care due to a lack of sexual health knowledge.
This study is one of the first efforts to explore the spatial association between sociodemographic characteristics, risky sexual behaviours and STDs based on geographical variables from a representative population-based sample in China. However, our study has limitations that need to be considered in the interpretation of the results. First, as is inherent with all cross-sectional studies, this study could neither establish temporality nor causality of the observed associations with the outcome. Second, there might be recall bias on risky sexual behaviours and STDs or under-reported information due to participants’ privacy concerns. In order to maximally reduce bias, we used tablet devices to conduct the interviews, the respondents can read and respond privately, which maintained the privacy during the interviews. The data were uploaded to a secure university server immediately by the participants themselves so that interviewers were not able to review the responses. Therefore, compared with traditional face-to-face and paper-and-pencil interview, participants in our research study may have been less worry about disclosure of personal information. Furthermore, this is a pilot study with limited human, material and financial resources so it was confined to only two districts in Guangzhou. Our results might not be generalisable to other geographical locations so caution is needed when making comparisons with other regions and cities.
CONCLUSION
The present study provides useful information about risky sexual behaviours to inform STD control programmes in Guangzhou related to identifying high-risk geographies. Current sexual health intervention programmes and research mostly focus on key populations, but this spatial analysis highlights the need to better identify, screen and intervene on a general population level, including migrants and college students in Guangzhou. Identification of these clusters is critical for targeted intervention to optimise health and prevent a generalised epidemic of STDs.
Supplementary Material
Key messages.
-
▸
Identifying clusters of risky sexual behaviours is critical for optimising surveillance and targeting control measures for specific risk locations.
-
▸
The geographical distribution of short-term migrants and college students overlapped with risky sexual behaviours.
-
▸
Improved sexually transmitted diseases-prevention interventions should be developed to reduce the risk of risky sexual behaviours among short-term migrant and student populations.
Acknowledgments
The authors wish to thank Professor Michael Emch from Department of Geography and Department of Epidemiology, School of Public Health, University of North Carolina at Chapel Hill for his kind support in spatial sampling strategy. In addition, we thank all the study participants and the team of interviewers from Sun Yat-sen University and the University of Macau.
Funding This project is funded by Fogarty Global Health Fellows Program Consortium comprised of the University of North Carolina, John Hopkins Bloomberg School of Public Health, Morehouse and Tulane (5R25TW009340-02, 1R25TW009340-01). BJH received additional support from grant SRG2014-00001-FSS awarded by the R&DAO, University of Macau and JDT received support from National Institutes Health (grant 1R01AI114310-01, 5P30AI050410-13, 1D43TW009532-02).
Footnotes
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/sextrans-2015-052268).
Handling editor Jackie A Cassell
Contributors WC, BJH, JDT and LL designed the study. WC, FZ and BJH did the data collection. FZ and WC also did statistical analysis, literature search and wrote the first draft of the manuscript. All authors contributed to the data interpretation and manuscript development. All authors reviewed and approved the final version of the manuscript.
Competing interests None declared.
Patient consent Obtained.
Ethics approval Both the Institutional Review Board at Guangdong Provincial Skin Diseases and STI (5-39593) Control Center and University of North Carolina at Chapel Hill (12-2457) approved the study protocol.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.World Health Organization. Sexually transmitted infections (STIs) 2013 http://www.who.int/mediacentre/factsheets/fs110/en/ (accessed Jul 2015)
- 2.Woodhall S, Ramsey T, Cai C, et al. Estimation of the impact of genital warts on health-related quality of life. Sex Transm Infect. 2008;84:161–6. doi: 10.1136/sti.2007.029512. [DOI] [PubMed] [Google Scholar]
- 3.Chen XS, Gong XD, Liang GJ, et al. Epidemiologic trends of sexually transmitted diseases in China. Sex Transm Dis. 2000;27:138–42. doi: 10.1097/00007435-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 4.National center for AIDS/STD control and prevention, China CDC. Chinese National Notifiable Disease Reported 2014. 2015 http://ncaids.chinacdc.cn/yqjc/blbg/201503/t20150302_111777.htm (accessed Mar 2015)
- 5.Xiao Z, Mehrotra P, Zimmerman R. Sexual revolution in China: implications for Chinese women and society. AIDS Care. 2011;23(Suppl 1):105–12. doi: 10.1080/09540121.2010.532537. [DOI] [PubMed] [Google Scholar]
- 6.Pan S, Huang Q, Shi M, et al. The accomplishment of a sexuality revolution in China—oreliminary report of a comparison study between 2000 and 2006. Gaoxiong: Universal Press; 2008. [Google Scholar]
- 7.Huang Y, Abler L, Pan S, et al. Population-based sexual behavior surveys in China: Liuzhou compared with other prefectural cities. AIDS Behav. 2014;18(Suppl 2):S118–25. doi: 10.1007/s10461-013-0645-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.State Statistics Bureau, People’s Republic of China. The statistical bulletin of national economic and social development in 2014. 2015 http://www.stats.gov.cn/tjsj/zxfb/201502/t20150226_685799.html (accessed Jul 2015)
- 9.He N. Sociodemographic characteristics, sexual behavior, and HIV risks of rural-to-urban migrants in China. Biosci Trends. 2007;1:72–80. [PubMed] [Google Scholar]
- 10.Han Y, Kim H, Ma J. School bonds and the onset of substance use among Korean youth: an examination of social control theory. Int J Environ Res Public Health. 2015;12:2923–40. doi: 10.3390/ijerph120302923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson FG. Social isolation and bereavement. Lancet. 1970;296:1356–7. doi: 10.1016/s0140-6736(70)92374-3. [DOI] [PubMed] [Google Scholar]
- 12.Zou X, Chow EP, Zhao P, et al. Rural-to-urban migrants are at high risk of sexually transmitted and viral hepatitis infections in China: a systematic review and meta-analysis. BMC Infect Dis. 2014;14:490. doi: 10.1186/1471-2334-14-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paavonen J, Westrom L, Eschenbach D, et al. Pelvic inflammatory disease. In: Holmes KK, Sparling PF, Stamm WE, et al., editors. Sex transm dis. 4th. New York City: McGraw-Hill; 2008. pp. 1017–50. [Google Scholar]
- 14.Parish WL, Laumann EO, Cohen MS, et al. Population-based study of chlamydial infection in China: a hidden epidemic. JAMA. 2003;289:1265–73. doi: 10.1001/jama.289.10.1265. [DOI] [PubMed] [Google Scholar]
- 15.Kulldorff M. Commentary: geographical distribution of sporadic Creutzfeldt-Jakob Disease in France. Int J Epidemiol. 2002;31:495–6. [PubMed] [Google Scholar]
- 16.Wang W, Wei C, Buchholz ME, et al. Prevalence and risks for sexually transmitted infections among a national sample of migrants versus non-migrants in China. Int J STD AIDS. 2010;21:410–5. doi: 10.1258/ijsa.2009.008518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearline RV, Tucker JD, Yuan LF, et al. Sexually transmitted infections among individuals over fifty years of age in China. AIDS Patient Care STDS. 2010;24:345–7. doi: 10.1089/apc.2009.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai R, Richardus JH, Looman CW, et al. Trends in high-risk sexual behaviors among general population groups in China: a systematic review. PLoS ONE. 2013;8:e79320. doi: 10.1371/journal.pone.0079320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kriesel JD, Bhatia AS, Barrus C, et al. Multiplex PCR testing for nine different sexually transmitted diseases. Int J STD AIDS. 2015 doi: 10.1177/0956462415615775. Published Online First. Epub Date: Nov 6 2015. [DOI] [PubMed] [Google Scholar]
- 20.Wallace R. Traveling waves of HIV infection on a low dimensional ‘socio-geographic’ network. Soc Sci Med. 1991;32:847–52. doi: 10.1016/0277-9536(91)90311-y. [DOI] [PubMed] [Google Scholar]
- 21.Saggurti N, Mahapatra B, Swain SN, et al. Male migration and risky sexual behavior in rural India: is the place of origin critical for HIV prevention programs? BMC Public Health. 2011;11(Suppl 6):S6. doi: 10.1186/1471-2458-11-S6-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tucker JD, Henderson GE, Wang TF, et al. Surplus men, sex work, and the spread of HIV in China. AIDS. 2005;19:539–47. doi: 10.1097/01.aids.0000163929.84154.87. [DOI] [PubMed] [Google Scholar]
- 23.Xu H. HIV prevention and control among migrant population in China. J China AIDS/STD Prev Control. 2001;7:376–7. [Google Scholar]
- 24.Ali H, Guy RJ, Fairley CK, et al. Understanding trends in genital Chlamydia trachomatis can benefit from enhanced surveillance: findings from Australia. Sex Transm Infect. 2012;88:552–7. doi: 10.1136/sextrans-2011-050423. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention in United States. Trends in the Prevalence of Sexual Behaviors and HIV Testing National Youth Risk Behavior Survey (YRBS): 1991–2013. 2014 http://www.cdc.gov/healthyyouth/data/yrbs/pdf/trends/us_sexual_trend_yrbs.pdf (accessed Jul 2015)
- 26.Long L, Yuan T, Wang M, et al. Factors associated with condom use among male college students in Wuhan, China. PLoS ONE. 2012;7:e51782. doi: 10.1371/journal.pone.0051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National center for AIDS/STD control and prevention, China CDC. The number of 15∼24 years of young students infected with HIV increased year by year in Yunnan Province, China. 2014 http://ncaids.chinacdc.cn/yqjc/blbg/201411/t20141126_106989.htm (accessed Nov 2014)
- 28.Wand H, Ramjee G. Targeting the hotspots: investigating spatial and demographic variations in HIV infection in small communities in South Africa. J Int AIDS Soc. 2010;13:41. doi: 10.1186/1758-2652-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu X, Tucker JD, Hong F, et al. Multilevel and spatial analysis of syphilis in Shenzhen, China, to inform spatially targeted control measures. Sex Transm Infect. 2012;88:325–9. doi: 10.1136/sextrans-2011-050397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong Y, Li X, Stanton B, et al. Too costly to be ill: healthcare access and health-seeking behaviours among rural-to-urban migrants in China. World Health Popul. 2006;8:22–34. doi: 10.12927/whp.2006.18280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


