Abstract
This paper reviews the importance of mesenchymal-epithelial interactions in development and gives detailed technical protocols for investigating these interactions. Successful analysis of mesenchymal-epithelial interactions requires knowing the ages in which embryonic, neonatal and adult organs can be separated into mesenchymal and epithelial tissues. Methods for separation of mesenchymal and epithelial and preparation of tissue recombinants are described.
Keywords: Mesenchymal-epithelial interactions, Mesenchyme epithelium, Differentiation, Development, Mesenchyme, Epithelium, Tissue recombinants
1. Introduction
Interactions between epithelium and connective tissue play a central role in development of all organs composed of an epithelial parenchyma whether the epithelium is derived from ectoderm, endoderm and mesoderm. Such interactions are involved in development of the integument (ectoderm) and all of its derivatives such as the mammary gland, salivary glands, pituitary gland, preputial glands, hair, teeth, nails, feathers, claws and other derivatives. Likewise, the development of the endodermal gastrointestinal system and its derivatives (lungs, liver, pancreas, urinary bladder, prostate, gall bladder and the urethra) are dependent upon mesenchymal-epithelial interactions. Organs composed of mesoderm-derived epithelium (kidney, epididymis, vas deferens, seminal vesicle, uterus, cervix and vagina) also owe their development to mesenchymal-epithelial interactions. Thus, interactions between mesenchyme and epithelium are one of the fundamental developmental mechanisms responsible for the development of the majority of organs in the body.
In the embryo these interactions are called mesenchymal-epithelial interactions, signifying interactions between epithelium and undifferentiated embryonic connective tissue (mesenchyme). Such interactions between epithelium and connective tissue continue throughout life, but postnatally are called stromal-epithelial interactions (Cunha et al., 1985). The significance of embryonic mesenchymal-epithelial interactions is that during development the mesenchyme induces and specifies epithelial identify, and development (Cunha et al., 1983a; Haffen et al., 1987; Saxen, 1987; Birchmeier and Birchmeier, 1993; Baskin et al., 1996; Cunha and Hom, 1996), a process that includes specifying the types of secretory products produced by the epithelium (Cunha et al., 1995; Aboseif et al., 1999).
There are two types of tissue recombinants: homotypic and heterotypic. Homotypic tissue recombinants are composed of epithelium and mesenchyme derived from the same organ. For homotypic tissue recombinants the expectation is that the epithelium and mesenchyme will continue their normal development provided that both tissues are derived from wild-type mice. Of course, for homotypic tissue recombinants one tissue may be derived from a mutant mouse to assess the role of gene knockout on development. Heterotypic tissue recombinants, composed of epithelium and mesenchyme derived from different organ rudiments, are constructed with the expectation that the mesenchyme may reprogram epithelial development (Table 1). Reprogramming of epithelial development by heterotypic mesenchyme has been shown to involve: (a) Epithelial morphogenesis, (b) epithelial cytodifferentiation, (c) specification of keratin expression, (d) regulation of epithelial proliferation and apoptosis, (e) specification of secretory proteins, (f) induction of epithelial androgen receptor, (g) epithelial expression of heparin sulfate proteoglycans, (h) epithelial expression of p63, (i) epithelial expression of Hox genes, and (j) regulation of epithelial progesterone receptor (Cunha et al., 1997; Kurita et al., 2001a; Kurita and Cunha,2001; Kurita et al., 2001b). Surprisingly, there appears to be germ layer restrictions on the ability of an epithelium to respond to a heterotypic mesenchyme. For example, urinary bladder epithelium is derived from endoderm and when combined with mesenchyme from either the urogenital sinus (a prostate inductor) or seminal vesicle, the result is prostatic differentiation (Table 1). Seminal vesicle and urogenital sinus mesenchyme are closely related anatomically and both are glandular inductors. While seminal vesicle mesenchyme can induce glandular differentiation in endodermal bladder epithelium, the endpoint is prostate and not seminal vesicle, perhaps because prostatic differentiation is within the development repertoire of endoderm, while seminal vesicle is not. Likewise, urogenital sinus mesenchyme induces seminal vesicle differentiation when combined with mesoderm-derived epithelia of the epididymis, ductus deferens and ureter (Table 1), again perhaps because seminal vesicle differentiation is within the development repertoire of mesoderm, while prostate is not. Thus, while the result in such tissue recombinants in a dramatic reprogramming of epithelial differentiation, the result is specified by the germ layer origin of the epithelium. An apparent exception to this rule is the induction of preputial gland differentiation by preputial gland mesenchyme in bladder epithelium (Taylor et al., 2009).
Table 1.
Heterotypic inductions in which the mesenchyme elicits a new developmental fate in the epithelium.
| Mesenchyme | Epithelium | Epithelial germ layer | Induced epithelial differentiation | Reference |
|---|---|---|---|---|
| Urogenital sinus | Adult bladder | Endoderm | Prostate | (Cunha et al., 1983b) |
| Urogenital sinus | Urethra | Endoderm | Prostate | (Boutin et al., 1991) |
| Seminal vesicle | Bladder | Endoderm | Prostate | (Donjacour and Cunha, 1988) |
| Seminal vesicle | Adult ureter | Mesoderm | Seminal vesicle | (Cunha et al., 1991; Lipschutz et al., 1996) |
| Seminal vesicle | Adult ductus deferens | Mesoderm | Seminal vesicle | (Cunha et al., 1991) |
| Seminal vesicle | Wolffian duct | Mesoderm | Seminal vesicle | (Higgins et al., 1989) |
| Seminal vesicle | Adult epididymis | Mesoderm | Seminal vesicle | (Turner et al., 1989; Cunha et al., 1992) |
| Uterus | Vagina | Mesoderm | Uterine | (Cunha, 1976) |
| Vagina | Uterus | Mesoderm | Vagina | (Cunha, 1976) |
| Mammary gland | Epidermis | Ectoderm | Mammary gland | (Cunha et al., 1995) |
| Rectum | Bladder | Endoderm | Rectum | (Li et al., 2000) |
| Back skin | Plantar foot skin | Ectoderm | Hair follicles | (Kollar, 1970) |
| Tooth | Plantar foot skin | Ectoderm | Tooth | (Kollar and Baird, 1970) |
| Duodenum | Stomach | Endoderm | Duodenum | (Ishizuya-Oka and Mizuno, 1984; Yasugi and Mizuno, 2008) |
| Preputial gland | Bladder | Endoderm | Preputial gland | (Taylor et al., 2009) |
Based upon decades of research on mesenchymal-epithelial interactions, the following concepts have emerged. (a) Embryonic epithelial development and differentiation is impaired or completely abrogated in the absence of mesenchyme even though an appropriate extracellular matrix can substitute for living mesenchyme for certain aspects of epithelial development. (b) Mesenchyme induces and specifies epithelial development. (c) Mesenchyme itself undergoes a differentiation process, which is dependent upon an interaction with epithelium (Cunha et al., 1989; DiSandro et al., 1998). Thus, mesenchymal-epithelial interactions involve reciprocal signaling of mesenchyme to epithelium and epithelium to mesenchyme. (d) Temporal factors play an important role in morphogenetic mesenchymal-epithelial interactions, in so far as initially the epithelium is undifferentiated and not committed to a particular differentiation endpoint. Subsequently, as a result of mesenchymal induction, epithelial identity is covertly specified, that is, determined but not yet expressed. Continued mesenchymal-epithelial interactions promote specific morphogenetic programs such as ductal branching morphogenesis followed by specific forms of cytodifferentiation and ultimately the production of epithelial specific proteins, such as tissue-specific secretory proteins (Higgins et al., 1989; Cunha et al., 1995; Aboseif et al., 1999).
Mesenchymal specification of epithelial identity requires that the epithelium is uncommitted in regard to a particular differentiation endpoint, that is, capable to responding to inductive cues from heterotypic mesenchyme. The typical scenario is that early in development, the epithelium is developmentally plastic, uncommitted and thus capable of being reprogrammed by heterotypic mesenchyme. For example, Fig. 1 illustrates a series of tissue recombinants between neonatal epithelium and mesenchyme for the mouse uterus and vagina, each of which exhibits a unique histodifferentiation and a unique molecular profile. In this experiment uterine mesenchyme induced vaginal epithelium to undergo uterine differentiation, and vaginal mesenchyme induced uterine epithelium to undergo vaginal differentiation (Cunha, 1976). Subsequent studies have demonstrated that the newly induced uterine and vaginal cytodifferentiation is associated with expression of vaginal- and uterine-specific molecular markers (Kurita, 2011). The initial mesenchyme-induced covert commitment to a new phenotype may be an irreversible event in so far as the covertly determined epithelium may continue to express its normal differentiation program even when recombined with a heterotypic mesenchyme. For example, when 13-day embryonic salivary gland epithelium is associated with seminal vesicle mesenchyme, salivary gland development continues (Cunha, 1972). Likewise, association of embryonic mammary gland epithelium with salivary gland mesenchyme (SgM + MgE) results in development of a ductal branching pattern that is distinctly salivary gland, but the epithelium maintains mammary gland identity and produces milk proteins (Sakakura et al., 1976). The commitment of the epithelium to a particular endpoint typically becomes fixed over a specific time course. For example, vaginal and uterine epithelial differentiation is gradually specified by inductive cues from mesenchyme in the early neonatal period. This was determined by examining tissue recombinants composed of vaginal epithelium plus uterine mesenchyme (UtM + VgE) and uterine epithelium plus vaginal epithelium (VgM + UtE) (Fig. 1). When UtM + VgE and VgM + UtE were prepared with epithelium derived from mice 0- to 5-days-old, all UtM + VgE tissue recombinants underwent uterine conversion of the original vaginal epithelium, and all VgM + UtE tissue recombinants underwent vaginal conversion of the original uterine epithelium. However, when the uterine and vaginal epithelia were derived from mice > 5 days of age, there was a progressive loss of responsiveness to the heterotypic mesenchymes, and thus the original epithelial differentiation was maintained even in the presence of heterotypic mesenchyme (Cunha, 1976). Such concepts have been derived by varying the age of the interacting tissues (heterochronic tissue recombinants), which provide important clues concerning the developmental process.
Fig. 1.
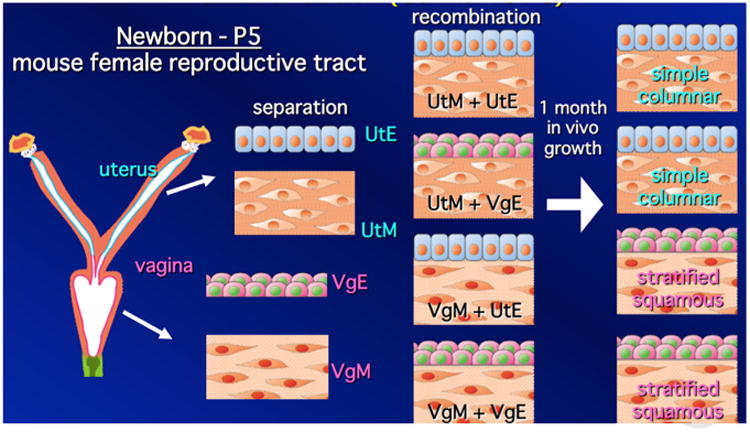
Mesenchymal specification of uterine and vaginal epithelial differentiation. To determine which tissue (mesenchyme or epithelium) specifies uterine and vaginal epithelial differentiation, four tissue recombinants were prepared with neonatal tissues (≤ 5 days postpartum) as indicated and grown under the renal capsule for 1 month in female hosts. Homotypic tissue recombinants (UtM + UtE and VgM + VgE) gave the expected differentiation with maintenance of epithelial prospective fate. Heterotypic tissue recombinants (VgM + UtE and UtM + VgE) demonstrated that mesenchyme specifies epithelial cytodifferentiation. Subsequent studies have shown that mesenchyme-altered epithelial cytodifferentiation is associated with expected molecular markers of uterine and vaginal epithelial differentiation (Kurita, 2011). (Figure drawn by Takeshi Kurita and used with permission).
Surprisingly, there are several examples in which an adult epithelium can be completely reprogrammed as a result of interaction with heterotypic mesenchyme of a different organ. The first example of such an extraordinary result involved tissue recombinants composed of urogenital sinus mesenchyme (UGM, a prostatic inductor) and epithelium derived from the adult urinary bladder (UGM + Adult BLE) (Fig. 2 and Table 1). In such tissue recombinants the adult urinary bladder epithelium was induced by UGM to undergo prostatic development and to produce prostatic secretory proteins and many other molecular markers indicative of prostatic epithelium (Cunha et al., 1983b; Donjacour and Cunha, 1993; Hayward et al., 1996; Aboseif et al., 1999). Since then, several more examples of this type of result have emerged (Table 1). Today, such results might be interpreted as involving the retention of undifferentiated stem cells in adult epithelia, even though evidence suggests that the prostatic epithelial cells emerging in UGM + BLE recombinants are derived from partially differentiated supra-basal cells in the adult bladder epithelium (Li et al., 2009)
Fig. 2.
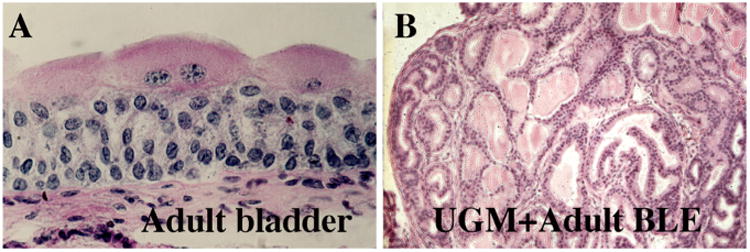
Histology of the adult mouse urinary bladder (A) and a tissue recombinant composed of 16 day embryonic urogenital sinus mesenchyme plus adult urinary bladder epithelium (UGM + Adult BLE). Note that the stratified non-glandular adult urinary bladder epithelium has differentiated into glandular tissue resembling prostate when grown in association with urogenital sinus mesenchyme. Further analysis has demonstrated the expression of many prostate-specific molecular markers in the prostate-induced adult bladder epithelium (Cunha et al., 1987).
2. Methods
2.1. Equipment and supplies
Dissection of embryonic/neonatal rudiments from donor animals requires the following items: Dissecting microscope, dissecting instruments (scissors of various sizes, forceps of various sizes including #5 Dumont forceps [Fine Science Tools, Foster City, CA], van Graefe microdissecting knife [Fine Science Tools, Foster City, CA], spring-loaded Vannas scissors [Fine Science Tools, Foster City, CA]), Pasteur pipettes, DMEM/Ham's F12 medium, Maximov depression slides (Fisher, Pittsburgh, PA) and plastic Petri dishes. A dissecting microscope with illumination capabilities both above and below the specimen stage is necessary. Intact embryonic/ neonatal organs, when viewed with transmitted light from below the stage, reveal not only the mesenchyme but also the internal epithelium (Fig. 3).
Fig. 3.
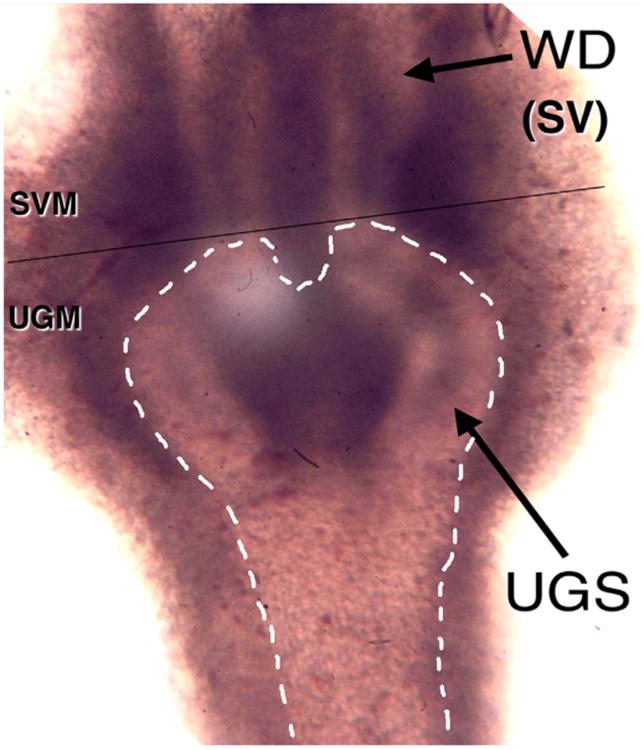
Wholemount image of a 16-day embryonic urogenital sinus (UGS) using transmitted light. The urogenital sinus epithelium is outlined with dashed lines and is surrounded with urogenital sinus mesenchyme (UGM). Above the UGS the two epithelial Wolffian ducts (WDs) can be seen, which are surrounded by seminal vesicle mesenchyme (SVM). The thin black line indicates where the specimen must be transected to isolate the WDs from the UGS.
3. Selection of age of developing rudiment and dissection
The objective of the tissue separation procedure is to isolate epithelium free of mesenchymal contamination and mesenchyme free of epithelial contamination. This can only be achieved if the morphological complexity of the interface between epithelium and mesenchyme is simple. Embryonic back skin, prior to the emergence of hair follicles, is a good example as the embryonic epidermis is essentially a flat epithelial sheet, and the epithelial-mesenchymal interface is straight and uncomplicated (Fig. 4A). Embryonic mammary glands and embryonic salivary glands can be separated into epithelium and mesenchyme when these organ rudiments are in the “light bulb” stage before initiation of branching morphogenesis (Fig. 4B). Thus, to select the appropriate age of the rudiment requires knowledge of the organogenetic process. In the examples above, the ideal age to obtain clean separation of embryonic mouse back skin is 14 days of gestation, one day before the appearance of hair follicles. While epidermis can be isolated from back skin at day 15, the delicate hair follicles will break off and be retained as contaminants of the dermis. Mammary gland rudiments can be separated cleanly from embryonic day 12 to about day 17, before branching morphogenesis begins, with day 13 to 14 being ideal (Cunha et al., 2000; Cunha, 2013a). Salivary gland rudiments should be dissected at day 13, the “light bulb” stage, before branching morphogenesis. Neonatal uterus should be obtained from birth to 5 or 6 days postnatal, before the delicate uterine glands emerge. In all cases the ideal ages to carry out epithelial-mesenchymal separations is before the interface between epithelium and mesenchyme becomes complex due to ductal branching, epithelial folding or outgrowth of epithelial appendages such as hair follicles or glands. Table 2 gives the ages in which clean separations can be achieved for a variety of organ rudiments. Note that embryonic mesenchymal-epithelial interactions become stromal-epithelial interactions in adulthood and that for certain adult organs (cornea, vagina, urinary bladder, esophagus, plantar surface skin) the epithelial-stromal interface remains simple into adulthood and thus allows for clean separations.
Fig. 4.
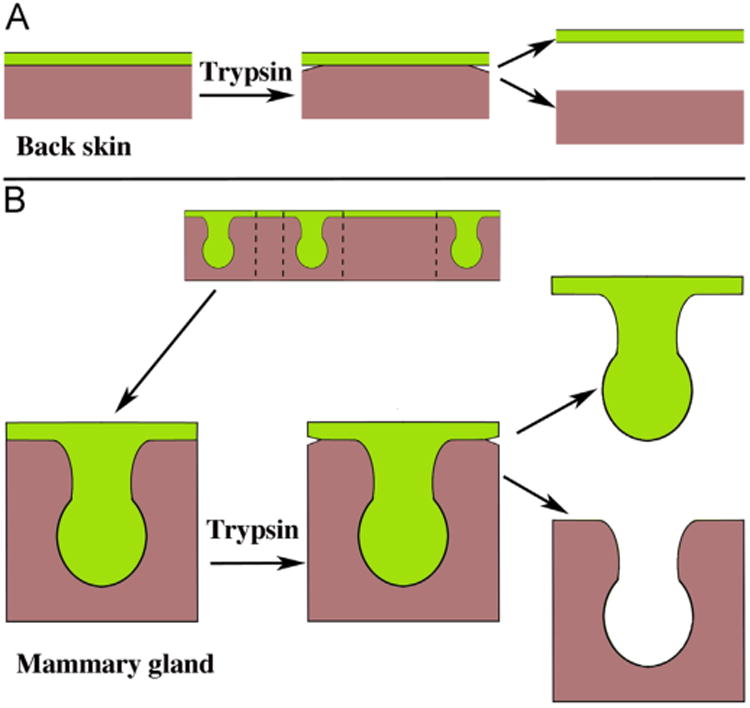
Schematics of 14-day embryonic back skin (A) and 13-day embryonic mammary glands (B). The flat planar epidermis shows evidence of “loosening” following trypsinization so that clean separation of epithelium and mesenchyme can be achieved (A). A strip of skin with three mammary epithelial buds is shown and in turn trimmed to size (B). Following trypsinization, the edges of the epidermis are separating, which indicates that the tryptic digestion was successful, thus allowing clean separation of the epithelium and mesenchyme. Mesenchyme = brown, epithelium = green. (Adapted from (Cunha, 2013b) with permission). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Optimal ages to achieve epithelial-mesenchymal separations for mouse organs.
| Organ | Age |
|---|---|
| Tooth | E12-13 |
| Vibrissae | E12 |
| Salivary gland | E13 |
| Back skin | E14 |
| Preputial gland | E14-16 |
| Urogenital sinus | E14-16 |
| Seminal vesicle | E16-P0 |
| Mullerian duct | E16-19 |
| Wolffian duct | E16-19 |
| Uterus | P0-P5 |
| Vagina | P0-adult |
| Urinary Bladder | E14-adult |
| Esophagus | E16-adult |
| Cornea | P0-adult |
| Plantar skin | E14-adult |
| Metanephric kidney | E11-12 |
| Ureter | P0-adult |
| Ductus deferens | P0-adult |
| Lung | E11-12 |
For gastrointestinal tract consult reviews leaders in the field (Haffen et al., 1987; Simon-Assmann et al., 1990; Yasugi and Mizuno, 2008).
After organ rudiments (or in some cases adult organs) are grossly dissected, they must be trimmed to an optimal size to allow for tissue separation. Gross dissection of blocks of skin containing mammary rudiments should be trimmed to the configuration indicated in Fig. 4B (Cunha et al., 2000; Cunha, 2013a), which is similar to that of 13-day salivary gland rudiments. For planar organs such as cornea, E14-day back skin, adult esophagus, adult urinary bladder, adult vagina, large flat tissue sheets should be trimmed to 1.5 to 2 mm square fragments. Of course, for adult tubular organs (adult esophagus, adult urinary bladder, and adult vagina), this first entails cutting them open to convert tubular morphology into planar morphology. For small tubular organ rudiments such as neonatal uterus, vas deferens, or embryonic Wolffian duct or Mullerian duct, the grossly dissected specimens should be trimmed to a length of 1.5 to 2 mm. Embryonic urogenital sinus should be trimmed as described previously (Staack et al., 2003) (Fig. 3). The actual separation of the above structures into epithelium and mesenchyme is somewhat unique for each specimen and will be described in detail below.
4. Separation of epithelium and mesenchyme/stroma
4.1. Supplies
Culture dishes and tubes (Becton-Dickinson, Franklin Lake, NJ), pipettes, Difco TM Trypsin 1/250 (Difco, Detroit, MI), calcium-magnesium-free Hanks salt solution, fetal calf serum, Dulbecco's modified Eagle's medium (DMEM). In a general sense once embryonic/neonatal rudiments or adult organs are isolated and trimmed to size, they can be separated into their epithelial and mesenchymal components following incubation for about 1.5 h in 1% Difco TM trypsin (1/250) in calcium-magnesium-free Hanks salt solution at 0 to 4 °C. After trypsinization the enzyme is neutralized with 10% fetal bovine serum in medium. Following tryptic digestion of planar organ rudiments, the edges of the epithelium should be separating and curling away from the mesenchyme at the peripheral cut edges (Fig. 4). Such obvious detachment of the epithelium from the mesenchyme is the clue that the enzymatic incubation has been successful. Simple teasing with fine forceps allows complete detachment of the intact epithelial sheet from the mesenchymal sheet. If the two tissues are still firmly adherent centrally, then additional trypsin digestion is required. For “light bulb like” organ rudiments such as mammary gland and salivary gland, a similar detachment of epithelium and mesenchyme should be evident at the cut edges of the specimen, in which case the mesenchyme can be “pinned” with forceps to the bottom of the dish and a second instrument used to tear the mesenchyme so that the epithelial “light bulb” can be teased out from its surrounding mesenchyme. During the separation process for both planar rudiments and glandular rudiments the epithelium should remain intact and should not fragment. Separated epithelium and mesenchyme should be stored in small tissue culture dishes prior to recombination in 10% fetal calf serum in medium.
An alternative method for separating epithelium and mesenchyme is to incubate the trimmed specimens in 0.02M EDTA in calcium-magnesium free (CMF) medium at 37 °C. The 0.02M EDTA can be made from a 0.5M EDTA stock solution. Prior to incubation with EDTA, wash the tissues 3 times (1 min each) in CMF medium then incubate in 0.02M EDTA in CMF medium for about 20 min at 37 degrees centigrade. When the epithelium and mesenchyme shows evidence of separating, fine forceps are used to complete the tissue separation, which should be carried out in CMF medium. After the tissues have been separated they should be stored briefly in complete medium containing calcium and magnesium. This is the preferred method for separating adult urinary bladder into its epithelial and stromal elements as the trypsin method results in severe fragmentation of the epithelium.
For small tubular organ rudiments such as neonatal uterus or vas deferens, the procedure is to trim the specimen to a length of 1.5 to 2 mm. The most efficient method of cutting embryonic or neonatal rudiments to size is a “scissors” technique in which the tip of the forceps is brought into contact with the bottom of the Maximov depression slide. In turn the van Graefe knife is brought into contact with the bottom of the dish and in contact with the forceps with the specimen in between (Fig. 5A). As the knife is slid towards the tip of the forceps, the specimen is compressed and is cut as the space between the two instruments and the bottom of the dish is reduced to zero. Obviously when a tube such as the neonatal uterus or vas deferens is transected, the epithelial tube and the surrounding mesenchymal tube are the same length (Fig. 5B). However, after trypsinization it will be noted that the ends of the epithelial tube extend beyond the ends of the mesenchymal tube as indicated (Fig. 5C). This means that the enzymatic digestion has been successful, and loosening of the adhesion between epithelium to mesenchyme has occurred, presumably because the trypsin has digested the basement membrane linking the epithelial tube to the mesenchymal tube, and the mesenchyme has contracted somewhat. To achieve separation of uterine epithelium and mesenchyme, cut across the trypsinized specimen at about 3/4s of the distance from the end by using a “scissor” technique described (Fig. 5A and D). The result is that the epithelial tube will break at the site of the cut (Fig. 5D), and will be extruded from the mesenchymal tube. After the extruded epithelial tube has been teased free of the mesenchyme, the mesenchymal tube can then be opened using the van Graefe knife, and the inner surface where the epithelium was present can be inspected and any residual epithelial pieces removed.
Fig. 5.
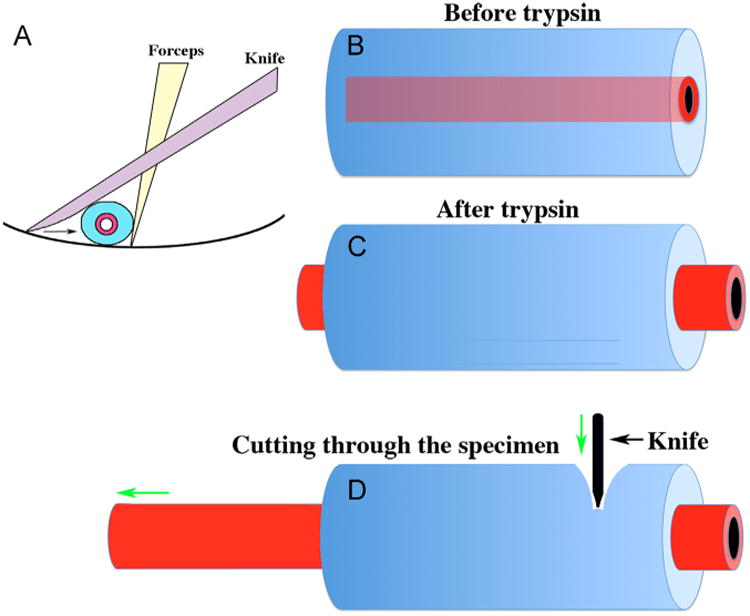
Method for cutting embryonic/neonatal organ rudiments (A) and separating epithelium and mesenchyme from tubular organs such as the neonatal uterus, ductus deferens or ureter (B–D). An optimal method of cutting organ rudiments to size involves a modified “scissor” technique (A) in which the specimen is positioned between forceps, a dissecting knife and the bottom of the dish Drawing the knife towards the forceps compresses the organ rudiment and results in a clean cut. Separating epithelium and mesenchyme from tubular organs is achieved after it is noted that the epithelial tube extends beyond the mesenchymal tube (C). Tissue separation is achieved by cutting acros the specimen using the technique in (A) following trypsination. This causes the epithelium to break cleanly at the point of the knife with extrusion of the epithelial tube out of the mesenchymal tube (D).
For even smaller tubular organs such as embryonic Mullerian or Wolffian ducts the procedure is initially similar. Trim the specimen to a length of 1.5 to 2 mm. After trypsinization, the epithelial tube will extend beyond the mesenchymal tube as described above for uterus. Then heat a Pasteur pipette to soften the glass and pull to create a smaller diameter tip. Introduce the “pulled” pipette into the flame of an alcohol burner to “fire polish” the tip. The goal is to create a pipette with a distal opening about the diameter of the epithelial Mullerian duct or Wolffian duct. It will take several tries to get this right, but once accomplished attach a length of tubing to the pipette for oral control. The separation procedure involves sucking the epithelial tube out of the mesenchymal tube. The resultant epithelial tube should remain intact and should be of a similar length to that of the original specimen. The ends of the mesenchymal tube can be trimmed away as any epithelial contamination will be at the ends of the mesenchymal tube.
5. Preparation of tissue recombinants
5.1. Materials
Finely drawn-out Pasteur pipettes, 35 or 60 mm culture dishes, Dumont #5 forceps, fetal calf serum, Bacto-Agar (Difco), 2× DMEM H16 medium, L-glutamine, penicillin, streptomycin, dissecting microscope equipped for trans-illumination of the specimen. To make the 1% Bacto-Agar in 1× DMEM, 1 gram of agar is dissolved in 50 ml of boiling distilled water. Cool the dissolved agar to about 50 degrees centigrade and then add 50 ml of 2× DMEM. Mix quickly and aliquot into 25 ml lots in small Erlenmeyer flasks. To make the final 0.5% Bacto-Agar plates, liquefy the 1% agar by heating to about 50 °C, dilute with 1× DMEM and dispense a layer of 0.5% agar about 3 mm in depth into 35 ml plastic dishes.
In summary tissue recombinant experiments begin with dissection and trimming of the rudiments as described above. Trimmed rudiments are then incubated in trypsin at 4 °C. Following neutralization of the enzyme with 10% fetal bovine serum in medium, the epithelium and mesenchyme are separated as described above and stored briefly in separate dishes. The pieces of mesenchyme are then transferred by pipette in a minimum volume of medium to culture dishes containing the solidified agar medium. Using a drawn out Pasteur pipette, excess liquid medium is removed from the surface of the agar by suction using either a manually or an orally controlled drawn out pipette. The mesenchymes are then pushed into aggregates of 2–4 mesenchymes (the amount of mesenchyme should be ≧ than that of the original rudiment). Again, any excess fluid on the surface of the agar is removed by suction using a drawn out Pasteur pipette. Tissue recombinants are prepared by transferring 1 or 2 epithelial rudiments with an orally controlled holding micropipette onto a mesenchymal aggregate on the agar gel. The epithelial rudiments should be placed on top of or surrounded laterally by mesenchyme. Following overnight culture at 37 °C in a humidified CO2 incubator, the tissues become firmly adherent to each other forming a cohesive tissue recombinant. For best results the tissue recombinants should be transplanted beneath the renal capsule of syngeneic or athymic nude mouse hosts. The technique of renal capsule grafting can be found at http://mammary.nih.gov/tools/mousework/Cunha001/index.html and is described in this volume. Following 1–4 weeks of in vivo growth, the grafts can be harvested for analysis.
For hormone target organs the hormonal status of the host must be appropriate, so choice of male versus female hosts is critical. Using nude mouse hosts, it is possible to prepare tissue recombinants with embryonic mesenchyme and epithelium from different genetic backgrounds (gene knockouts) or even from different species (rat-mouse, mouse-human, etc.). When in vivo grafting is part of the experimental design, immunologic considerations are paramount. If possible carry out experiments in which epithelium, mesenchyme and recipient host are of the same inbred strain. Cross strain or cross species (rat/mouse, mouse/human) tissue recombinants must be transplanted into immunedeficient hosts.
6. Validation of results
Whenever possible, one of the interacting tissues (preferably the epithelium) should be marked with GFP, LacZ, etc. By this means a single tissue recombinant can yield definitive results. In heterotypic tissue recombinants such as uterine mesenchyme plus vaginal epithelium (UtM + VgE), the presence of uterine epithelial differentiation as an endpoint result can be explained in two ways: (1) Artifact due to contamination of the uterine mesenchyme with its own uterine epithelium, or (2) that the uterine mesenchyme actually induced and reprogrammed the vaginal epithelium to differentiate into uterine epithelium. If the vaginal epithelium was derived from a “GFP mouse”, and the resultant harvested tissue recombinant contained simple columnar uterine epithelium expressing GFP, the interpretation is clear. When genetically marked tissues are not available, rat-mouse heterospecific tissue recombinants can be prepared as these interactions proceed across species lines (Cunha et al., 1983c; Aboseif et al., 1999). In this case the harvested tissue recombinants are embedded in paraffin and sectioned. After removal of the paraffin, stain with Hoechst dye 33258 as described (Cunha and Vanderslice, 1984)). Mouse cell nuclei have many bright fluorescent spots, whereas rat or human nuclei stain homogeneously.
Additional technical descriptions can be found in the literature (Cunha and Donjacour, 1987; Cunha et al., 2000; Cunha, 2013a).
7. Discussion
The study of tissue recombinants as a method to assess the role of mesenchymal-epithelial interactions has been applied to developing mammalian organs for over 50 years, with the first study of its kind being that of Clifford Grobstein (Grobstein, 1953), well before the availability of gene knockout mice. A particularly important use of tissue recombinant technology is determining the actual tissue responsible for the phenotype in germline knockout mice. In germline knockout mice, the gene of interest is deleted simultaneously in both epithelium and mesenchyme, and thus the phenotype of the knockout mouse could be due to absence of the gene in either the epithelium or mesenchyme (or both). Even though the phenotype of the mouse may be expressed in the epithelium, the altered epithelial phenotype may be due to impaired signaling from the mesenchyme. Thus, the answer to this question is paramount in understanding the mutant phenotype. This question was first addressed by Klaus Kratochwil who examined testosterone-induced destruction of the embryonic mouse mammary epithelium. To address this issue, Kratochwil prepared 4 tissue recombinants with embryonic epithelium and mesenchyme from wild-type and androgen-insensitive Tfm (testicular feminization) mice (wild-type mesenchyme + wild-type epithelium, wild-type mesenchyme + Tfm epithelium, Tfm mesenchy-me + wild-type epithelium, and Tfm mesenchyme + Tfm epithelium). This experiment was carried out before it was shown that the Tfm locus encodes the androgen receptor. All tissue recombinants were treated in vitro with testosterone. Destruction of the mammary epithelium occurred when wild-type mesenchyme was utilized (wild-type mesenchyme + wild-type epithelium, wild-type mesenchyme + Tfm epithelium). As expected, the mammary epithelium survived testosterone treatment in Tfm mesenchyme + Tfm epithelium tissue recombinants, but also survived in Tfm mesenchyme + wild-type epithelium tissue recombinants. The interpretation of these pioneering experiments is that testosterone-induced destruction of embryonic mouse mammary epithelium is due to a paracrine mechanism requiring the presence of androgen receptors in the mesenchyme (Kratochwil, 1971). In similar fashion Tfm/wild-type experiments have been carried out in developing prostate and have demonstrated that androgen-induced prostatic development requires the presence of androgen receptors in the mesenchyme (Cunha and Lung, 1978). Similar experiments have analyzed the phenotype of germline mutant mice lacking estrogen receptor alpha, the progesterone receptor and the arylhydrocarbon receptor. In all cases deficiencies in epithelial hormonal response has been shown to result from the absence of mesenchyme/stromal hormone receptors (Cooke et al., 1997; Kurita et al., 1998; Cunha et al., 2004). This body of mesenchymal-epithelial interaction research is the basis of paracrinology as an important mechanistic scenario in endocrinology. As more knockout mice come on line, the use of tissue recombinant research can contribute an important piece of the puzzle of development by determining the tissue (epithelium or mesenchyme) responsible for mutant phenotypes, thus chartering the course of future research.
Acknowledgments
This work was supported by NIH grant RO1 DK0581050.
Abbreviations
- DES
Diethylstilbestrol
- BPA
bis-phenol A
References
- Aboseif S, El-Sakka A, Young P, Cunha G. Mesenchymal reprogramming of adult human epithelial differentiation. Differ Res Biol Divers. 1999;65:113–118. doi: 10.1046/j.1432-0436.1999.6520113.x. [DOI] [PubMed] [Google Scholar]
- Baskin LS, Hayward SW, Sutherland RA, DiSandro MJ, Thomson AA, Goodman J, Cunha GR. Mesenchymal-epithelial interactions in the bladder. World J Urol. 1996;14:301–309. doi: 10.1007/BF00184602. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W. Molecular aspects of mesenchymal-epithelial interactions. Annu Rev Cell Biol. 1993;9:511–540. doi: 10.1146/annurev.cb.09.110193.002455. [DOI] [PubMed] [Google Scholar]
- Boutin EL, Battle E, Cunha GR. The response of female urogenital tract epithelia to mesenchymal inductors is restricted by the germ layer origin of the epithelium: prostatic inductions. Differ Res Biol Divers. 1991;48:99–105. doi: 10.1111/j.1432-0436.1991.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Buchanan D, Young P, Setiawan T, Brody J, Korach K, Taylor J, Lubahn D, Cunha GR. Stromal estrogen receptors (ER) mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR. Tissue interactions between epithelium and mesenchyme of urogenital and integumental origin. Anat Rec. 1972;172:529–542. doi: 10.1002/ar.1091720307. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Stromal induction and specification of morphogenesis and cytodifferentiation of the epithelia of the Mullerian ducts and urogenital sinus during development of the uterus and vagina in mice. J Exp Zool. 1976;196:361–370. doi: 10.1002/jez.1401960310. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Tissue recombination techniques for embryonic mammary gland. J Mammary Gland Biol Neoplasia. 2013a;18:221–225. doi: 10.1007/s10911-013-9295-3. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Tissue recombination techniques for mouse embryonic mammary glands. J Mammary Gland Biol Neoplasia. 2013b;18:221–225. doi: 10.1007/s10911-013-9295-3. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Lung B. The possible influences of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J Exp Zool. 1978;205:181–194. doi: 10.1002/jez.1402050203. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Vanderslice KD. Identification in histological sections of species origin of cells from mouse, rat and human. Stain Technol. 1984;59:7–12. doi: 10.3109/10520298409113823. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Donjacour AA. Mesenchymal-epithelial interactions: technical considerations. In: Coffey DS, Bruchovsky N, Gardner WA, Resnick MI, Karr JP, editors. Assessment of Current Concepts and Approaches to the Study of Prostate Cancer. A.R. Liss; New York: 1987. pp. 273–282. [Google Scholar]
- Cunha GR, Hom YK. Role of mesenchymal-epithelial interactions in mammary gland development. J Mammary Gland Biol Neoplasia. 1996;1:21–35. doi: 10.1007/BF02096300. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Sekkingstad M, Meloy BA. Heterospecific induction of prostatic development in tissue recombinants prepared with mouse, rat, rabbit, and human tissues. Diff Res Biol Divers. 1983c;24:174–180. doi: 10.1111/j.1432-0436.1983.tb01317.x. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Young P, Brody JR. Role of uterine epithelium in the development of myometrial smooth muscle cells. Biol Reprod. 1989;40:861–871. doi: 10.1095/biolreprod40.4.861. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Turner T, B LE, Donjacour AA. Role of mesenchyme in the development of the urogenital tract. In: Colborn T, Clement C, editors. Chemically-Induced Alterations in Sexual and Functional Development: The Wildlife/Human Connection. Princeton Scientific Publishing Co., Inc.; Princeton: 1992. [Google Scholar]
- Cunha GR, Donjacour AA, Hayward SW. Mesenchymal-epithelial interactions in the development of the male reproductive system. In: Motta PM, editor. Microscopic and Reproductive Development: A Dynamic Approach. Kluwer Academic Publishers; New York: 1997. IN PRESS. [Google Scholar]
- Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417–434. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Bigsby RM, Cooke PS, Sugimura Y. Stromal-epithelial interactions in adult organs. Cell Diff. 1985;17:137–148. doi: 10.1016/0045-6039(85)90481-6. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Young P, Higgins SJ, Cooke PS. Neonatal seminal vesicle mesenchyme induces a new morphological and functional phenotype in the epithelia of adult ureter and ductus deferens. Development. 1991;111:145–158. doi: 10.1242/dev.111.1.145. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Hom YK, Young P, Brody J. Transplantation and tissue recombination techniques to study mammary gland biology. In: Ip MM, Asch BB, editors. Methods in Mammary Gland Biology and Breast Cancer Research. Kluwer Academic Publishers; New York: 2000. pp. 289–306. [Google Scholar]
- Cunha GR, Chung LW, Shannon JM, Taguchi O, Fujii H. Hormone-induced morphogenesis and growth: role of mesenchymal-epithelial interactions. Recent Prog Horm Res. 1983a;39:559–598. doi: 10.1016/b978-0-12-571139-5.50018-5. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Fujii H, Neubauer BL, Shannon JM, Sawyer LM, Reese BA. Epithelial-mesenchymal interactions in prostatic development. I. Morphological observations of prostatic induction by urogenital sinus mesenchyme in epithelium of the adult rodent urinary bladder. J Cell Biol. 1983b;96:1662–1670. doi: 10.1083/jcb.96.6.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, Sugimura S. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987;8:338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Young P, Christov K, Guzman R, Nandi S, Talamantes F, Thordarson G. Mammary phenotypic expression induced in epidermal cells by embryonic mammary mesenchyme. Acta Anat. 1995;152:195–204. doi: 10.1159/000147698. [DOI] [PubMed] [Google Scholar]
- DiSandro MJ, Li Y, Baskin LS, Hayward S, Cunha G. Mesenchymal-epithelial interactions in bladder smooth muscle development: epithelial specificity. J Urol. 1998;160:1040–1046. doi: 10.1097/00005392-199809020-00022. discussion 1079. [DOI] [PubMed] [Google Scholar]
- Donjacour AA, Cunha GR. Seminal vesicle mesenchyme induces prostatic morphology and secretion in urinary bladder epithelium. J Cell Biol. 1988;107:609a. doi: 10.1242/dev.121.7.2199. [DOI] [PubMed] [Google Scholar]
- Donjacour AA, Cunha GR. Assessment of prostatic protein secretion in tissue recombinants made of urogenital sinus mesenchyme and urothelium from normal or androgen-insensitive mice. Endocrinology. 1993;131:2342–2350. doi: 10.1210/endo.132.6.7684975. [DOI] [PubMed] [Google Scholar]
- Grobstein C. Epithelio-mesenchymal specificity in the morphogenesis of mouse submandibular rudiments in vitro. J Exp Zool. 1953;124:383–444. [Google Scholar]
- Haffen K, Kedinger M, Simon-Assmann P. Mesenchyme-dependent differentiation of epithelial progenitor cells in the gut. J Pediatr Gastroenterol Nutr. 1987;6:14–23. doi: 10.1097/00005176-198701000-00005. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Cunha GR, Dahiya R. Normal development and carcinogenesis of the prostate: a unifying hypothesis. Ann N Y Acad Sci. 1996;784:50–62. doi: 10.1111/j.1749-6632.1996.tb16227.x. [DOI] [PubMed] [Google Scholar]
- Higgins SJ, Young P, Cunha GR. Induction of functional cytodifferentiation in the epithelium of tissue recombinants. II. Instructive induction of Wolffian duct epithelia by neonatal seminal vesicle mesenchyme. Development. 1989;106:235–250. doi: 10.1242/dev.106.2.235. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Mizuno T. Intestinal cytodifferentiation in vitro of chick stomach endoderm induced by the duodenal mesenchyme. J Embryol Exp Morphol. 1984;82:163–176. [PubMed] [Google Scholar]
- Kollar EJ. Induction of hair follicles by embryonic dermal papillae. J Invest Dermatol. 1970;55:374–378. doi: 10.1111/1523-1747.ep12260492. [DOI] [PubMed] [Google Scholar]
- Kollar EJ, Baird GR. Tissue interactions in embryonic mouse tooth germs. II. The inductive role of the dental papilla. J Embryol Exp Morphol. 1970;24:173–186. [PubMed] [Google Scholar]
- Kratochwil K. In vitro analysis of the hormonal basis for the sexual dimorphism in the embryonic development of the mouse mammary gland. J Embryol Exp Morphol. 1971;25:141–153. [PubMed] [Google Scholar]
- Kurita T. Normal and abnormal epithelial differentiation in the female reproductive tract. Diff Res Biol Divers. 2011;82:117–126. doi: 10.1016/j.diff.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Cunha GR. Roles of p63 in differentiation of Mullerian duct epithelial cells. Ann N Y Acad Sci. 2001;948:9–12. doi: 10.1111/j.1749-6632.2001.tb03982.x. [DOI] [PubMed] [Google Scholar]
- Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (Müllerian) epithelial differentiation. Dev Biol. 2001a;240:194–211. doi: 10.1006/dbio.2001.0458. [DOI] [PubMed] [Google Scholar]
- Kurita T, Young P, Brody J, Lydon JP, O'Malley BW, Cunha GR. Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell (UtE) proliferation. Endocrinology. 1998;139:4708–4713. doi: 10.1210/endo.139.11.6317. [DOI] [PubMed] [Google Scholar]
- Kurita T, Wang YZ, Donjacour AA, Zhao C, Lydon JP, O'Malley BP, Isaacs JT, Dahiya R, Cunha GR. Paracrine regulation of apoptosis by steroid hormones in the male and female reproductive system. Cell Death Differ. 2001b;8:192–200. doi: 10.1038/sj.cdd.4400797. [DOI] [PubMed] [Google Scholar]
- Li X, Wang Y, Sharif-Afshar AR, Uwamariya C, Yi A, Ishii K, Hayward SW, Matusik RJ, Bhowmick NA. Urothelial transdifferentiation to prostate epithelia is mediated by paracrine TGF-beta signaling. Diff Res Biol Divers. 2009;77:95–102. doi: 10.1016/j.diff.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu W, Hayward SW, Cunha GR, Baskin LS. Plasticity of the urothelial phenotype: effects of gastro-intestinal mesenchyme/stroma and implications for urinary tract reconstruction. Diff Res Biol Divers. 2000;66:126–135. doi: 10.1046/j.1432-0436.2000.660207.x. [DOI] [PubMed] [Google Scholar]
- Lipschutz JH, Young P, Taguchi O, Cunha GR. Urothelial transformation into functional glandular tissue in situ by instructive mesenchymal induction. Kidney Int. 1996;49:59–66. doi: 10.1038/ki.1996.8. [DOI] [PubMed] [Google Scholar]
- Sakakura T, Nishizuka Y, Dawe CJ. Mesenchyme-dependent morphogenesis and epithelium-specific cytodifferentiation in mouse mammary gland. Science. 1976;194:1439–1441. doi: 10.1126/science.827022. [DOI] [PubMed] [Google Scholar]
- Saxen L. Organogenesis of the kidney. Cambridge University Press; New York: 1987. [Google Scholar]
- Simon-Assmann P, Simo P, Bouziges F, Haffen K, Kedinger M. Synthesis of basement membrane proteins in the small intestine. Digestion. 1990;46:12–21. doi: 10.1159/000200362. [DOI] [PubMed] [Google Scholar]
- Staack A, Donjacour AA, Brody J, Cunha GR, Carroll P. Mouse urogenital development: a practical approach. Diff Res Biol Divers. 2003;71:402–413. doi: 10.1046/j.1432-0436.2003.7107004.x. [DOI] [PubMed] [Google Scholar]
- Taylor RA, Wang H, Wilkinson SE, Richards MG, Britt KL, Vaillant F, Lindeman GJ, Visvader JE, Cunha St GR, John J, Risbridger GP. Lineage enforcement by inductive mesenchyme on adult epithelial stem cells across developmental germ layers. Stem Cells. 2009;27:3032–3042. doi: 10.1002/stem.244. [DOI] [PubMed] [Google Scholar]
- Turner T, Young P, Cunha GR. Seminal vesicle induction of adult mouse epididymal epithelium by newborn mouse and rat seminal vesicle mesenchyme. J Cell Biol. 1989;109:69a. Abstract. [Google Scholar]
- Yasugi S, Mizuno T. Molecular analysis of endoderm regionalization. Dev Growth Differ. 2008;50(Suppl. 1):S79–S96. doi: 10.1111/j.1440-169X.2008.00984.x. [DOI] [PubMed] [Google Scholar]


