Abstract
Endothelial cells (ECs) line the interior of blood and lymphatic vessels and experience spatially varying wall shear stress (WSS) as an intrinsic part of their physiological function. How ECs, and mammalian cells generally, sense spatially varying WSS remains poorly understood, due in part to a lack of convenient tools for exposing cells to spatially varying flow patterns. We built a multiplexed device, termed a 6-well impinging flow chamber, that imparts controlled WSS gradients to a six-well tissue culture plate. Using this device, we investigated the migratory response of lymphatic microvascular ECs, umbilical vein ECs, primary fibroblasts, and epithelial cells to WSS gradients on hours to days timescales. We observed that lymphatic microvascular ECs migrate upstream, against the direction of flow, a response that was unique among all the cells types investigated here. Time-lapse, live cell imaging revealed that the microtubule organizing center relocated to the upstream side of the nucleus in response to the applied WSS gradient. To further demonstrate the utility of our device, we screened for the involvement of canonical signaling pathways in mediating this upstream migratory response. These data highlight the importance of WSS magnitude and WSS spatial gradients in dictating the cellular response to fluid flow.
Keywords: Mechanotransduction, Cell migration, Vascular biology, Fluid mechanics
INTRODUCTION
Living cells undergo directed migration in response to gradients in chemical concentration (chemotaxis), substrate stiffness (durotaxis), ligand density (hapto-taxis), and mechanical force (mechanotaxis). However, while a great deal is known about cell migration arising from chemotaxis, haptotaxis, and durotaxis, less is known about the mechanisms underlying mechano-taxis. Mechanotaxis plays important roles in morphogenesis, during which cell-generated forces lead to organized collective cell migration and proper tissue organization,40 and is likewise involved in the malignant phenotype of cancer cells derived from the mammary epithelium.24,32 At present, mechanotaxis remains difficult to study owing to a lack of methods that apply controlled gradients of mechanical stress in a format that is compatible with commonly used cell culture and live-cell fluorescent microscopy techniques.
Gradients in fluid shear stress represent a particularly important mechanical stress gradient. Vessel branches, bends, and expansions each result in significant alterations in wall shear stress (WSS) and WSS gradients (WSSGs). Accordingly, the endothelial cells (ECs) that line the blood circulatory and lymphatic systems are thought to be sensitive to WSS and WSSGs. Cues from WSS and WSSGs are required for vascular remodeling of the mouse yolk sac16 and for proper heart formation during development,12 and are associated with vascular disease, including atherosclerosis in adulthood.2,19
Several approaches have been used to study EC response to WSSGs. The vertical step flow geometry produces fluid flows with spatial inhomogeneities in WSS, and has been used to study EC migration, proliferation, and signaling.3,7,8,27,36 Converging–diverging width and T-junction channels have been used to study EC response to WSSGs.9,15,20,29–31,34,38 Additional studies have used microfluidic techniques for imposing controllable WSSs.21 These studies have each provided insight into EC response to spatially or temporally varying WSS.
To our knowledge, a medium- to high-throughput device that applies controlled gradients in WSS and that maintains compatibility with standard cell-culture dishes has not been described. Such a device would be useful not only to understand EC response to fluid flow, but also for applying controlled mechanical stresses to cell types that are not sensitive to WSS per se, but that do respond to mechanical stress. Examples of mechanical guidance include the migration of Xenopus mesendodermal cells away from an externally applied force,40 and the attraction of two isolated ECs toward each other in response to tugging forces transmitted through the intervening matrix.26
We previously demonstrated that lymphatic human microvascular ECs (HMVECs) migrate upstream in the presence of an impinging flow field.22 We here refer to the migrational or orientation response of cells to flow as rheotaxis, a term originally used to describe behavioral orientation to water current, as observed in fish1 and subsequently in cells.22,25 In our previous work we used a single-well impinging flow chamber (IFC) device that imposed controlled WSSGs onto a 60-mm cell culture dish. We found that lymphatic HMVECs exposed to WSSGs with a peak shear of 72 dynes/cm2 migrated upstream, against the flow direction. While our previous results suggest the lymphatic HMVECs sense and respond to spatial gradients, the study lacked the throughput needed for systematic investigation of flow-dependent intracellular signaling mechanisms.
Here we demonstrate a system for studying the the response of cells to controlled WSSGs that is compatible with standard 6-well tissue culture dishes and high resolution, live-cell imaging. Using this device, we found that lymphatic HMVECs upstream migration requires cell–cell contacts. Further, we observed that sub-confluent lymphatic HMVECs, human umbilical vein ECs, Madin-Darby canine kidney epithelial cells (MDCKs) and fibroblast cells moved downstream in response to the same WSSG. We used the 6-well IFC in combination with live-cell fluorescent imaging to track microtubule dynamics and Golgi relocation in migrating lymphatic HMVECs. Finally, we performed a small-molecule inhibitor screen to probe the role of known flow-sensing pathways in controlling upstream rheotaxis in lymphatic HMVECs. In combination, these data illustrate the usefulness of the 6-well IFC platform as a flexible, parallelizable and robust tool to characterize the response of a wide variety of cell types to WSSGs of controlled orientation and magnitude.
MATERIALS AND METHODS
Primary Cell Culture
Primary lymphatic human microvascular ECs (HMVECs; CC-2810) and primary human umbilical vein endothelial cells (HUVECs; CC-2517) purchased from Lonza Corporation (Walkersville, MD) were grown in EGM-2 basal medium (Lonza CC-3156) containing supplements and growth factors (Lonza CC-4147) that include 5% FBS (Fetal Bovine Serum), hEGF, VEGF, hFGF-B, R3-IGF-1, hydrocortisone, and ascorbic acid. 50 units/mL of penicillin and 50 μg/mL streptomycin (Life Technologies, Carlsbad, CA) were added to the medium. Cells were used between passages 6–10. 3–5 days before the experiment, cells were plated onto a 6-well cell culture dish with a #1.5 glass coverslip bottom (In Vitro Scientific, Sunnyvale, CA). These dishes were pre-coated with 0.2% gelatin (Sigma-Aldrich, Saint Louis, MO) for 24 h. The gelatin was then aspirated, cells were plated at 0.5–1.5 × 105 cells per dish and incubated at 37 °C and 5% CO2. Prior to imaging, the medium was exchanged to Leibovitz's L-15 medium (Life Technologies) to allow for CO2 independent imaging. The L-15 medium included 5% FBS, the endothelial growth factor kit from Lonza (CC-4147), 50 units/mL of penicillin and 50 μg/mL streptomycin (Life Technologies). Human foreskin fibroblasts (HFFs, CCD-1070Sk) were purchased from ATCC (CRL-2091), and MDCK epithelial cells were a gift from Dr. James Nelson (Stanford University). Fibroblast, epithelial and higher density EC experiments were performed with cells at surface coverage (fraction of the coverslip covered by cells) of greater than 95% and cell confluencies (fraction of maximum cell density) of 80% to ensure that cells had sufficient contact with neighboring cells. Experiments utilizing low density lymphatic HMVECs were performed with surface coverage of ~20% (cell confluency of 16%) such that most cells lacked cell–cell contacts.
Fluid Dynamics
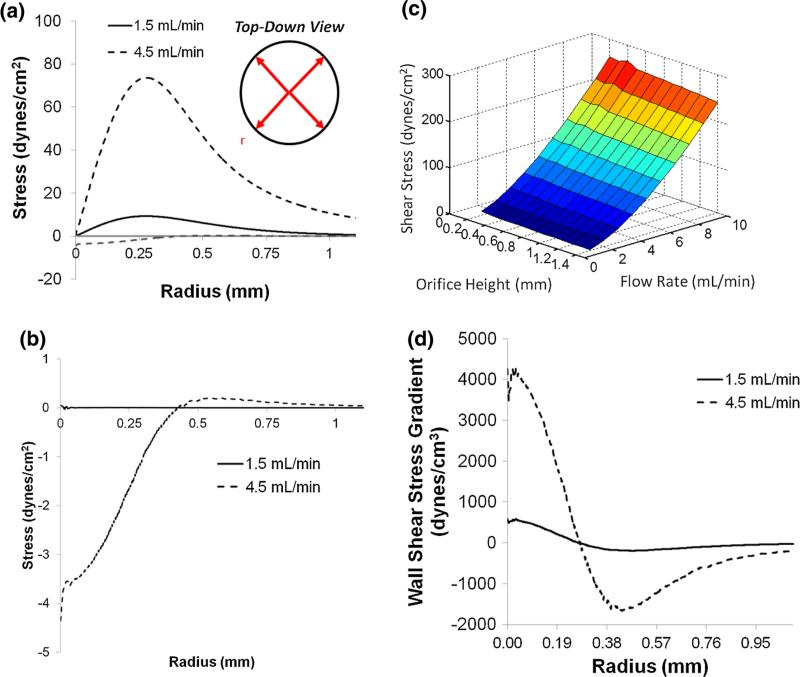
Numerical calculations were performed on the full Navier–Stokes equations using the commercial finite-element analysis package COMSOL Multiphysics 3.5a to derive the WSSs and normal stresses in the IFC. A two-dimensional axisymmetric geometry (cylindrical coordinates) was chosen where a cross-section of the jet was modeled, from the stagnation point (center of impinging jet orifice, r = 0) radially outward and upward in the z-direction towards the orifice height. The no-slip condition was applied to the walls of the IFC orifice and the surface of flow impingement. At the return channel, where fluid was drawn into the pump, the flow rate was specified with a uniform velocity distribution. The pressure was set to ambient conditions. Lagrange quadratic shape functions were used to approximate the solution to the differential equations on each mesh element and refined until the numerical values of the WSS changed by less than 0.1%. These triangular elements, which were chosen to provide adequate r and z resolution for the mesh in the fluid space, had an average area of 1500 μm2. This resulted in a z-resolution of on average 68 elements from the cell surface to the orifice wall. The Reynolds numbers were calculated by using the diameter of the exit orifice as the length scale, the viscosity of the medium and the average velocity calculated from the flow rates used in the experiment. All Reynolds numbers were in the laminar flow regime such that the finite element calculations are sufficient to provide a faithful simulation of the experimental flow fields. Peak shear stress was calculated for heights ranging from 0.4 to 1.4 mm, as well as for flow rates between 1 and 10 mL/min.
6-well Impinging Flow Chamber Design and Experimental Setup
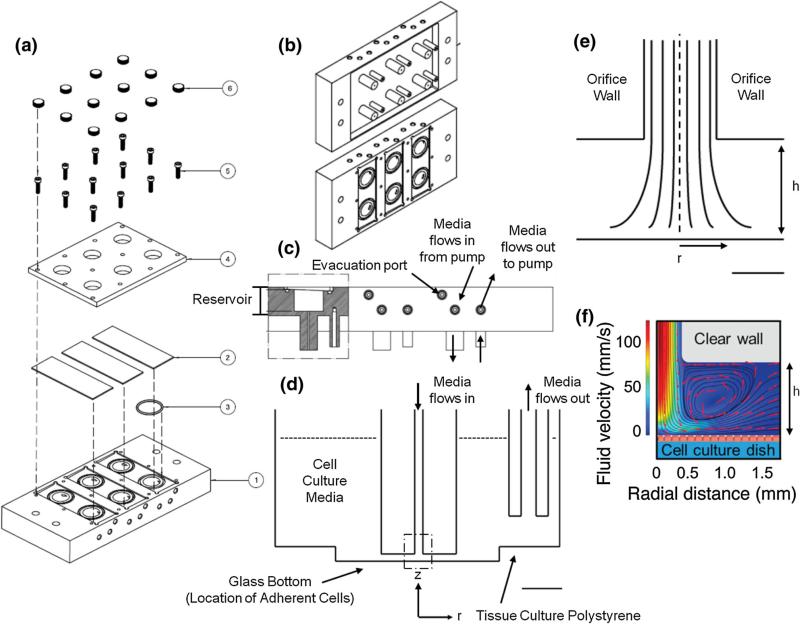
The 6-well IFC was designed to sit on top of a standard glass-bottom 6-well dish in place of a lid. The 6-well IFC was fabricated out of polished acrylic to enable overhead illumination during time-lapse brightfield experiments, minimizing the interference to the illumination path. The 6-well IFC contains vertical posts that extend down into each well of the cell culture dish with dimensions that maintain a 1 mm spacing between the flow exit and the cell culture dish surface (Figs. 1d and 1e). Fluid is delivered with a 9-roller dampened peristaltic pump (Idex, Oak Harbor, WA). The fluid leaves each well through the orifice of another vertical post located in a non-imaged region to the side of the well and 13 mm away from the inlet post, such that the exit does not perturb the impinging flow region. While the inner diameter of the inlet port is 0.7 mm, the diameter of the exit port is 2 mm and is located 1 mm above the surface of the cell culture dish such that the exit velocity and shear stress are minimized in comparison to the primary flow of interest at the impinging flow orifice.
FIGURE 1.
A six-well IFC for producing a radially symmetric WSSG. The system is designed as a lid that sits directly on top of a standard 6-well glass bottom tissue culture dish. (a) An overhead view of the components of the IFC (1). 6 o-rings (3) fit into grooves in the IFC device, which holds 3 glass microscope slides in place (2) to create the top surface of the flow reservoir, with one slide covering 2 reservoirs. A Plexiglas lid (4) secured with 12 screws with caps (5 & 6) covers the glass slides to seal the reservoirs. (b) An angled view of the bottom of the IFC, showing 6 pairs of inlet and outlet orifices to dispense and collect media respectively, and the top of the IFC. The inlet and outlet orifices are submerged in a glass-bottom 6-well dish containing cell culture media. (c) A side view of the IFC. The reservoir collects bubbles and is filled with medium to equilibrate in temperature and to trap any bubbles. Bubbles can be evacuated through an evacuation port, which is directly connected to each of the 6 reservoirs. There are two other ports through which media flows into the device and out from the device. (d) A cross-sectional schematic showing to scale the location of the two jet orifices, which are submerged in media inside each well. The orifice that applies impinging flow is 1 mm away from the glass bottom of each well, while the orifice that removes media from the well is 4 mm from the polystyrene bottom of the well. (Scale bar = 5 mm) (e) The dashed region of impinging flow in (d) is expanded to scale, where the dashed line indicates the axis of symmetry at r = 0. The velocity flow profile and thus WSS profile are axisymmetric about r = 0. At z = 0, the glass bottom of the well, there is a stagnation point in the flow, and h indicates the orifice height from the glass bottom of the well to the orifice wall (h = 1 mm). (Scale bar = 500 μm) (F) Finite element simulation shows the cross-sectional fluid velocity profile (jet height is 1 mm) and fluid streamlines (red arrows) indicating that the cells (red) experience a spatially varying, axisymmetric flow. An orifice wall height of 1 mm was used in this simulation, which is identical to the IFC impinging jet orifice height from the glass bottom of each well.
Experiments were performed with a Nikon TE microscope for brightfield imaging using a 4× Nikon objective and a Flea 3 camera (Point Grey, BC, Canada) or with a Nikon Ti (Nikon Corporation, Tokyo, Japan) microscope for live-cell fluorescence imaging with an Andor Neo camera (Andor Technology, Belfast, UK). Both microscopes were equipped with a temperature control chamber to maintain the environmental temperature at 37 °C.
Before the start of the flow experiment the 6-well IFC was primed with the cell culture medium to ensure the removal of bubbles and a continuous supply of cell culture medium. To accomplish priming, a 6-well plate that did not contain the cells was filled with 10 mL of medium in each well. The 6-well IFC was positioned on top of the 6-well dish. The pump was turned on and medium was allowed to circulate through the tubing. Once the lines were filled, the pump was turned off and remaining air in the chamber was evacuated through the evacuation port using a syringe. The evacuation port is located at a high side of the chamber such that any bubbles present are removed. After priming the device, the 6-well IFC is positioned onto the 6-well dish containing the cells of interest. The flow rate in all experiments was 4.5 mL/min, corresponding to a peak WSS of 72 dynes/cm2. Cleaning of the 6-well IFC is performed analogously to the priming step described above. Two 6-well dishes are filled with 10 mL of sterile water and flowed through the tubing and jet apparatus. Bubbles are evacuated through the evacuation port with a syringe. Once all bubbles are removed, water is flowed through the IFC for 10 min each wash. Subsequently, 6-well dishes containing 10 mL of 10% hydrogen peroxide and 2 6-well dishes containing 10 mL sterile water each well are placed under the IFC for 30, 10 and 10 min respectively, and bubbles were removed from each reservoir prior to proceeding to the next step.
Inhibitor Experiments
LY294002 was purchased from Calbiochem (San Diego, CA). PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine), nocodazole and PF5733228 were purchased from Sigma-Aldrich. One hour prior to the start of the experiment, stock solutions of inhibitors were diluted in L-15 medium and added to the cells. The inhibitor was also included in the recirculating medium to maintain its concentration throughout the flow system. Each well of the 6-well IFC is connected to a separate reservoir of medium, allowing multiple independent concentrations or experiments simultaneously.
Typically, six simultaneous experiments were performed for each inhibitor within one 6-well plate, generally four inhibitor concentrations and two control samples (cells cultured under identical conditions, experiencing the same flow, but lacking inhibitor). Concentrations were determined based on published IC50s (if available) and/or concentrations used in previously published experiments. Lymphatic HMVECs were exposed to PP2, LY294002 and 18β-glycyrrhetinic acid at 1, 5, 10, and 50 μM, to PF573228 at 0.5, 1, 3, and 10 μM and to nocodazole at 0.1, 0.2, 0.5, and 1 μM. If the inhibitor had an effect on cell migration, data were plotted for a representative concentration, if the inhibitor had no effect on cell migration, data were plotted for the highest concentration evaluated (18β-glycyrrhetinic acid).
Immunofluorescence Staining
Samples were washed with phosphate buffer saline (PBS), fixed with 4% paraformaldehyde (Fluka, Sigma Aldrich) in PBS (w%) for 15 min, permeabilized with 0.5% Triton X-100 (Fluka) in PBS (v%) for 10 min at room temperature and washed with PBS. Samples were then incubated for 1 h with 1% bovine serum albumin (Sigma Aldrich) in PBS (w%). Cells were incubated overnight with an antibody that marks the Golgi apparatus (1:200) produced in mouse and specific for the 58 kDa Golgi protein formiminotransferase cyclodeaminase (ab27043, Abcam, Cambridge, UK), and with TRITC-phalloidin (1:1000) for 10 min, (Sigma Aldrich.) Samples were then stained with Hoechst 34580 (10 μg/mL) for 5 min at room temperature, followed by two rinses of PBS. The fluorescently stained samples were visualized on a Nikon Ti microscope using 20× or 40× air objectives, and imaged using an Andor Neo camera.
Live-Cell Fluorescent Microcopy
We used time-lapse live-cell imaging to characterize HMVEC rheotaxis in response to WSSGs. The Golgi apparatus and microtubules were labeled using Cell-Light RFP-Golgi marker N-acetylgalactosaminyltransferase, and CellLight Emerald GFP-tubulin, respectively (1:60 dilutions each; Life Technologies). Time-lapse movies were collected for 24 h in L-15 medium, with one frame recorded every 10 min. Data were collected on a Nikon TiE microscope equipped with a 40× air objective, Heliophor light source, and Andor Neo camera.
Analysis of Cell Migration
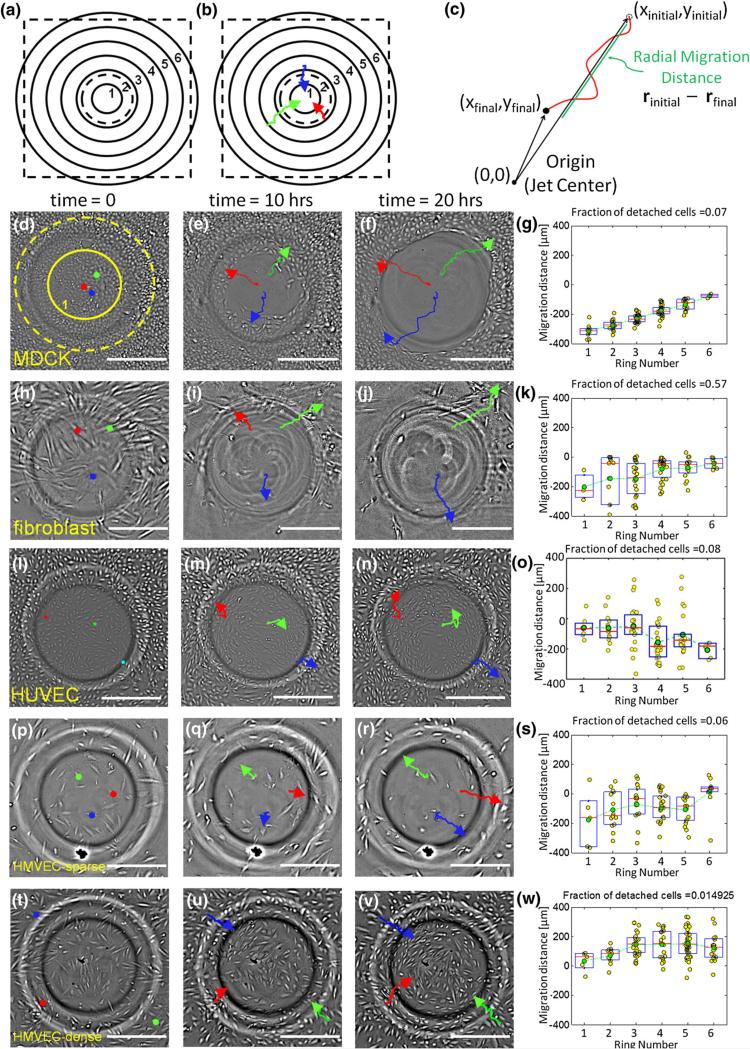
Bright-field movies were processed with Fiji software33 and analyzed using custom Matlab routines. All image files were bandpass filtered in Fiji using high and low frequency cutoffs at 2 and 20 pixels (approx. 2.5 and 25 μm). Cell migration was tracked over 20 h using the Manual Tracking plugin in Fiji for about 100 cells per well. Cellular x and y positions were determined every 20 min and input into a custom Matlab script. Total displacement was calculated in the radial direction (Fig. 3c). To plot displacements the field of view was divided into concentric annular regions with a width of 185 μm. Cells within a ring experienced similar shear stress due to the radial symmetry of the 6-well IFC. The total radial displacement for each cell, calculated via the final and initial radial distance from the jet center, was plotted as a function of the region that contained the cell's starting position.
FIGURE 3.
Cell-type specific response to WSSGs. (a) Regions concentric to the jet orifice center (flow stagnation point) within the microscope field of view (dashed black square) are binned in 185 μm radius rings, with ring 1 closest to the jet center. The impinging jet radius (black dashed circle) and ring 1 are also overlaid, to scale, on d. (b) Quantification of cell migration in response to the WSSG gradient. The positions of representative cells (red, blue, green) were manually determined at 20 min intervals during the 20 h movies. In this illustration cells move inward toward the position on the dish directly beneath the impinging flow. (c) The radial cell migration distance is defined by calculating the difference between the initial and final radial distances from the jet center. Here, a positive migration distance indicates movement toward the jet center (against the flow direction), while a negative migration distance indicates movement away from the jet center (with the flow direction). Response to an impinging flow field (4.5 mL/min, peak WSS 72 dynes/cm2) for MDCKs (d–g), HFFs (h–k), HUVECs seeded at a density that establishes cell–cell contacts (l–o), sparsely seeded lymphatic HMVECs (p–s), and lymphatic HMVECs seeded at a density that establishes cell–cell contacts (t–w). d, h, l, p, and t show the cells before the onset of flow. Three cells are highlighted with colored circles. e, i, m, q, and u show the images of cells after 10 h of exposure to the flow, and f, j, n, r and v show the same fields of view after 20 h. The migration paths of cells are tracked with colored lines. Final cell positions are shown with an arrowhead. g, k, o, s and w show total displacements over 20 h for 100 cells as a function of their initial position (see panel B). MDCKs, HFFs, HUVECs and sparsely seeded HMVECs migrate with the flow direction. In contrast, more densely seeded HMVECs migrate upstream, against the flow direction. (Scale bar = 300 μm).
RESULTS
Design of the 6-Well Impinging Flow Chamber
We sought to design a device that would impart a controlled WSSG while maintaining compatibility with standard cell culture formats and live-cell imaging in both brightfield and fluorescent microcopy. The 6-well IFC apparatus fits on standard 6-well tissue culture plates, fulfilling this requirement (Fig. 1). In addition, our design is experimentally robust: finite element modeling reveals that WSSs and WSSGs are insensitive to small variations in the spacing between the 6-well IFC apparatus and coverslip surface (Figs. 2a and 2c). The chamber immediately above the IFC orifice catches bubbles in the medium and helps to damp out any pressure fluctuations generated by the peristaltic pump. This combination of features makes this device convenient to use.3,7,8,15,20,27,29,30,34,36,38
FIGURE 2.
Finite element simulation of hydrodynamic stress. (a) WSS (i.e., shear experienced at the cell surface) and normal stress (b) plotted as a function of the radial distance from the stagnation point situated directly below the center of the flow orifice. Maximum WSSs are 9 and 72 dyn/cm2 at flow rates of 1.5 and 4.5 mL/min, respectively. WSS reaches a maximum at r ~ 0.28 mm regardless of flow rate. Inset: a top-down, schematic view indicating that the flow is directed radially outward from the stagnation point at the jet center. (c) Computational simulation of maximum WSS as function of the impinging jet orifice height (h, Figs. 1e and 1f) and volumetric flow rate. (d) WSSG plotted as a function of radial distance from the stagnation point directly below the center of the flow orifice. The WSSG is positive and increases to a maximum at 21 μm, then decreases to zero at the maximum WSS (r ~ 0.28 mm). The WSSG becomes negative and reaches a minimum (r ~ 440 μm) before decaying to zero.
The flow profile generated by the 6-well IFC has several useful attributes. The magnitude of the WSS and WSSGs can be easily tuned across a wide range of values by changing the flow rate (Fig. 2a). Importantly, the position of maximum WSS, slightly past the edge of the IFC orifice, remains approximately constant over a large range of flow rates, thus enabling facile setup and imaging under a wide variety of WSSs (Fig. 2c).22 Our WSS calculations assume a negligible cell height along the glass bottom of the well. Since the average cell height (5–10 μm) is three orders of magnitude smaller than the orifice height, h (1 mm), the WSS profile is essentially unchanged by the presence of adherent cells.
Shear Stress Response is Cell-Type Specific
In previous work, we observed that lymphatic HMVECs migrated inward, against the direction of flow for applied WSSGs with maximal WSS values of either 9 or 72 dynes/cm2, with the major difference being the timescale of the response.22 Here we expanded our assay to determine if other cell types might exhibit similar upstream migration. In order to accentuate any possible phenotypes, we chose to use flow conditions that resulted in a WSSG spanning ~10 dynes/cm2 at the edge of the field of view to 72 dynes/cm2 at the WSS maximum. This choice of conditions allowed us to observe cellular responses to a wide range of WSS magnitudes, while the relatively large maximal WSS value accentuated responses to flow, if present, on an experimentally tractable timescale.
We used the 6-well IFC to expose different classes of cells, including fibroblasts, MDCKs, HUVECs and lymphatic HMVECs to the impinging flow field (Fig. 3). MDCK cells, moved downstream (Figs. 3d–3g), but were able to maintain adhesion to the surface (~7% detachment). Fibroblasts also moved downstream (Figs. 3h–3k), with a significant fraction (57%) detaching from the surface within the 20-h experiment. HUVECs likewise migrated with the flow direction, downstream (Figs. 3l–3o).
Interestingly, the direction of lymphatic HMVEC migration appeared to depend on cell–cell contact. Sparsely seeded cells, (~20% confluency) such that most lymphatic HMVECs lacked visible contacts to neighbors, migrated downstream in the large majority of cases (Figs. 3p–3s). Cells that did migrate against the flow were found to be in contact with neighboring cells (data not shown). In contrast, lymphatic HMVECs seeded such that the cells maintained cell–cell contact migrated upstream, consistent with our previous work (Figs. 3t–3w).22
Use of the 6-Well IFC for Small Molecule Inhibitor Screening
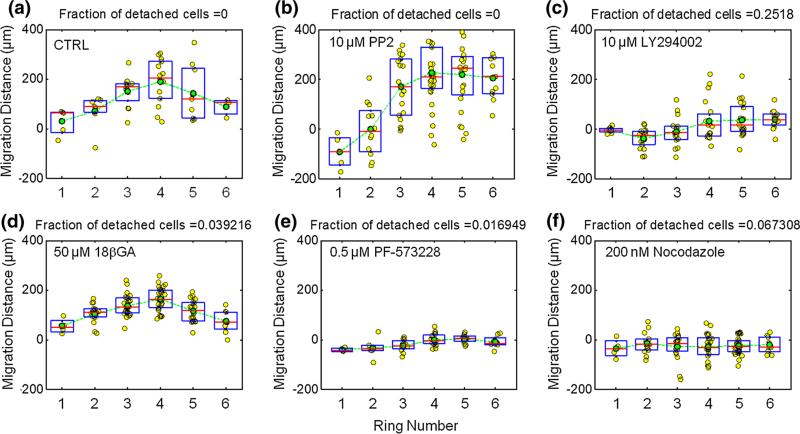
Here we demonstrate that signaling pathways related to flow sensing and migration can be interrogated with the 6-well IFC. We first investigated the role of PI3K and Src, which have been previously described to mediate shear stress sensing.39 Lymphatic HMVECs exposed to the Src inhibitor PP2 migrated downstream in the region directly under the impinging flow (regions 1 and 2), while cells in regions 3, 4, 5 and 6 migrated upstream (Fig. 4b). This result differs only subtly from the control experiment in which cells from all 6 regions migrate upstream (Fig. 4a). PI3K inhibition with LY294002 repressed migration more effectively: cells in region 1 showed nearly no net displacement, while those in region 2, which contains the peak in shear stress, migrated downstream (Fig. 4c). Cells in regions 3, 4, 5 showed a small displacement upstream. Gap junctions have also been implicated in EC flow sensing.6 However, lymphatic HMVECs exposed to impinging flow maintained the upstream migration phenotype in the presence of 18β-glycyrrhetinic acid (50 μm) (Fig. 4d), which has been proposed to mediate gap junction disassembly, possibly through connexin 43 dephosphorylation.4,11
FIGURE 4.
Effect of small molecule inhibitors for Src, PI3K, connexin 43, FAK, and microtubule assembly on directed lymphatic HMVEC migration in response to WSSGs. Plots show the radial distance traveled by individual cells (yellow dots) as a function of the ring number corresponding to the cell's starting location (Fig. 3b). Each ring is an annulus with a thickness of 185 μm. Also shown are the mean (green circles), median (red lines) and 25th and 75th percentiles (boxes). (a) Control. (b) 10 μM Src inhibitor PP2. (c) 10 μM PI3K inhibitor LY294002. (d) 50 μM connexin 43 inhibitor 18β-glycyrrhetinic acid (18βGA). (e) 0.5 μM FAK inhibitor PF-573228. (f) 200 nM microtubule polymerization inhibitor nocodazole.
Focal adhesion kinase (FAK) and microtubules have been previously implicated in directional cell migration.13,23,37 FAK inhibitor PF573228 significantly inhibited both directional cell migration as well as random movement, consistent with previous results in which mouse embryo fibroblast FAK knockout cells demonstrated reduced cell motility (Fig. 4e).13 However, in contrast to PI3K inhibition, the lymphatic HMVECs treated with PF573228 remained strongly adhered with only 1.7% of cells detaching. We next inhibited microtubule polymerization with a low concentration of nocodazole (200 nM) and found that nocodazole treatment decreased directed upstream migration as well as migration velocity (Fig. 4f).
Live-Cell Fluorescent Imaging Under Shear Stress Gradient Conditions
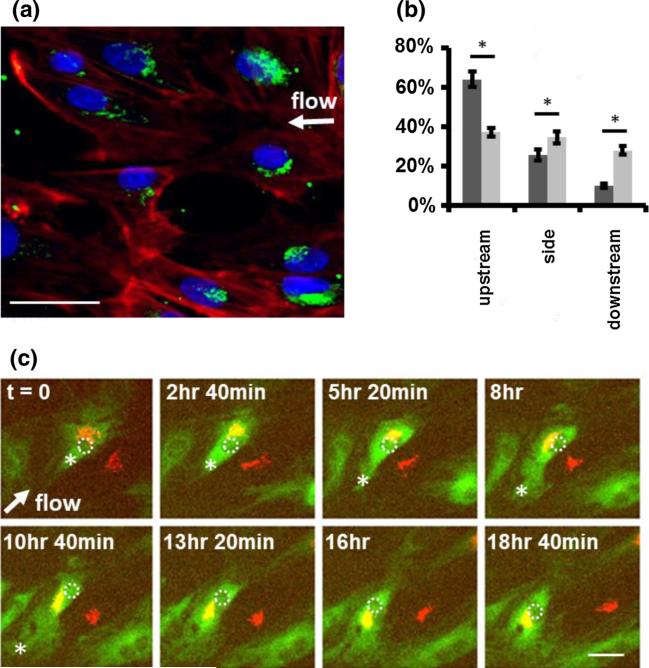
The location of the microtubule organizing center (MTOC) is an important component of directed cell migration. Because the MTOC is small and difficult to visualize, we chose to track the Golgi apparatus and use its location as a proxy for the MTOC (Fig. 5a).5 We found that the Golgi apparatus oriented preferentially to the upstream part of the cell, in the direction of migration (Fig. 5b).
FIGURE 5.
Cell polarization precedes MTOC relocation in response to shear stress gradients. (a) Lymphatic HMVECs were fixed and stained with anti-formiminotransferase cyclodeaminase antibody (green) to mark the Golgi apparatus and phalloidin (red) to stain filamentous actin. (b) The location of the Golgi relative to the nucleus in cells exposed to WSSG for 20 h (dark grey bars) was compared to static controls (no imposed flow; light gray bars). The location of the Golgi was defined as upstream, downstream, or to the side of the nucleus with respect to the direction of flow for each cell. In cells exposed to a WSSG, the Golgi was predominantly located on the upstream side of the cells (p value < 0.05). Data are for a 832 by 702 μm2 region containing HMVECs in which the flow stagnation point is approximately at the center of the field of view. Bars show the population standard deviation. (c) Live cell imaging of lymphatic HMVEC migration and Golgi relocation in response to a WSSG. The flow direction is shown with a white arrow. Lymphatic HMVECs were transfected with Emerald GFP-tubulin (green) and a Golgi marker (RFP-N-acetylgalactosaminyltransferase; red) and imaged every 160 min. Three cells are in the field of view. One cell's nucleus is highlighted with a dashed white circle for clarity, and the edge of microtubule extension is marked with an asterisk. Time-lapse images show that cell migration was initiated by microtubule extension in the upstream direction (asterisk) followed by Golgi relocation toward the upstream direction. Scale bars are 50 μm.
We asked whether MTOC relocation initiates upstream migration or whether lamellipodial extension occurs first. To determine the sequence of events, we labeled lymphatic HMVECs using both CellLight Tubulin-GFP and CellLight RFP-Golgi marker (Life Technologies), and performed time-lapse imaging on cells exposed to the WSSG generated by the IFC. After the onset of flow, we observed microtubule dynamics consistent with active membrane protrusion at the upstream side (Fig. 5c). The Golgi apparatus relocated to the upstream side of the nucleus at later time points (Fig. 5c).
DISCUSSION
While cell migration in response to chemical gradients or matrix stiffness gradients has been well studied, much less is known about how cells react to spatial variations in WSS. The 6-well IFC provides a simple and robust means to study the response of cells to WSS and WSSGs in a higher throughput manner than was previously feasible.
With this device we are able to apply mechanical force in the form of fluid shear to a wide variety of cell types. We found that MDCK epithelial cells and HFFs, two canonical epithelial and fibroblastic cell lines, moved downstream. Interestingly, we also noted that the fibroblasts detached from the surface at a higher frequency as compared to lymphatic HMVECs or MDCK epithelial cells, perhaps suggesting that both cell types have evolved to resist shear-mediated detachment. The observed differences in adhesive stability among lymphatic HMVECs, MDCKs, and HFFs highlight the potential utility of the IFC as a means of studying the response of different cell types to mechanical stress, here applied via fluid flow.
We observed that lymphatic HMVECs at moderate density migrated upstream in the flow field produced by the 6-well IFC. Upstream migration required cell–cell contact, as sparsely seeded lymphatic HMVECs migrated downstream. These observations are striking, especially given the observation that HUVECs migrated downstream even at densities that allowed cell–cell contacts. Previous studies also reported that HUVECs migrated with the flow direction in the presence of uniform WSS.14,41 The mechanistic origin of the unusual, upstream migration specifically by lymphatic HMVECs is thus unclear. However, it is possible that this observation may be biologically relevant, as MTOC reorientation and migration against the direction of blood flow have both been observed in vivo.28
The relatively high throughput enabled by the 6-well IFC allowed us to probe multiple signaling pathways thought to be involved in flow sensing and cell migration. Surprisingly, we found that the Src-family kinase inhibitor PP2 exerted a relatively modest effect on HMVEC upstream migration. Inhibition of FAK, a key regulator of cell migration,18 led to a dramatic decrease in cell migration in both the upstream and downstream directions, suggesting that FAK inhibition blocks cell motility generally, as opposed to directional migration specifically. In contrast, inhibition of PI3K and partial disruption of the microtubule cytoskeleton seemed to alter the directionality of cell migration, though with simultaneous effects on cell adhesion (PI3K inhibition) and cell velocity (microtubule disruption).
We found that cell–cell contact was required for lymphatic HMVEC upstream migration. Perhaps relatedly, previous work demonstrated the importance of cell–cell contact in chemotaxis by neural crest cells.35 The gap junction protein connexin 43 is implicated in directional cell migration by regulating microtubule dynamics in mouse embryonic fibrob-lasts.10 However, we found that treatment with 18β-glycyrrhetinic acid, a gap-junction inhibitor, had no effect on lymphatic HMVEC rheotaxis. However, more work would be required to rule out a role for gap junction mediated signaling in our system.
Importantly, the shear stress produced by the 6-well IFC can also be used to assess relative adhesion strength. PI3K inhibition resulted in 25% detachment in response to impinging flow, implying that PI3K inhibition attenuated the cells’ ability to adhere to the surface, possibly due to an effect on integrin activation or recruitment. Cells that remained attached were able to migrate upstream in regions with a lower magnitude and gradient of shear stress (Fig. 4c) although at an attenuated level relative to controls.
The 6-well IFC is compatible with fluorescent microscopy, a capability that facilitated the observation that upstream migration is initiated by microtubule extension and membrane protrusion at the upstream side, followed by Golgi relocation to the upstream side of the cell (Fig. 5c). Previous studies have shown that biased lamellipodia develop prior to MTOC reorientation, followed by endothelial cell migration downstream in response to uniform shear stress.17 Interestingly, in vivo studies located the MTOC on the upstream side of the nucleus toward the heart.28
CONCLUSION
We built a flexible, multiplexed device to expose cells to gradients of shear stress in a manner that is compatible with live-cell imaging in existing 6-well tissue culture formats. We found that the epithelial cells, fibroblasts and low-density lymphatic HMVECs moved with the flow direction in this device. In contrast, lymphatic HMVECs seeded at densities that allow cell–cell contacts migrated upstream, against the flow direction. Our study illustrates the potential usefulness of the 6-well IFC in interrogating signaling pathways involved in cellular flow sensing. We also demonstrated its utility in imaging spatiotemporal changes in cytoskeletal dynamics that occur in response to shear stress gradients. More broadly, the 6-well IFC offers a useful means of applying spatial gradients in mechanical force to cells that are not expected to be sensitive to fluid flow specifically, but that sense and respond to external forces as part of their physiological function. We therefore anticipate that the 6-well IFC will provide a useful means of studying the cellular response to fluid flow in a wide variety of circumstances.
ACKNOWLEDGMENTS
This work was supported in part by a National Institutes of Health (NIH) New Innovator Award 1DP2OD007078-01 (A.R.D.), NIH R01HL128779 (A.R.D. and G.G.F.), and a Burroughs-Wellcome Career Award at the Scientific Interface (A.R.D.).
REFERENCES
- 1.Allee WC. An experimental analysis of the relation between physiological states and rheotaxis in isopoda. J. Exp. Zool. 1912;13:269–344. [Google Scholar]
- 2.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 2011;91(1):327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiu JJ, Wang DL, Chien S, Skalak R, Usami S. Effects of disturbed flow on endothelial cells. J. Biomech. Eng. 1998;120(1):2–8. doi: 10.1115/1.2834303. [DOI] [PubMed] [Google Scholar]
- 4.Chung TH, Wang SM, Chang YC, Chen YL, Wu JC. 18beta-glycyrrhetinic acid promotes src interaction with connexin43 in rat cardiomyocytes. J. Cell. Biochem. 2007;100(3):653–664. doi: 10.1002/jcb.21018. [DOI] [PubMed] [Google Scholar]
- 5.Coan DE, Wechezak AR, Viggers RF, Sauvage LR. Effect of shear stress upon localization of the Golgi apparatus and microtubule organizing center in isolated cultured endothelial cells. J. Cell Sci. 1993;104(Pt 4):1145–1153. doi: 10.1242/jcs.104.4.1145. [DOI] [PubMed] [Google Scholar]
- 6.DePaola N, Davies PF, Pritchard WF, Jr, Florez L, Harbeck N, Polacek DC. Spatial and temporal regulation of gap junction connexin43 in vascular endothelial cells exposed to controlled disturbed flows in vitro. Proc. Natl. Acad. Sci. USA. 1999;96(6):3154–3159. doi: 10.1073/pnas.96.6.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Depaola N, Gimbrone MA, Davies PF, Dewey CF., Jr. Vascular endothelium responds to fluid shear-stress gradients. Arterioscler. Thromb. 1992;12(11):1254–1257. doi: 10.1161/01.atv.12.11.1254. [DOI] [PubMed] [Google Scholar]
- 8.Depaola N, Gimbrone MA, Davies PF, Dewey CF., Jr. Vascular endothelium responds to fluid shear-stress gradients (Vol 12, Pg 1254–1257, 1992). Arterioscler. Thromb. 1993;13(3):465–465. doi: 10.1161/01.atv.12.11.1254. [DOI] [PubMed] [Google Scholar]
- 9.Dolan JM, Meng H, Singh S, Paluch R, Kolega J. High fluid shear stress and spatial shear stress gradients affect endothelial proliferation, survival, and alignment. Ann. Biomed. Eng. 2011;39(6):1620–1631. doi: 10.1007/s10439-011-0267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis R, Xu X, Park H, Wei CJ, Chang S, Chatterjee B, Lo C. Connexin43 modulates cell polarity and directional cell migration by regulating microtubule dynamics. PLoS One. 2011;6(10):e26379. doi: 10.1371/journal.pone.0026379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan X, Wilson S, Schlender KK, Ruch RJ. Gap-junction disassembly and connexin 43 dephosphorylation induced by 18 beta-glycyrrhetinic acid. Mol. Carcinog. 1996;16(3):157–164. doi: 10.1002/(SICI)1098-2744(199607)16:3<157::AID-MC6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 12.Hove JR, Köster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421(6919):172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 13.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377(6549):539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 14.Koo MA, Kang JK, Lee MH, Seo HJ, Kwon BJ, You KE, Kim MS, Kim D, Park JC. Stimulated migration and penetration of vascular endothelial cells into poly (l-lactic acid) scaffolds under flow conditions. Biomater. Res. 2014;18(7) doi: 10.1186/2055-7124-18-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaMack JA, Friedman MH. Individual and combined effects of shear stress magnitude and spatial gradient on endothelial cell gene expression. Am. J. Physiol.-Heart C. 2007;293(5):H2853–H2859. doi: 10.1152/ajpheart.00244.2007. [DOI] [PubMed] [Google Scholar]
- 16.Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134(18):3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masuda M, Fujiwara K. The biased lamellipodium development and microtubule organizing center position in vascular endothelial cells migrating under the influence of fluid flow. Biol. Cell. 1993;77(3):237–245. doi: 10.1016/s0248-4900(05)80193-5. [DOI] [PubMed] [Google Scholar]
- 18.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005;6(1):56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 19.Mohamied Y, Rowland EM, Bailey EL, Sherwin SJ, Schwartz MA, Weinberg PD. Change of direction in the biomechanics of atherosclerosis. Ann. Biomed. Eng. 2014;43:16–25. doi: 10.1007/s10439-014-1095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohan S, Mohan N, Valente AJ, Sprague EA. Regulation of low shear flow-induced HAEC VCAM-1 expression and monocyte adhesion. Am. J. Physiol. 1999;276(5 Pt 1):C1100–C1107. doi: 10.1152/ajpcell.1999.276.5.C1100. [DOI] [PubMed] [Google Scholar]
- 21.Muthard RW, Diamond SL. Side view thrombosis microfluidic device with controllable wall shear rate and transthrombus pressure gradient. Lab Chip. 2013;13(10):1883–1891. doi: 10.1039/c3lc41332b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostrowski MA, Huang NF, Walker TW, Verwijlen T, Poplawski C, Khoo AS, Cooke JP, Fuller GG, Dunn AR. Microvascular endothelial cells migrate upstream and align against the shear stress field created by impinging flow. Biophys. J. 2014;106(2):366–374. doi: 10.1016/j.bpj.2013.11.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palazzo AF, Eng CH, Schlaepfer DD, Marcantonio EE, Gundersen GG. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science. 2004;303(5659):836–839. doi: 10.1126/science.1091325. [DOI] [PubMed] [Google Scholar]
- 24.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Polacheck WJ, German AE, Mammoto A, Ingber DE, Kamm RD. Mechanotransduction of fluid stresses governs 3D cell migration. Proc. Natl. Acad. Sci. USA. 2014;111(7):2447–2452. doi: 10.1073/pnas.1316848111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinhart-King CA, Dembo M, Hammer DA. Cell–cell mechanical communication through compliant substrates. Biophys. J. 2008;95(12):6044–6051. doi: 10.1529/biophysj.107.127662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remuzzi A, Dewey CF, Jr, Davies PF, Gimbrone MA., Jr Orientation of endothelial cells in shear fields in vitro. Biorheology. 1984;21(4):617–630. doi: 10.3233/bir-1984-21419. [DOI] [PubMed] [Google Scholar]
- 28.Rogers KA, McKee NH, Kalnins VI. Preferential orientation of centrioles toward the heart in endothelial cells of major blood vessels is reestablished after reversal of a segment. Proc. Natl. Acad. Sci. USA. 1985;82(10):3272–3276. doi: 10.1073/pnas.82.10.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rouleau L, Farcas M, Tardif JC, Mongrain R, Leask RL. Endothelial cell morphologic response to asymmetric stenosis hemodynamics: effects of spatial wall shear stress gradients. J. Biomech. Eng.-T Asme. 2010;8:081013. doi: 10.1115/1.4001891. [DOI] [PubMed] [Google Scholar]
- 30.Sakamoto N, Saito N, Han X, Ohashi T, Sato M. Effect of spatial gradient in fluid shear stress on morphological changes in endothelial cells in response to flow. Biochem. Biophys. Res. Commun. 2010;395(2):264–269. doi: 10.1016/j.bbrc.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Sato M, Saito N, Sakamoto N, Ohashi T. High wall shear stress gradient suppress morphological responses of endothelial cells to fluid flow. IFMBE Proc. 2010;25:312–313. [Google Scholar]
- 32.Schedin P, Keely PJ. Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb. Perspect. Biol. 2011;3(1):a003228. doi: 10.1101/cshperspect.a003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szymanski MP, Metaxa E, Meng H, Kolega J. Endothelial cell layer subjected to impinging flow mimicking the apex of an arterial bifurcation. Ann. Biomed. Eng. 2008;36(10):1681–1689. doi: 10.1007/s10439-008-9540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, Mayor R. Collective chemotaxis requires contact-dependent cell polarity. Dev. Cell. 2010;19(1):39–53. doi: 10.1016/j.devcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ting LH, Jahn JR, Jung JI, Shuman BR, Feghhi S, Han SJ, Rodriguez ML, Sniadecki NJ. Flow mechanotransduction regulates traction forces, intercellular forces, and adherens junctions. Am. J. Physiol. Heart Circ. Physiol. 2012;302(11):H2220–H2229. doi: 10.1152/ajpheart.00975.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomar A, Schlaepfer DD. Focal adhesion kinase: switching between GAPs and GEFs in the regulation of cell motility. Curr. Opin. Cell Biol. 2009;21(5):676–683. doi: 10.1016/j.ceb.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsou JK, Gower RM, Ting HJ, Schaff UY, Insana MF, Passerini AG, Simon SI. Spatial regulation of inflammation by human aortic endothelial cells in a linear gradient of shear stress. Microcirculation. 2008;15(4):311–323. doi: 10.1080/10739680701724359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437(7057):426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 40.Weber GF, Bjerke MA, DeSimone DW. A mechanoresponsive cadherin–keratin complex directs polarized protrusive behavior and collective cell migration. Dev. Cell. 2012;22(1):104–115. doi: 10.1016/j.devcel.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshikawa N, Ariyoshi H, Aono Y, Sakon M, Kawasaki T, Monden M. Gradients in cytoplasmic calcium concentration ([Ca 2+] i) in migrating human umbilical vein endothelial cells (HUVECs) stimulated by shear-stress. Life Sci. 1999;65(24):2643–2651. doi: 10.1016/s0024-3205(99)00533-0. [DOI] [PubMed] [Google Scholar]