Abstract
Objective
to evaluate the short- and long-term effects of acupuncture on vasomotor symptoms (VMS) and quality of life-related measures.
Methods
A total of 209 perimenopausal and postmenopausal women aged 45-60 experiencing ≥4 VMS per day recruited from the community and randomized to receive up to 20 acupuncture treatments within the first 6 months (acupuncture group) or the second 6 months (waitlist control group) of the 12-month study period. The primary outcome was mean daily frequency of VMS. Secondary outcomes were VMS interference with daily life, sleep quality, depressive symptoms, somatic and other symptoms, anxiety, and quality of life.
Results
VMS frequency declined by 36.7% at 6 months in the acupuncture group and increased by 6.0% in the control group (p<0.001 for between-group comparison). At 12 months, the reduction from baseline in the acupuncture group was 29.4% (p<0.001 for within-group comparison from baseline to 12 months), suggesting that the reduction was largely maintained post treatment. Statistically significant clinical improvement was observed after 3 acupuncture treatments and maximum clinical effects occurred after a median of 8 treatments. Persistent improvements were seen in many quality of life-related outcomes in the acupuncture group relative to the control group.
Conclusions
We found that a course of acupuncture treatments was associated with significant reduction in VMS, as well as several quality of life measures, compared with no acupuncture, and that clinical benefit persisted for at least 6 months beyond the end of treatment.
Keywords: menopause, acupuncture, vasomotor symptoms, hot flashes
Vasomotor symptoms (VMS), which include hot flashes and night sweats, are the most common and troubling symptoms associated with menopause.1,2 Although some women report experiencing the menopausal transition without any VMS,3 for other women, these symptoms can be frequent and severe and interfere with daily activities and quality of life.4-12 Recent estimates suggest that frequent menopausal VMS can continue for a median of 7.4 years.13 Further, VMS are the chief menopause-related problems for which US women seek medical attention.14-16
Estrogen therapy, alone or in combination with progesterone, is currently the most effective treatment of VMS. Hormone therapy (HT), however, is associated with a number of risks such as thromboembolic events and breast cancer, and some troublesome side effects such as breast tenderness and irregular bleeding.17-19,19-21 Many women seek alternatives such as other pharmaceutical agents, herbal or dietary remedies, or behavioral therapies.22-25 Unfortunately, many of these agents also have side effects and/or have not been shown to be effective.24,26-29
A number of randomized controlled trials have shown that acupuncture can be effective at reducing the frequency and severity of VMS.30-35 A recent meta-analysis of 12 RCTs concluded that acupuncture improves VMS frequency and severity and menopause-related symptoms in women experiencing natural menopause, with clinical effects lasting up to 3 months.36 However, there is a paucity of evidence about longer term effects of acupuncture, the effects of acupuncture on health-related quality of life, and optimal dosing strategies for acupuncture among women with VMS.
Although the mechanisms of hot flashes are not well understood, one possible cause is the reduction in β-endorphins in the hypothalamus resulting from low concentrations of estrogen. Theories of neurophysiological and neurohumoral mechanisms, as well as concepts of Traditional Chinese Medicine, suggest that acupuncture may be an effective method to control hot flashes through its effect on endorphins. Changes in level of β-endorphins and other neurotransmitters affect the thermoregulatory center in the hypothalamus and acupuncture may alter these central neuromodulators.37-41
We conducted a pragmatic, randomized clinical trial to evaluate the effectiveness of acupuncture for the treatment of menopause-related VMS. The purpose of this study was to provide an unbiased estimate of short- and medium-term clinical benefit or harms that a woman who is experiencing frequent VMS might expect from undergoing a course of acupuncture treatments, relative to not treating VMS with acupuncture.
METHODS
Study Design Overview
This was a pragmatic 2-site, 2-arm clinical trial of perimenopausal and postmenopausal women experiencing an average of ≥4 VMS per day who were randomized to either: 1) a 6-month course of up to 20 acupuncture treatments, or 2) a waitlist control group consisting of usual care for 6 months followed by the same 6-month course of acupuncture treatments administered to study participants in the intervention. Because our aim was to estimate the magnitude and nature of the effects of acupuncture relative to no acupuncture, and because sham acupuncture is neither physiologically inert nor available to patients outside the context of experimental, sham-controlled clinical trials, we did not use a sham acupuncture control. The rationale for this methodological approach is further justified in part by a recent meta-analysis of acupuncture for menopausal hot flashes that concluded that sham acupuncture could induce a treatment effect comparable to true acupuncture in the reduction of hot flash frequency.36
The trial aimed to generate an unbiased estimate of short- and medium-term clinical benefit or harms that a person with VMS might expect from undergoing a course of acupuncture treatments, relative to not treating VMS with acupuncture. Primary outcomes were frequency and severity of VMS assessed by daily diaries. Secondary outcomes included a variety of quality of life-related measures. The study was approved by the Wake Forest Health Sciences Institutional Review Boards for both sites. Recruitment occurred between April 2011-July 2013 at Wake Forest School of Medicine and Chapel Hill Doctors Healthcare Center in North Carolina. Follow-up continued through July 2014.
Study Participants
Eligible participants were perimenopausal or postmenopausal women aged 45-60 experiencing ≥4 VMS/day. Perimenopause was defined as three or more months of self-defined amenorrhea; postmenopause was defined as amenorrhea for 12 or more months.42 Women who had a bilateral oophorectomy and/or hysterectomy prior to cessation of menses were classified as having surgical menopause. Exclusion criteria were: initiation or change in dose of any treatment for VMS in the last 4 weeks, initiation or change in dose of an antidepressant in the last 3 months, having received acupuncture for any indication in the prior 4 weeks or having received acupuncture from one of the study acupuncturists in the prior 6 months, self-reported health status as poor or fair on the telephone screener, or a diagnosis of hemophilia.
Procedures
Women were recruited through newspaper advertisements, radio announcements, and community postings. Initial eligibility was determined by a telephone screener. Eligible women were scheduled for a baseline study visit, at which time they were consented and completed a self-administered questionnaire and received instructions on keeping a 2-week hot flash diary (described under Measures), which was then mailed back to study personnel and used to verify eligibility.
Following completion of the 2-week diary, all women who met the criteria of an average of ≥4 VMS per day were scheduled for their first acupuncture treatment visit to occur within the following 3 weeks. At this visit, the acupuncturist provided a traditional Chinese medicine (TCM) diagnosis to obtain a baseline diagnosis on all women. Following diagnosis, acupuncturists logged into the study website and completed the randomization process to identify group assignment. Women randomized to the acupuncture group received their first treatment at that time. Control group participants were told that their first acupuncture treatment would be in 6 months.
All study participants returned for a study visit at 2, 4, 6, 9, and 12 months post randomization to complete study questionnaires, including an update of medications. Women were allowed to continue or initiate other treatments during this time. Eleven (6%) women in the acupuncture group and two women in the control group (5%) began other behavioral treatments (e.g., mindfulness, mindfulness, yoga) post randomization. Study staff who administered questionnaires were blinded as to group assignment. Study participants were paid $35 for their baseline visit and $25 for completion of each of the follow-up visits. Those who completed all 6 visits were paid an additional $50.
Randomization
Eligible women were randomized to one of the two groups, stratified by site and menopause status, using a block randomization with block size unknown to the investigators and staff to ensure balanced accrual to each group of the study. Randomization sequences were prepared at Wake Forest. Study participants were randomized using a 4:1 distribution to optimize statistical power for identifying possible clinical effects up to 6 months after completion of the 6-month treatment period for participants randomized to the intervention group.
Acupuncture Treatment
Participants assigned to acupuncture were allowed to have up to 20 acupuncture treatments by one of four study acupuncturists over a 6-month period. The frequency of treatments was determined by the acupuncturist and study participant. All study acupuncturists were licensed acupuncturists with 8 to 33 years of practice experience prior to the start of this clinical trial. Acupuncturists ascribed a primary TCM diagnosis and up to two secondary TCM pattern diagnoses to each study participant. Study acupuncturists elicited a symptom history from participants and conducted a physical exam that included pulse palpation and tongue inspection. Information from the history and physical examination informed participants’ diagnoses, which in turn informed acupuncture point selection and individualized treatment plans. TCM diagnoses were reassessed (and changed, if indicated) at each subsequent treatment session. Study acupuncturists were permitted to change acupuncture point selection and other aspects of treatment if clinically indicated at each treatment session.
Sterile, disposable acupuncture needles were inserted through the skin to a depth of from one-half to three centimeters based on anatomical location. Acupuncturists attempted to achieve a “de Qi” sensation upon insertion of acupuncture needles (a sensation of soreness, numbness, heaviness or distention around the needled acupuncture point) whenever possible. No restrictions were placed on duration of each treatment, or on the application of other treatment modalities such as moxibustion or manual or electrical stimulation of acupuncture needles at selected acupuncture point locations, but prescribing Chinese herbal remedies was not permitted.
Control Group
Participants in this group did not receive any form of acupuncture needling during the first 6 months of study participation. Individualized TCM diagnoses and treatment plans were developed at that time based on participants’ clinical presentation at the time that they began their first acupuncture treatment.
Measures
The primary study outcome was the change in frequency and severity of hot flashes and night sweats as measured by the Daily Diary of Hot Flashes (DDHF). The DDHF records the frequency and severity (using a 4- point scale from mild to very severe) of each VMS and allows the investigator to calculate VMS frequency and a VMS index score (the sum of the number of VMS multiplied by each level of severity).43 Women were asked to record the number and severity of VMS each day during the treatment phase of the trial and were provided tally counters to help them keep track of the number of hot flashes throughout the day. Baseline VMS frequency and the index score were calculated using the last fourteen days of diaries available prior to randomization.
Secondary Outcomes
Secondary study outcomes included hot flash interference, sleep quality, menopause related symptoms, depressive symptoms, anxiety, perceived stress, and health-related quality of life.
Hot flash interference was assessed by Hot Flash Related Daily Interference Scale (HFRDIS) which measures the degree that hot flashes interfere with nine daily activities (work, social activities, leisure activities, sleep, mood, concentration, relation with others, sexuality, and enjoyment of life) and overall quality of life within the past week.44 Sleep was assessed using the Pittsburgh Sleep Quality Index (PSQI) 45 and the PROMIS short form Sleep Disturbance measure. The Women's Health Questionnaire (WHQ) measures physical and emotional health of women aged 40 to 60 years and consists of 36 items covering 9 domains: depressed mood, somatic symptoms, anxiety/fears, vasomotor symptoms, sleep problems, sexual behavior, menstrual symptoms, memory/concentration, and attractiveness .46 We excluded the domain of menstrual symptoms in analyses since this domain was not relevant to the vast majority of women in the study. Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression scale, short form (CESD-10).47 Anxiety symptoms were measured with the General Anxiety Disorder (GAD-7),48 and the PROMIS short form Anxiety. Perceived Stress was assessed by the 4-item Perceived Stress Scale (PSS).49
Two measures of quality of life were included: a single global 100 mm visual analogue scale (VAS) and the Physical and Mental Health Component scores of the Medical Outcomes Study (MOS) 36-Item Short Form Health Survey (SF-36).50
Covariates
Covariates included in analyses were previous use of acupuncture sometime in the past but still meeting eligibility requirements (yes/no), current use of HT (yes/no), menopause status (perimenopausal/naturally postmenopausal/surgical post), and season of randomization (winter/spring/summer/fall).
Statistical Analysis
Descriptive statistics of baseline measures were reported, and the two study groups were compared using t-tests for continuous measures and chi-square tests for categorical measures. For the primary analysis comparing change in VMS between study groups, a repeated measures ANCOVA model was used. Included in the model were study group, time after randomization, interaction of study arm and time, baseline VMS frequency (or the Index score), season of randomization, site, acupuncture use prior to the study, use of HT, and baseline menopause status. Self-reported VMS data were collected daily and assigned a time variable used in the model. The time variable was defined as number of weeks after randomization. Least square means from the group by time interaction were estimated and plotted for a visual representation of the results of the study. Two group t-tests for differences of the least square means between groups were performed at 6 months and 12 months to address the primary aims of the study. These t-tests were also performed within each study group to assess the difference from 6 months to 12 months. Additional exploratory tests were then done each week for the first 6 months and every 4 weeks from 6 to 12 months. The same modeling approach was used to evaluate the Index (i.e. frequency × severity) and secondary outcomes. For the secondary outcomes, the time variable in models was defined as the time points of data collection (Months 2, 4, 6, 9, and 12). Using the models, tests for differences in study groups were done at Months 2, 6, and 12. During the treatment period for the acupuncture group, we report the median of the cumulative number of treatments received up to a given week. All analyses were done as intent-to-treat analysis and were performed using SAS version 9.4 software (SAS Institute Inc., Cary, NC).
RESULTS
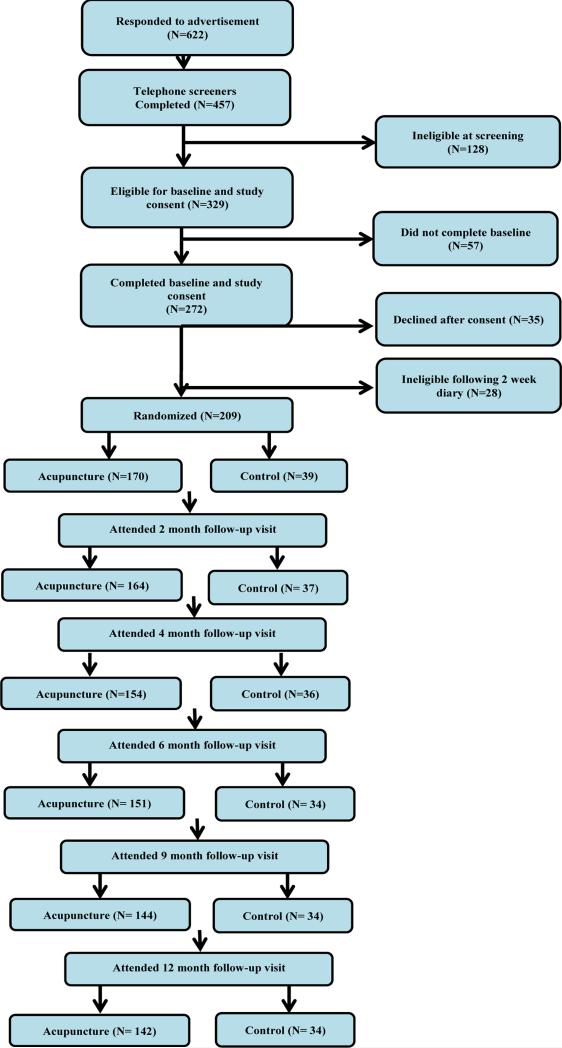
Participant data are shown in Figure 1. Of the 329 screener-eligible women, 272 completed an initial baseline study visit. Of these, 28 were subsequently determined ineligible based on too few VMS after their 2-week hot flash diary and 35 declined further participation. 209 women were entered into the study and randomized. Study retention was excellent, with 89% remaining in the study at 6 months and 84% at 12 months.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram.
Sample characteristics
Sample characteristics by group assignment are shown in Table 1. There were no significant group differences in baseline characteristics. Participants had a mean number of 9.5 (SD=5.0; median = 8.5) VMS/day at baseline. The majority of the sample (76%) was white; 22% participants were African-American. The majority of participants (57%) were naturally postmenopausal and 27% had undergone a surgical menopause (38 women had a hysterectomy only or a hysterectomy with one ovary removed; 16 women had both ovaries removed; and 2 women had a hysterectomy, but did not know whether or not they had any ovaries removed). Twenty-four percent of women had received acupuncture at some point in the past. Two participants in the acupuncture group and no control participants were using hormone therapies at randomization..
TABLE 1.
Baseline characteristics of study sample
| Characteristic | Total Sample N=209 | Acupuncture N=170 | Control N=39 | P-value |
|---|---|---|---|---|
| Age at randomization, mean (SD),y | 53.8 (3.5) | 53.8 (3.5) | 53.6 (3.6) | 0.65 |
| No. VMS/day at baseline,a mean (SD) | 9.5 (5.0) | 9.4 (5.1) | 9.8 (5.0) | 0.68 |
| Race, No. (%) | 0.27 | |||
| Black or African American (non-Hispanic) | 46 (22.0) | 38 (22.4) | 8 (20.5) | |
| Hispanic, American Indian or Alaskan Native | 4 (1.9) | 2 (1.2) | 2 (5.1) | |
| White (non-Hispanic) | 159 (76.1) | 130 (76.5) | 29 (74.4) | |
| Marital Status, No. (%) | 0.74 | |||
| Married or living in a marriage-like relationship | 162 (77.5) | 131 (77.1) | 31 (79.5) | |
| Never married, divorced, separated or widowed | 47 (22.5) | 39 (22.9) | 8 (20.5) | |
| Education, No. (%) | 0.39 | |||
| Less than college | 92 (44.0) | 75 (44.1) | 17 (43.6) | |
| College degree | 47 (22.5) | 41 (24.1) | 6 (15.4) | |
| Beyond college degree | 70 (33.5) | 54 (31.8) | 16 (41.0) | |
| Menopausal status, No. (%) | 0.94 | |||
| Perimenopause | 35 (16.7) | 29 (17.1) | 6 (15.4) | |
| Postmenopause | 118 (56.5) | 95 (55.9) | 23 (59.0) | |
| Surgical menopause | 56 (26.8) | 46 (27.1) | 10 (25.6) | |
| Baseline* VMS (average/day) | 0.85 | |||
| < 5 | 36 (17.2) | 30 (17.6) | 6 (15.4) | |
| 5-9.9 | 99 (47.4) | 82 (48.2) | 17 (43.6) | |
| 10-14.9 | 49 (23.4) | 39 (22.9) | 10 (25.6) | |
| 15 + | 25 (12.0) | 19 (11.2) | 6 (15.4) | |
| On Hormone Therapy (HT) at randomization | 2 (1.0) | 2 (1.2) | 0 | 0.99b |
| Prior use of acupuncture, No. (%) | 50 (23.9) | 41 (24.1) | 9 (23.1) | 0.89 |
VMS, vasomotor symptoms.
Baseline refers to the 2 weeks of hot flash diaries completed just prior to randomization.
p-value from Fisher's Exact test.
Reduction of Vasomotor Symptoms
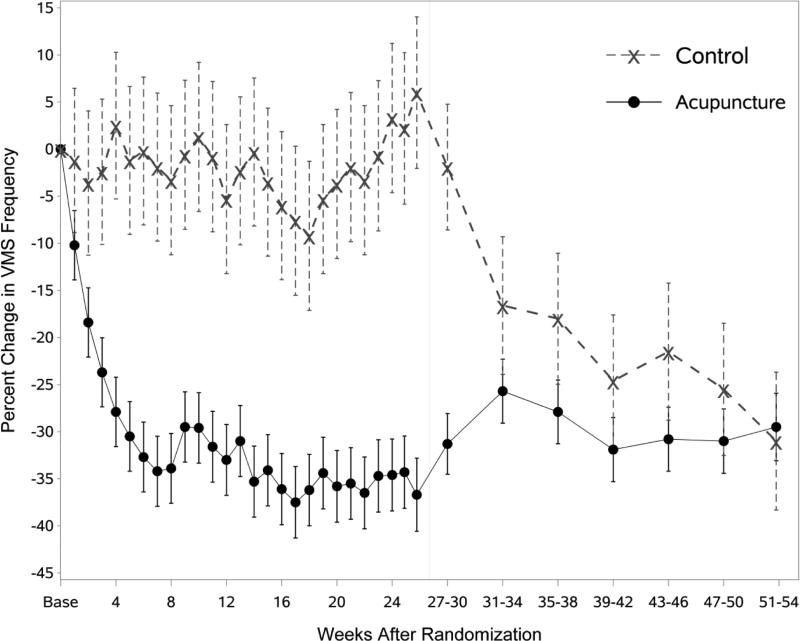
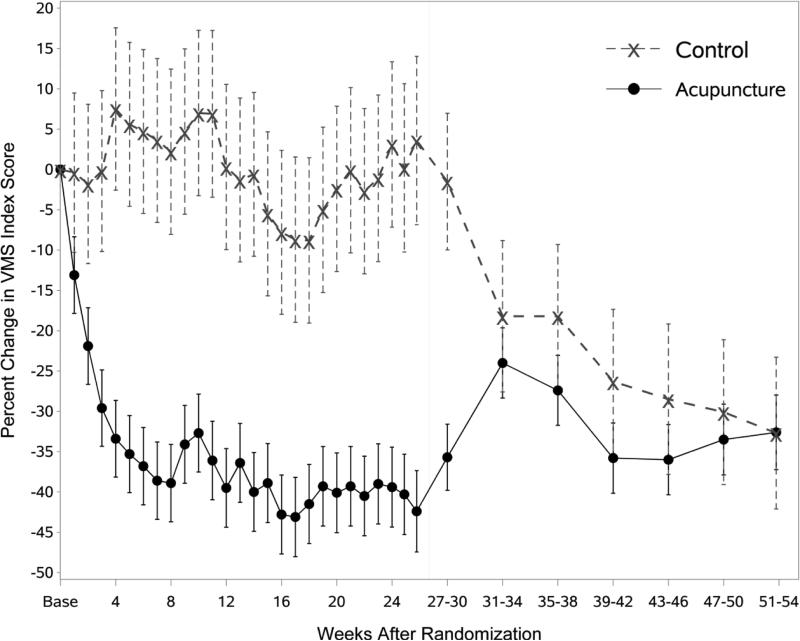
Percent change in total VMS frequency is shown in Figure 2 and Table 1. There was a significant group effect (p<0.001) with VMS frequency declining by 36.7% at 6 months in the acupuncture group and increasing by 6.0% in the control group. Week 3 was the first week where the percent change in VMS frequency was significantly different from controls (p=0.01), with decreases of 23.7% and 2.4% of baseline frequency in the acupuncture and control groups, respectively (Table 2). At 12 months, the reduction from baseline in the acupuncture group was 29.5% (p<0.001), suggesting that the reduction in VMS was largely maintained for 6 months after discontinuing acupuncture treatments. The control group began receiving treatments at 6 months; between 6 and 12 months, VMS frequency in this group decreased to 31.0% of baseline (p<0.001). Results of a sensitivity analysis that censored participant data at the time a participant started a new behavioral therapy were similar to the primary analysis (data not shown). Results for the VMS index score (Figure 3) are very similar to the effects seen in the VMS frequency analysis.
Figure 2.
Estimated percent change in VMS frequency by group and weeks from randomization, controlling for baseline VMS frequency, season of randomization, site, prior use of acupuncture, use of hormone therapy, and menopause status.
TABLE 2.
VMS change in frequency by week from baseline and comparisons between groups and from baseline
| Acupuncture |
Control |
P-values |
|||||
|---|---|---|---|---|---|---|---|
| Week | Na | Change in VMS Estimatesb | Na | Change in VMS Estimatesb | Acupuncture vs Control | Acupuncture vs no change | Control vs no change |
| 1 | 167 | −10.2 (3.7) | 39 | −1.2 (7.7) | 0.29 | 0.006 | 0.87 |
| 2 | 168 | −18.4 (3.7) | 39 | −3.6 (7.7) | 0.08 | <.001 | 0.64 |
| 3 | 169 | −23.7 (3.7) | 38 | −2.4 (7.7) | 0.01 | <.001 | 0.75 |
| 4 | 168 | −27.9 (3.7) | 37 | 2.5 (7.8) | <.001 | <.001 | 0.75 |
| 5 | 165 | −30.5 (3.7) | 36 | −1.2 (7.9) | <.001 | <.001 | 0.88 |
| 6 | 164 | −32.7 (3.7) | 36 | −0.2 (7.9) | <.001 | <.001 | 0.98 |
| 7 | 162 | −34.2 (3.7) | 36 | −1.9 (7.9) | <.001 | <.001 | 0.81 |
| 8 | 163 | −33.9 (3.7) | 35 | −3.3 (7.9) | <.001 | <.001 | 0.68 |
| 9 | 160 | −29.5 (3.7) | 35 | −0.6 (7.9) | 0.001 | <.001 | 0.94 |
| 10 | 159 | −29.6 (3.7) | 35 | 1.3 (7.9) | <.001 | <.001 | 0.87 |
| 11 | 156 | −31.6 (3.8) | 34 | −0.8 (8.0) | <.001 | <.001 | 0.92 |
| 12 | 155 | −33.0 (3.8) | 35 | −5.3 (7.9) | 0.002 | <.001 | 0.51 |
| 13 | 153 | −31.0 (3.8) | 36 | −2.3 (7.9) | 0.001 | <.001 | 0.77 |
| 14 | 154 | −35.3 (3.8) | 36 | −0.3 (7.9) | <.001 | <.001 | 0.97 |
| 15 | 152 | −34.1 (3.8) | 36 | −3.5 (7.9) | <.001 | <.001 | 0.65 |
| 16 | 153 | −36.1 (3.8) | 36 | −6.0 (7.9) | <.001 | <.001 | 0.44 |
| 17 | 151 | −37.5 (3.8) | 35 | −7.6 (7.9) | <.001 | <.001 | 0.34 |
| 18 | 151 | −36.2 (3.8) | 35 | −9.2 (7.9) | 0.002 | <.001 | 0.25 |
| 19 | 150 | −34.4 (3.8) | 35 | −5.3 (7.9) | <.001 | <.001 | 0.50 |
| 20 | 149 | −35.8 (3.8) | 35 | −3.7 (7.9) | <.001 | <.001 | 0.64 |
| 21 | 150 | −35.5 (3.8) | 35 | −1.9 (7.9) | <.001 | <.001 | 0.81 |
| 22 | 149 | −36.5 (3.8) | 35 | −3.3 (7.9) | <.001 | <.001 | 0.67 |
| 23 | 145 | −34.7 (3.8) | 34 | −0.7 (8.0) | <.001 | <.001 | 0.93 |
| 24 | 147 | −34.6 (3.8) | 35 | 3.3 (7.9) | <.001 | <.001 | 0.68 |
| 25 | 145 | −34.3 (3.8) | 33 | 2.2 (8.0) | <.001 | <.001 | 0.78 |
| 26 | 139 | −36.7 (3.9) | 33 | 6.0 (8.0) | <.001 | <.001 | 0.46 |
| End of treatment for acupuncture group and start of treatment for control group | |||||||
| 27-30 | 142 | −31.3 (3.2) | 35 | −1.9 (6.7) | <.001 | <.001 | 0.78 |
| 31-34 | 143 | −25.7 (3.4) | 34 | −16.6 (7.3) | 0.26 | <.001 | 0.02 |
| 35-38 | 140 | −27.9 (3.4) | 33 | −18.0 (7.0) | 0.20 | <.001 | 0.01 |
| 39-42 | 141 | −31.9 (3.4) | 34 | −24.6 (7.0) | 0.35 | <.001 | <.001 |
| 43-46 | 141 | −30.8 (3.4) | 33 | −21.5 (7.3) | 0.25 | <.001 | 0.003 |
| 47-50 | 138 | −31.0 (3.4) | 33 | −25.5 (7.0) | 0.48 | <.001 | <.001 |
| 51-54 | 115 | −29.5 (3.6) | 29 | −31.0 (7.3) | 0.85 | <.001 | <.001 |
VMS, vasomotor symptoms.
Number of participants with a diary in a given period.
Adjusted for baseline VMS, season of randomization, site, acupuncture use prior to the study, use of hormone therapy, and baseline menopause status.
Figure 3.
Estimated percent change in VMS frequency and cumulative number of acupuncture treatments by group and weeks from randomization, controlling for baseline VMS frequency, season of randomization, site, prior use of acupuncture, use of hormone therapy, and menopause status.
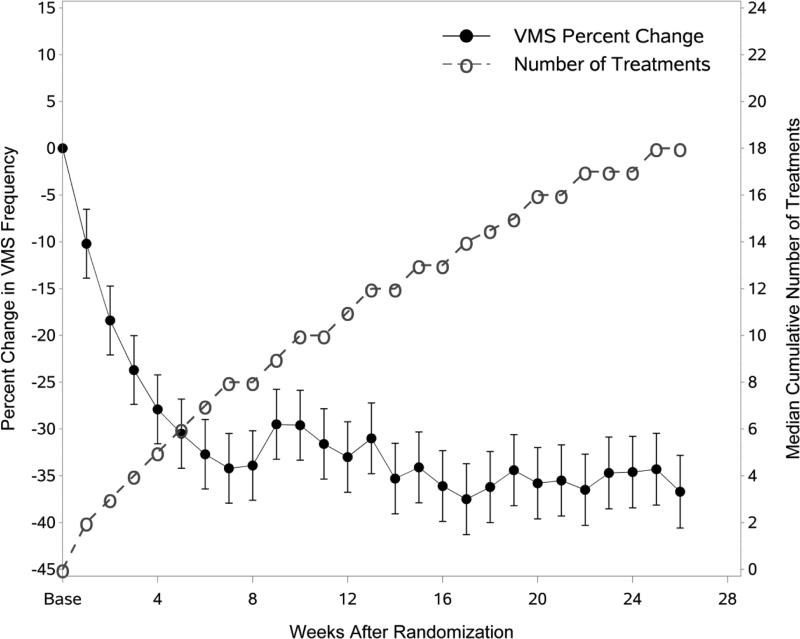
Figure 4 shows the median cumulative number of acupuncture treatments for the acupuncture group superimposed on Figure 2 with the control group removed. As seen in this figure, the maximum reduction in hot flashes occurred at Week 7, which corresponds to a median of 8 acupuncture treatments. Following Week 8, the median cumulative number of treatments continued to increase to a median of 18 at week 26, whereas the percent change in VMS frequency did not. The control group used a median of 16 treatments during their treatment period (months 6 to 12).
Figure 4.
Estimated percent change in VMS Index Score by group and weeks from randomization, controlling for baseline VMS frequency, season of randomization, site, prior use of acupuncture, use of hormone therapy, and menopause status.
Secondary Outcomes
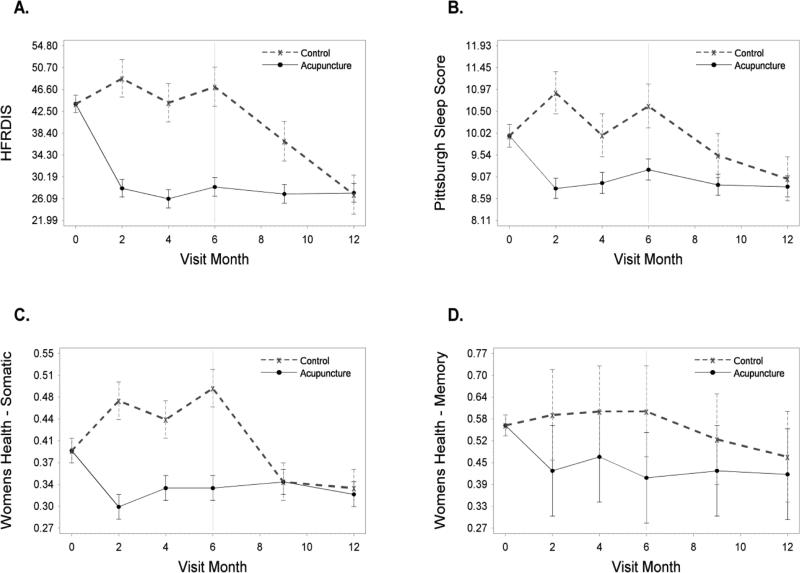
At the 6-month follow-up, the acupuncture group was 19 points lower than the controls on the hot flash interference scale in adjusted analyses (p<0.001). Participants in the acupuncture group also reported significantly fewer sleep problems on all three measures: the Pittsburgh sleep score; sleep domain of the WHQ; and PROMIS sleep measure as compared to women in the control group (Table 3, Figure 5). Women in the acupuncture group also reported significantly fewer symptoms on the WHQ: vasomotor, anxiety, somatic symptoms, and memory. At 12 months, the effects of acupuncture were maintained in the acupuncture group for all of these outcomes. The control group showed similar improvement on these outcomes and in addition, had reduced depressive symptoms from 6 to 12 months.
TABLE 3.
Mean scores and standard errors on secondary outcomes
| Study Visit Monthb |
||||||
|---|---|---|---|---|---|---|
| Outcome | Initial Visita | 2 | 6 | 12 | P-value 6 vs 12 Months | |
| Hot Flash Related Daily Interference Scale | Acupuncture | 44.44 (1.86) | 28.01 (1.67) | 28.27 (1.73) | 27.16 (1.76) | 0.53 |
| Control | 41.24 (3.66) | 48.65 (3.54) | 47.08 (3.68) | 26.83 (3.68) | <.001 | |
| Diff P-valuec | 0.46 | <.001 | <.001 | 0.93 | ||
| CESD | Acupuncture | 8.05 (0.41) | 7.44 (0.35) | 7.62 (0.36) | 7.31 (0.37) | 0.39 |
| Control | 7.77 (0.78) | 8.79 (0.73) | 8.97 (0.75) | 7.38 (0.75) | 0.03 | |
| Diff P-value | 0.76 | 0.10 | 0.10 | 0.93 | ||
| WHQ Depression | Acupuncture | 0.17 (0.01) | 0.16 (0.01) | 0.18 (0.01) | 0.16 (0.02) | 0.20 |
| Control | 0.22 (0.04) | 0.13 (0.03) | 0.22 (0.03) | 0.13 (0.03) | 0.004 | |
| Diff P-value | 0.20 | 0.43 | 0.20 | 0.42 | ||
| WHQ Anxiety | Acupuncture | 0.23 (0.02) | 0.21 (0.03) | 0.19 (0.03) | 0.17 (0.03) | 0.54 |
| Control | 0.23 (0.04) | 0.35 (0.04) | 0.29 (0.05) | 0.21 (0.05) | 0.06 | |
| Diff P-value | 0.98 | 0.001 | 0.02 | 0.46 | ||
| WHQ Attractiveness | Acupuncture | 0.36 (0.03) | 0.36 (0.03) | 0.34 (0.03) | 0.35 (0.03) | 0.69 |
| Control | 0.37 (0.06) | 0.52 (0.05) | 0.40 (0.06) | 0.32 (0.06) | 0.24 | |
| Diff P-value | 0.80 | 0.01 | 0.30 | 0.72 | ||
| WHQ Somatic | Acupuncture | 0.38 (0.02) | 0.30 (0.02) | 0.33 (0.02) | 0.32 (0.02) | 0.8165 |
| Control | 0.43 (0.03) | 0.47 (0.03) | 0.49 (0.03) | 0.33 (0.03) | <.001 | |
| Diff P-value | 0.2426 | <.001 | <.001 | 0.83 | ||
| WHQ Memory | Acupuncture | 0.57 (0.03) | 0.43 (0.13) | 0.41 (0.13) | 0.42 (0.13) | 0.77 |
| Control | 0.51 (0.06) | 0.59 (0.13) | 0.60 (0.13) | 0.47 (0.13) | 0.01 | |
| Diff P-value | 0.39 | 0.004 | 0.001 | 0.33 | ||
| WHQ Sexuality | Acupuncture | 0.54 (0.03) | 0.48 (0.03) | 0.43 (0.03) | 0.45 (0.03) | 0.53 |
| Control | 0.55 (0.06) | 0.54 (0.05) | 0.56 (0.05) | 0.52 (0.05) | 0.46 | |
| Diff P-value | 0.89 | 0.29 | 0.04 | 0.27 | ||
| WHQ Vasomotor | Acupuncture | 0.94 (0.01) | 0.73 (0.03) | 0.77 (0.03) | 0.74 (0.03) | 0.22 |
| Control | 0.95 (0.02) | 0.93 (0.06) | 0.97 (0.06) | 0.70 (0.06) | <.001 | |
| Diff P-value | 0.87 | 0.001 | 0.002 | 0.59 | ||
| WHQ Sleep | Acupuncture | 0.50 (0.03) | 0.37 (0.02) | 0.42 (0.02) | 0.38 (0.02) | 0.21 |
| Control | 0.49 (0.05) | 0.55 (0.05) | 0.60 (0.05) | 0.45 (0.05) | 0.005 | |
| Diff P-value | 0.83 | <.001 | <.001 | 0.22 | ||
| Pittsburgh Sleep Score | Acupuncture | 9.85 (0.27) | 8.81 (0.22) | 9.22 (0.23) | 8.85 (0.23) | 0.14 |
| Control | 10.41 (0.68) | 10.90 (0.46) | 10.61 (0.48) | 9.02 (0.48) | 0.002 | |
| Diff P-value | 0.39 | <.001 | 0.01 | 0.75 | ||
| GAD7 | Acupuncture | 5.39 (0.41) | 4.17 (0.71) | 4.17 (0.71) | 4.15 (0.72) | 0.94 |
| Control | 4.56 (0.75) | 5.93 (0.91) | 5.64 (0.92) | 4.29 (0.92) | 0.06 | |
| Diff P-value | 0.38 | 0.01 | 0.05 | 0.84 | ||
| Perceived Stress Scale | Acupuncture | 4.30 (0.26) | 4.07 (0.22) | 4.34 (0.23) | 4.00 (0.24) | 0.15 |
| Control | 3.95 (0.53) | 5.28 (0.47) | 5.16 (0.49) | 3.77 (0.49) | 0.01 | |
| Diff P-value | 0.56 | 0.02 | 0.13 | 0.67 | ||
| PROMIS – Anxiety t-score | Acupuncture | 51.75 (0.65) | NA | 50.53 (0.62) | 49.77 (0.64) | 0.23 |
| Control | 50.35 (1.52) | NA | 53.17 (1.31) | 49.89 (1.31) | 0.01 | |
| Diff P-value | 0.37 | 0.07 | 0.93 | |||
| PROMIS – Depression t-score | Acupuncture | 49.30 (0.60) | NA | 48.77 (0.56) | 48.51 (0.57) | 0.67 |
| Control | 49.33 (1.35) | NA | 49.70 (1.17) | 48.08 (1.17) | 0.21 | |
| Diff P-value | 0.98 | 0.48 | 0.74 | |||
| PROMIS – Sleep t-score | Acupuncture | 55.72 (0.67) | NA | 52.83 (0.58) | 51.99 (0.59) | 0.17 |
| Control | 56.85 (1.19) | NA | 57.10 (1.22) | 51.78 (1.22) | <.001 | |
| Diff P-value | 0.46 | 0.002 | 0.87 | |||
| SF-36 PCS | Acupuncture | 53.62 (0.54) | NA | 53.59 (0.44) | 53.35 (0.45) | 0.61 |
| Control | 53.03 (1.18) | NA | 52.13 (0.91) | 53.31 (0.91) | 0.23 | |
| Diff P-value | 0.64 | 0.15 | 0.97 | |||
| SF-36 MCS | Acupuncture | 50.22 (0.69) | NA | 49.81 (0.84) | 50.60 (0.85) | 0.28 |
| Control | 49.59 (1.75) | NA | 47.36 (1.44) | 48.82 (1.44) | 0.33 | |
| Diff P-value | 0.70 | 0.10 | 0.23 | |||
| Global QOL | Acupuncture | 70.83 (1.49) | 73.49 (1.27) | 71.09 (1.31) | 69.77 (1.35) | 0.38 |
| Control | 67.42 (3.08) | 68.80 (2.65) | 67.61 (2.74) | 70.02 (2.74) | 0.44 | |
| Diff P-value | 0.32 | 0.11 | 0.25 | 0.93 | ||
WHQ, Women's Health Questionnaire; CESD, Center for Epidemiological Studies Depression Scale; GAD7, General Anxiety Disorder Scale; PROMIS, Patient Reported Outcomes Measurement Information System (t-score rescales raw score into a standardized score with mean=50 and standard deviation=10); PCS = Physical Component Summary; MCS = Mental Component Summary.
Values for initial visit are raw group means (standard error). T-tests were used for comparison between groups.
Values for other visits are lsmean (standard error). P-values are for difference between treatment arms at given time point. These are results from a repeated measures ANCOVA model adjusting for baseline value, site, acupuncture use prior to the study, use of hormone therapy medications, and baseline menopause status.
Diff P-value refers to difference between Acupuncture and Control Group.
Figure 5.
Estimated mean scores on secondary outcomes by group and month shown for: a) Hot Flash Interference, b) Pittsburgh Sleep Score, c) WHQ- Somatic, and d) WHQ- Memory.
DISCUSSION
The findings from this study support previous research showing a significant effect of acupuncture on VMS reduction,30-36 with a sustained effect lasting 6 months after the end of treatment. Acupuncture had a significant positive effect on hot flash interference, sleep, somatic and memory symptoms, and anxiety and these benefits were maintained 6 months following the end of treatment. Other studies have also found beneficial effects on similar outcomes for shorter periods of time.36 However, it is not clear from our study, as well as those of others, whether the benefits on sleep and other symptoms are direct effects of acupuncture or secondary to the reduction of vasomotor symptoms. This study also goes beyond previous research by including a longer post-treatment follow-up than most studies.32,33,35
Our findings provide information that can be used to estimate the number of weeks and treatments needed for acupuncture to be effective. We found that a statistically significant reduction in VMS frequency in the acupuncture group relative to controls was observed during the third week of acupuncture treatments, which represents fewer treatments than the number of treatments used in previous trials with predetermined treatment schedules.30-35,51,52 We also found that maximum reduction in VMS frequency occurred after 7 weeks of treatment, corresponding to a median of 8 treatments. It is important to note that this study was not designed to formally compare different doses of acupuncture, and that unmeasured confounders may have biased these estimates. For example, it is possible that women who experienced relief of symptoms after one or two treatments may have been more likely to schedule their third or fourth treatment sooner. It is also possible that certain patient characteristics influenced acupuncturists’ treatment decisions. These unknown and unmeasured potential confounders in our study are likely to be similarly present in clinical practice.
It is worth comparing our results to studies of selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), which have been viewed as viable alternatives to hormone therapy. The Menopause Strategies: Finding Lasting Answers for Symptoms and Health (MsFLASH) network found a 47% reduction in VMS from baseline with escitalopram (compared to 33% in the placebo group),53 but that one-third of women whose VMS had improved relapsed within 3 weeks after discontinuation of escitalopram.54 MsFLASH also found a 47.6% reduction from baseline in VMS frequency with venlafaxine, but did not look at effects post treatment.55 A recent systematic review concluded that although venlafaxine was shown to provide beneficial effects on reducing VMS, trials have also reported significant adverse effects.56 In the present study, only three women reported adverse effects: two reported pain during treatment and one reported a feeling of numbness. Another review concluded that paroxetine, which is the only SSRI approved by the FDA for VMS, had the highest probability for reducing VMS.57 Both reviews conclude that, while effective for reducing VMS, both SSRIs and SNRIs can have adverse side effects. Although the 35% reduction in hot flash frequency observed in our study was less than in these trials, the low adverse event rate of 1.4% suggests that acupuncture is safe, and persistent clinical benefits were observed up to the final follow-up assessment 6 months after end of treatments.
Strengths of this current study include the simulation of clinical practice where acupuncturists were allowed to individualize treatment and participants experienced a course of acupuncture treatments that was similar to what they might expect outside of the context of a clinical trial. The study had good retention of participants and spanned all seasons to avoid seasonal effects of hot flashes. A limitation of the study is the potential unreliability and risk of bias associated with self-reporting of VMS, despite the fact that daily diaries represent the standard outcome measure used in most clinical trials of VMS treatment studies. It is also possible that women who perceived little benefit from acupuncture were more likely to drop out of the study. In addition, although we did not find an additional benefit beyond 8 acupuncture treatments, it is possible that the additional treatments helped to provide a maintenance effect.
The present study was designed to simulate clinical practice in community settings where the number, frequency, and timing of acupuncture treatments are allowed to vary according to acupuncturists' recommendations and patients’ preferences. Our study design reflects the priorities proposed by comparative effectiveness researchers who argue that pragmatic or effectiveness trials with good external validity are generally better suited to inform clinical practice than efficacy trials with good internal validity. For example, our study design meets criteria for 7 of the 10 domains identified by the pragmatic-explanatory continuum indicator summary (PRECIS) assessment tool58 as being important for effectiveness trials: 1) broad patient eligibility criteria; 2) experimental intervention flexibility; 3) experimental intervention practitioner expertise with “only ordinary attention to dose setting”; 4) usual practice for a comparison intervention; 5) primary trial outcomes that are meaningful to study participants; 6) no obtrusive measurement of participant compliance; 7) no obtrusive measurement of practitioner adherence to study protocol; and 8) analysis of primary outcomes “to see if the treatment works under the usual conditions, with all of the noise inherent.” A similar set of domains has been proposed by a different group of comparative effectiveness researchers who developed a tool to distinguish efficacy from effectiveness studies.59 An important feature of both of these tools is their emphasis on not using placebo- or sham-controlled comparison groups. The movement away from the use of placebo or sham control groups is a defining feature of the emerging field of comparative effectiveness research, which generally strives to prioritize answering the question, “Can this intervention work under usual conditions?” (effectiveness studies) rather than “Does this intervention work under ideal conditions?” (efficacy studies).58
Movement towards conducting more pragmatic clinical studies without placebo controls notwithstanding, the fact that we did not include a sham acupuncture procedure as a control group precludes knowing to what extent nonspecific effects may have contributed to the observed clinical response reported by study participants. It is possible that most, or even all, of the clinical benefits observed by participants who received acupuncture relative to those in the waitlist group are attributable to patients’ expectations of benefit, or the additional care and attention that they received by healthcare providers, or other factors not directly related to physiological responses to the insertion of acupuncture needles. It is also possible that the sustained reduction in hot flash frequency and improvement in quality of life related measures after completion of the course of acupuncture treatments are attributable to nonspecific effects. The purpose of this study, however, was not to estimate the magnitude of specific effects associated with acupuncture, but, rather, to estimate the magnitude of clinical benefit (and harms) that women with frequent hot flashes might expect from a course of acupuncture treatments. The findings from our study, combined with the findings from prior research, provides compelling evidence that a course of acupuncture treatments can reduce hot flash frequency and severity and can improve health-related quality of life. Future research can explore the mechanisms of action and the relative contributions of specific and nonspecific effects of acupuncture for this clinical condition.
CONCLUSIONS
Acupuncture as practiced in clinical settings can have a positive benefit on reducing hot flashes and improving sleep and other symptoms relative to no acupuncture. The clinical benefits are observed after just a few acupuncture treatments and appear to persist for many months after acupuncture treatments have been discontinued.
Acknowledgments
Funding Support: Research supported by grant R01AT005854 from the National Center for Complementary and Integrative Health (NCCIM).
Footnotes
Conflict of interest/financial disclosure: Dr. Coeytaux has a financial interest in two organizations involved in recruiting study subjects and administering acupuncture treatments at one of the two study sites. A conflict of interest management plan was developed by Duke University and is available upon request.
Trial Registration: clinical trials.gov identifier: NCT01276028
References
- 1.Avis NE, Brockwell S, Colvin A. A universal menopausal syndrome? Am J Med. 2005;118(Suppl 12B):37–46. doi: 10.1016/j.amjmed.2005.09.057. [DOI] [PubMed] [Google Scholar]
- 2.Kronenberg F. Hot flashes: Epidemiology and physiology. Ann NY Acad Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. [DOI] [PubMed] [Google Scholar]
- 3.Avis NE, Crawford SL, McKinlay SM. Psychosocial, behavioral, and health factors related to menopause symptomatology. Womens Health. 1997;3:103–120. [PubMed] [Google Scholar]
- 4.Carpenter JS, Johnson D, Wagner L, Andrykowski M. Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Oncol Nurs Forum. 2002;29:E16–E25. doi: 10.1188/02.ONF.E16-E25. [DOI] [PubMed] [Google Scholar]
- 5.Daly E, Gray A, Barlow D, McPherson K, Roche M, Vessey M. Measuring the impact of menopausal symptoms on quality of life. Brit Med J. 1993;307:836–840. doi: 10.1136/bmj.307.6908.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuh JL, Wang SJ, Lee SJ, Lu SR, Juang KD. Quality of life and menopausal transition for middle-aged women on Kinmen island. Qual Life Res. 2003;12:53–61. doi: 10.1023/a:1022074602928. [DOI] [PubMed] [Google Scholar]
- 7.Hays J, Ockene JK, Brunner RL, et al. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2003;348:1839–1854. doi: 10.1056/NEJMoa030311. [DOI] [PubMed] [Google Scholar]
- 8.Kumari M, Stafford M, Marmot M. The menopausal transition was associated in a prospective study with decreased health functioning in women who report menopausal symptoms. J Clin Epidemiol. 2005;58:719–727. doi: 10.1016/j.jclinepi.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Ledesert B, Ringa V, Breart G. Menopause and perceived health status among the women of the French GAZEL cohort. Maturitas. 1994;20:113–120. doi: 10.1016/0378-5122(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 10.Avis NE, Ory M, Matthews KA, Schocken M, Bromberger J, Colvin A. Health-related quality of life in a multiethnic sample of middle-aged women: Study of Women's Health Across the Nation (SWAN). Med Care. 2003;41:1262–1276. doi: 10.1097/01.MLR.0000093479.39115.AF. [DOI] [PubMed] [Google Scholar]
- 11.Williams RE, Levine KB, Kalilani L, Lewis J, Clark RV. Menopause-specific questionnaire assessment in US population-based study shows negative impact on health-related quality of life. Maturitas. 2009;62:153–159. doi: 10.1016/j.maturitas.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Blumel JE, Chedraui P, Baron G, et al. A large multinational study of vasomotor symptom prevalence, duration, and impact on quality of life in middle-aged women. Menopause. 2011;18:778–785. doi: 10.1097/gme.0b013e318207851d. [DOI] [PubMed] [Google Scholar]
- 13.Avis NE, Crawford SL, Greendale G, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531–539. doi: 10.1001/jamainternmed.2014.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johannes CB, Crawford SL, Posner JG, McKinlay SM. Longitudinal patterns and correlates of hormone replacement therapy use in middle-aged women. Am J Epidemiol. 1994;140:439–452. doi: 10.1093/oxfordjournals.aje.a117266. [DOI] [PubMed] [Google Scholar]
- 15.Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Fehnel SE, Clark RV. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas. 2007;58:348–358. doi: 10.1016/j.maturitas.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson WK, Ellison SA, Grason H, Powe NR. Patterns of ambulatory care use for gynecologic conditions: a national study. Am J Obstet Gynecol. 2001;184:523–530. doi: 10.1067/mob.2001.111795. [DOI] [PubMed] [Google Scholar]
- 17.Barnabei VM, Grady D, Stovall DW, et al. Menopausal symptoms in older women and the effects of treatment with hormone therapy. Obstet Gynecol. 2002;100:1209–1218. doi: 10.1016/s0029-7844(02)02369-4. [DOI] [PubMed] [Google Scholar]
- 18.Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 19.Writing Group for the Women's Health Initiative Investigators Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 20.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. New Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 21.Wassertheil-Smoller S, Hendrix S, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 22.Beal MW. Women's use of complementary and alternative therapies in reproductive health care. J Nurse Midwifery. 1998;43:224–234. doi: 10.1016/s0091-2182(98)00009-3. [DOI] [PubMed] [Google Scholar]
- 23.Kessel B, Kronenberg F. The role of complementary and alternative medicine in management of menopausal symptoms. Endocrinol Metab Clin North Am. 2004;33:717–739. doi: 10.1016/j.ecl.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Nedrow A, Miller J, Walker M, Nygren P, Huffman LH, Nelson HD. Complementary and alternative therapies for the management of menopause-related symptoms: a systematic evidence review. Arch Intern Med. 2006;166:1453–1465. doi: 10.1001/archinte.166.14.1453. [DOI] [PubMed] [Google Scholar]
- 25.Newton KM, Buist DS, Keenan NL, Anderson LA, LaCroix AZ. Use of alternative therapies for menopause symptoms: results of a population-based survey. Obstet Gynecol. 2002;100:18–25. doi: 10.1016/s0029-7844(02)02005-7. [DOI] [PubMed] [Google Scholar]
- 26.Huntley AL, Ernst E. A systematic review of herbal medicinal products for the treatment of menopausal symptoms. Menopause. 2003;10:465–476. doi: 10.1097/01.GME.0000058147.24036.B0. [DOI] [PubMed] [Google Scholar]
- 27.Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: A review of randomized, controlled trials. Ann Intern Med. 2002;137:805–813. doi: 10.7326/0003-4819-137-10-200211190-00009. [DOI] [PubMed] [Google Scholar]
- 28.Loprinzi CL, Kubler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomized controlled trial. Lancet. 2000;356:2059–2063. doi: 10.1016/S0140-6736(00)03403-6. [DOI] [PubMed] [Google Scholar]
- 29.Nagamani M, Kelver ME, Smith ER. Treatment of menopausal hot flashes with transdermal administration of clonidine. Am J Obstet Gynecol. 1987;156:561–565. doi: 10.1016/0002-9378(87)90050-0. [DOI] [PubMed] [Google Scholar]
- 30.Avis NE, Legault C, Coeytaux RR, et al. A randomized, controlled pilot study of acupuncture treatment for menopausal hot flashes. Menopause. 2008;15:1070–1078. doi: 10.1097/gme.0b013e31816d5b03. [DOI] [PubMed] [Google Scholar]
- 31.Borud EK, Alraek T, White A, et al. The acupuncture on hot flushes among menopausal women (ACUFLASH) study, a randomized controlled trial. Menopause. 2009;16:484–493. doi: 10.1097/gme.0b013e31818c02ad. [DOI] [PubMed] [Google Scholar]
- 32.Nedeljkovic M, Tian L, Ji P, et al. Effects of acupuncture and Chinese herbal medicine (Zhi Mu 14) on hot flushes and quality of life in postmenopausal women: results of a four-arm randomized controlled pilot trial. Menopause. 2014;21:15–24. doi: 10.1097/GME.0b013e31829374e8. [DOI] [PubMed] [Google Scholar]
- 33.Kim KH, Kang KW, Kim DI, et al. Effects of acupuncture on hot flashes in perimenopausal and postmenopausal women-a multicenter randomized clinical trial. Menopause. 2010;17:269–280. doi: 10.1097/gme.0b013e3181bfac3b. [DOI] [PubMed] [Google Scholar]
- 34.Painovich JM, Shufelt CL, Azziz R, et al. A pilot randomized, single blind, placebo-controlled trial of traditional acupuncture for vasomotor symptoms and mechanistic pathways of menopause. Menopause. 2012;19:54–61. doi: 10.1097/gme.0b013e31821f9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vincent A, Barton DL, Mandrekar JN, et al. Acupuncture for hot flashes: a randomized, sham-controlled clinical study. Menopause. 2007;14:45–52. doi: 10.1097/01.gme.0000227854.27603.7d. [DOI] [PubMed] [Google Scholar]
- 36.Chiu HY, Pan CH, Shyu YK, Han BC, Tsai PS. Effects of acupuncture on menopause-related symptoms and quality of life in women on natural menopause: a meta-analysis of randomized controlled trials. Menopause. 2015;22:234–244. doi: 10.1097/GME.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 37.Casper RF, Yen SS. Neuroendocrinology of menopausal flushes: an hypothesis of flush mechanism. Clin Endocrinol (Oxf ) 1985;22:293–312. doi: 10.1111/j.1365-2265.1985.tb03243.x. [DOI] [PubMed] [Google Scholar]
- 38.Chang HT. Neurophysiological interpretation of acupuncture analgesia. Endeavour. 1980;4:92–96. doi: 10.1016/0160-9327(80)90054-x. [DOI] [PubMed] [Google Scholar]
- 39.Han JS, Terenius L. Neurochemical basis of acupuncture analgesia. Annu Rev Pharmacol Toxicol. 1982;22:193–220. doi: 10.1146/annurev.pa.22.040182.001205. [DOI] [PubMed] [Google Scholar]
- 40.Pomeranz B. Scientific basis of Acupuncture. In: Stux G, Pomeranz B, editors. Basics of Acupuncture. 4th ed. Springer; Berlin: 1998. pp. 6–47. [Google Scholar]
- 41.Yano T, Kato B, Fukuda F, et al. Alterations in the function of cerebral dopaminergic and serotonergic systems following electroacupuncture and moxibustion applications: possible correlates with their antistress and psychosomatic actions. Neurochem Res. 2004;29:283–293. doi: 10.1023/b:nere.0000010457.00855.8c. [DOI] [PubMed] [Google Scholar]
- 42.Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of reproductive aging workshop (STRAW). Fertil Steril. 2001;76:874–878. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 43.Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19:4280–4290. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 44.Carpenter JS. The Hot Flash Related Daily Interference Scale: a tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manage. 2001;22:979–989. doi: 10.1016/s0885-3924(01)00353-0. [DOI] [PubMed] [Google Scholar]
- 45.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiat Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 46.Hunter MS. The Women's Health Questionnaire (WHQ): Frequently Asked Questions (FAQ). Health Qual Life Outcomes. 2003;1:41. doi: 10.1186/1477-7525-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: Evaluation of a short form of the CES-D. Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 48.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 49.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983:385–396. [PubMed] [Google Scholar]
- 50.Ware JE, Jr, Sherbourne CD. The MOS 36-item short form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 51.Venzke L, Calvert JF, Gilbertson B. A randomized trial of acupuncture for vasomotor symptoms in post-menopausal women. Complement Ther Med. 2010;18:59–66. doi: 10.1016/j.ctim.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Park JE, Lee MS, Jung S, et al. Moxibustion for treating menopausal hot flashes: a randomized clinical trial. Menopause. 2009;16:660–665. doi: 10.1097/gme.0b013e318198cdf7. [DOI] [PubMed] [Google Scholar]
- 53.Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305:267–274. doi: 10.1001/jama.2010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joffe H, Guthrie KA, Larson J, et al. Relapse of vasomotor symptoms after discontinuation of the SSRI Escitalopram: results from the MsFLASH research network. Menopause. 2013;20:261–268. doi: 10.1097/GME.0b013e31826d3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joffe H, Guthrie KA, LaCroix AZ, et al. Low-dose estradiol and the serotoninnorepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA. 2014;174:1058–1066. doi: 10.1001/jamainternmed.2014.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Handley AP, Williams M. The efficacy and tolerability of SSRI/SNRIs in the treatment of vasomotor symptoms in menopausal women: A systematic review. J Am Assoc Nurse Pract. 2014;27:54–61. doi: 10.1002/2327-6924.12137. [DOI] [PubMed] [Google Scholar]
- 57.Shams T, Firwana B, Habib F, et al. SSRIs for hot flashes: a systematic review and meta-analysis of randomized trials. J Gen Intern Med. 2014;29:204–213. doi: 10.1007/s11606-013-2535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol. 2009;62:464–475. doi: 10.1016/j.jclinepi.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 59.Gartlehner G, Hansen RA, Nissman D, Lohr KN, Carey TS. A simple and valid tool distinguished efficacy from effectiveness studies. J Clin Epidemiol. 2006;59:1040–1048. doi: 10.1016/j.jclinepi.2006.01.011. [DOI] [PubMed] [Google Scholar]