SUMMARY
Sensing of lipopolysaccharide (LPS) in the cytosol triggers caspase-11 activation and is central to host defense against Gram-negative bacterial infections and to the pathogenesis of sepsis. Most Gram-negative bacteria that activate caspase-11 however are not cytosolic and the mechanism by which LPS from these bacteria gains access to caspase-11 in the cytosol remains elusive. Here we identify outer membrane vesicles (OMV) produced by Gram-negative bacteria as a vehicle that delivers LPS into the cytosol triggering caspase-11-dependent effector responses in vitro and in vivo. OMV are internalized via endocytosis, and LPS is released into the cytosol from early endosomes. The use of hypovesiculating bacterial mutants, compromised in their ability to generate OMV, reveal the importance of OMV in mediating the cytosolic localization of LPS. Collectively, these findings demonstrate a critical role for OMV in enabling the cytosolic entry of LPS and consequently caspase-11 activation during Gram-negative bacterial infections.
INTRODUCTION
The compartmentalization of the innate immune system enables it to mount customized immune responses to pathogens based on the subcellular localization of microbial products (Brubaker et al., 2015). Toll-like receptors (TLRs) sense microbial products at the cell surface and within endosomes inducing NFκB/IRF3-mediated transcriptional responses. A second set of receptors in the cytosol recognizes the same microbial products, but triggers markedly distinct responses, the hallmark of which is the activation of inflammasomes. Inflammasomes are multi-protein complexes comprised of a sensor such as an NLR protein, an adaptor protein, ASC, and the protease caspase-1. A multitude of microbial signals such as toxins, flagellin, and nucleic acids can trigger inflammasome assembly resulting in caspase-1 activation (Rathinam et al., 2012a). Caspase-1 processes pro-IL-1β and pro-IL-18 into active forms and cleaves gasdermin D to trigger pyroptosis, an inflammatory cell death (He et al., 2015; Kayagaki et al., 2015; Shi et al., 2015).
The latest addition to the repertoire of immune surveillance mechanisms in the cytosol is a new LPS sensing pathway. LPS was considered to be exclusively detected at the cell surface by TLR4, in combination with MD2 and CD14, leading to transcriptional regulation of gene expression (Park et al., 2009; Poltorak et al., 1998). Recent studies have discovered that the host is capable of TLR4-independent recognition of LPS in the cytosol (Hagar et al., 2013; Kayagaki et al., 2013). Inflammatory caspases namely caspase-11 in mice and caspase-4 and -5 in humans are considered as the receptors for cytosolic LPS (Shi et al., 2014). Caspase-11 binds cytosolic LPS leading to its own activation, which together with NLRP3 and ASC triggers caspase-1 activation. Interestingly, caspase-11 also directly cleaves gasdermin D mediating pyroptosis in a NLRP3- and caspase-1-independent manner (Kayagaki et al., 2015; 2011; Shi et al., 2014; 2015). The cytosolic entry of LPS thus stimulates responses that are entirely distinct from the responses to TLR4-recognition of extracellular LPS and therefore, LPS accessing the cytosol is a critical event. During infections with cytosolic bacteria such as Burkholderia and Shigella, LPS may gain access to the cytosol owing to the cytosolic niche of bacteria. However, most Gram-negative bacteria that activate caspase-11 including enterohaemorrhagic Escherichia coli (EHEC), Vibrio cholerae, Citrobacter rodentium, and Haemophilus influenzae are not cytosolic (Gurung et al., 2012; Kailasan Vanaja et al., 2014; Meunier et al., 2014; Rathinam et al., 2012b) raising an intriguing question of how LPS from these Gram-negative bacteria gains access to the cytosol.
Gram-negative bacteria secrete outer membrane vesicles (OMV), which are of 20-250 nanometers in diameter. OMV are not a byproduct of bacterial cell wall damage or lysis but bona fide secretory vesicles produced in a programmed fashion (Ellis and Kuehn, 2010). LPS is one of the most abundant components of OMV. Bacteria secrete OMV at increased levels upon exposure to stressful conditions such as host milieu, and OMV enable bacteria to communicate with host cells and deliver their products intracellularly to modulate cellular functions. Therefore, OMV, similar to eukaryotic exosomes/microvesicles, are an efficient inter-cellular communication mechanism (Demuth et al., 2003; Galka et al., 2008; Kesty et al., 2004). Previous studies showed that purified OMV are immunostimulatory (Kim et al., 2013; Park et al., 2010), and OMV-associated peptidoglycan has been shown to activate NOD signaling and NF-κB activation (Bonham and Kagan, 2014; Kaparakis et al., 2010). Furthermore, OMV produced by gut microbiota play an immunomodulatory role in mucosal immunity (Hickey et al., 2015; Shen et al., 2012). Thus, OMV facilitate the trans-kingdom or inter-kingdom communication between the host and microbes in pathogenic as well as symbiotic contexts.
Here, we identified OMV as the vehicle that mediates the cytosolic localization of LPS during extra cellular Gram-negative bacterial infections. Following their clathrin-mediated endocytic uptake, OMV deliver LPS into the cytosol from early endocytic compartments. Consequently, OMV activate cytosolic LPS sensing pathway leading to pyroptosis and caspase-1 activation. Demonstrating a necessary role for OMV for intracellular LPS release during bacterial infections, genetic attenuation of bacterial OMV production diminishes their ability to activate caspase-11-dependent cell death and IL-1 responses. Collectively these findings reflect OMV as a biologically relevant means by which LPS enters the cell during bacterial infections. In broader terms, our data reveal a fundamental mechanism that delivers pathogen-associated molecular patterns (PAMP) to the cytosol to alert the immune system. In order to mount optimal immune responses, the host should be able to recognize not only PAMP but also cues indicative of harmful infections (Vance et al., 2009). Since OMV are produced by live replicating bacteria and alert cytosolic surveillance mechanisms, OMV with immunostimulatory PAMP can be considered as a critical contextual cue for infections that pose serious threat to the host.
RESULTS
LPS Gains Access to the Cytosol During Extracellular Gram-negative Bacterial Infections
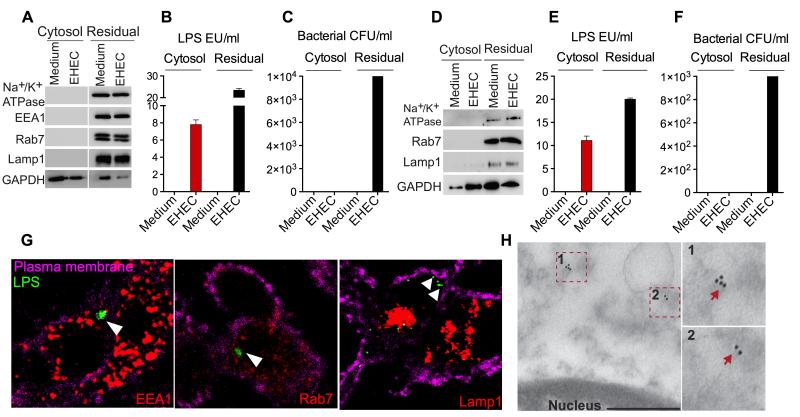
LPS entry into the cytosol is a critical step in the caspase-11-driven noncanonical inflammasome responses to Gram-negative bacteria. However, the majority of the Gram-negative bacteria such as EHEC that activate this cytosolic LPS sensing pathway are extracellular and excluded from the cytosol owing to their susceptibility to the bactericidal mechanisms of phagolysosomes (Kailasan Vanaja et al., 2014). Given this, we sought to determine if LPS actually enters the cytosol during an extracellular Gram-negative bacterial infection by employing four independent approaches. First, we extracted cytosol from uninfected or EHEC-infected bone marrow-derived macrophages (BMDM) using digitonin and assessed LPS levels with the limulus amebocyte lysate (LAL) assay. Digitonin is commonly used to isolate cytosol (Ramsby and Makowski, 2005), and the use of an extremely low concentration of digitonin (0.005%) for a very short duration allowed extraction of cytosol devoid of plasma membrane, early and late endosomes and lysosomes (Na+/K+ ATPase, EEA1, Rab7, and Lamp1, respectively; Fig. 1A). By subjecting the cytosol fraction and the residual non-cytosolic fraction (which includes the plasma membrane and the organelles such as endosomes and lysosomes) to LAL assay, we found that LPS was present in the cytosol of infected but not uninfected cells (Fig. 1B). In contrast, we did not recover any bacteria from the cytosol by agar plating (Fig. 1C) indicating that LPS detected in the cytosol is not from bacteria escaping into the cytosol. On the other hand, the residual non-cytosolic fraction contained a considerable number of bacteria and consequently a significant amount of LPS (Fig. 1B-C). Similar results were obtained for the cytosol extracted using a standard non-detergent method (Sun et al., 2013) in which cells were mechanically lysed in hypotonic buffer (Fig. 1D-F). Additionally, the cytosolic level of LPS increased over time and a significantly higher amount of LPS was observed in the cytosol at 16 h compared to 4 h (Fig. S1A) resulting in a proportional increase in cell death, a direct outcome of cytosolic LPS-mediated caspase-11 activation (Fig. S1B).
Figure 1. LPS Gains Access to the Cytosol During Extracellular Gram-Negative Bacterial Infections.
(A-C) Immunoblots for Na+/K+ ATPase, EEA1, Rab7, LAMP1 and GAPDH (A), LAL assay for LPS (EU, endotoxin units; B), and agar plating for bacterial counts (C) in the cytosolic and residual fractions of uninfected BMDM or BMDM infected with EHEC for 4 h at an MOI of 10 obtained by digitonin fractionation.
(D-F) Immunoblots for Na+/K+ ATPase, Rab7, LAMP1 and GAPDH (D), LAL assay for LPS (E) and agar plating for bacterial counts (F) in the cytosolic and residual fractions of uninfected or EHEC-infected BMDM obtained by Dounce homogenization in hypotonic buffer.
(G) Confocal microscopy of BMDM infected with EHEC at an MOI of 10 for 4 h. LPS was visualized with an anti-LPS antibody (green), EEA1, Rab7 and LAMP1 with the respective antibodies (red) and plasma membrane with cholera toxin B (magenta).
(H) Transmission electron microscopy (TEM) of BMDM infected with EHEC for 4 h at an MOI of 10 following immunogold labeling with an anti-LPS antibody. Scale bar = 0.5 μM.
Data are presented as mean±SEM from one experiment representative of three experiments for A-C and two experiments for D-H. See also Figure S1.
To visualize the cytosolic localization of LPS during EHEC infection, BMDM infected with EHEC were stained with antibodies against LPS or Lipid A as well as endosomal or lysosomal markers and analyzed using confocal microscopy. At 4 h of infection distinct LPS or lipid A foci in the cytosol that did not associate with early endosomes, late endosomes or lysosomes were found (Fig. 1G and S1C). Approximately 30-40% of the infected cells contained these cytosolic LPS foci whereas no LPS or lipid A foci were detected in uninfected cells (Fig. S1D-E). Furthermore, transmission electron microscopy (TEM) of EHEC-infected cells following immunogold staining for LPS showed the presence of LPS in the cytosol (outside membrane-bound organelles; Fig. 1H) and bacteria in phagosome-like structures but not in the cytosol (Fig. S1F). Collectively, these data clearly demonstrate the cytosolic localization of LPS but not bacteria during an extracellular Gram-negative bacterial infection.
Bacterial OMV Deliver LPS into the Cytosol
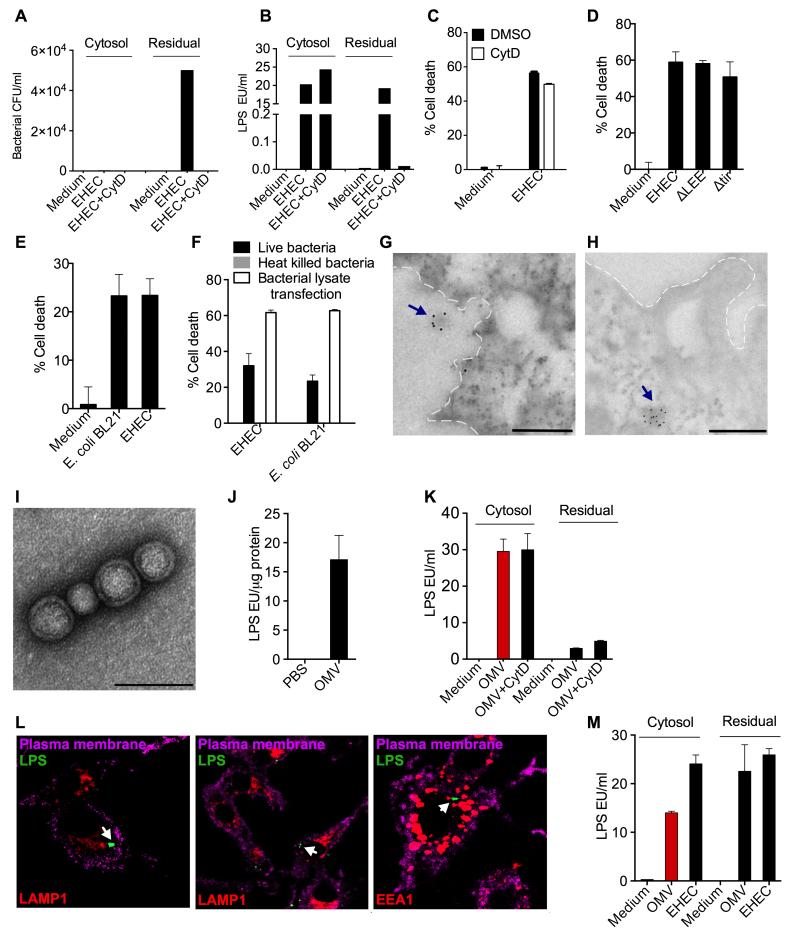
The above observations raise an intriguing question: how does LPS from extracellular bacteria such as EHEC enter the cytosol? To test if bacterial phagocytosis is required for cytosolic LPS entry in EHEC infection, LPS was quantified in the cytosol fraction extracted from cells treated with cytochalasin D prior to infection with EHEC. Cytochalasin D treatment completely inhibited EHEC attachment and phagocytosis as no bacteria were recovered from cytosolic or non-cytosolic residual fractions (Fig. 2A). Contrastingly, cytochalasin D treatment did not affect LPS levels in the cytosol (Fig. 2B) showing that LPS entry into the cytosol occurs regardless of bacterial phagocytosis. Further confirming this, EHEC-induced caspase-11-dependent cell death was not altered by cytochalasin D pretreatment (Fig. 2C and Fig. S2A).
Figure 2. Bacterial OMV Deliver LPS into the Cytosol.
(A-B) Bacterial count (A) and LPS quantity (B) as determined by agar plating and LAL assay, respectively, in the cytosolic and residual fractions from uninfected or EHEC-infected BMDM pretreated with DMSO or 2 μM cytochalasin D (cytD) 45 min prior to infection.
(C) Cell death in uninfected or EHEC-infected BMDM pretreated with DMSO or 2 μM cytochalasin D (cytD) 45 min prior to infection as determined by Cell titer glo assay at 16 h post-infection (p.i).
(D-E) Cell death in BMDM following infection with the indicated bacterial strains as determined by LDH assay at 16 h p.i.
(F) Cell death in BMDM following stimulation with live or heat killed bacteria or transfection with bacterial lysate as determined by LDH assay at 16 h p.i.
(G-H) Transmission electron microscopy (TEM) of BMDM infected with EHEC for 4 h at an MOI of 10 following immunogold labeling with an anti-LPS antibody. Plasma membrane is depicted by dotted lines. Scale bar = 0.5 μM.
(I-J) Negative staining TEM of purified OMV (I) and LAL assay for LPS in purified OMV (J). Scale bar = 0.1 μM.
(K) LPS levels in the cytosolic and residual fractions of BMDM treated with 50 μg OMV for 4 h as assessed by LAL assay.
(L) Confocal images of BMDM treated with 25 μg of OMV for 4 h. Cells were stained for LPS, EEA1 or LAMP1 with corresponding antibodies and for plasma membrane with cholera toxin B. Arrows indicate LPS staining.
(M) LPS levels in the cytosolic and residual fractions of HeLa cells treated with 200 μg OMV or EHEC at an MOI of 300 for 6 h as assessed by LAL assay.
Data are presented as mean±SEM of one experiment representative of two experiments. See also Figure S2.
It is possible that LPS may be released from bacteria in the extracellular space and this free LPS diffuses across the plasma membrane into the cytosol. However, when purified free LPS is added to the macrophage culture, it does not enter the cytosol (Fig. S2B) and it has to be introduced into the cytosol by transfection to activate the cytosolic LPS sensing pathway and subsequent cell death (Fig. S2C). Alternately, LPS could be delivered into the cytosol by bacterial type III secretion system (T3SS), which injects bacterial factors into the host cell via a needle-like structure. However, EHEC mutants lacking T3SS (ΔLEE) or Tir (Δtir), a key T3SS protein required for the initiation of the needle-like structure, induced equivalent levels of cell death as wild-type EHEC arguing against this possibility (Fig. 2D). Furthermore, E. coli BL21, a laboratory strain of E. coli that does not contain T3SS or other virulence factors, induced comparable levels of cell death as EHEC further confirming the dispensability of T3SS and other virulence factors in LPS delivery (Fig. 2E). In contrast, heat killed EHEC and E. coli BL21 failed to induce cell death indicating that viability of bacteria is essential for cytosolic LPS entry (Fig. 2F). This inability of heat killed bacteria to activate cytosolic LPS sensing was attributable more to failure to deliver LPS into the cell than to lack of LPS itself as heat killed bacteria have functional LPS (Sander et al., 2011). Supporting this, transfection of heat killed bacterial lysate into the macrophages induced cell death similar to live bacteria (Fig. 2F). These observations strongly suggest that a live bacteria-specific carrier-like mechanism is responsible for the intracellular entry of LPS.
Extracellular vesicles (EV) produced by eukaryotic and prokaryotic cells are highly specialized secretory mechanisms that mediate cell-to-cell transfer of protein, lipid, and nucleic acid contents (Kulp and Kuehn, 2010). EV secreted by Gram-negative bacteria known as outer membrane vesicles (OMV), owing to their size, shape and membrane composition, interact tightly with plasma and endocytic membranes of host cells and efficiently transfer their content across these membranes (Jäger et al., 2015; Kuehn and Kesty, 2005). OMV are rich in LPS, and OMV-associated LPS is believed to be highly potent (Ellis and Kuehn, 2010). Taking all these into account, we hypothesized that OMV secreted by Gram-negative bacteria function as a vehicle to deliver LPS into the cytosol.
During EHEC infection of macrophages, vesicle-like LPS-containing structures with a size similar to that of OMV were observed by immunoelectron microscopy both in the extracellular (Fig. 2G) and intracellular (Fig. 2H) space, suggesting a role for OMV in the intracellular transport of LPS. To test this, OMV from E. coli BL21 was purified using established methods (Chutkan et al., 2013), and verified to be devoid of bacterial contamination by electron microscopy (Fig. 2I) and agar plating (data not shown). OMV had the characteristic lipid bilayer structure (Fig. 2I) and contained a significant amount of LPS (Fig. 2J). BMDM were treated with purified E. coli OMV and monitored for the presence of LPS in the cytosol by LAL assay and confocal microscopy. Cytosolic fractions from OMV-treated BMDM contained substantial amounts of LPS (Fig. 2K). Furthermore, OMV delivery of LPS was more efficient with a relatively faster kinetics than that by chemical transfection (Fig. S2D). Similar to EHEC infection (Fig. 2B), LPS release in the cytosol by OMV was not affected by pretreatment of cells with cytochalasin D (Fig. 2K). Likewise, confocal microscopy demonstrated LPS and lipid A specks in the macrophage cytosol that were not associated with early endosomes, lysosomes or cell membrane following OMV treatment (Fig. 2L and Fig. S2E). More than 50% of the cells contained LPS specks in the cytosol 4 h after OMV treatment. We also observed colocalization of these intracellular LPS specks with caspase-11 in cells stimulated with OMV or EHEC (Fig. S2F). Cytosolic LPS sensing also exists in nonphagocytic cells such as HeLa (Shi et al., 2014), where bacteria like EHEC remain extracellular and OMV might become more important in enabling intracellular transport of LPS. In line with this idea, subcellular fractionation followed by LAL assay showed the presence of substantial amounts of LPS in the cytosol of HeLa cells upon treatment with OMV or EHEC (Fig. 2M).
Bacterial OMV Activate Cell Death and IL-1 Responses
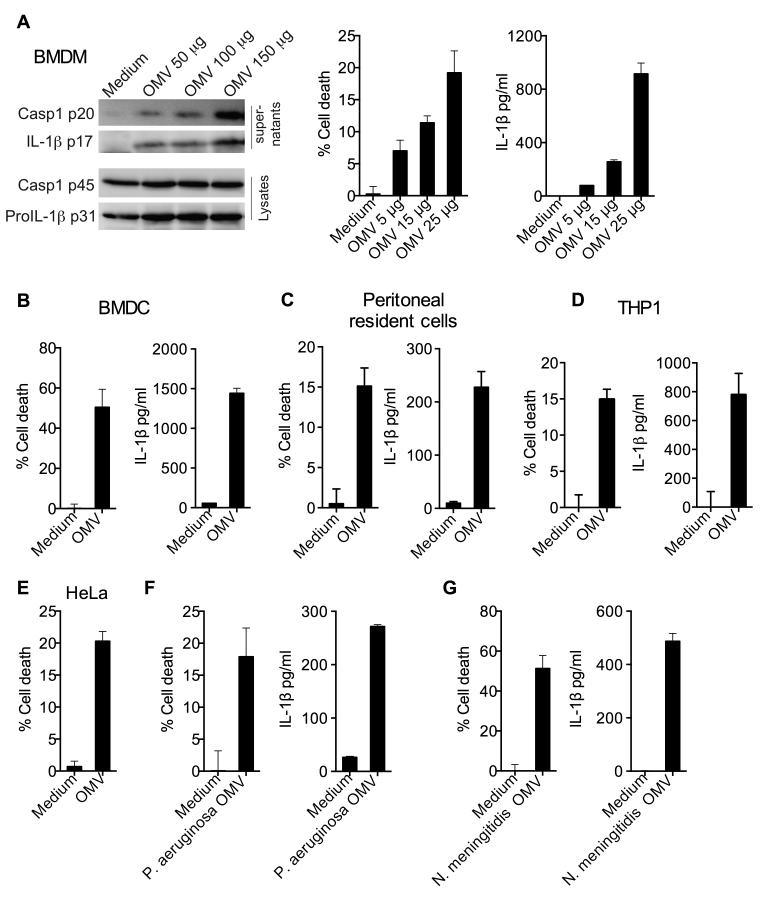
Consistent with the delivery of LPS into the cytosol by OMV, treatment of BMDM with purified OMV triggered caspase-1 and IL-1β proteolytic maturation, IL-1β secretion and more importantly, cell death in a dose-dependent manner (Fig. 3A). Furthermore, OMV elicited pyroptosis and/or IL-1β release in BMDCs, mouse peritoneal resident cells, THP1 macrophages and HeLa cells indicating that OMV can intracellularly deliver LPS in an array of cell types including human cells (Fig. 3B-E). We next tested if the phenomenon of cytosolic LPS delivery and caspase-11 activation by OMV is common to extracellular Gram-negative bacteria. Two clinically important bacteria that are well documented to produce OMV during infections are Neisseria meningitidis and Pseudomonas aeruginosa (Mashburn and Whiteley, 2005; Namork and Brandtzaeg, 2002). Like E. coli OMV, both N. meningitidis and P. aeruginosa OMV triggered pyroptotic cell death and IL-1β secretion in BMDM (Fig. 3F-G). Overall, OMV are sufficient for LPS delivery into the cytosol leading to induction of pyroptosis and IL-1 maturation.
Figure 3. Bacterial OMV Activate Cell Death and IL-1 Responses.
(A) Immunoblots for indicated proteins, LDH assay for cell death and ELISA for IL-1β in wild-type BMDM treated with indicated doses of OMV for 16 h.
(B-G) Cell death and/or IL-1β secretion by indicated cell types treated with 25 μg of indicated OMV for 16 h.
Data are presented as mean±SEM of one experiment representative of two experiments.
OMV-mediated Activation of Cell Death and IL-1 is Dependent on LPS and Caspase-11
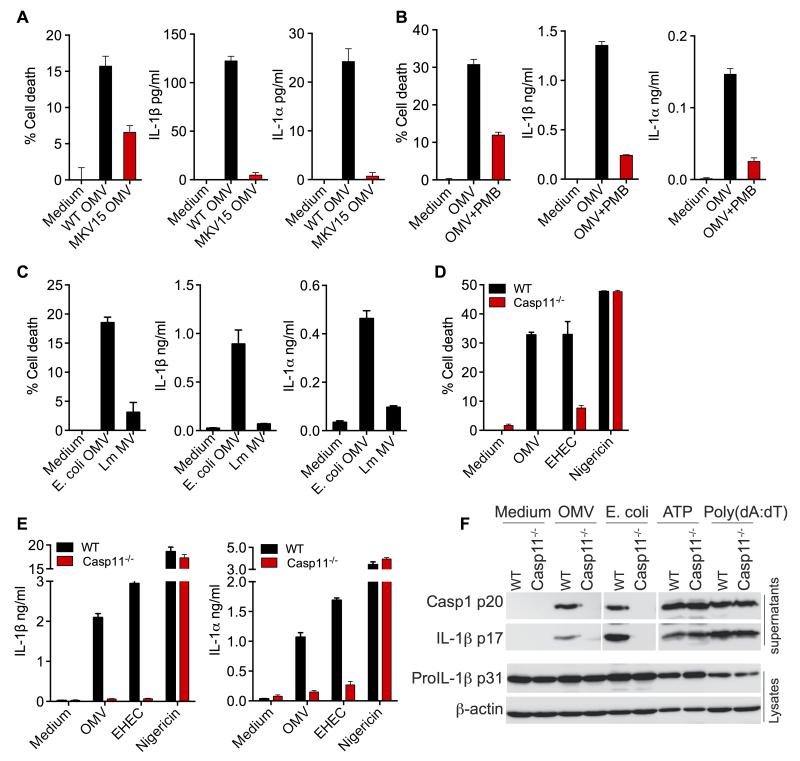
LPS is one of the most abundant components of OMV and is displayed on the outer surface of OMV. In addition OMV are also loaded with outer membrane proteins, lipoproteins, nucleic acids to name a few. To verify that OMV-mediated cell death and IL-1 responses are elicited by LPS and not other bacterial components in OMV, BMDM were stimulated with OMV purified from wild-type E. coli or an E. coli mutant (MKV15) that lacks functional hexa-acylated lipid A and thus caspase-11 activating capacity (Fig. S3). OMV from LPS mutant E. coli failed to induce strong cell death and IL-1α and IL-1β secretion (Fig. 4A). Alternately to address if LPS is the functional moiety of OMV that elicit these responses, we pretreated OMV with polymyxin B, an antibiotic that neutralizes LPS, and tested its effect on inflammasome activation. As expected polymyxin B-pretreatment of OMV reduced their capacity to trigger pyroptotic and IL-1 responses (Fig. 4B). Additionally, membrane vesicles prepared from the Gram-positive bacterium, Listeria monocytogenes, which lacks LPS, did not induce cell death or production of IL-1β and IL-1α (Fig. 4C). These data clearly show that LPS is the key ingredient of OMV that activates cell death and IL-1 cytokines. More importantly, cell death, production of IL-1β and IL-1α as well as cleavage of caspase-1 and IL-1β elicited by OMV were absolutely dependent on caspase-11 (Fig. 4D-F). These data clearly demonstrate that OMV are indeed activating the caspase-11-mediated LPS sensing pathway in the cytosol.
Figure 4. Activation of Cell Death and IL-1 by OMV is Dependent on LPS and Caspase-11.
(A-C) Cell death and IL-1β and IL-1α secretion by BMDM treated with OMV from wild-type E. coli or MKV15 strain, which lacks a functional hexa-acylated lipid A (A) or OMV pretreated with 10 μg/ml polymyxin B (PMB) (B) or membrane vesicles from L. monocytogenes (Lm MV) (C).
(D-E) Cell death and secretion of IL-1β and IL-1α by wild-type or caspase-11−/− BMDM stimulated as indicated for 16 h.
(F) Cleaved caspase-1 p20 and IL-1β p17 in the supernatants and proIL-1β and β-actin in the lysates of wild-type or caspase-11−/− BMDM stimulated as indicated for 16 h.
Data are presented as mean±SEM of one experiment representative of two experiments for A-C and F and three experiments for D-E. See also Figure S3.
Bacterial OMV Attain Intracellular Access through Endocytosis
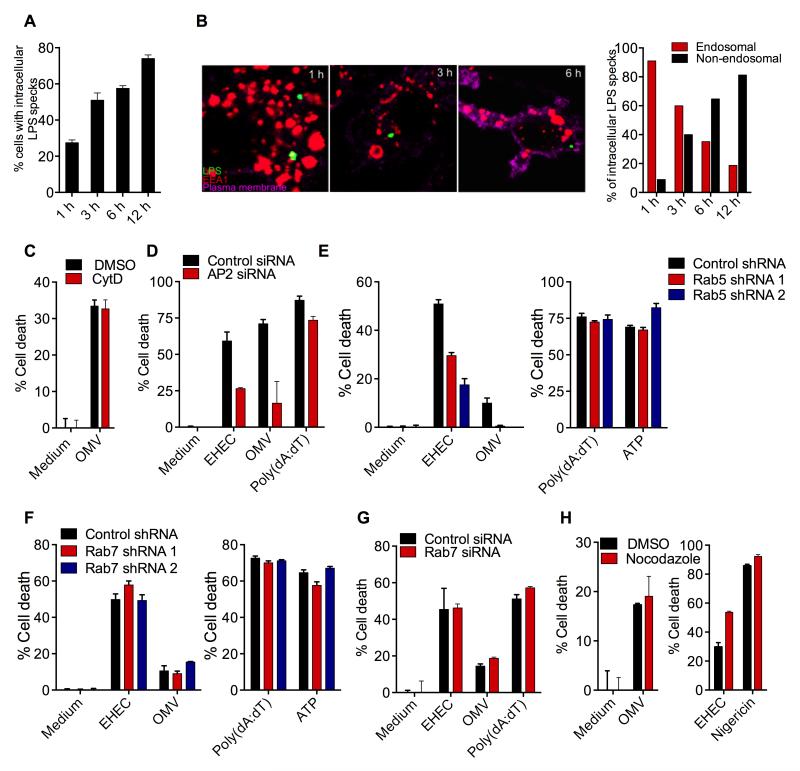
To gain insight into the mechanism by which OMV enable cytosolic access of LPS, the localization of OMV was tracked at various time points following exposure to BMDM with confocal microscopy. We stained OMV-treated BMDM with an anti-LPS antibody as a marker of OMV. Confocal imaging revealed that OMV bound to the cells and were internalized within 30 min of treatment. After 1 h of treatment ~25% of the cells contained intracellular LPS specks indicating OMV internalization (Fig. 5A). The proportion of cells containing LPS specks increased over time and by 12 h ~80% of cells contained OMV (Fig. 5A). To delineate the distribution of these intracellular LPS specks in the cytosol versus membrane bound organelles, the cells were also stained with anti-EEA1 or anti-LAMP2 antibodies to visualize early endosomes and lysosomes, respectively, as well as fluorescent cholera toxin B to label cell membrane. At 1 h of treatment nearly all of the internalized OMV colocalized with early endosomal compartments (Fig. 5B) suggesting that OMV enter the cells through endocytosis. Notably, albeit at very low frequency, LPS specks were observed outside endosomes in the cytosol starting from 1 h. The frequency of these cytosolic LPS specks increased over time and at 12 h > 75% of the intracellular LPS specks were in the cytosol (Fig. 5B). In contrast to its predominant localization in EEA1-stained compartments, LPS specks colocalized with LAMP2-stained lysosomal compartments at low frequency (Fig. S4A).
Figure 5. Bacterial OMV Attain Intracellular Access through Endocytosis.
(A-B) BMDM were treated with OMV for the indicated time points and were stained with antibodies against LPS and EEA1 as well as cholera toxin B for plasma membrane. Confocal microscopy-based quantification of % of cells with intracellular LPS (A) or % of intracellular LPS in the endosomal vs nonendosomal compartments (B). Quantification was done by counting 50 fields containing ~10 cells each.
(C and H) Cell death in BMDM pretreated with 2 μM cytochalasin D (cytD) or 10 μM nocodazole 45 min prior to stimulation with the indicated treatments.
(D) Cell death in immortalized BMDM (iBMDM) transfected with control or AP2 siRNA and treated as indicated.
(E-F) Cell death in iBMDM expressing control, Rab5A (E), or Rab7 (F) shRNAs in response to indicated stimulations.
(G) Cell death in iBMDM transfected with control or Rab7 siRNA and treated as indicated.
Data are presented as mean±SEM of one experiment representative of two experiments. See also Figure S4.
Given this uptake of OMV by macrophages, we sought to determine the mechanism underlying the cellular entry of OMV. Cytosolic LPS delivery by OMV appears to occur independent of TLR4 and CD14 as pyroptosis and secretion of IL-1β and IL-18 upon OMV treatment were intact in TLR4−/− and CD14−/− cells (Fig. S4B). Pretreatment with cytochalasin D did not affect cell death induced by OMV implying that actin polymerization-dependent internalization was not required for OMV uptake (Fig. 5C). Since OMV are of appropriate cargo size for clathrin-mediated endocytosis (CME) (McMahon and Boucrot, 2011), it was reasoned that OMV may enter the cells via CME. Immunofluorescence staining revealed the colocalization of OMV with clathrin near the plasma membrane implying their uptake in clathrin coated pits (Fig. S4C). Importantly, we performed siRNA-mediated silencing of the expression of μ2 subunit of the AP2 adaptor complex, which is essential for CME (Doherty and McMahon, 2009) and assessed its effect on OMV-driven caspase-11 activation. AP2 knockdown markedly reduced the pyroptotic response to both OMV and EHEC, without affecting polydA:dT-induced AIM2-dependent cell death (Fig. 5D and see S4D for knockdown efficiency).
Endosomes are directed towards lysosomes to degrade their cargo raising the question of from where LPS exits the endosomal pathway to reach the cytosol. In our confocal imaging analysis LPS was observed at a remarkably higher frequency in early endosomes than in lysosomes (Fig. 5B and S4A) prompting us to consider if OMV-associated LPS escapes from the early endosome into the cytosol. To test this, the early endosome formation was inhibited by stable knockdown of Rab5 (key GTPase involved in this process) using two independent shRNAs. Induction of cell death by EHEC and OMV but not by poly(dA:dT) and ATP was substantially reduced by Rab5 knockdown (Fig. 5E and see S4E for knockdown efficiency). Contrastingly, blocking the maturation of early endosomes to late endosomes by shRNA or siRNA knockdown of Rab7, which is critical for early-to-late endosome transition, did not affect OMV- and EHEC-induced pyroptosis (Fig. 5F-G and see S4F for knockdown efficiency). Similarly, OMV- and EHEC-induced pyroptosis was insensitive to nocodazole (Fig. 5H and S4G), which blocks microtubule-dependent progression of early to late endosome. Taken together, the requirement for AP2 complex and Rab5 but not Rab7 reveals that OMV enter the cells by clathrin-mediated endocytosis and importantly LPS access the cytosol from early endosomes.
OMV are Essential for Cytosolic LPS Access and Caspase-11 Activation during Bacterial Infection
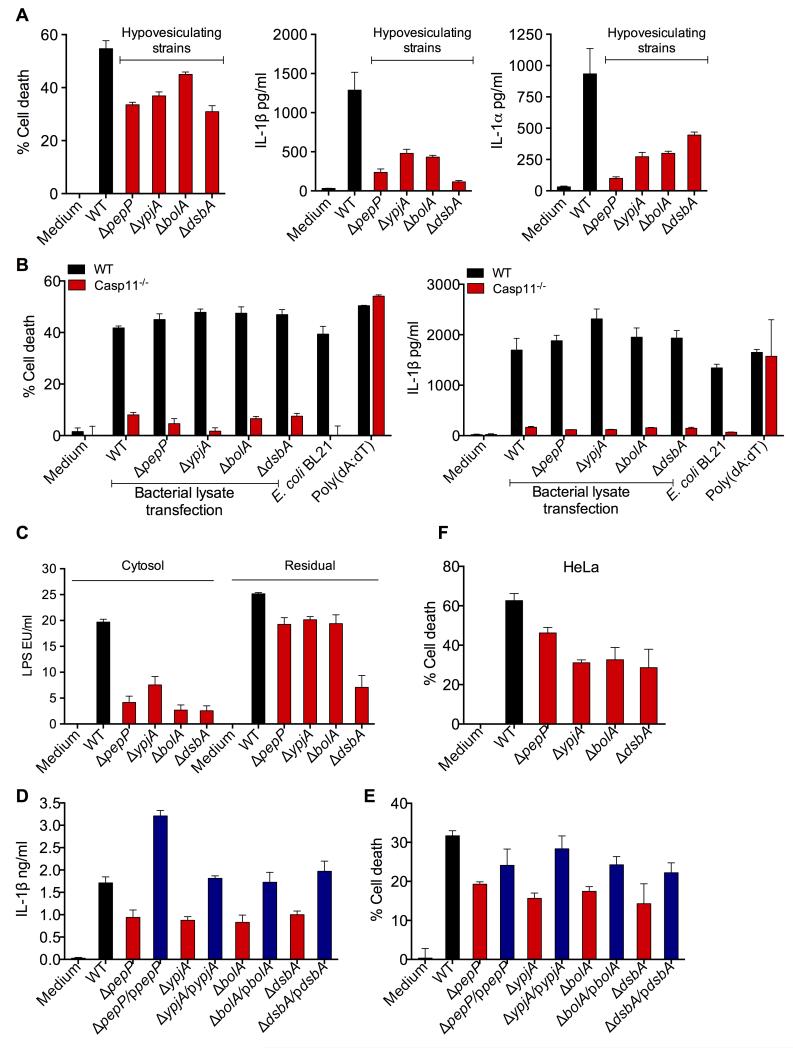
Having shown that OMV are sufficient for cytosolic LPS delivery and caspase-11 activation, we next tested if OMV are required for LPS to gain access to the cytosol and trigger caspase-11 activation during actual extracellular Gram-negative bacterial infections to assess its biological relevance. The release of OMV by Gram-negative bacteria is a programmed process with specific bacterial factors coordinating this process. Mutations in genes that encode proteins involved in OMV production such as pepP, ypjA, bolA, and dsbA result in hypovesiculating E. coli strains that minimally produce OMV without a major growth defect (Kulp and Kuehn, 2010; McBroom and Kuehn, 2007; McBroom et al., 2006; Schwechheimer and Kuehn, 2013). It should be noted that hypovesiculating strains do produce OMV but at reduced levels. To directly test if OMV are essential for caspase-11 activation during infections, BMDM were infected with wild-type and hypovesiculating strains at same MOI, and cell death and IL-1 cytokine levels were measured. Remarkably, hypovesiculating strains induced markedly decreased levels of caspase-11-dependent cell death, IL-1β and IL-1α compared to wild-type bacteria (Fig. 6A and S5A). We verified that cells were infected with equivalent numbers of wild-type or mutant bacteria by agar plating (Fig. S5B). Furthermore, the inducible expression of proIL-1β, IFN-β, CCL5, TNF, CXCL10, and CCL2 as well as TLR4-dependent secretion of TNF and IL-6 were comparable between wild-type and mutant bacterial strains (Fig S5C-E). This indicates that mutant bacteria are not globally defective for innate immune activation and the defect was specific to cytosolic LPS-driven responses (Fig 6A). Consistent with our hypothesis, transfection of lysates of these mutants into the cytosol induced caspase-11-dependant cell death and IL-1β secretion at levels similar to wild-type bacterial lysate further confirming that the LPS in these mutants is fully functional and the defective caspase-11 activation observed during infection with live hypovesiculating mutants is attributable to impaired cytosolic LPS delivery (Fig. 6B). Directly supporting this, LPS levels detected in the cytosol of cells infected with hypovesiculating strains were considerably lower compared to that of wild-type bacteria-infected cells (Fig. 6C and S5F). Notably, complementation of each hypovesiculating mutant with plasmids encoding the corresponding gene restored IL-1β and cell death close to wild-type levels (Fig. 6D-E). Furthermore, these hypovesiculating mutants induced lower levels of cell death compared to wild-type E. coli in HeLa cells (Fig. 6F). In sum, these data substantiate that optimal OMV production is necessary for delivering LPS into the cytosol and activating caspase-11 during extracellular Gram-negative bacterial infections.
Figure 6. OMV Production is Essential for E. coli-induced Caspase-11 Activation.
(A) Cell death and secretion of IL-1β and IL-1α by unprimed BMDM stimulated with wild-type or indicated hypovesiculating strains of E. coli at an MOI of 25 for 16 h.
(B) Cell death and secretion of IL-1β by wild-type or caspase-11−/− BMDM transfected with lysates from wild-type or indicated hypovesiculating strains of E. coli or stimulated with E. coli or poly(dA:dT) for 16 h.
(C) LPS levels, as determined by LAL assay, in the cytosol and residual fractions of BMDM infected with wild-type or indicated hypovesiculating strains of E. coli for 4 h.
(D-E) Secretion of IL-1β and cell death in BMDM infected with wild-type or indicated hypovesiculating strains or their corresponding complements expressing the deleted gene on a plasmid (blue bars) at an MOI of 25 for 16 h.
(F) Cell death in HeLa cells stimulated with wild-type or indicated hypovesiculating strains of E. coli at an MOI of 300 for 16 h.
Data are presented as mean±SEM of one experiment representative of three experiments for AB and two experiments for C-F. See also Figure S5.
OMV Activate Cytosolic LPS Sensing Pathway in vivo
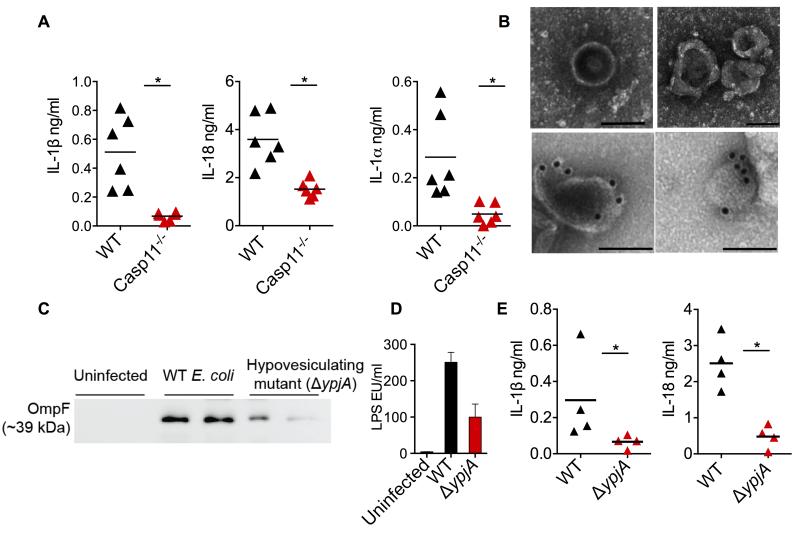
To determine if OMV can activate the cytosolic LPS sensing pathway in vivo, wild-type and caspase-11−/− mice were injected with purified OMV and caspase-11 activation was assessed by measuring IL-1β, IL-1α, and IL-18 levels in plasma. OMV elicited IL-1β, IL-18 and IL-1α cytokine responses in wild-type mice and these responses were significantly reduced in caspase-11−/− mice (Fig. 7A) demonstrating that OMV activate caspase-11 in vivo.
Figure 7. OMV are Essential for the Activation of Cytosolic LPS Sensing in vivo.
(A) IL-1β, IL-18, and IL-1α levels in the plasma of wild-type and caspase-11−/− mice injected i.p. with 100 μg of OMV. Mice were first primed with 200 μg poly(I:C) for 6 h (i.p.). Cytokine levels were assessed 6 h post OMV injection (n=6).
(B) Negative staining (top row) and LPS immunogold (bottom row) TEM for OMV in the peritoneal lavages from wild-type mice infected i.p with 109 CFU of E. coli for 12 h (images were from two different mice). Scale bar = 0.1 μM.
(C-D) Immunoblot for OmpF (C) and LAL assay for LPS (D) in OMV isolated from the peritoneal lavages of mice infected with 109 CFU of wild-type or ΔypjA strain of E. coli for 12 h. Each lane represents one mouse.
(E) IL-1β and IL-18 levels in the plasma of wild-type mice infected with 109 CFU of wild-type or ΔypjA strain of E. coli for 12 h (n=4).
Data are presented as mean±SEM of one experiment representative of two experiments. See also Figure S6.
The production of OMV during a wide range of human bacterial infections is clearly evident from the growing body of literature (Ellis and Kuehn, 2010). Particularly, OMV have been found in the serum and body fluids of patients with lethal meningococcal sepsis (Namork and Brandtzaeg, 2002; Stephens et al., 1982). We first assessed OMV production during enteric infections using a Citrobacter rodentium infection model that closely resembles human EHEC infection (Mallick et al., 2012). LPS-immunogold staining and TEM identified bacterial OMV in the cecum (Fig. S6A-C). Strikingly, these LPS-containing vesicle-like structures closely interacted with the microvilli of enterocytes (Fig. S6D) and appear to be taken up by enterocytes (Fig. S6E). More importantly, TEM also revealed the intracellular localization of LPS in the intestinal epithelial cells (Fig. S6F-G).
A similar approach employed to evaluate OMV secretion during systemic E. coli infection identified OMV in the peritoneal cavity of infected mice (Fig. 7B and S6H). Immunogold staining using antibodies against LPS and OmpF, an outer membrane protein of E. coli and a marker for OMV, confirmed the bacterial origin of these OMVs (Fig. 7B and S6I). Finally, to directly evaluate if OMV production is essential for eliciting caspase-11-mediated responses during bacterial infection in vivo, a hypovesiculating ΔypjA E. coli strain was utilized. The reduced production of OMV by this hypovesiculating mutant in vivo was confirmed by lower levels of OmpF and LPS (OMV markers) in the OMV preparations from the peritoneal lavages of mice infected with ΔypjA mutant relative to wild-type E. coli (Fig. 7C-D). More importantly, plasma levels of IL-1β and IL-18 following infection with this ΔypjA E. coli were markedly lower compared to that of wild-type E. coli-infected mice (Fig. 7E). This data confirmed that the optimal OMV production is a critical requirement for cytosolic LPS localization and eliciting caspase-11-mediated responses during bacterial infections in vivo as well.
DISCUSSION
Sensing of LPS in the cytosol by inflammatory caspases such as caspase-11 has emerged as a central mechanism of innate immune activation during Gram-negative bacterial infections as well as sepsis (Hagar et al., 2013; Kayagaki et al., 2013; Shi et al., 2014). Here, we provide key mechanistic insights into a previously unknown yet critical upstream event in this pathway, the entry of LPS into the cytosol. We demonstrate that OMV secreted by Gram-negative bacteria function as vehicles that deliver LPS into the cytosol and activate caspase-11-mediated responses in immune cells and mice. Bacteria that poorly produce OMV elicit weak cell death and IL-1 maturation showing that OMV is a biologically relevant and essential mechanism by which LPS gains access to the cytosol during Gram-negative bacterial infections.
Our data suggest that OMV are endocytosed by macrophages, and OMV release LPS likely from the early endosomal compartments into the cytosol. This proposed mechanism of early endosomal exit of OMV-LPS is consistent with the idea that early endosomes are the sorting stations for internalized cargoes; cargoes destined for degradation progress to lysosomes via late endosomes whereas other cargoes are routed to the cell surface or cytosol, where they exert their biological activity. Early endosomal escape allows OMV-bound LPS to reach cytosol functionally intact and avoid complete degradation in the lysosomes. The biophysical characteristics inherent to OMV such as their surface lipid phase and membrane curvature perhaps permit them to fuse with the endosomal membranes ultimately leading to cytosolic access.
OMV, like eukaryotic exosomes/microvesicles, can tightly interact with cellular membranes. As a result, OMV-bound products, particularly hydrophobic and insoluble ones (like lipids), have greater capacity than their free form to translocate across cellular membranes (Kulp and Kuehn, 2010). Therefore, it is conceivable that OMV-associated LPS is a better candidate than free LPS for cytosolic access. Additionally, OMV may deliver LPS as large aggregates at high concentrations locally, which could serve as a platform facilitating caspase-11 oligomerization. Thus, OMV are at the critical interphase between host and microbes, and OMV offer bacterial products specific access to otherwise inaccessible cellular and tissue compartments.
According to the “patterns of pathogenesis” hypothesis, two critical determinants of the innate immune responses during an infection are the presence of live replicating bacteria and the location where PAMP are recognized (Vance et al., 2009). Bacteria-derived small molecules including cyclic-di-GMP and mRNA are considered as indicators of live bacterial infections (Sander et al., 2011; Vance et al., 2009). OMV are secreted by growing bacteria and importantly, OMV enable the cytosolic localization of PAMP including LPS as revealed by this study. Therefore, the innate immune recognition of inflammatory PAMP in the context of OMV, particularly in the cytosol, would license a strong inflammatory reaction from the host. Thus, OMV with the cargo of immunostimulatory PAMP may represent a cardinal sign of infection with not only viable but also multiplying bacteria.
Our finding of OMV activation of cytosolic LPS sensing pathway may have significant implications for vaccine development. Owing to their high immunogenicity, OMV are being considered as vaccine candidates against several infectious diseases. Notably, OMV-based vaccines have been licensed for meningococcal disease caused by N. meningitides in several countries (Acevedo et al., 2014). Additionally, OMV-based vaccines against a number of Gram-negative bacterial infections are currently under development. However, an unresolved key issue in the development of OMV vaccines is their LPS content because the contribution of LPS to immunogenicity vs toxicity of OMV vaccines is not yet clear. Therefore, future experimental evaluation of whether the activation of cytosolic LPS sensing by OMV is beneficial or harmful in OMV vaccine applications will be key in the rational design of OMV-based vaccines and in improving their safety and adjuvanticity.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6 mice from The Jackson Laboratory (Bar Harbor, ME) and Caspase-11−/− mice (kind gift of Vishva Dixit and Kate Fitzgerald) were bred and maintained in specific pathogen–free conditions at the UConn Health. Animal protocols were carried out in accordance with the guidelines set forth by the Institutional Animal Care and Use Committee.
Bacterial Strains and Growth Conditions
Bacterial strains and their sources are described in Supplemental Experimental Procedures. Bacterial strains were grown over-night in LB broth and the macrophages were infected at an MOI of 25 unless otherwise indicated.
Purification and Characterization of Bacterial OMV
OMV were purified from E. coli BL21 or P. aeruginosa PAK strain as described previously with modifications (Chutkan et al., 2013). See Supplemental Experimental Procedures for details.
Cell Culture and Stimulations
Bone-marrow derived macrophages (BMDM) and dendritic cells (BMDC) were generated as described previously (Rathinam et al., 2010). Cells used to assess the inflammasome and cell death responses were primed with Pam3CSK4 (Invivogen) for 3 h unless otherwise indicated. Cells were infected with bacteria at indicated MOIs and supernatants were collected 16 h post-stimulation unless otherwise indicated. See Supplemental Experimental Procedures for details.
Isolation of Cytosol Fraction from BMDM and HeLa cells
Subcellular fractionation of BMDM was conducted by a digitonin-based fractionation method as described previously with modifications (Ramsby and Makowski, 2005). Briefly, 4 × 106 cells were infected with EHEC or treated with OMV or left untreated, washed thoroughly and treated with 0.005% digitonin extraction buffer for 8 min to collect the supernatant containing cytosol. The residual cell fraction containing cell membrane, organelles and nucleus was collected in 0.1% CHAPS buffer. See Supplemental Experimental Procedures for details.
siRNA and shRNA knockdown
ON-TARGETplus SMARTpool non-targeting control, mouse AP2m1, and mouse Rab7 siRNAs (250 nM; GE Dharmacon) were reverse transfected into immortalized BMDM (iBMDM; 104 cells) using 1 μl Interferin (Polyplus). The transfection was repeated next day and cells were subjected to experiments or western blotting to assess the knockdown efficiency 48 h later. TRC lentiviral pLKO.1 vectors (GE Dharmacon) encoding eGFP (control; RHS4459), Rab5A (clones TRCN0000100795 and TRCN0000100797), or Rab7 (clones TRCN0000100880 and TRCN0000100881) shRNAs were used for stable knockdown of corresponding genes. Briefly, iBMDM transduced with lenti virus supernatant obtained from 293T cells (72-96 h after transfection with pLKO.1 and packaging plasmids) were puromycin-selected. The knockdown efficiency was assessed by quantitative PCR or western blotting.
Confocal Microscopy
BMDM were stimulated with EHEC or OMV or left untreated for various durations as indicated and processed for confocal imaging. See Supplemental Experimental Procedures for details.
Transmission Electron Microscopy (TEM)
TEM for OMV and BMDM infected with EHEC were performed according to standard procedures. See Supplemental Experimental Procedures for details.
ELISA and Cell Death Assay
IL-1β and IL-1α levels were assessed by Ready-Set-Go!® ELISA kits (eBioscience). IL-18 ELISA was performed as described previously (Bossaller et al., 2012). Cell death was assessed by LDH cytotoxicity detection kit (Clontech) or CellTiter-Glo® assay (Promega).
Immunoblotting and Antibodies
Immunoblotting was performed as described (Rathinam et al., 2012b) with the antibodies listed in the Supplemental Experimental Procedures.
In Vivo OMV Stimulation and Infection
Eight to 16 weeks old C57BL/6 and caspase-11−/− mice were primed with intraperitoneal (i.p.) administration of 200 μg of poly(I:C) (high molecular weight; Invivogen) for 6 h prior to i.p injection with 100 μg of purified OMV. Cytokine levels in the plasma were analyzed at 6 h post-OMV injection. Additionally, C57BL/6 mice were infected with 109 CFU of wild-type or ΔypjA E. coli strains at exponential phase of growth and plasma cytokine levels were analyzed at 12 h post-infection.
Statistical Analysis
In vitro data were analyzed for statistical significance by One-way or Two-way analysis of variance followed by the Sidak’s post-test with Prism Software. Data from in vivo experiments were analyzed by Mann Whitney test. P values of less than 0.05 were considered significant.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Kate Fitzgerald and Vishwa Dixit for caspase-11−/− mice; the Central Electron Microscopy facility at UConn Health for TEM; Kate Fitzgerald, Kamal Khanna and Anthony Vella for critical review of the manuscript; Meta Kuehn, Yale E. coli Genetic Stock Center, John Leong, and Egil Lien for bacterial strains; Sanjay Ram, Dan Granoff, and Serena Giuntini, Jonathan Kagan, Justin Radolf, Arvind Anand and Jorge Cervantes for reagents or technical help. This work was supported by funds from UConn Health, the Charles Hood Child Health Research Award by the Charles H. Hood Foundation, and the National Institutes of Health (AI119015) to V.R.
Footnotes
AUTHOR CONTRIBUTIONS
V.R. and S.K.V. conceived the study, designed the experiments and wrote the manuscript; S.K.V., A.R., B.B., I.B., M.Y., S.D., and V.R., performed the experiments and analyzed the data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acevedo R, Fernández S, Zayas C, Acosta A, Sarmiento ME, Ferro VA, Rosenqvist E, Campa C, Cardoso D, Garcia L, et al. Bacterial outer membrane vesicles and vaccine applications. Front. Immunol. 2014;5:121. doi: 10.3389/fimmu.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham KS, Kagan JC. Endosomes as Platforms for NOD-like Receptor Signaling. Cell Host & Microbe. 2014;15:523–525. doi: 10.1016/j.chom.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossaller L, Chiang P-I, Schmidt-Lauber C, Ganesan S, Kaiser WJ, Rathinam VAK, Mocarski ES, Subramanian D, Green DR, Silverman N, et al. Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner. The Journal of Immunology. 2012;189:5508–5512. doi: 10.4049/jimmunol.1202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutkan H, Macdonald I, Manning A, Kuehn MJ. Quantitative and qualitative preparations of bacterial outer membrane vesicles. Methods Mol Biol. 2013;966:259–272. doi: 10.1007/978-1-62703-245-2_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth DR, James D, Kowashi Y, Kato S. Interaction of Actinobacillus actinomycetemcomitans outer membrane vesicles with HL60 cells does not require leukotoxin. Cell Microbiol. 2003;5:111–121. doi: 10.1046/j.1462-5822.2003.00259.x. [DOI] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mechanisms of Endocytosis. Annu. Rev. Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 2010;74:81–94. doi: 10.1128/MMBR.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galka F, Wai SN, Kusch H, Engelmann S, Hecker M, Schmeck B, Hippenstiel S, Uhlin BE, Steinert M. Proteomic characterization of the whole secretome of Legionella pneumophila and functional analysis of outer membrane vesicles. Infect. Immun. 2008;76:1825–1836. doi: 10.1128/IAI.01396-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P, Malireddi RKS, Anand PK, Demon D, Vande Walle L, Liu Z, Vogel P, Lamkanfi M, Kanneganti T-D. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-β (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J Biol Chem. 2012;287:34474–34483. doi: 10.1074/jbc.M112.401406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W-T, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang Z-H, Zhong C-Q, Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey CA, Kuhn KA, Donermeyer DL, Porter NT, Jin C, Cameron EA, Jung H, Kaiko GE, Wegorzewska M, Malvin NP, et al. Colitogenic Bacteroides thetaiotaomicron Antigens Access Host Immune Cells in a Sulfatase-Dependent Manner via Outer Membrane Vesicles. Cell Host & Microbe. 2015;17:672–680. doi: 10.1016/j.chom.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger J, Keese S, Roessle M, Steinert M, Schromm AB. Fusion of Legionella pneumophila outer membrane vesicles with eukaryotic membrane systems is a mechanism to deliver pathogen factors to host cell membranes. Cell Microbiol. 2015;17:607–620. doi: 10.1111/cmi.12392. [DOI] [PubMed] [Google Scholar]
- Kailasan Vanaja S, Rathinam VAK, Atianand MK, Kalantari P, Skehan B, Fitzgerald KA, Leong JM. Bacterial RNA:DNA hybrids are activators of the NLRP3 inflammasome. Proceedings of the National Academy of Sciences. 2014;111:7765–7770. doi: 10.1073/pnas.1400075111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaparakis M, Turnbull L, Carneiro L, Firth S, Coleman HA, Parkington HC, Le Bourhis L, Karrar A, Viala J, Mak J, et al. Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell Microbiol. 2010;12:372–385. doi: 10.1111/j.1462-5822.2009.01404.x. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signaling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, et al. Noncanonical Inflammasome Activation by Intracellular LPS Independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- Kesty NC, Mason KM, Reedy M, Miller SE, Kuehn MJ. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. 2004;23:4538–4549. doi: 10.1038/sj.emboj.7600471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Yoon YJ, Lee J, Choi E-J, Yi N, Park K-S, Park J, Lötvall J, Kim Y-K, Gho YS. Outer Membrane Vesicles Derived from Escherichia coli Up-Regulate Expression of Endothelial Cell Adhesion Molecules In Vitro and In Vivo. PloS One. 2013;8:e59276. doi: 10.1371/journal.pone.0059276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19:2645–2655. doi: 10.1101/gad.1299905. [DOI] [PubMed] [Google Scholar]
- Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick EM, McBee ME, Vanguri VK, Melton-Celsa AR, Schlieper K, Karalius BJ, O'Brien AD, Butterton JR, Leong JM, Schauer DB. A novel murine infection model for Shiga toxin-producing Escherichia coli. J. Clin. Invest. 2012;122:4012–4024. doi: 10.1172/JCI62746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashburn LM, Whiteley M. Membrane vesicles traffic signals and facilitate group activities in a prokaryote. Nature. 2005;437:422–425. doi: 10.1038/nature03925. [DOI] [PubMed] [Google Scholar]
- McBroom AJ, Kuehn MJ. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Molecular Microbiology. 2007;63:545–558. doi: 10.1111/j.1365-2958.2006.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J. Bacteriol. 2006;188:5385–5392. doi: 10.1128/JB.00498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier E, Dick MS, Dreier RF, Schürmann N, Kenzelmann Broz D, Warming S, Roose-Girma M, Bumann D, Kayagaki N, Takeda K, et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature. 2014;509:366–370. doi: 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- Namork E, Brandtzaeg P. Fatal meningococcal septicaemia with “blebbing” meningococcus. 2002;360:1741. doi: 10.1016/S0140-6736(02)11721-1. [DOI] [PubMed] [Google Scholar]
- Park BS, Song DH, Kim HM, Choi B-S, Lee H, Lee J-O. The structural basis of lipopolysaccharide recognition by the TLR4–MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- Park K-S, Choi K-H, Kim Y-S, Hong BS, Kim OY, Kim JH, Yoon CM, Koh G-Y, Kim Y-K, Gho YS. Outer Membrane Vesicles Derived from Escherichia coli Induce Systemic Inflammatory Response Syndrome. PloS One. 2010;5:e11334. doi: 10.1371/journal.pone.0011334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Ramsby ML, Makowski GS. The Proteomics Protocols Handbook. 2005:37–48. [Google Scholar]
- Rathinam VAK, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nature Immunology. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VAK, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nature Immunology. 2012a;13:333–332. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VAK, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA. TRIF Licenses Caspase-11-Dependent NLRP3 Inflammasome Activation by Gram-Negative Bacteria. Cell. 2012b;150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander LE, Davis MJ, Boekschoten MV, Amsen D, Dascher CC, Ryffel B, Swanson JA, Müller M. Detection of prokaryotic mRNA signifies microbial viability and promotes immunity. Nature. 2011;474:385–389. doi: 10.1038/nature10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer C, Kuehn MJ. Synthetic effect between envelope stress and lack of outer membrane vesicle production in Escherichia coli. J. Bacteriol. 2013;195:4161–4173. doi: 10.1128/JB.02192-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host & Microbe. 2012;12:509–520. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- Stephens DS, Edwards KM, Morris F, McGee ZA. Pili and outer membrane appendages on Neisseria meningitidis in the cerebrospinal fluid of an infant. J Infect Dis. 1982;146:568. doi: 10.1093/infdis/146.4.568. [DOI] [PubMed] [Google Scholar]
- Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host & Microbe. 2009;6:10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.