Abstract
We sought to determine the effects of exogenous administration of human coagulation factors following pig-to-baboon liver xenotransplantation (LXT) using GalT-KO donors. Post-LXT, baboons received either: no coagulation factors (historical control, n=1), bolus administration of a human prothrombin concentrate complex (hPCC) (2.5 mL/kg, n = 2), continuous infusion of hPCC (1.0 ml/hr, n=1) or a continuous infusion of human recombinant Factor VIIa (1 mcg/kg/hr, n=3). The historical control recipient demonstrated persistent thrombocytopenia despite platelet administration post-transplant along with widespread thrombotic microangiopathy (TMA). In contrast, platelet levels were maintained in bolus hPCC recipients, however these animals quickly developed large vessel thrombosis and TMA leading to graft failure with shortened survival. Recipients of continuous coagulation factor administration experienced either stabilization or an increase in their circulating platelets with escalating doses. Furthermore, transfusion requirements were decreased and hepatic TMA was noticeably absent in recipients of continuous coagulation factor infusions when compared to the historical control and bolus hPCC recipients. This effect was most profound with a continuous, escalating dose of Factor VIIa. Further studies are warranted as this regimen may allow for prolonged survival following LXT.
Introduction
Liver transplantation remains the primary therapy for end-stage liver failure, metabolic diseases, and non-metastatic hepatic malignancies. With over 15,000 patients currently active on the waitlist and with demand outnumbering the supply, the transplant community has been forced to seek alternative sources for organs besides extended criteria and living liver donors. Xenotransplantation utilizing pigs may serve as an ideal option to address the widespread shortage of liver grafts if the immunological and coagulation hurdles can be addressed [1].
Advances in achieving successful xenotransplantation have been made through the development of genetically engineered alpha-1,3-galactosyltransferase knock out (GalT-KO) swine. Utilization of these and other transgenic swine donors have helped to resolve the initial complications of hyperacute rejection and have been shown to prolong survival in renal [2] and cardiac [3] xenotransplantation models. However, survival after liver xenotransplantation (LXT) still remains shortened, with the longest reported worldwide survival lasting up-to 9 days [4]. Current barriers to successful LXT include profound thrombocytopenia seen immediately following graft reperfusion and lasting until death, despite the administration of platelets and pro-coagulants [4–6]. This thrombocytopenia, which has also been experienced in a model of both kidney [7] and heart [8,9] xenotransplantation has been associated with severe bleeding complications as well as profound graft thrombotic microangiopathy (TMA) [5] and eventual graft loss.
Previous work in our laboratory [4] has noted that despite adequate hepatic synthetic function after xenotransplantation, detection of circulating swine coagulation factor production post-transplantation, most notably Factor VII, did not seem to reach pre-transplant levels. To address this dilemma, in this study we investigated for the first time the effects of exogenous administration of human coagulation factors on the ability to improve bleeding, thrombocytopenia, TMA and survival in a model of pig-to-baboon LXT.
Materials and Methods
Pig-to-Baboon Liver Xenotransplantation
Baboon recipients (Papio hamadryus, n=7) were purchased from the Mannheimer Foundation (Homestead, Fl, USA). After acclimatization, including jacket and tether training, orthotopic LXT was performed with GalT-KO miniature swine donors which were derived through breeding at our own facility via homologous recombination and nuclear transfer as previously described [10]. Donor and recipient details are provided in Table 1.
Table 1.
Recipient and Donor Details
| Group | Recipient Baboon ID | Recipient Weight (kg)/Liver Weight (g) | Donor Pig ID | Donor Weight (kg)/Liver Weight (g) | Immunosuppressive Regimen | Coagulation Factor Protocol | Survival (POD) |
|---|---|---|---|---|---|---|---|
| 1 | B353-O | 8.4/247 | 21672 | 8.8/208 | ATG+FK506+CVF+CS | Octaplex (Bolus) | 1 |
| 1 | B356-O | 8.3/240 | 21880 | 8.0/240 | ATG+FK506+CVF+CS | Octaplex (Bolus) | 3 |
| 1 | B397-O | 6/184 | 22832 | 7.7/218 | ATG+FK506+CVF+CS | Octaplex (Continuous) | 6 |
| 2 | B368-N | 7.6/235 | 22242 | 4.3/190 | ATG+FK506+CVF+CS | NovoSeven | 7 |
| 2 | B365-N | 7.0/226 | 22514 | 6.9/187 | ATG+FK506+CVF+CS | NovoSeven | 5 |
| 2 | B381-N | 6.6/213 | 22606 | 8.9/179 | ATG+FK506+CVF+CS | NovoSeven | 5 |
| 3 | B274-C | 9.2/213 | 19234 | 7.1/205 | ATG+LcCd2b+CVF+Anti-CD154+FK506+CS | None | 6 |
Table 1 provides detailed information on the weights of the baboon recipients and swine donors, including the liver. Additionally, the immunosuppression and coagulation factor protocol is provided along with the survival that was achieved.
Donor Surgery
After sedation with Telazol (2 mg/kg), donor pigs were brought into the operating room where general endotracheal anesthesia was achieved with Isoflurane. A midline laparotomy was performed to gain access into the peritoneal cavity. The liver was mobilized and the hilar structures were isolated. After exposure and cannulation of the infra-renal abdominal aorta, heparin was administered and the aorta was cross-clamped above the celiac axis. The liver was then flushed with cold Lactated Ringer's solution followed by cold UW solution (Barr Pharmaceuticals, Pomona, NY, USA). Lastly, after division of the vessels and the bile duct, the liver was excised, weighed and placed on ice, where a donor cholecystectomy and preparation of the vessels for transplantation was performed.
Recipient Surgery
Three days prior to transplantation recipient baboons underwent indwelling tunneled line placement into the left internal and external jugular veins using 18-gauge Tygon® tubing (Saint Gobain People Corp, Valley Forge, PA, USA). On the morning of transplantation recipients were sedated with Atropine (0.01 mg/kg) and Ketamine (10 mg/kg) and brought to the operating room. Following endotracheal intubation and general anesthesia under Isoflurane, a small-bore percutaneous arterial catheter was placed into the right femoral artery under sterile technique. The abdomen was entered through a midline incision, a splenectomy and subsequent recipient hepatectomy was performed. The donor xenoliver was transplanted using a bicaval technique. The suprahepatic vena caval anastomosis was completed using 5-0 Prolene (Ethilon, Somerville, NJ, USA), followed by the infrahepatic vena caval anastomosis using 6-0 Prolene and the portal vein anastomosis using 7-0 Prolene. The liver was reperfused and then the donor hepatic artery was anastomosed to the recipient hepatic artery in an end-to-end fashion via a branch patch technique using interrupted 8-0 Prolene. The bile duct was then reconstructed in a duct-to-duct fashion using 6-0 Prolene. The abdomen was closed, the animal was extubated and subsequently returned to its cage for full recovery.
All animals were cared for adhering to the Principles of Laboratory Animal Care as described by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals (NIH publication No. 86-23, revised 1985). All experimental protocols were approved and in accordance with the Massachusetts General Hospital Institutional Animal Care and Use Committee (IACUC) (2014N000276, 2009N000004).
Immunosuppression
Immunosuppression consisted of Thymoglobulin induction (10 mg/kg) (generously provided by Genzyme, Cambridge, MA, USA) as well as Cobra Venom Factor (100 ug/kg) (Complement Technology, Tyler, TX, USA) for complement factor (CH50) depletion on Day −1. Maintenance immunosuppression consisted of a continuous FK-506 infusion (0.20 mg/kg/day) (target serum levels 10-20 ng/ml) starting on Day −2 and a methylprednisone taper starting on Day 0. Additionally, the historical control (B274) also received LoCd2b (rat anti-primate CD2 IgG2b) and anti-CD154 (25 mg/kg).
Experimental Groups
A total of 7 liver xenotransplants (Table 1) were performed and grouped as follows: Group 1: bolus administration of Octaplex®, a second-generation human prothrombin complex concentrate (hPCC) containing the coagulation factors II, VII, IX, X and the inhibitors of coagulation, Proteins C and S (generously provided by Octapharma, Lachen, CH) at 2.5 mL/kg (n=2) and a continuous infusion of Octaplex® at 1.0 ml/hr (n=1). Group 2: a continuous infusion of NovoSeven®, recombinant activated Factor VIIa, (Novo Nordisk, Plainsboro, NJ, USA) at 1 mcg/kg/hr (n=3) and further titrated to maintain Factor VII activity to >60%. Group 3: no exogenous coagulation factors (historical control, n=1). For those animals receiving continuous coagulation factors, infusions began six hours following reperfusion of the porcine liver. This latter initiation time was intended to allow for supplementation of baboon circulating factors, with Factor VII having the shortest half-life of all factors (3-6 hours). In addition, the time delay was to also prevent any potential immediate post-transplant graft thrombosis.
Clinical Monitoring
Blood was analyzed daily for complete blood count (Hemavet 950 FS, Drew Scientific Group; Waterbury, CT, USA), chemistry (Catalyst Dx, IDEXX, Holliston, MA, USA), serum FK-506 levels (Architect i1000SR, Abbott Diagnostics, Abbott Park, IL, USA) and for a comparative coagulation profile at the Clinical Special Coagulation Laboratory (Massachusetts General Hospital, Boston, MA) and the Animal Health Diagnostic Center (Cornell University College of Veterinary Medicine, Ithaca, NY, B397-O only) according to human blood protocols.
Tissue Sample Collection, Preparation and Histological Evaluation
Liver biopsies were obtained prior to hepatectomy, immediately before reperfusion, at 30 minutes post-reperfusion, at 1 hour post-reperfusion, during exploratory laparotomy and at necropsy. Tissue samples were stored in 10% formalin and paraffin blocks were prepared in 4-μm sections with hematoxylin and eosin (H&E) staining. Images were viewed at varying magnification and captured using a Nikon confocal microscope (Nikon D-ECLIPSE C1, Tokyo, Japan).
Results
Clinical Course
Group 1
All three baboons had an uneventful intra-operative course and recovered immediately after surgery. B353-O received bolus Octaplex® six hours post-LXT at a dose of 20 mL every eight hours, and required euthanasia at 30 hours post-LXT due to severe intra-hepatic thrombosis. Given these findings, for B356-O bolus Octaplex® administration was changed to 20 mL every 24 hours for the first two days, and then decreased to 10 mL every 24 hours thereafter. Despite this decrease, the animal developed severe graft thrombosis and was euthanized on post-operative day (POD) 3. The third animal in this group, B397-O, received a continuous infusion of Octaplex® starting at 0.3 ml/hr which was quickly increased up to 1.0 ml/hr, based on serum factor activity. The animal required re-exploration on POD 4 due to respiratory difficulty, during which time the liver appeared pink, viable and without evidence of thrombosis. The animal unfortunately developed multi-drug resistant Enterococcus bacteremia causing disseminated intravascular coagulation (DIC) on POD 6 and was euthanized.
Group 2
All three baboons receiving NovoSeven® had uneventful intra-operative courses and began NovoSeven® infusion six hours post-operatively at a continuous rate of 1.0 mcg/kg/hr. Given the short half-life of NovoSeven® (approximately two hours), the dose was empirically increased approximately every 18-24 hours in an effort to maintain stable activity. B368-N developed pre-transplant thrombocytopenia following Thymoglobulin induction lasting throughout the post-operative period. NovoSeven® was increased to 1.5 mcg/kg/hr on POD 3 and re-exploration was required on POD 4 and 6 due to worsening ascites and respiratory difficulty. During both times a healthy appearing liver was found. The animal was euthanized on POD 7 due to bleeding, renal and respiratory failure. B365-N began NovoSeven® at 1.0 mcg/kg/hr, which was increased to 1.5 mcg/kg/hr on POD 2 and then to 2.0 mcg/kg/hr on POD 3. On POD 3, the animal experienced an unexpected leukocytosis and elevated liver function tests (LFT's) along with bloody diarrhea suspicious for Clostridium difficile colitis. Metronidazole and a methylprednisone taper were administered with resolution of the diarrhea and improvement in LFT's. The animal required re-exploration on POD 4 at which time the liver appeared pink and viable, however worsening leukocytosis, metabolic acidosis and multi-system organ failure necessitated euthanasia on POD 5, likely due to sepsis from Staphylococcus epidermidis from peritoneal cultures. B381-N required brief post-operative re-intubation and mechanical ventilation for two hours to allow for full recovery after surgery. NovoSeven® was increased to 1.5 mcg/kg/hr due to persistent anemia despite red blood cell transfusions. Re-exploration on POD 2 demonstrated bleeding from the stump of the previously ligated splenic vein, which was repaired. NovoSeven® was further increased to 2.0 mcg/kg/hr on POD 3 and to 4.0 mcg/kg/hr on POD 4 (Fig. 2D). Repeat re-exploration on POD 4 demonstrated diffuse oozing from the retroperitoneum, however, the liver appeared pink and healthy. The animal developed renal and respiratory failure on POD 5 and was euthanized.
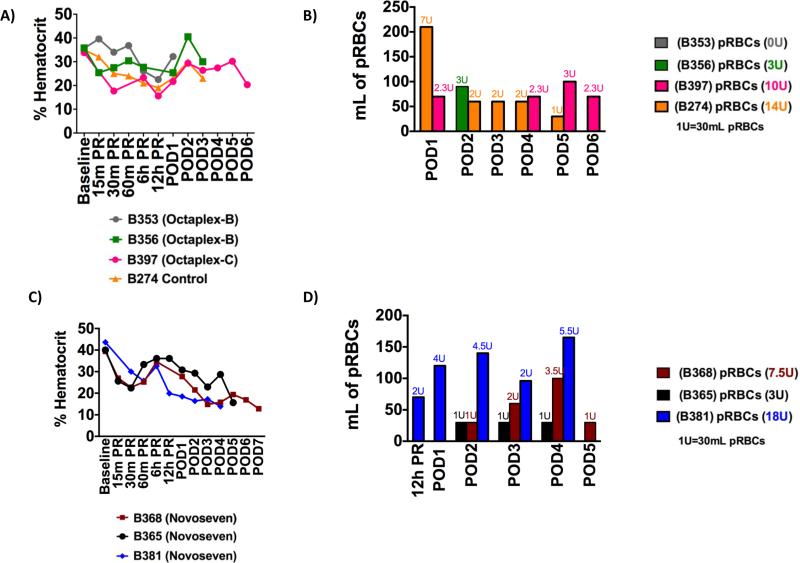
Figure 2. Transfusion Requirements.
A) Hematocrit and B) Transfusion requirements for Octaplex® and control recipients and C) Hematocrit and D) Transfusion requirements for NovoSeven® recipients. Overall transfusion requirements were decreased in all recipients when compared to the historical control recipient.
Group 3
The post-operative course for the historical control animal, B274, has been previously reported. In summary, the animal was re-explored on POD 2 and POD 5 due to deteriorating LFT's and persistent bleeding despite platelet administration. The animal required euthanasia on POD 6 due to diffuse bleeding [4].
Post-Transplant Liver Function Tests
LFT's temporarily increased in all animals due to ischemia-reperfusion injury following LXT, but improved in a time dependent manner for the continuous coagulation factor recipients (Figs. 1A & B). With the exception of B365-N, where the LFT's increased on POD 3 possibly due to a mild rejection crisis, the remainder of the NovoSeven® recipients demonstrated resolving LFT's until the time of graft failure and euthanasia (Figs. 1A & B). This was similarly the case with B397-O where the LFT's normalized following LXT, but increased on POD 5 correlating with the development of sepsis/DIC and graft failure. In contrast, all baboons receiving bolus Octaplex® administration demonstrated early and persistently elevated LFT's consistent with graft failure (Figs. 1A & B).
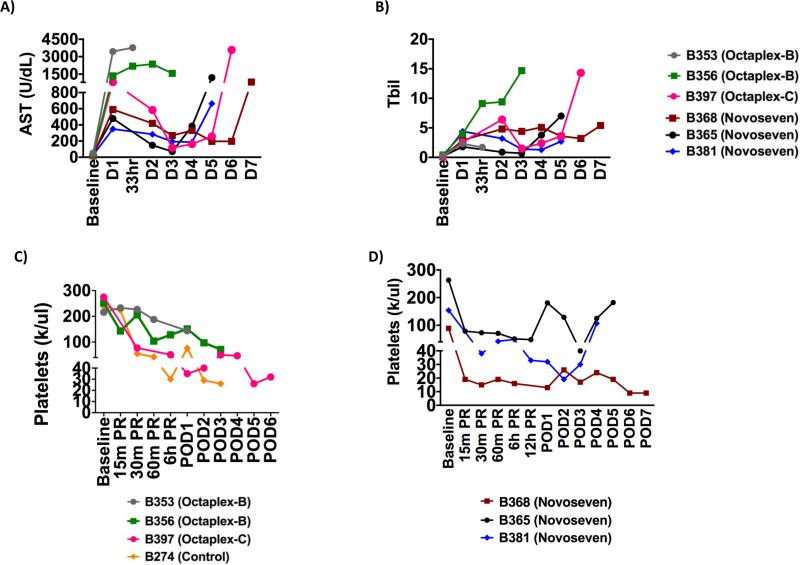
Figure 1. Liver Function Tests and Platelet Levels.
A) AST, B) Bilirubin and C & D) platelets results for all recipient baboons with respect to the administration of either Octaplex® or NovoSeven®. Octaplex-B denotes bolus Octaplex®, Octaplex-C denotes continuous Octaplex®. Continuous coagulation factor recipients demonstrated resolution of liver function until euthanasia and improvement in circulating platelet counts.
Platelet Counts Following Transplantation
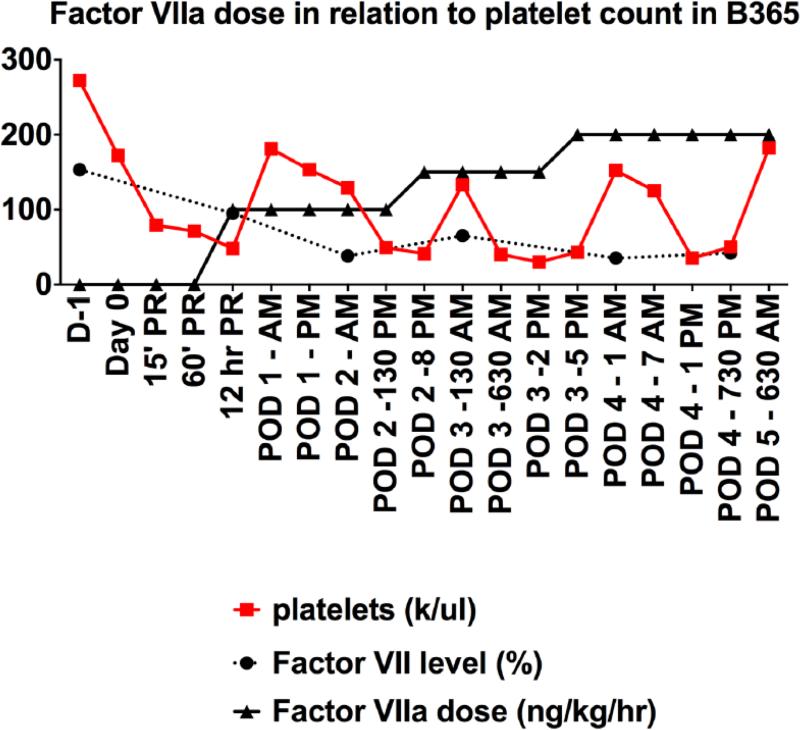
Bolus Octaplex® animals, (B353-O and B356-O) did not experience the rapid development of severe thrombocytopenia previously observed after LXT (Fig. 1C), however the shortened survival made it difficult to make further evaluations. B397-O also developed thrombocytopenia following reperfusion of the xenograft, but maintained stable platelet counts without excessive bleeding when administered continuous, low-dose Octaplex®. In comparison, NovoSeven® baboons developed severe thrombocytopenia, with platelet counts <50,000 within six hours following liver reperfusion, at which time NovoSeven® was initiated and titrated to maintain Factor VII activity at >60%. Escalating doses of NovoSeven® were observed to correlate with an increase in circulating platelets in both B365-N (Fig. 3) and B381-N. B368-N however, who developed pre-transplant thrombocytopenia following Thymoglobulin induction, did not demonstrate an increase in circulating platelets but did maintain stable platelet counts in the absence of any exogenous platelet administration (Fig. 1D).
Figure 3. Escalating Doses of NovoSeven® Correlate with an Increase in Circulating Platelets.
Escalating doses of NovoSeven® administration, aimed to maintain Factor VII activity >50%, were observed to correlate with increases in circulating platelet counts (B365 shown).
Transfusion Requirements Following Transplantation
Baboons receiving bolus Octaplex® (B353-O and B356-O) maintained a stable hematocrit (Fig. 2A) and did not require significant blood transfusions (Fig. 2B) during their shortened survival. B397-O, continuous Octaplex®, had an initial minimal transfusion requirement, but was subsequently able to maintain a stable hematocrit until POD 4 without any additional transfusions (Fig. 2B). In comparison, with the exception of B381-N who experienced post-operative surgical bleeding from the splenectomy bed, baboons receiving continuous NovoSeven® administration maintained a stable hematocrit and demonstrated a delay in the initial onset of transfusion requirements. This was most evident in B365-N, who only required 90 mL of packed red blood cells during the post-operative course (Figs. 2C & 2D).
Coagulation Factor Activity in Liver Xenograft Recipients
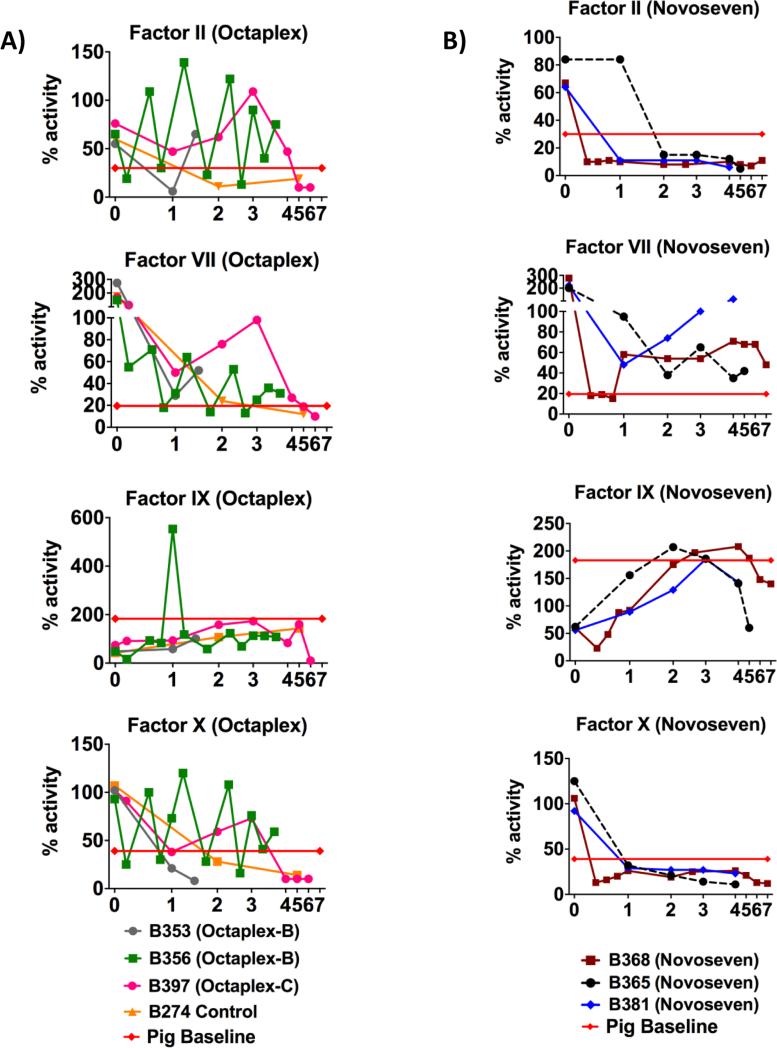
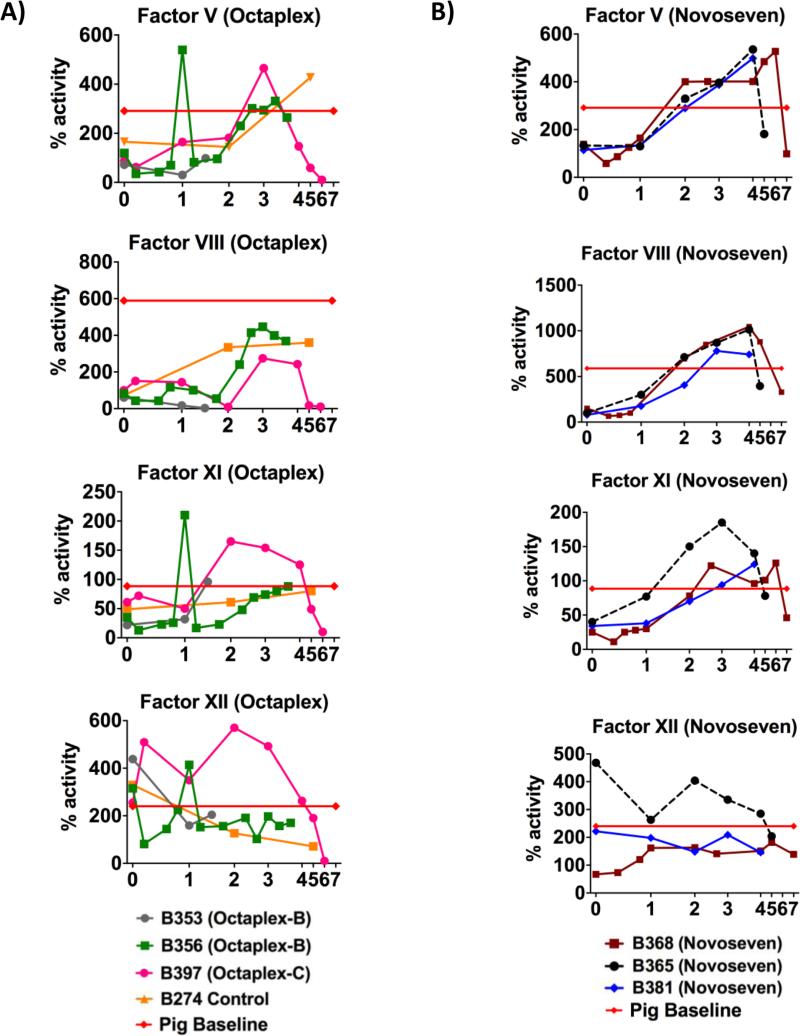
B353-O and B356-O demonstrated transient increases in Factors II, VII, IX and X associated with bolus Octaplex® administration (Fig. 4A). Factors V, VIII, XI, and XII, which are not present in Octaplex®, were initially low but increased in a time dependent manner, approaching baseline pig levels (Fig. 5A). In contrast, when Octaplex® was administered in a continuous fashion to B397-O, coagulation factor activity close to pig baseline levels were achieved through POD 5, until a sudden decrease in all factor activity occurred in relation to the animal developing sepsis and subsequent graft failure (Fig. 4A). Similarly, all NovoSeven® recipients (B368-N, B365-N and B381-N) initially demonstrated low Factor II and X activity (Fig. 4B) while Factors V, VII, IX, XI, and XII had activity which approached or even surpassed pig baseline (Figs. 4B & 5B). Factor VII activity was sufficiently maintained between 50-100% by titrating NovoSeven® as needed based off daily activity.
Figure 4. Vitamin-K Dependent Factors.
Vitamin K dependent Coagulation factor activity for A) Octaplex® recipients and B) NovoSeven® recipients with the administration of exogenous coagulation factors following transplantation. Octaplex-B denotes bolus Octaplex® and Octaplex-C denotes continuous Octaplex®. Red line indicates pig baseline levels.
Figure 5. Non-Vitamin-K Dependent Factors.
Non-vitamin K dependent coagulation factor activity for A) Octaplex® recipients and B) NovoSeven® recipients with the administration of exogenous coagulation factors following transplantation. Octaplex-B denotes bolus Octaplex® and Octaplex-C denotes continuous Octaplex®. Red line indicates pig baseline levels.
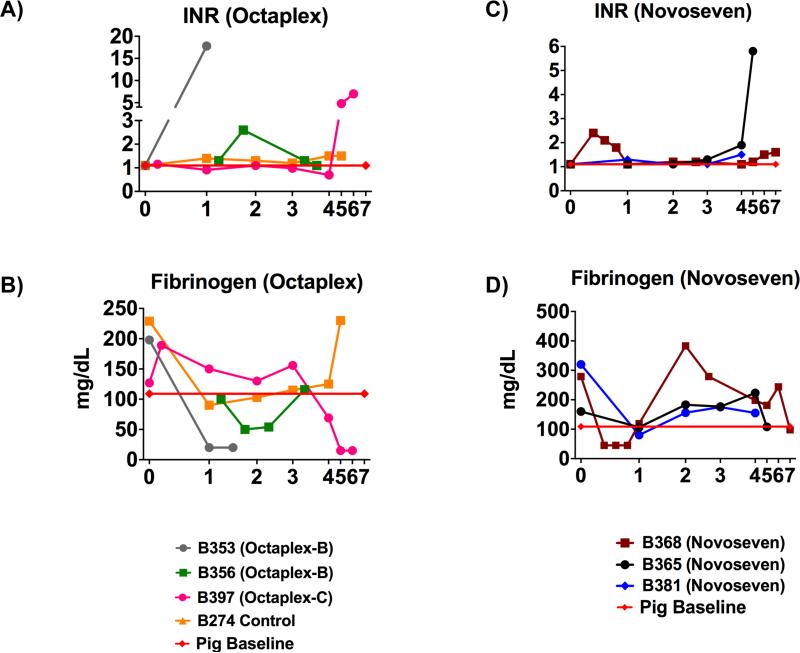
Following LXT, international normalized ratio (INR) and fibrinogen levels demonstrated strong evidence for sufficient synthetic liver function in all recipients receiving continuous coagulation factors, as the INR remained below 2.0 up until graft failure (Figs. 6A & 6C). Furthermore, with a half-life of 3-4 days, in-vivo detection of fibrinogen beyond POD 3 in the continuous coagulation factor recipients suggests that production was in part due to the xenoliver (Fig. 6D). This is in stark contrast to the baboons receiving bolus Octaplex® administration where severe consumptive coagulopathy along with a decrease in fibrinogen production and an increase in PT/INR were observed immediately following administration (Figs. 6A & 6B).
Figure 6. Post-Transplant Synthetic Function in Xenolivers.
Values for INR in A) Octaplex® and control and recipients and C) NovoSeven® recipients, demonstrating the ability to maintain a stable INR with continuous factor administration until graft failure. Fibrinogen values for B) Octaplex® and control recipients as well as D) NovoSeven® recipients, demonstrating the ability of xenolivers receiving continuous coagulation factors to maintain adequate synthetic function until graft failure. Octaplex-B denotes bolus Octaplex® and Octaplex-C denotes continuous Octaplex®. Red line indicates pig baseline levels. INR, International Normalized Ratio.
Macroscopic and Microscopic Findings on Open Liver Biopsy
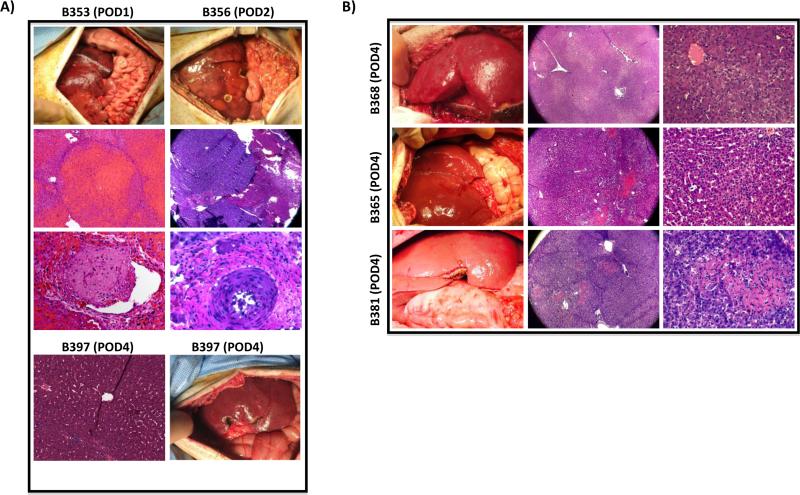
Bolus Octaplex® baboons (B353-O and B356-O) underwent exploratory laparotomy and open liver biopsy on POD 1 and 3, respectively. B353-O demonstrated an intact hepatic parenchyma with rare centrilobular necrosis in the right lobe, lobular necrosis of the left lobe, severe occlusive and non-occlusive thrombi in the right and left portal vein branches consistent with graft thrombosis, as well as widespread TMA (Fig. 7A - left panels). B356-O demonstrated patchy necrosis of the liver interspaced with normal healthy tissue as well as several occlusive and non-occlusive thrombi consistent with graft thrombosis and widespread TMA (Fig. 7A - right panels). In contrast, the continuous Octaplex® recipient (B397-O) demonstrated a healthy appearing liver on POD 4 with mild portal triad inflammation and focal hepatocyte damage without any evidence of graft thrombosis or TMA (Fig. 7A - bottom panels).
Figure 7. Findings on Intra-Operative Biopsy.
A) Left, B353; Right, B356, both demonstrating a necrotic, non-viable liver with evidence of vascular thrombosis and TMA during post-operative re-exploration. In comparison, B397, bottom, demonstrates a pink, healthy liver graft without evidence of vascular thrombosis or TMA. B) Top, Middle, Bottom; B368, B365 and B381 respectively, demonstrating a pink, healthy liver without evidence of vascular thrombosis or TMA. TMA, Thrombotic Microangiopathy.
NovoSeven® baboons in Group 2 (B368-N, B365-N and B381-N) all underwent open liver biopsy on POD 4 revealing a normal appearing liver graft with histology demonstrating patchy areas of ischemic changes observed around the central vein surrounded by normal appearing parenchyma (B368-N) (Fig. 7B - top panel) and evidence of mild inflammation around the hepatic sinusoids (B365-N & B381-N) (Fig. 7B - middle and bottom panels), but without evidence of graft thrombosis or TMA. Additionally, B365-N demonstrated early features of possible acute rejection as evidenced by polymorphonuclear cellular infiltration around the hepatic sinusoids, correlating with the animal's clinical course.
Macroscopic and Microscopic Findings on Necropsy
Bolus Octaplex® baboons (B353-O POD 1 and B356-O POD 3) demonstrated diffuse skin and small bowel petechiae as well as hemorrhagic fluid in the peritoneal, pleural and pericardial cavities. Both livers demonstrated extensive necrosis, interspersed with relatively normal tissue along with diffuse vascular thrombi, consistent with widespread TMA (not shown). In contrast, the continuous Octaplex® recipient (B397-O POD 6) demonstrated hemorrhagic ascites which was absent 48 hours earlier. Furthermore, the liver was firm and dark with histology confirming diffuse hemorrhage and necrosis, but without the development of TMA.
NovoSeven® baboons (B368-N POD 7, B365-N POD 5, B381-N POD 5) all demonstrated a similarly appearing dark, firm and congested liver without gross necrosis on necropsy. All three livers demonstrated some degree of parenchymal hemorrhage on histology, which was most noticeable in B368-N and B365-N (not shown). Further analysis in B368-N revealed minimal inflammation with mild centrilobular necrosis. The early features of acute cellular rejection observed in B365-N on POD 4 were again observed on necropsy along with areas of necrosis, hemorrhage and moderate inflammation around the hepatic sinusoids and portal triads. Histology in B381-N demonstrated an intact hepatic architecture with minimal necrosis. Most encouragingly however was the absence of TMA in all NovoSeven® recipients. Lastly, all three animals were found to have hemorrhagic pulmonary effusions correlating with their deteriorating respiratory condition at the time of euthanasia.
Preliminary Results of a Subsequent Xenoliver Transplant with Continuous Coagulation Factor Administration
On the basis of these findings, we have recently performed another xenoliver transplantation utilizing continuous exogenous administration of coagulation factors plus several procedural modifications intended to mitigate infectious complications and have achieved a 25-day survival of the recipient baboon. Important with regard to the results reported above for platelet levels following continuous factor administration, thrombocytopenia in this animal resolved completely starting on day 11. The details for this experiment will be reported elsewhere (Shah et al., Annals of Surgery, In Press).
Discussion
Although liver xenotransplantation (LXT) represents a potential therapy to cure or bridge patients with liver failure, there still remain significant barriers to success [11,12]. The development of genetically engineered pigs has significantly protected xenografts from acute humoral and cellular immune responses [3], but has not improved survival sufficiently to consider it a successful human therapy at this time [4,11]. This is mainly due in part to the coagulation dysregulation that occurs presumably due to molecular incompatibilities between pigs and primates that promote, or fail to regulate, pathological clotting [12–14]. The latter is manifested not only as thrombosis in the graft, but also as a severe systemic consumptive coagulopathy and severe thrombocytopenia [14–17]. Therefore, in addition to further genetic modifications to dampen the robust immunological response, the use of exogenous systemic pharmacological agents may provide us with insights into the coagulation barriers that exist. In this study, utilizing a large animal model of pig-to-baboon liver xenotransplantation, we analyzed the effects of exogenous administration of human recombinant coagulation factors using two different pharmacological agents: Octaplex®, a second-generation human prothrombin complex concentrate (hPCC) containing the factors II, VII, IX, and X, as well as Proteins C and S, and NovoSeven®, human recombinant activated Factor VII.
Profound thrombocytopenia has been well documented as a major complication following pig-to-baboon liver xenotransplantation [18], baboon-to-human liver xenotransplantation [19] and following the transplantation of dog livers into pigs [20]. In previous recipients from our center who underwent LXT without coagulation factor administration, platelet transfusions were required when circulating platelet counts fell below 20,000. Additionally, these historical recipients required the administration of anti-fibrinolytic agents (Amicar) in order to maintain an average platelet count above 20,000. Although Amicar administration was used to prevent further thrombocytopenia, limit the progression of DIC and to help stabilize the hematocrit [4–6], these animals had ongoing blood transfusion requirements and ultimately succumbed to fatal hemorrhage within 9 days of LXT. In contrast, in the present study, we observed stabilization of platelets counts with the administration of Octaplex®, as well as the prevention of severe thrombocytopenia and improvement in circulating platelet counts with the infusion of escalating doses of NovoSeven® in two out of three baboons. Unlike the historical recipients, these results were achieved without the need for platelet transfusions or the administration of anti-fibrinolytic agents. Additionally, in a third animal that developed pre-transplant thrombocytopenia, NovoSeven® appeared to maintain stable platelets counts, preventing any further thrombocytopenia.
In our study, the continuous administration of human coagulation factors also substantially decreased the transfusion requirements, with recipients on average requiring 208 mL of blood during their post-operative course. Notably, the historical recipients required on average 620 mL of blood during a similar post-operative time period [4]. This reduced transfusion requirement seen with animals receiving continuous exogenous factor administration has also been previously reported in cases following human orthotopic liver transplantation in which NovoSeven® was administered, as well as in patients with congenital Factor VII deficiency [21,22]. Although in this study we cannot determine the exact mechanism by which the baboon recipients receiving continuous exogenous factor administration experienced a decreased transfusion requirement, evidence in the literature suggests that administration of recombinant Factor VIIa reduces bleeding by significantly shortening the prolonged bleeding time, even in the presence of severe thrombocytopenia, through a potential increase in the generation of thrombin [23]. To investigate this, Hendricks et al., studied the effects of recombinant Factor VIIa administration on coagulation, as measured by thromboelastography (TEG), during human orthotopic liver transplantation. It was determined that even a single bolus injection of recombinant factor VIIa had an effect on all hemostatic variables. Recombinant Factor VIIa was also observed to improve the speed of clot formation and at supra-therapeutic levels improved the activation of Factor X, leading to further activation of the clotting cascade [24]. In concordance with their results, we similarly observed improvements in several features of coagulation parameters such as PT, PTT (data not shown) and INR, which were consistently maintained within normal ranges for the first 72 hours following LXT (Figs. 6A & 6C).
The infusion of activated Factor VIIa in the presence of thrombocytopenia has also demonstrated the ability to normalize the time required to reach maximum thrombin generation, as well as accelerate the velocity of platelet activation by reducing the onset time of platelet activation and the time required to reach maximum platelet activation [25,26]. This could explain why in our studies we observed an increase in circulating platelet levels immediately following an increase in the dose of NovoSeven® administration. It appears that by having activated platelets available more rapidly, less consumption of platelets would be required to achieve hemostasis. Moreover, since thrombin is known to further activate platelets, NovoSeven® may reduce the time required for thrombin generation, resulting in additional thrombin-dependent acceleration of platelet activation and possible acceleration of the amplification phase of thrombin generation. Theoretically, these effects should be sufficient for the initial formation of the hemostatic plug and the temporary cessation of bleeding. However, this process requires the preservation of the xenograft to attain adequate hepatic function, as measured by the production of coagulation factors and fibrinogen
While fibrinogen production was adequate in animals undergoing continuous infusions, indicating functional xenografts and/or decreased fibrinogen consumption, bolus Octaplex® animals demonstrated early loss of fibrinogen production signifying early graft failure, while control animals maintained stable, but low levels of fibrinogen production that did not demonstrate improvement over the post-transplant period (Figs. 6B & 6D). Additionally, we observed that with the continuous infusion of low-dose exogenous coagulation factors we were able to achieve a more consistent and stable level of coagulation factor activity when compared to the bolus and control recipients who had fluctuating and diminishing activity, consistent with early graft loss (in the case of the bolus Octaplex® recipients) and with increased bleeding (in the case of the control animals) (Figs. 4 & 5). Although the administration of Octaplex® should provide the factors necessary for adequate coagulation, we saw in our results that the bolus administration of exogenous coagulation factors dose produced significant liver thrombosis and profound TMA (Fig. 7), while a continuous low-dose infusion not only seemed to avoid thrombosis, but also provided sufficient amounts of coagulation factors necessary for the preservation of the xenograft for a longer duration of time. This was best evident by the detection of sufficient fibrinogen production for the first four post-operative days (Figs. 6B & 6D) and with sufficient coagulation factor activity (Figs. 4 & 5).
The prevention of TMA must also be further investigated if pig-to-primate xenotransplantation is to be successful. This phenomenon is not only limited to the liver, but has also been observed in other models such as the heart [8] and the kidney [7]. Shimizu et al. [8] have previously postulated that despite the initial elimination of hyperacute rejection (HAR) by transplanting GalT-KO swine, acute humoral xenograft rejection (AHXR) caused by anti-non-Gal antibodies may still be responsible for the activation of endothelial cell mediated microvascular thrombosis and subsequent microangiopathy [6,8,13]. Detection of circulating IgM and IgG were seen as early as one hour post-transplantation, with the subsequent development of increasing levels of IgM, IgG, C3 and C4d within the graft vessels throughout the post-operative period, which was related to the development of TMA within the xenografts [8]. Although we did not observe any evidence of AHXR, we still found TMA in both the historical control baboon (B274) and those baboons receiving bolus dosing of Octaplex® (B353-O and B356-O), which demonstrated widespread vascular thrombosis on necropsy. These findings were likely attributed to endothelial cell activation and the profound stimulation of the clotting cascade secondary to the bolus administration of supra-therapeutic doses of human coagulation factors.
In comparison, baboons receiving continuous low-dose infusions of exogenous coagulation factors (B368-N, B365-N, B381-N and B397-O) did not demonstrate evidence of TMA in their xenografts. Furthermore, serum FACS antibody analysis failed to detect any evidence of circulating baboon anti-pig IgM or IgG throughout the post-operative period. Although data exists to support non-gal-antibody mediated AHXR as a source for TMA [7,8,13], the fact that these animals did not demonstrate any signs of TMA could be due to a possible unknown mechanism of platelet stabilization or to an interaction between Factor VIIa with tissue factor (TF) to modulate endothelial cell activation. The mechanism by which TMA is prevented through continuous factor administration remains a promising area of further investigation, as previous reports of prolonged xenograft survival have all been limited by the development of TMA [27]. Indeed, while still requiring further investigation of the basis of its prolonged survival, the normalization of platelets in a subsequent recipient baboon (see preliminary results above), would appear to support our hypothesis of the relationship between coagulation factor administration, platelet stabilization and avoidance of TMA (Shah et al., Annals of Surgery, In Press).
Conclusion
Exogenous, low-dose, continuous coagulation factor infusion (Octaplex® or NovoSeven®) decreases the overall transfusion requirements following pig-to-baboon LXT, and may allow for prolonged graft survival. Although our study is limited by a small cohort size, this is a function of the complexity of the large animal xenotransplantation model, but is similar to previously published data at other centers. For the first time, we demonstrate prevention in the development of TMA following LXT in recipients receiving continuous coagulation factor administration as well as the stabilization or improvement in thrombocytopenia. Further work is needed to determine the mechanisms by which TMA is prevented and thrombocytopenia is improved as this data is encouraging in furthering our understanding of the coagulation barriers limiting successful xenotransplantation.
Acknowledgments
The authors would like to thank Christian Schuetz for his assistance in the organization of data and review of the manuscript. Jigesh A. Shah is supported by a National Institute of Allergy and Infectious Disease, NIAID, 2T32AI007529-16A1 Training Grant.
Abbreviations
- AHXR
Acute Humoral Xenograft Rejection
- DIC
Disseminated Intra-Vascular Coagulation
- GalT-KO
alpha-1,3-galactosyltransferase knock out
- H&E
hematoxylin and eosin
- hPCC
Human Prothrombin Concentrate Complex
- HAR
Hyper-Acute Rejection
- IACUC
Institutional Animal Care and Use Committee
- INR
International Normalized Ratio
- KG
Kilogram
- LFT
Liver Function Test
- LXT
Liver Xenotransplantation
- MCG
Microgram
- NIH
National Institute of Health
- PTT
Partial Thromboplastin Time
- POD
Post-Operative Day
- PT
Prothrombin Time
- TEG
Thromboelastography
- TMA
Thrombotic Microangiopathy
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Sachs DH. The pig as a xenograft donor. Pathol. Biol. (Paris) 1994;42:217–219. [PubMed] [Google Scholar]
- 2.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked Prolongation of Porcine Renal Xenograft Survival in Baboons Through the Use of alpha1,3-galactosyltransferase Gene-Knockout Donors and the Cotransplantation of Vascularized Thymic Tissue. Nat. Med. 2005;11:32–34. doi: 10.1038/nm1172. doi:10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 3.Tseng Y-L, Kuwaki K, Dor FJMF, Shimizu A, Houser S, Hisashi Y, et al. alpha1,3-Galactosyltransferase gene-knockout pig heart transplantation in baboons with survival approaching 6 months. Transplantation. 2005;80:1493–1500. doi: 10.1097/01.tp.0000181397.41143.fa. doi:10.1097/01.tp.0000181397.41143.fa. [DOI] [PubMed] [Google Scholar]
- 4.Kim K, Schuetz C, Elias N, Veillette GR, Wamala I, Varma M, et al. Up to 9-day survival and control of thrombocytopenia following alpha1,3-galactosyl transferase knockout swine liver xenotransplantation in baboons. Xenotransplantation. 2012;19:256–264. doi: 10.1111/j.1399-3089.2012.00717.x. doi:10.1111/j.1399-3089.2012.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekser B, Long C, Echeverri GJ, Hara H, Ezzelarab M, Lin CC, et al. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants. Am. J. Transplant. 2010;10:273–285. doi: 10.1111/j.1600-6143.2009.02945.x. doi:10.1111/j.1600-6143.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- 6.Ekser B, Echeverri GJ, Hassett AC, Yazer MH, Long C, Meyer M, et al. Hepatic function after genetically engineered pig liver transplantation in baboons. Transplantation. 2010;90:483–493. doi: 10.1097/TP.0b013e3181e98d51. doi:10.1097/TP.0b013e3181e98d51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimizu A, Yamada K, Yamamoto S, Lavelle JM, Barth RN, Robson SC, et al. Thrombotic microangiopathic glomerulopathy in human decay accelerating factor-transgenic swine-to-baboon kidney xenografts. J. Am. Soc. Nephrol. 2005;16:2732–2745. doi: 10.1681/ASN.2004121148. doi:10.1681/ASN.2004121148. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu A, Hisashi Y, Kuwaki K, Tseng Y-L, Dor FJMF, Houser SL, et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from alpha1,3-galactosyltransferase gene-knockout pigs in baboons. Am. J. Pathol. 2008;172:1471–1481. doi: 10.2353/ajpath.2008.070672. doi:10.2353/ajpath.2008.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kozlowski T, Shimizu A, Lambrigts D, Yamada K, Fuchimoto Y, Glaser R, et al. Porcine kidney and heart transplantation in baboons undergoing a tolerance induction regimen and antibody adsorption. Transplantation. 1999;67:18–30. doi: 10.1097/00007890-199901150-00004. doi:10.1097/00007890-199901150-00004. [DOI] [PubMed] [Google Scholar]
- 10.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc. Natl. Acad. Sci. U. S. A. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. doi:10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox A, Zhong R. Current advances in xenotransplantation. Hepatobiliary Pancreat. Dis. Int. 2005;4:490–494. [PubMed] [Google Scholar]
- 12.Hammer C, Thein E. Physiological aspects of xenotransplantation, 2001. Xenotransplantation. 2002;9:303–305. doi: 10.1034/j.1399-3089.2002.02036.x. doi:10.1034/j.1399-3089.2002.02036.x. [DOI] [PubMed] [Google Scholar]
- 13.Lin CC, Cooper DKC, Dorling A. Coagulation dysregulation as a barrier to xenotransplantation in the primate. Transpl. Immunol. 2009;21:75–80. doi: 10.1016/j.trim.2008.10.008. doi:10.1016/j.trim.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowan PJ, Robson SC, D'Apice AJF. Controlling coagulation dysregulation in xenotransplantation. Curr. Opin. Organ Transplant. 2011;16:214–221. doi: 10.1097/MOT.0b013e3283446c65. doi:10.1097/MOT.0b013e3283446c65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwase H, Ezzelarab MB, Ekser B, Cooper DKC. The role of platelets in coagulation dysfunction in xenotransplantation, and therapeutic options. Xenotransplantation. 2014;21:201–220. doi: 10.1111/xen.12085. doi:10.1111/xen.12085. [DOI] [PubMed] [Google Scholar]
- 16.Cowan PJ, D'Apice AJF. The coagulation barrier in xenotransplantation: incompatibilities and strategies to overcome them. Curr. Opin. Organ Transplant. 2008;13:178–183. doi: 10.1097/MOT.0b013e3282f63c74. doi:10.1097/MOT.0b013e3282f63c74. [DOI] [PubMed] [Google Scholar]
- 17.Yeh H, Machaidze Z, Wamala I, Fraser JW, Navarro-Alvarez N, Kim K, et al. Increased transfusion-free survival following auxiliary pig liver xenotransplantation. Xenotransplantation. 2014;21:454–64. doi: 10.1111/xen.12111. doi:10.1111/xen.12111. [DOI] [PubMed] [Google Scholar]
- 18.Ramirez P, Chavez R, Majado M, Munitiz V, Muñoz A, Hernandez Q, et al. Life-supporting human complement regulator decay accelerating factor transgenic pig liver xenograft maintains the metabolic function and coagulation in the nonhuman primate for up to 8 days. Transplantation. 2000;70:989–998. doi: 10.1097/00007890-200010150-00001. doi:10.1097/00007890-200010150-00001. [DOI] [PubMed] [Google Scholar]
- 19.Starzl TE, Fung J, Tzakis A, Todo S, Demetris AJ, Marino IR, et al. Baboon-to-human liver transplantation. Lancet. 1993;341:65–71. doi: 10.1016/0140-6736(93)92553-6. doi:10.1016/0140-6736(93)93183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tector AJ, Fridell JA, Elias N, Watanabe T, Salazar J, Greinke D, et al. Aberrations in hemostasis and coagulation in untreated discordant hepatic xenotransplantation: Studies in the dog-to-pig model. Liver Transplant. 2002;8:153–159. doi: 10.1053/jlts.2002.30881. doi:10.1053/jlts.2002.30881. [DOI] [PubMed] [Google Scholar]
- 21.Hendriks HG, Meijer K, de Wolf JT, Klompmaker IJ, Porte RJ, de Kam PJ, et al. Reduced transfusion requirements by recombinant factor VIIa in orthotopic liver transplantation: a pilot study. Transplantation. 2001;71:402–405. doi: 10.1097/00007890-200102150-00011. [DOI] [PubMed] [Google Scholar]
- 22.Tran HTT, Tjønnfjord GE, Paus A, Holme PA. rFVIIa administered by continuous infusion during surgery in patients with severe congenital FVII deficiency. Haemophilia. 2011;17:764–770. doi: 10.1111/j.1365-2516.2011.02596.x. doi:10.1111/j.1365-2516.2011.02596.x. [DOI] [PubMed] [Google Scholar]
- 23.Tranholm M, Rojkjaer R, Pyke C, Kristensen AT, Klitgaard B, Lollike K, et al. Recombinant factor VIIa reduces bleeding in severely thrombocytopenic rabbits. Thromb. Res. 2003;109:217–223. doi: 10.1016/s0049-3848(03)00146-4. doi:10.1016/S0049-3848(03)00146-4. [DOI] [PubMed] [Google Scholar]
- 24.Hendriks HGD, Meijer K, de Wolf JTM, Porte RJ, Klompmaker IJ, Lip H, et al. Effects of recombinant activated factor VII on coagulation measured by thromboelastography in liver transplantation. Blood Coagul. Fibrinolysis. 2002;13:309–313. doi: 10.1097/00001721-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Gerotziafas GT, Chakroun T, Depasse F, Arzoglou P, Samama MM, Elalamy I. The role of platelets and recombinant factor VIIa on thrombin generation, platelet activation and clot formation. Thromb. Haemost. 2004;91:977–985. doi: 10.1160/TH03-10-0638. doi:10.1160/TH03-10-0638. [DOI] [PubMed] [Google Scholar]
- 26.Gerotziafas GT, Zervas C, Gavrielidis G, Tokmaktsis A, Hatjiharissi E, Papaioannou M, et al. Effective hemostasis with rFVIIa treatment in two patients with severe thrombocytopenia and life-threatening hemorrhage. Am. J. Hematol. 2002;69:219–222. doi: 10.1002/ajh.10056. doi:10.1002/ajh.10056. [DOI] [PubMed] [Google Scholar]
- 27.Dor FJMF, Kuwaki K, Tseng YL, Shimizu A, Houser SL, Yamada K, et al. Potential of aspirin to inhibit thrombotic microangiopathy in α1,3-galactosyltransferase gene-knockout pig hearts after transplantation in baboons. Transplant. Proc. 2005;37:489–490. doi: 10.1016/j.transproceed.2004.12.235. doi:10.1016/j.transproceed.2004.12.235. [DOI] [PubMed] [Google Scholar]