Abstract
Metabolic syndrome is associated with long-term morbidity and mortality after adult liver transplant (LT). Whether pediatric LT recipients have a higher prevalence of metabolic syndrome remains controversial. In a cross-sectional study, we evaluated pediatric LT recipients aged 8–30 years using National Health and Nutrition Examination Survey (NHANES) protocols. LT recipients were matched by gender, race/ethnicity, and age with controls from NHANES. Pediatric LT recipients (n=83), after adjusting for overweight/obesity and glucocorticoid use, had increased prevalence of pre-hypertension and hypertension, impaired glucose tolerance (IGT; 2-hour glucose after oral glucose tolerance test ≥ 140mg/dL), and low HDL than matched NHANES controls (n=235) despite a lower prevalence of overweight/obesity. Among LT recipients, the adjusted odds of IGT doubled for every 7.5 years on calcineurin-inhibitors (CNIs, OR 2.10, 95% CI 1.06–4.17 per 7.5 years on CNIs, p=0.03). Among all subjects with IGT, LT recipients had a lower prevalence of overweight/obesity and less insulin resistance (HOMA-IR) than controls with IGT. Among normal weight subjects, LT recipients were significantly more likely than controls to have pre-hypertension/hypertension, IGT, low HDL, and metabolic syndrome. Pediatric LT recipients have unique metabolic syndrome profiles and risk factors, and will require tailored screening and management protocols.
Introduction
Ten year survival after pediatric liver transplantation exceeds 80%. (1) To optimize outcomes in pediatric liver transplant (LT) recipients, attention to chronic medical conditions that impact long-term morbidity is crucial. Metabolic syndrome is a cluster of factors associated with long-term morbidity and mortality in adult LT recipients. (2) Whether pediatric LT recipients have a higher prevalence of metabolic syndrome and its components than non-transplanted peers has been debated, (3,4) but not yet investigated in a cohort with matched controls.
Strict definitions of metabolic syndrome for both adults and children include elevated waist circumference, hypertension, elevated serum triglycerides, low high-density lipoprotein (HDL), and impaired glucose metabolism as its components, although cutoffs have not been codified in children. (5,6) Overweight or obesity by body mass index (BMI) is sometimes substituted for waist circumference, especially in pediatric studies. (7,8)
Recent retrospective reviews of pediatric LT recipients have demonstrated relatively high prevalence of overweight/obesity and other components of metabolic syndrome.(3,9,10) However, these reports are largely based on data collected sporadically during clinical care rather than systematically for research purposes and have not included matched control groups. Furthermore, pediatric LT recipients do not typically undergo rigorous evaluation for pre-diabetes, an important component of metabolic syndrome tied to long-term morbidity. Pre-diabetes is defined by the American Diabetes Association as either elevated fasting glucose (fasting glucose; ≥100mg/dL) or impaired glucose tolerance (IGT; ≥140mg/dL 2 hrs after glucose load).(11)
This is the first study to investigate metabolic syndrome and glucose metabolism in pediatric LT recipients using standardized research protocols and matched controls. We hypothesized that overweight/obesity and exposure to immunosuppression agents, specifically glucocorticoids and calcineurin inhibitors (CNIs), would increase the prevalence of metabolic syndrome components among LT recipients compared to non-transplanted peers.
Methods
This study was approved by UCSF’s Committee on Human Research (IRB 12-10290). Our LT cohort was evaluated in a cross-sectional study of pediatric LT recipients aged 8–30 years at the time of study visit. All subjects underwent first LT prior to age 18, were at least 1 year from last LT, were on stable immunosuppressive regimens, and had no known diabetes at time of enrollment. After age-appropriate consent and assent were obtained, subjects were evaluated in UCSF’s Pediatric Clinical Research Center or during inpatient admission for a surveillance liver biopsy. Patients who underwent surveillance biopsy were at least 5 years from transplant with no rejection in more than 1 year (n=39)All had height, weight, and anthropometrics measured using the National Health and Nutrition Examination Survey (NHANES) 2011 Anthropometry Procedures protocols (http://www.cdc.gov/nchs/nhanes/nhanes2011-2012/manuals11_12.htm). Waist circumference was measured twice to enable calculation of a mean value. Blood pressure was measured three times sitting, using a digital sphygmomanometer, with at least 5 minutes of rest preceding each measurement, also following NHANES 2011 protocols; a mean value was calculated for both systolic and diastolic blood pressure. Fasting serum was obtained after at least an 8-hour fast. Oral glucose tolerance testing was performed with weight-based glucose load (1.75 gram/kg to maximum 75 grams), following the NHANES 2011 Oral Glucose Tolerance Testing (OGTT) protocols (http://www.cdc.gov/nchs/nhanes/nhanes2011-2012/manuals11_12.htm).
LT recipients were matched by gender, race/ethnicity, and age (± 1 year) with 3 controls from NHANES 2009–2010 and 2011–2012 cohorts. NHANES is a bi-annual, nationally representative cross-sectional study of children and adults in the U.S. administered by the Centers for Disease Control and Prevention. We used publically available, person-level data from the most recent surveys available at the time of data analysis (http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm, accessed 8/14/2015). Four LT had only two matched controls, and five had one matched control because of limited availability of younger controls whose race/ethnicity was classified as Asian or Other.
NHANES only performs fasting serum samples and oral glucose tolerance testing on children 12 years and older. Thus, children younger than 11 were matched with 12 year-old controls and sensitivity analyses excluding them from the modeling were performed; results of this sensitivity analysis are reported when they differ significantly from whole-cohort results. Only NHANES subjects with body mass index (BMI), blood pressure, and all laboratory values of interest available were included as potential controls. For both LT and NHANES subjects, only oral glucocorticoids were counted as current glucocorticoid use; topical and inhaled steroids were not included. NHANES protocols were approved by the National Center for Health Statistics, with informed consent obtained from all participants.
Abnormal values of metabolic syndrome components were defined on previously published standards that were age and other demographic specific whenever possible. (Supplemental Table 1) For subjects younger than 18 years at study visit, BMI percentile for age and gender was calculated based on 2000 CDC growth chart data. (12) Subjects were classified as overweight if their BMI percentile was 85th–94th percentile for age and gender and obese if their BMI percentile was ≥ 95th percentile. (13) Elevated waist circumference was considered ≥ 90th percentile for age and gender. (8,14) Systolic and diastolic hypertension were defined as use of anti-hypertensives or blood pressure greater than the 95th percentile for gender, age, and height; pre-hypertension included those with blood pressure percentiles 90–94th percentile. (8,15)
Obesity and hypertension in subjects 18 years or older were classified according to adult guidelines. Overweight was considered BMI 25–29.9kg/m2 and obese BMI ≥ 30 kg/m2. Elevated waist circumference was ≥ 88 cm for females and ≥ 102 cm for males.(14) Hypertension was defined as use of anti-hypertensives or systolic blood pressure ≥ 140 mmHg; diastolic ≥ 90mmHg. Pre-hypertension included those with systolic ≥ 120 mmHg; diastolic ≥ 80mmHg.(8)
Elevated lipids for all subjects represented values at or above the 75th percentile for children and young adults.(8) The cutoffs for elevated lipids were as follows: triglycerides ≥ 75mg/dL for children 9 or younger and ≥ 90 mg/dL for those 10 or older; low-density lipoprotein (LDL) > 110 mg/dL, and total cholesterol ≥ 170mg/dL. Low HDL was ≤ 40 mg/dL, which represents the 10th percentile. (8) Elevated fasting glucose was considered at least 100 mg/dL and IGT ≥ 140mg/dL two hours after glucose load, following American Diabetes Association definitions. (11)
HOMA-IR is a measure of insulin resistance, and was calculated as (fasting glucose, mg/dL × fasting insulin, mU/L)/405. (16) HOMA-IR > 3.16 is considered insulin resistant in children and adolescents. (17) HOMA-%B is a measure of pancreatic β-cell function, with 100% considered “normal,” and is calculated as = (360 × fasting insulin, mg/dL/fasting glucose, mU/L – 63). (16)
We defined metabolic syndrome as the presence of three or more of the following: (1) elevated waist circumference, (2) systolic or diastolic hypertension, (3) elevated triglycerides, (4) low HDL, (5) elevated fasting glucose or IGT. (7,8)
Statistical analyses
A p-value < 0.05 was considered statistically significant in all analyses. Categorical variables were compared using McNemar’s chi-squared tests; continuous variables were compared using Student’s t-test with unequal variances for normally distributed variables and Kruskal-Wallis tests for skewed variables. Conditional fixed-effects logistic regression was used for multivariate analysis to account for matching. Ordinal IGT risk by years on CNIs in the LT group was evaluated using a 1 degree-of-freedom test for trend. All statistical analysis was done with Stata 12 (College Station, TX).
Results
The cohort included 83 pediatric LT recipients and 235 matched NHANES controls. (TABLE 1) The LT subjects ranged in age from 8.0 to 29.0 years of age and from 1.0 to 23.6 years since transplant. The majority were on tacrolimus monotherapy. (TABLE 1) Of the 5% on glucocorticoids, all 4 were on ≤5mg prednisone daily.
Table 1.
Demographics and clinical characteristics*
| LT (n=83) |
NHANES controls (n=235) |
p | |
|---|---|---|---|
| Age at visit (years) | 15.6 ± 4.8 | 16.6 ± 4.1 | 0.11 |
| Female | 45% | 43% | 0.89 |
| Race/Ethnicity | |||
| White | 27% | 27% | 0.95 |
| Black | 9% | 9% | |
| Hispanic | 43% | 43% | |
| Asian | 13% | 13% | |
| Other/Multi-racial | 8% | 8% | |
| AST (IU/L) | 51 ± 73 | 25 ± 11 | 0.002 |
| ALT (IU/L) | 62 ± 102 | 22 ± 18 | <0.001 |
| GGT (IU/L) | 65 ± 204 | 17 ± 19 | 0.03 |
| Total bilirubin (mg/dL) | 1.0 ± 0.7 | 0.8 ± 0.3 | 0.002 |
| Creatinine (mg/dL) | 0.65 ± 0.23 | 0.71 ± 0.18 | 0.04 |
| Years since transplant | 11.2 ± 5.7 | ||
| Transplant indication¶ | |||
| Biliary atresia | 33% | ||
| Metabolic disease | 17% | ||
| Acute liver failure | 12% | ||
| Cholestatic conditions | 6% | ||
| Tumor | 2% | ||
| Other | 30% | ||
| Glucocorticoids at visit | 5% | 0.4% | 0.006 |
| CNI | |||
| Tacrolimus | 80% | ||
| Cyclosporine | 10% | ||
| Not on CNI | 10% | ||
| Tacrolimus trough at visit (n=66, μg/L) | 4.4 ± 2.2 | ||
| Mean recent tacrolimus trough† (n=66, μg/L) | 4.8 ± 2.2 | ||
| Cyclosporine trough at visit (n=8, μg/L) | 75 ± 52 | ||
| Mean recent cyclosporine trough† (n=8, μg/L) | 96 ± 63 | ||
Data represents proportion or mean ± SD. McNemar’s chi-squared test for categorical variables, t-test with unequal variances for continuous variables.
Metabolic disease includes alpha-1-antitrypsin deficiency, Crigler-Najjar syndrome, cystic fibrosis, glycogen storage disease, inborn errors in bile acid metabolism, neonatal hemochromatosis, primary hyperoxaluria, tyrosinemia, urea cycle defects, and Wilson’s disease. Cholestatic conditions includes Alagille syndrome, Byler disease, progressive intrahepatic cholestatic syndromes, total parenteral nutrition cholestasis, sclerosing cholangitis, and idiopathic cholestasis. Other diagnoses include congenital hepatic fibrosis, Budd-Chiari syndrome, autoimmune hepatitis cirrhosis, drug toxicity, hepatitis C cirrhosis, and unknown cirrhosis.
Mean of 3 most recent trough levels prior to study visit.
Only two LT recipients were on anti-hypertensives at study visit: one had controlled hypertension but the other, who was on steroids, exhibited systolic hypertension. Neither was overweight/obese. One LT recipient with normal weight developed insulin-dependent diabetes post-transplant; he had elevated fasting blood glucose but fasting glucose was not measured. One LT recipient was on sirolimus and had elevated LDL and total cholesterol, but normal HDL and triglycerides.
The cohort also included four liver-kidney transplant recipients, two transplanted for congenital hepatic fibrosis with polycystic kidney disease and two for primary hyperoxaluria. All four were receiving tacrolimus (mean trough 4.77 μg/L, SD 0.94 μg/L), and one was taking prednisone 5mg daily. None of the four liver-kidney recipients required anti-hypertensives or had systolic or diastolic hypertension at study visit.
Prevalence of metabolic syndrome components in LT and controls
In univariate analysis, LT recipients were less likely to be overweight/obese or have an elevated waist circumference than matched NHANES controls. They were more likely to have both systolic and diastolic pre-hypertension or hypertension and low HDL, but less likely to have elevated total cholesterol or LDL. (TABLE 2) Of all subjects with elevated waist circumference, 93% of LT recipients and 100% of NHANES controls were also overweight/obese by BMI.
Table 2.
Metabolic syndrome components in LT recipients versus matched NHANES controls
| LT (n=83) |
NHANES controls (n=235) |
OR for LT, unadjusted* (95% CI) |
p | OR for LT,* adjusted for overweight/obesity and glucocorticoids (95% CI) |
p | |
|---|---|---|---|---|---|---|
| Weight status by BMI/BMI %ile | ||||||
| Overweight | 18% | 24% | 0.48 (0.27–0.85)** | 0.01 | – | – |
| Obese | 8% | 19% | ||||
| Elevated waist circumference | 16% | 27% | 0.51 (0.27–0.99) | 0.05 | – | – |
| Systolic pre-hypertension or hypertension | 24% | 12% | 2.51 (1.25–5.04) | 0.01 | 2.94 (1.31–6.61)† | 0.009 |
| Diastolic pre-hypertension or hypertension | 12% | 5% | 2.71 (1.15–6.57) | 0.03 | 4.84 (1.49 – 15.63)† | 0.008 |
| Elevated fasting glucose (≥100mg/dL) | 19% | 19% | 0.93 (0.48–1.78) | 0.83 | 1.09 (0.55–2.18)† | 0.79 |
| IGT (2-hour glucose ≥ 140mg/dL) § | 30% | 6% | 6.70 (3.07–14.59) | <0.001 | 6.50 (2.76 – 15.30) | <0.001 |
| Low HDL | 38% | 24% | 1.79 (1.06–3.06) | 0.03 | 2.86 (1.50–5.44)† | 0.001 |
| Elevated triglycerides | 13% | 22% | 0.53 (0.26–1.07) | 0.08 | 0.56 (0.27–1.19)† | 0.13 |
| Elevated LDL | 2% | 14% | 0.17 (0.04–0.71) | 0.02 | 0.08 (0.01–0.64)† ‡ | 0.02 |
| Elevated total cholesterol | 2% | 22% | 0.09 (0.02–0.37) | 0.001 | 0.04 (0.01–0.33)‡ | 0.002 |
| Metabolic syndrome | 14% | 14% | 0.96 (0.46–2.01) | 0.93 | 1.69 (0.56–5.10)† | 0.35 |
ORs calculated using conditional fixed-effects logistic regression with adjustment for confounders as noted.
OR for overweight/obesity prevalence in LT compared to NHANES controls.
Overweight/obesity is also a statistically significant risk factor in conditional regression (data not shown).
Steroid use is also a statistically significant risk factor in conditional regression (data not shown).
Unable to calculate. All 3 controls with diastolic hypertension were overweight/obese. 1 of 3 LT recipients with diastolic hypertension was overweight/obese.
76 LT recipients had 2-hour glucose.
In LT recipients, blood glucose 2-hours after glucose challenge was significantly higher than in controls (127 ± 30 vs 99 ± 24 mg/dL, p<0.001), and IGT was significantly more common (30% vs 6%; p<0.001). (TABLE 2) However, there were no differences in fasting glucose levels (92 ± 7 vs 93 ± 8 mg/dL, p=0.24) or the prevalence of fasting glucose elevation (19% vs 19%; p=0.98). (TABLE 2) There was no difference in metabolic syndrome prevalence between LT recipients and controls. (TABLE 2)
Accounting for matching and adjusting for overweight/obesity and glucocorticoid use confirmed that LT recipients are at higher risk for systolic and diastolic pre-hypertension/hypertension, IGT, and low HDL despite a lower risk of overweight/obesity. (TABLE 2) LT recipients had lower adjusted odds of other dyslipidemias after adjustment. (TABLE 2)
In sensitivity analysis excluding liver-kidney recipients and their matched controls, unadjusted and adjusted odds ratios’ (OR) directionality, magnitude and significance did not change (data not shown). Sensitivity analysis excluding subjects with only 1–2 matched controls (n=9 LT, 13 controls) also revealed no changes in adjusted or unadjusted models (data not shown).
In subgroup analysis considering only LT recipients with AST and ALT ≤ 40 IU/L(n=50) and their matched controls (n=145), LT recipients still had a significantly higher prevalence of IGT (OR 5.59, 1.70–18.42, p=0.005) despite a lower risk of overweight/obesity (OR 0.41, 95% CI 0.19–0.87, 0.02) with no significant difference in prevalence of elevated fasting glucose (OR 1.32, 95% CI 0.54–3.21, p=0.54) after adjusting for overweight/obesity and corticosteroids,. In this smaller sample, there were no longer significant differences in the risk of low HDL (OR 2.08, 0.90–4.82, p=0.09), systolic pre-hypertension or hypertension (OR 1.29, 95% CI 0.40–4.15, p=0.67), or diastolic pre-hypertension or hypertension (OR 2.85, 95% CI 0.64–12.79, p=0.17) in adjusted models.
Risk of metabolic syndrome in LT compared to controls differs by weight status
In analysis restricted to normal weight subjects, LT recipients were significantly more likely than NHANES controls to have pre-hypertension/hypertension (28% vs. 10%, p=0.002), IGT (30% vs. 4%, p<0.001), low HDL (33% vs. 10%, p<0.001), and metabolic syndrome (7% vs. 1%, p=0.02). (FIGURE 1)
Figure 1.
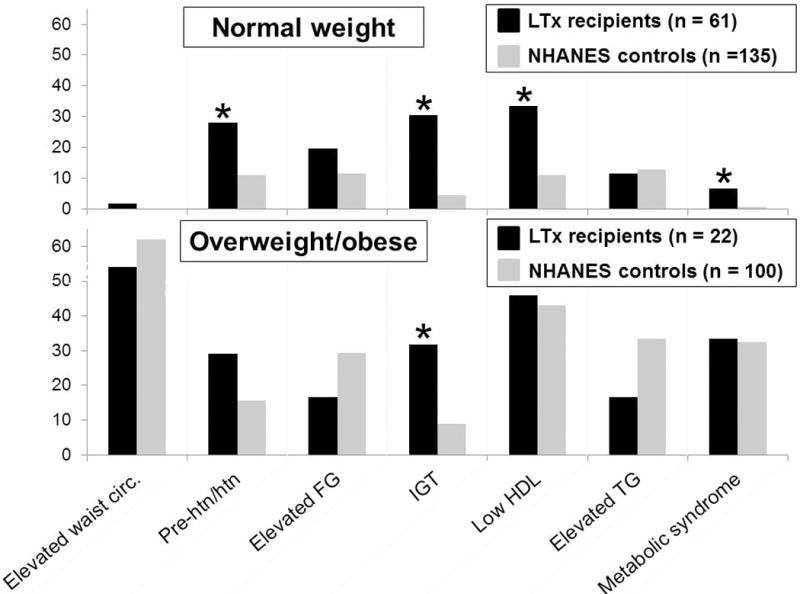
Prevalence of metabolic syndrome and its components in pediatric LT recipients and matched NHANES controls, by weight status. Of note, in both the normal weight and overweight/obese groups, there were not statistically significant differences between LT recipients and NHANES controls in gender, age, or race/ethnicity distribution. (*indicates statistically significant difference, p<0.05). LT, liver transplant; NHANES, National Health and Nutrition Examination Survey.
Among all overweight/obese subjects, a larger proportion of LT recipients than NHANES controls had IGT (30% vs. 9%, p=0.01), again without a significant difference in elevated fasting glucose prevalence (18% vs. 29%, p=0.30). The prevalence of metabolic syndrome was similar among LT and NHANES controls (36% vs 32%) (p=0.76). (FIGURE 1)
In both the normal weight and overweight/obese groups, LT recipients had a substantially lower prevalence than NHANES controls of both elevated total cholesterol (normal weight: 2% vs. 20%, p=0.001; overweight/obese: 5% vs. 24%, p=0.04) and elevated LDL (normal weight: 2% vs. 11%, p=0.03; overweight/obese: 5% vs. 18%, p=0.12).
Among the 50 LT recipients with AST and ALT ≤ 40 IU/L and their matched controls, normal weight LT recipients (n=34) still had a higher prevalence of IGT than normal weight controls (n=78) (26% vs. 3%, p<0.001) and low HDL (29% vs. 10%, p=0.01) with no significant differences in prevalence of pre-hypertension/hypertension (11% vs. 9%, p=0.68) and metabolic syndrome (6% vs. 1%, p=0.18). Overweight/obese LT recipients (n=15) had no significantly elevated prevalence of IGT (23% vs. 10%, p=0.21) or metabolic syndrome (27% vs. 34%, p=0.57) compared to overweight/obese controls (n=67).
Glucose tolerance profiles
LT recipients with IGT had higher 2-hour glucose levels than the controls with IGT. However, LT recipients with IGT had a lower prevalence of overweight/obesity (p=0.06) and lower fasting glucose, insulin, HOMA-IR, and Hemoglobin A1c than controls with IGT. (TABLE 3) Among all subjects with normal glucose tolerance, LT recipients still had significantly higher 2-hour glucose levels and hemoglobin A1c with no difference in fasting parameters. (TABLE 3)
Table 3.
Differences in glucose tolerance profile, LT vs. NHANES controls*
| Impaired glucose tolerance | Normal glucose tolerance | |||||
|---|---|---|---|---|---|---|
| LT recipients (n=23) |
NHANES controls (n=15) |
p | LT recipients (n=53) |
NHANES controls (n=220) |
p | |
| Female | 57% | 53% | 0.85 | 40% | 44% | 0.28 |
| Age (years) | 16.6 (13.2–20.2) | 14.0 (12.0–19.0) | 0.20 | 15.4 (10.6–18.2) | 16.0 (13.0–19.0) | 0.03 |
| Overweight or obese | 26% | 60% | 0.04 | 26% | 40% | 0.05 |
| Fasting glucose (mg/dL) | 91 (87–94) | 102 (100–108) | 0.003 | 92 (87–98) | 93 (88–97) | 0.79 |
| 2-hour glucose (mg/dL) | 156 (147–173) | 147 (140–166) | 0.09 | 114 (99–122) | 96 (81–109) | <0.001 |
| Fasting insulin (mU/mL) | 10.60 (7.00–13.60) | 21.04 (11.01–29.54) | 0.03 | 10.30 (7.60–15.90) | 11.72 (7.63–17.39) | 0.74 |
| HOMA-IR | 2.25 (1.56–3.59) | 5.25 (2.72–8.46) | 0.02 | 2.40 (1.68–3.67) | 2.67 (1.71–4.19) | 0.77 |
| HOMA-%B | 136 (105–253) | 201 (115–242) | 0.42 | 131 (105–205) | 138 (96–208) | 0.89 |
| Hemoglobin A1c | 4.9 (4.8–5.2) | 5.3 (5.1–5.5) | <0.001 | 4.9 (4.5–5.2) | 5.3 (5.1–5.5) | <0.001 |
Data represents median (interquartile range) or proportion, with p by Kruskal-Wallis or chi-squared testing, respectively.
Nine NHANES controls but no LT recipients had a hemoglobin A1c suggestive of pre-diabetes (≥ 5.7%, p=0.07). (11) Only two of 23 LT recipients with IGT also had high fasting glucose; 12 of 15 controls with IGT had high fasting glucose.
Risk factors for IGT and metabolic syndrome components among LT recipients
Among all LT recipients, IGT was more prevalent in those on glucocorticoids (3/4; 75%) but was also quite common in those not taking glucocorticoids (20/72; 28%, p=0.05). The odds of IGT doubled for every 7.5 years on CNIs after adjusting for overweight/obesity and glucocorticoid use (OR 2.10, 95% CI 1.06–4.17 per 7.5 years on CNIs, p=0.03).
Neither IGT nor elevated fasting glucose were associated with age, obesity, CNI type, Latino ethnicity, obesity, Tanner stage, transplant indication, or family history of diabetes in univariate analysis or after adjusting for current glucocorticoid use (data not shown). Elevated fasting glucose was not significantly associated with glucocorticoid use, years of immunosuppression, or years since transplant (data not shown).
The odds of systolic hypertension also increased for every 7.5 years on CNIs in adjusted analysis, although this did not reach statistical significance Years of CNI exposure was not significantly associated with systolic pre-hypertension/hypertension (OR 1.90, 95% CI 0.95–3.79, p=0.07), diastolic pre-hypertension/hypertension, low HDL, elevated triglycerides, or metabolic syndrome in the LT cohort (data not shown).
Of LT recipients on glucocorticoids at study visit (n=4), 2 were overweight/obese: three had IGT; one fulfilled criteria for metabolic syndrome while the other had two components (IGT + low HDL). Of the 2 normal weight recipients on glucocorticoids, one had metabolic syndrome with systolic hypertension, IGT, and low HDL; the other was hypertensive.
We had limited ability to study the impact of CNI type or being off CNIs due to small subgroup size. Children on tacrolimus were fewer years post-transplant at study visit than those on cyclosporine or off CNIs, although there was no difference in age at visit. (TABLE 4) None of the children off CNIs had hypertension, and prevalence in those on tacrolimus was lower than those on cyclosporine. There were no significant differences in elevated fasting glucose, IGT, low HDL or high triglycerides by CNI. (TABLE 4)
Table 4.
CNI impact on prevalence of metabolic syndrome components in LT recipients*
| Tacrolimus (n=67) |
Cyclosporine (n=8) |
Off CNIs (n=8) |
p | |
|---|---|---|---|---|
| Age at visit | 15.6 (11.5–18.3) | 18.1 (12.2–20.9) | 17.6 (13.8–18.3) | 0.46 |
| Years since transplant | 10.6 (5.2–14.6) | 17.1 (11.7–19.1) | 16.1 (11.3–17.7) | 0.004 |
| Overweight/Obese | 27% | 13% | 38% | 0.52 |
| Elevated waist circumference | 16% | 13% | 14% | 0.95 |
| Systolic pre-hypertension or hypertension | 24% | 50% | 0 | 0.07 |
| Diastolic pre-hypertension or hypertension | 12% | 25% | 0 | 0.1 |
| Elevated fasting glucose (≥100mg/dL) | 19% | 25% | 13% | 0.82 |
| IGT (2-hour glucose ≥ 140mg/dL) | 31% | 38% | 17% | 0.69 |
| Low HDL | 42% | 25% | 13% | 0.19 |
| Elevated triglycerides | 13% | 13% | 13% | 0.99 |
| Elevated LDL | 3% | 0 | 0 | 0.78 |
| Elevated total cholesterol | 3% | 0 | 0 | 0.78 |
| Metabolic syndrome | 15% | 13% | 13% | 0.97 |
Data represents median (interquartile range) or proportion, with p by Kruskal-Wallis or chi-squared testing, respectively.
Discussion
The prevalence of hypertension, low HDL, and IGT was significantly higher in pediatric LT recipients than matched peers. Increased risk of these conditions persisted after adjusting for overweight/obesity and glucocorticoid use. Our study is the first to compare metabolic syndrome in pediatric LT recipients to matched controls. Previous, retrospective studies have also identified hypertension, impaired fasting glucose, and low HDL as relatively common after pediatric LT, with an overall metabolic syndrome prevalence of 14–19%. (3,18) Our study verifies these findings and highlights the differences in metabolic syndrome risk between pediatric LT recipients and non-transplanted peers.
Overweight/obese LT recipients were more likely to have IGT but had a similar prevalence of metabolic syndrome components as overweight/obese NHANES controls. Normal weight LT recipients had a significantly higher risk of metabolic syndrome and its components than normal weight NHANES controls. The relatively high prevalence of metabolic syndrome and its components in normal weight LT recipients has been reported previously in adult cohorts, but our study is the first to investigate this phenomenon in pediatrics. (19, 20)
The most novel finding in our study is the high prevalence of IGT in pediatric LT recipients. IGT is caused by impaired pancreatic β-cell ability to respond to a glucose challenge and/or insulin resistance. IGT is considered a pre-diabetic state, and is a predictor of future diabetes even in those with normal fasting glucose. (11,21) LT recipients with IGT, compared to NHANES controls with IGT, had lower fasting insulin, less insulin resistance and reduced β-cell function. Thus, β-cell dysfunction may explain the increased prevalence of IGT in LT recipients.
IGT was associated with years of CNI use, and was not entirely explained by obesity or glucocorticoid use. We hypothesize that long-term exposure to CNIs contributes to IGT in LT recipients by impairing β-cell function. Tacrolimus is thought to cause glucose intolerance by impairing insulin secretion (22) and possibly inducing pancreatic β-cell apoptosis. (23) Cyclosporine seems to be less diabetogenic than tacrolimus but may still impair insulin secretion. (24) Reduced insulin secretion has been observed more consistently with long-term CNI use. (25,26) The increased risk of IGT with increasing years on CNIs in our cohort is consistent with this. A recent Finnish study reported less glucose intolerance (7% IGT, 14% elevated fasting glucose) than we observed, but the majority of their cohort was on cyclosporine. (3)
Also of clinical significance, fasting glucose did not correlate with IGT; no LT recipients had elevated HbA1c. Thus, fasting parameters and HbA1c may not be sensitive indicators of IGT after pediatric LT. Previous pediatric studies have shown HbA1c to be a less sensitive marker of glucose tolerance in children and adolescents than in adults, and appropriate cutoffs for children are not known. (27,28)
CNI exposure was also associated with systolic hypertension in LT recipients, which was expected based on their known direct nephrotoxicity, enhanced sodium reabsorption, and systemic vasoconstriction. (29) The high prevalence of low HDL seen in our cohort has been observed in other cohorts of pediatric LT recipients; (3, 9) this will be an important topic for future study, as low HDL in children is associated with poor VLDL and triglyceride clearance. The apparent protective effect of LT against other dyslipidemias has been reported in pediatric cohorts (3, 18) but not in adults. This finding again argues for further study of mechanism and allowance for screening protocols tailored to the metabolic profile of pediatric LT recipients.
The American Academy of Pediatrics categorizes pediatric renal and heart transplant recipients as high-risk for hypertension, dyslipidemia, and diabetes; they consequently recommend different screening and management guidelines for these children. (8) Our analyses suggest that pediatric LT recipients are also at increased risk for hypertension, low HDL and pre-diabetes, although they seem to be at lower risk for other dyslipidemias. Recent AASLD/AST guidelines support annual blood sugar and lipid monitoring with fasting blood samples for pediatric LT recipients. (30)
Our data suggests that oral glucose tolerance testing may be required to detect pre-diabetes after pediatric LT and supports dyslipidemia screening. However, longitudinal research in larger cohorts is needed before evidence-based recommendations on the utility of routine glucose tolerance testing in this population can be made. Study of prospective cohorts is needed to define risk factors for IGT in these children, to evaluate rate and risk factors for progression from the early IGT we identified, and to examine whether CNI minimization or other interventions may help prevent IGT, hypertension, and other metabolic syndrome components.
The limitations of our study include the single-center cohort of LT recipients studied at varying ages and times since transplant and the lack of concurrent, single-center controls. We matched multiple controls to each LT recipient to optimize statistical power and used NHANES protocols to screen the LT cohort to minimize bias in comparison to controls. Another limitation was lack of specific data in NHANES, forcing the use of 12 year old controls for 8–10 year old LT recipients. Sensitivity analysis, however, demonstrated that this modification did not bias our results. Our cohort was too small to fully investigate the impact of CNI type or being off CNIs. Larger, multi-center studies to allow more detailed investigation of risk factors will be needed.
Our data supports routine screening for metabolic syndrome components in pediatric LT recipients during long-term follow-up, even when they are normal weight and not on maintenance glucocorticoids. Further research is needed to define appropriate screening schedules, to understand what drives the development of these conditions in pediatric LT recipients and to optimize long-term management. As these conditions are well-established precursors to clinical morbidities like diabetes and cardiovascular events and are eminently treatable, early detection and optimized management may prevent long-term morbidity and mortality.
Acknowledgments
This work has been supported by NIH-NIDDK K23 DK0990253-A101 (Dr. Perito), the American Gastroenterological Association Emmet B. Keeffe Career Development Award in Liver Disease (Dr. Perito), UCSF Liver Center Pilot Funding (Dr. Perito, P30 DK026743), UCSF and by the NIH-National Center for Advancing Translational Sciences (UCSF-CTSI Grant UL1 TR000004). The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the NIH, AGA, or other agencies.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BMI
Body mass index
- CDC
Centers for Disease Control
- CNI
Calcineurin inhibitor
- FG
Fasting glucose
- GGT
Gamma-glutamyl transpeptidase
- HOMA-%B
Homeostatic model assessment – Percent beta-cell function
- HOMA-IR
Homeostatic model assessment – Insulin Resistance
- IGT
Impaired glucose tolerance
- IQR
Interquartile range
- LT
Liver transplant
- NHANES
National Health and Nutrition Examination Survey
- OGTT
Oral glucose tolerance test
- OR
Odds ratio
- TG
Triglycerides
Footnotes
Disclosure: The authors have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Ng VL, Alonso EM, Bucuvalas JC, Cohen G, Limbers CA, Varni JW, et al. Health Status of Children Alive 10 Years after Pediatric Liver Transplantation Performed in the US and Canada: Report of the Studies of Pediatric Liver Transplantation Experience. J Pediatr. 2011 Dec 20; doi: 10.1016/j.jpeds.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laish I, Braun M, Mor E, Sulkes J, Harif Y, Ben Ari Z. Metabolic syndrome in liver transplant recipients: prevalence, risk factors, and association with cardiovascular events. Liver Transpl. 2011 Jan;17(1):15–22. doi: 10.1002/lt.22198. [DOI] [PubMed] [Google Scholar]
- 3.Kosola S, Lampela H, Makisalo H, Lohi J, Arola J, Jalanko H, et al. Metabolic syndrome after pediatric liver transplantation. Liver Transpl. 2014 Jun 13; doi: 10.1002/lt.23931. [DOI] [PubMed] [Google Scholar]
- 4.Perito ER, Rosenthal P. Delineating definitions and risk factors for metabolic syndrome after pediatric liver transplantation. Liver Transpl. 2014 Oct;20(10):1280. doi: 10.1002/lt.23946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberger J, Daniels SR, Eckel RH, Hayman L, Lustig RH, McCrindle B, et al. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009 Feb 3;119(4):628–647. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 6.Weiss R, Bremer AA, Lustig RH. What is metabolic syndrome, and why are children getting it? Ann N Y Acad Sci. 2013 Apr;1281:123–140. doi: 10.1111/nyas.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009 Oct 20;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 8.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011 Dec;128(Suppl 5):S213–56. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baskar S, George PL, Eghtesad B, Radhakrishnan K, Hupertz V, Aziz PF, et al. Cardiovascular risk factors and cardiac disorders in long-term survivors of pediatric liver transplantation. Pediatr Transplant. 2015 Feb;19(1):48–55. doi: 10.1111/petr.12388. [DOI] [PubMed] [Google Scholar]
- 10.Perito ER, Lau1 A, Rhee S, Roberts JP, Rosenthal P. Post-transplant metabolic syndrome in children and adolescents after liver transplant: a systematic review. Liver Transpl. 2012 May 29; doi: 10.1002/lt.23478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012 Jan;35(Suppl 1):S64–71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002 May;11(246):1–190. (246) [PubMed] [Google Scholar]
- 13.Barlow SE, Expert Committee Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007 Dec;120(Suppl 4):S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 14.Cook S, Auinger P, Huang TT. Growth curves for cardio-metabolic risk factors in children and adolescents. J Pediatr. 2009 Sep;155(3):S6.e15–26. doi: 10.1016/j.jpeds.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004 Aug;114(2 Suppl):555–576. 4th Report. [PubMed] [Google Scholar]
- 16.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004 Jun;27(6):1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 17.Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005 Apr;115(4):e500–3. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- 18.Dagher M, Ng VL, Carpenter A, De Angelis M, Avitzur Y, Mouzaki M. Overweight, central obesity, and cardiometabolic risk factors in pediatric liver transplantation. Pediatr Transplant. 2015 Mar;19(2):175–81. doi: 10.1111/petr.12425. [DOI] [PubMed] [Google Scholar]
- 19.Laish I, Braun M, Mor E, Sulkes J, Harif Y, Ben Ari Z. Metabolic syndrome in liver transplant recipients: Prevalence, risk factors, and association with cardiovascular events. Liver Transpl. 2011;17(1):15–22. doi: 10.1002/lt.22198. [DOI] [PubMed] [Google Scholar]
- 20.Laryea M, Watt KD, Molinari M, et al. Metabolic syndrome in liver transplant recipients: Prevalence and association with major vascular events. Liver Transpl. 2007;13(8):1109–1114. doi: 10.1002/lt.21126. [DOI] [PubMed] [Google Scholar]
- 21.Kanat M, Mari A, Norton L, Winnier D, DeFronzo RA, Jenkinson C, et al. Distinct beta-cell defects in impaired fasting glucose and impaired glucose tolerance. Diabetes. 2012 Feb;61(2):447–453. doi: 10.2337/db11-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Hooff JP, Christiaans MH, van Duijnhoven EM. Evaluating mechanisms of post-transplant diabetes mellitus. Nephrol Dial Transplant. 2004 Dec;19(Suppl 6):vi8–vi12. doi: 10.1093/ndt/gfh1063. [DOI] [PubMed] [Google Scholar]
- 23.Ozbay LA, Smidt K, Mortensen DM, Carstens J, Jorgensen KA, Rungby J. Cyclosporin and tacrolimus impair insulin secretion and transcriptional regulation in INS-1E beta-cells. Br J Pharmacol. 2011 Jan;162(1):136–146. doi: 10.1111/j.1476-5381.2010.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heisel O, Heisel R, Balshaw R, Keown P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant. 2004 Apr;4(4):583–595. doi: 10.1046/j.1600-6143.2003.00372.x. [DOI] [PubMed] [Google Scholar]
- 25.Duijnhoven EM, Boots JM, Christiaans MH, Wolffenbuttel BH, Van Hooff JP. Influence of tacrolimus on glucose metabolism before and after renal transplantation: a prospective study. J Am Soc Nephrol. 2001 Mar;12(3):583–588. doi: 10.1681/ASN.V123583. [DOI] [PubMed] [Google Scholar]
- 26.Ozbay LA, Moller N, Juhl C, Bjerre M, Carstens J, Rungby J, et al. Calcineurin inhibitors acutely improve insulin sensitivity without affecting insulin secretion in healthy human volunteers. Br J Clin Pharmacol. 2012 Apr;73(4):536–545. doi: 10.1111/j.1365-2125.2011.04118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JM, Wu EL, Tarini B, Herman WH, Yoon E. Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr. 2011 Jun;158(6):947–952. e1–3. doi: 10.1016/j.jpeds.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowicka P, Santoro N, Liu H, Lartaud D, Shaw MM, Goldberg R, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care. 2011 Jun;34(6):1306–1311. doi: 10.2337/dc10-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watt KD. Metabolic syndrome. Is immunosuppression to blame? Liver Transpl. 2011 Jul 14; doi: 10.1002/lt.22386. [DOI] [PubMed] [Google Scholar]
- 30.Kelly DA, Bucuvalas JC, Alonso EM, Karpen SJ, Allen U, Green M, et al. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013 Aug;19(8):798–825. doi: 10.1002/lt.23697. [DOI] [PubMed] [Google Scholar]


