Abstract
The aim of the study is to compare the efficacy of SPIO as a tracer in sentinel node biopsy (SNB) in breast cancer with Tc and patent blue in a multicentre prospective study and perform a meta-analysis of all published studies. It also aims to follow skin discoloration after SPIO injection and describe when and how it resolves. Totally 206 patients with early breast cancer were recruited. Tc and patent blue were administered in standard fashion. Patients were injected with SPIO (Sienna+) preoperatively. SNB was performed and detection rates were recorded for both methods. Skin discoloration was followed and documented postoperatively. Data extraction and subsequent meta-analysis of all previous studies were also performed. SN detection rates were similar between standard technique succeeded and SPIO both per patient (97.1 vs. 97.6 %, p = 0.76) as well as per node (91.3 vs. 93.3 %, p = 0.34), something which was not affected by the presence of malignancy. Concordance rates were also consistently high (98.0 % per patient and 95.9 % per node). Discoloring was present in 35.5 % of patients postoperatively, almost exclusively in breast conservation. It fades slowly and is still detectable in 8.6 % of patients after 15 months. Meta-analysis depicted similar detection rates (p = 0.71) and concordance rates (p = 0.82) per patient. However, it seems that SPIO is characterized by higher nodal retrieval (p < 0.001). SPIO is an effective method for the detection of SN in patients with breast cancer. It is comparable to the standard technique and seems to simplify logistics. Potential skin discoloration is something of consideration in patients planned for breast conservation.
Keywords: Sentinel node, Super paramagnetic iron oxide, Breast cancer
Introduction
Sentinel node biopsy (SNB) currently constitutes the standard of care for staging of the clinically and radiologically negative axilla in breast cancer surgery [1, 2]. Nowadays, it is accepted as the sole surgical procedure in the axilla when the sentinel node (SN) is proven negative or, in some cases even in the presence of metastases [3]. Additionally, neoadjuvant treatment for breast cancer has further widened the indications of SNB [4].
Identification of the SN is based on the use of a radioactive tracer, most often in combination with a vital dye injected interstitially, either close to the areola or around the tumor. The combination of these two methods has been established as the gold standard for the detection of the SN. Their simultaneous use has been clearly shown to increase the detection rate in figures up to 96–97 %, as depicted in the ALMANAC and AMAROS trials [1, 5].
There are, however, some drawbacks. The use of an isotope demands a nuclear medicine department. The short half-life (6 h) of Technetium 99 m (Tc) limits its usefulness and also confers possible hazards to the patient and staff. Moreover, complicated legislation and restrictions regulate the handling and disposal of radioactive material. All these limit the access to the method. Interestingly, recent literature reports that only 60 % of patients in developed countries have access to the procedure, with this figure dropping down to 5 % in China and even lower in the rest of the world [6]. The vital dye is allergenic, and has been associated with a few serious events [7, 8]. These facts stress the need for non-radioactive and non-allergenic tracers with an ability to detect the SN comparable to the traditional methods.
Superparamagnetic iron oxide nanoparticles (SPIO) have been used in the past as contrast agents for magnetic resonance imaging (MRI). Sienna+ is a brown solution containing dextran-coated SPIO particles with a particle size of 59 nm. After injection in the breast, Sienna+ drains through the lymphatics and accumulates in the SN. A handheld probe, SentiMag, is used to identify the SN, in the same way as the gamma probe is used for detection of isotope-containing nodes. The solution is dark brown, and the nodes are often colored, which can help the surgeon identify the SN visually as well. The nodes marked as sentinels can be visualized with MRI [9]. The method has been compared to the standard technique with results verifying non-inferiority [10–16]. However, the tracer has been noted to have a tendency to discolor the skin at the injection site in the breast, similar to what is found after injection of blue dye [12–16].
The aims of the present study were to compare detection rate with SPIO versus conventional technique, to describe the frequency and duration of discoloration, and to perform a meta-analysis of published data on detection and concordance between the different techniques.
Materials and methods
Study design
The Nordic SentiMag study is an international prospective multicenter trial, recruiting patients in 5 Swedish and 2 Danish hospitals with experience in SNB. All participating surgeons were trained in the methodology of the study and the function of the device and the surgical technique was thereafter standardized.
Patient selection
Patients were eligible for recruitment if they were older than 18 years, diagnosed with breast cancer or ductal carcinoma in situ, with clinically and ultrasonographically negative axilla, and scheduled for SNB. Exclusion criteria were hypersensitivity to dextran compounds, iron or Sienna+ , isotope intolerance, iron overload disease, pregnancy, pacemaker or other implantable metallic devices close to the axilla, or mental condition rendering the patient incapable of giving informed consent. All patients had to be available for postoperative follow-up. Appropriate candidates were identified from case presentations in the multidisciplinary rounds. All patients received oral and written information and signed informed consent. The ethics committee in Uppsala University in Sweden and Videnskabsetiske Komité, Region Midt in Denmark approved the study.
Operative protocol
Patients were injected with the radioactive tracer (99mTc), usually 40–60 mBq, either on the day of surgery or the day before. Injection could be made subareolarly, subdermally, or subcutaneously above the tumor according to local standards. Lymphoscintigraphy was not performed routinely. A vital blue dye (1–2 ml of Patent Blue V®) was injected in standard fashion after the onset of anesthesia. Two ml of Sienna+ diluted with 3 ml saline was injected subareolarly either shortly before or after induction of anesthesia. The injection site was massaged for 5 min, and the operation was not to start until at least 20 min had elapsed.
During operation, a handheld probe (SentiMag®, Endomagnetics Ltd, UK) was first used to detect magnetic uptake. A short incision was made in the axilla over the area with the greatest uptake, and the sentinel node was sought for primarily using the SentiMag probe. Metal retractors and instruments were removed and plastic ones were used at that point. Thereafter the finding was confirmed with the gamma probe and the SN(s) removed. All SNs were excised until the counts were lower than 10 % of the highest count or a maximum of four nodes per patient were removed. Blue and/or brown nodes were also regarded as SNs. Magnetic and gamma counts were registered before the skin incision; in situ and ex vivo in the SNs; and in the remaining axilla.
Patients were followed postoperatively and any discoloration was registered and measured repeatedly.
Pathology
All nodes were examined with hematoxylin–eosin staining. Frozen section was performed according to hospital policy. Immunohistochemistry was used in cases where no metastases were found with hematoxylin–eosin. Additional techniques were used according to the pathologist’s discretion.
Statistical methods: study endpoints
The study assumes a 97 % proportion detected by conventional SNB and SPIO SNB, a limit difference for equivalence of −4 % and the expected difference between the proportions detected under both arms as 0 %. This means that equivalence is accepted if the proportion detected under the SPIO arm is as low as 93 %. Detection rate was additionally tested in a right-sided binominal test with the alternative hypothesis that the proportion of successful SNBs was greater than 93 % for each tracer. Prospective sample size for a paired test with a 0.05 one-sided significance level and 80 % power to reject the null hypothesis was 214 cases.
The primary endpoint of the study was the proportion of successful SNBs (detection rate per case) with either the standard (radioisotope ± blue dye) or the magnetic technique (SPIO). Secondary endpoints included the proportion of SN detected (nodal detection rate) as well as the proportion of pathologically positive results (malignancy rate) per case and per node with either technique. Moreover, the concordance and reverse concordance for successful detections (per patient and per node, overall and in terms of malignancy) are compared. Concordance is defined as the number of both standard (radioisotope and blue dye or radioisotope alone) and SPIO-positive patients or nodes, divided by the number of patients or nodes marked by the standard method (standard+ and SPIO+/standard+). Reverse concordance is defined as the number of both standard- and SPIO- positive patients or nodes, divided by the number of patients or nodes marked by the SPIO tracer (standard+ and SPIO+/SPIO+). Only tumor positive patients and nodes are included in the respective malignancy concordance rates. Additionally, the false negatives of the method and the overlap between the conventional and the magnetic technique were addressed. Finally, skin discoloration and concomitant transcutaneous magnetic activity were followed and registered.
For all data, a 95 % Bayes confidence interval (95 %CI) was calculated on the basis of binominal distribution. Means (95 % Confidence Intervals, 95 %CI) or medians (interquartile range, IQR) are presented as appropriate after Kolmogorov–Smirnov and Shapiro–Wilk tests of normality are performed. Rates are presented with 95 % CI. A p value of <0.05 will indicate that the null hypothesis was rejected. Values were calculated using SPSS (V 22.0. Armonk, NY: IBM Corp.).
While the Nordic study was undertaken, several other comparative studies were published. A meta-analysis was undertaken to extract definitive results. Study selection and data extraction was performed independently by two authors (Fig. 1). Endpoints from the data extraction and calculation included detection rates per case and per node, including malignancy, where available. Heterogeneity among studies was assessed (I2). Dichotomous data were analyzed estimating the pooled odds ratio (OR), according the Mantel–Haenszel method. Concordance rates were also recalculated and presented. Rate comparison was performed using the inverse variance method. All studies included in the meta-analysis were evaluated according the revised MINORS criteria [17] by an author which was not involved in the Nordic study (Table 1). Analysis was conducted using the RevMan 5.3 software.
Fig. 1.
PRISMA flow chart for the conduct of the meta-analysis
Table 1.
Evaluation of the studies included in the meta-analysis according to the revised MINORS criteria Scoring
| Douek et al. [10] | Thill et al. [11] | Rubio et al. [12] | Pinero-Madrona et al. [13] | Ghilli et al. [14] | Houpeau et al. [15] | Nordic study | |
|---|---|---|---|---|---|---|---|
| A clearly stated aim | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Inclusion of consecutive patientsa | 1 | 1 | 2 | 2 | 2 | 1 | 2 |
| Prospective collection of data | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Endpoints appropriate to the aim of the study | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Unbiased assessment of the study endpointb | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Follow-up period appropriate to the aim | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Loss to follow up less than 5 %c | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Prospective calculation of the study size | 2 | 1 | 2 | 1 | 2 | 2 | 2 |
| An adequate control group | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Contemporary groups | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Baseline equivalence of groups | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Adequate statistical analyses | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Total | 23 | 22 | 24 | 23 | 24 | 23 | 24 |
Not reported:0, Reported but inadequate:1, Reported and adequate:2
aNo exclusion or details about the reasons for exclusion
bIf not blind, it has to be explained
cIf important for primary endpoint
Results
A total of 206 patients and procedures were included in the study. Mean age was 61.7 years (60.1, 63.4) and median BMI 25.4 (7.1). Mean tumor size was 19.0 mm (16.9, 21.2) with values ranging from 1 to 150 mm. [Blue was used in 127 patients (61.7 %)]. Patient and tumor characteristics are shown in Table 2.
Table 2.
Patient characteristics
| n | % | |
|---|---|---|
| Menopausal status | ||
| Premenopausal | 30 | 14.6 |
| Postmenopausal | 140 | 68.0 |
| Perimenopausal | 6 | 2.9 |
| Not assessed | 30 | 14.6 |
| Type of surgery | ||
| Mastectomy | 52 | 25.2 |
| Breast conserving surgery | 154 | 74.8 |
| pT | ||
| Tis | 10 | 4.9 |
| T1 | 126 | 61.1 |
| T2 | 56 | 27.2 |
| T3 | 7 | 3.4 |
| Not assessed | 7 | 3.4 |
| pN | ||
| N0 | 152 | 73.8 |
| N1mi | 20 | 9.7 |
| N1 | 27 | 13.1 |
| N2 | 6 | 2.9 |
| N3 | 1 | 0.5 |
| Not assessed | 0 | 0.0 |
| Grade | ||
| G1 | 37 | 18.0 |
| G2 | 74 | 35.9 |
| G3 | 32 | 15.5 |
| Not assessed | 62 | 30.1 |
| Ki67 % | ||
| >15 % | 106 | 51.5 |
| <15 % | 60 | 29.1 |
| =15 % | 18 | 8.7 |
| Not assessed | 22 | 10.7 |
The transcutaneous detection with Tc was successful in 202 of 206 patients (98.1 %; 94.8, 99.4), and with Sienna+ only in 189 (91.7 %; 86.9, 95.0), resulting in a 6.4 % difference (p = 0.0036). Correlation analysis showed that the value of the transcutaneous signal in both methods was associated with BMI both for Tc (Spearman’s ρ: −0.167, p < 0.05) and for Sienna+ (Spearman’s ρ: −0.191, p < 0.01). Age, tumor size, time between injection and operation and previous surgery did not correlate with transcutaneous detection.
SN detection with the standard technique succeeded in 200 patients (97.1 %; 93.5, 98.8) and 201 with Sienna+ (97.6 %; 94.1, 99.1), displaying no difference (p = 0.76). Both techniques were successful concomitantly in 196 cases (95.1 %; 91.0, 97.5). Subsequently, per patient concordance was 98.0 % (94.6, 99.3) and reverse concordance was 97.5 % (94, 99.1). Total failure for both techniques occurred in only one patient (0.48 %), who had multiple macrometastases. Metastases were noted in 54 patients (26.2 %; 20.5, 32.9). These were detected by both methods in 52 cases (96.3 %; 86.2, 99.4), Tc in 53 (98.1 %; 88.8, 99.9) and Sienna+ in 52 (96.3 %; 86.2, 99.4) resulting in concordance and reverse concordance per malignant case of 98.1 % (88.8, 99.9) and 100 % (91.3, 100), respectively. Per patient data of the Nordic study and studies included in the meta-analysis are presented in Tables 3 and 4.
Table 3.
Per patient figures from the studies used in the meta-analysis. nd: not defined, na: not applicable
| Study (Ref.) | Total cases | Fail SN | Successful standard technique | Successful SPIO technique | Both techniques successful | Malignant cases | Malignant cases detected by standard technique | Malignant cases detected by SPIO technique | Malignant cases detected by both techniques | Malignant cases in which both techniques failed |
|---|---|---|---|---|---|---|---|---|---|---|
| Douek et al. [10] | 160 | 3 | 152 | 151 | 146 | 39 | nd | nd | nd | nd |
| Thill et al. [11] | 150 | 2 | 146 | 147 | 145 | 34 | 31 | 33 | 31 | 2 |
| Rubio et al. [12] | 120 | 2 | 113 | 116 | 111 | 36 | 33 | 34 | 32 | 1 |
| Pineiro et al. [13] | 181 | 3 | 178 | 177 | 177 | 60 | 53 | 55 | 52 | 4 |
| Ghilli et al. [14] | 197 | nd | 195 | 193 | 187 | 57 | 56 | 55 | 54 | 1 |
| Houpeau et al. [15] | 108 | 2 | 103 | 105 | 102 | 46 | 44 | 45 | 43 | 1 |
| Nordic study | 206 | 1 | 200 | 201 | 196 | 54 | 53 | 52 | 52 | 1 |
Table 4.
Rates per patient from the studies used in the meta-analysis. nd: not defined, na: not applicable
| Study (Ref.) | Fail SLN rate (95 %CI) | Standard technique detection rate (95 %CI) | SPIO detection rate (95 %CI) | Rate difference [p-value] | Malignancy rate (95 %CI) | Malignant cases detected by standard technique | Malignant cases detected by SPIO technique | Rate difference for malignant cases [p-value] | Malignant cases detected by both techniques | Malignant cases in which both techniques failed |
|---|---|---|---|---|---|---|---|---|---|---|
| Douek et al. [10] | 1.9 (0.5, 5.8) | 95.0 (90.1, 97.7) | 94.4 (89.3, 97.2) | 0.6 [1] | 24.4 (18.1,31.9) | na | na | na | na | na |
| Thill et al. [11] | 1.3 (0.2, 5.2) | 97.3 (92.9, 99.1) | 98.0 (93.8, 99.5) | 1.0 [1] | 22.7 (16.4,30.4) | 20.7 (14.7, 28.2) | 22.0 (15.8, 29.6) | 1.3 [0.89] | 20.7 (14.7, 28.2) | 1.3 (0.02, 5.2) |
| Rubio et al. [12] | 1.7 (0.3,6.5) | 94.2 (87.9, 97.4) | 96.7 (91.2, 98.9) | 2.5 [0.54] | 30.0 (22.2, 39.2) | 27.5 (19.9, 36.5) | 28.3 (20.7, 37.4) | 0.5 [1] | 26.7 (19.2, 35.7) | 0.8 (0.04, 5.2) |
| Pineiro et al. [13] | 1.7 (0.4, 5.2) | 98.3 (94.8, 99.6) | 97.8 (94.1, 99.3) | 0.5 [1] | 33.2 (26.5, 40.6) | 29.3 (22.9, 36.6) | 30.4 (23.9, 37.7) | 1.1 [0.9] | 28.7 (22.4, 36) | 2.2 (0.7, 5.9) |
| Ghilli et al. [14] | na | 99.0 (95.8, 99.8) | 98.0 (94.5, 99.4) | 1.0 [0.69] | 28.9 (22.8, 36) | 28.4 (22.4, 35.4) | 27.9 (21.9, 34.8) | 0.5 [0.9] | 27.4 (21.4, 34.3) | 0.5 (0.03, 3.2) |
| Hopeau et al. [15] | 1.9 (0.3, 7.2) | 95.4 (89.5, 98.5) | 97.2 (92.1, 99.4) | 1.8 [0.72] | 42.6 (33.2, 52.5) | 40.7 (31.5, 50.6) | 41.7 (32.4, 51.6) | 1.0 [1] | 39.8 (30.6, 49.7) | 0.9 (0.05, 5) |
| Nordic study | 0.5 (0.03, 3.1) | 97.1 (93.5, 98.8) | 97.6 (94.11, 99.10) | 0.5 [1] | 26.2 (20.5, 32.9) | 25.7 (20, 32.4) | 25.2 (19.6, 31.8) | 0.5 [1] | 25.2 (19.6, 31.8) | 0.5 (0.03,3.1) |
The denominator is always the total of patients per study. 95 % CI: 95 % confidence intervals using the Wilson procedure with a correction for continuity. Rate differences are given as|Standard-SPIO|. Fisher’s exact test is performed and 2-tailed p values are given. p values <0.05 are considered significant
A total of 403 SNs were retrieved. Out of these, 353 were detected by both techniques, [87.6 % (83.9, 90.6)]; 368 with the standard technique, [mean 1.79 (1.65, 1.92) and detection rate 91.3 % (88.0, 93.8)]; 376 with Sienna+ , [mean 1.83 (1.69, 1.96) and detection rate 93.3 % (90.3, 95.5)], (p = 0.34). The nodal concordance and reverse concordance were 95.9 % (93.2, 97.6) and 93.9 % (90.8, 96.0), respectively. Out of the total 403 nodes, 68 (16.9 %; 13.4, 21.0) were malignant. Tc detected 63 (92.6 %; 83.0, 97.3) and Sienna+ 62 (91.2 %; 81.1, 96.4), whereas both succeeded simultaneously in 60 nodes (88.2 %; 77.6, 94.4). The concordance and reverse concordance for malignant nodes were 95.2 % (85.6, 98.8) and 96.8 % (87.7, 99.4). Per node data of the Nordic study and studies included in the meta-analysis are presented in Tables 5 and 6.
Table 5.
Per node figures from the studies used in the meta-analysis. nd: not defined, na: not applicable
| Study (Ref.) | Total nodes | Successful standard technique | Successful SPIO technique | Both techniques successful | Both techniques failed | Malignant nodes | Malignant nodes detected by standard technique | Malignant nodes detected by SPIO | Malignant nodes detected by both techniques | Malignant nodes in which both techniques failed |
|---|---|---|---|---|---|---|---|---|---|---|
| Douek et al. [10] | 404 | 297 | 323 | 268 | 52 | nd | nd | nd | nd | nd |
| Thill et al. [11] | 291 | 267 | 283 | 263 | nd | 45 | 41 | 43 | 41 | 4 |
| Rubio et al. [12] | 287 | 230 | 264 | nd | nd | nd | nd | nd | nd | nd |
| Pineiro et al. [13] | 321 | 277 | 292 | 260 | 12 | 76 | 67 | 69 | 65 | 5 |
| Ghilli et al. [14] | 380 | 360 | 364 | 344 | nd | 77 | 72 | 73 | 68 | 5 |
| Hopeau et al. [15] | 214 | 193 | 208 | 188 | 1 | 61 | 54 | 60 | 53 | 1 |
| Nordic study | 403 | 368 | 376 | 353 | 12 | 68 | 63 | 62 | 60 | 2 |
Table 6.
Rates per node from the studies used in the meta-analysis. nd: not defined, na: not applicable
| Study (Ref.) | Fail SLN rate with both techniques (95 %CI) | Standard technique detection rate (95 %CI) | SPIO detection rate (95 %CI) | Rate difference [p-value] | Malignancy rate (95 %CI) | Malignant nodes detected by standard technique | Malignant nodes detected by SPIO technique | Rate difference for malignant nodes [p/value] | Malignant nodes detected by both techniques | Malignant nodes in which both techniques failed |
|---|---|---|---|---|---|---|---|---|---|---|
| Douek et al. [10] | 12.9 (9.8, 16.6) | 73.5 (68.9, 77.7) | 80.0 (75.7, 83.6) | 6.5 [0.03] | na | na | na | na | na | na |
| Thill et al. [11] | na | 91.8 (87.8, 94.5) | 97.3 (94.5, 97.7) | 5.5 [0.1] | 15.5 (11.6, 20.3) | 14.1 (10.4, 18.6) | 14.8 (11, 19.5) | 0.7 [0.9] | 14.1 (10.4, 18.6) | 1.4 (0.4, 3.7) |
| Rubio et al. [12] | na | 80.1 (75, 84.5) | 92 (88, 94.8) | 11.6 [<0.0001] | na | na | na | na | na | nd |
| Pineiro et al. [13] | 3.7 (2, 6.6) | 86.3 (81.9, 89.8) | 91 (87.2, 93.8) | 4.7 [0.08] | 23.8 (19.2, 28.8) | 20.3 (16.1, 25.2) | 21.5 (17.2, 26.5) | 0.6 [0.9] | 20.3 (16.1, 25.2) | 1.6 (0.6, 3.8) |
| Ghilli et al. [14] | nd | 94.7 (91.9, 96.7) | 95.8 (93.1,97.5) | 1.1 [0.6] | 20.3 (16.4, 24.7) | 19 (15.2, 23.3) | 19.2 (15.4, 23.6) | 0.2 [1] | 17.9 (14.2, 22.2) | nd |
| Hopeau et al. [15] | 0.5 (0.03,3.4) | 90.2 (85.2, 93,7) | 97.2 (93.7, 98.9) | 7 [0.005] | 28.5 (22.7, 35.1) | 25.2 (19.7, 31.7) | 28 (22.2, 34.7) | 2.8 [0.58] | 24.8 (19.3, 31.2) | 0.5 (0.03,3.4) |
| Nordic study | 3 (1.6, 5.3) | 91.3 (88,93.8) | 93.3 (90.3, 95.5) | 2 [0.35] | 16.9 (13.4, 21) | 15.6 (12.3, 19.6) | 15.4 (12.1, 19.4) | 0.2 [1] | 13.2 (10.1, 16.9) | 0.5 (0.1, 2) |
The denominator is always the total of nodes per study. 95 % CI: 95 % confidence intervals using the Wilson procedure with a correction for continuity. Rate differences are given as|Standard-SPIO|. Fisher’s exact test and p values are given. p values <0.05 are considered significant
In cases where SNB failed, no risk factors were identified regardless of technique, neither in our material nor in the rest of the studies included in the meta-analysis.
Discoloration
Follow-up data were available in 186 of 206 patients (90.3 %). The initial protocol stated a follow-up visit after 6 months, but since a marked discoloration was found in a considerable amount of women, the follow-up period was extended. Thus, median follow-up was 310 days (IQR 182). Correlation analysis showed that the incidence of discoloring was strongly associated with breast conserving surgery (Kendall tau = −0.416, p < 0.001), since 95.6 % of patients with discoloration had been treated with breast conserving surgery (BCS). Age, BMI, or the incidence of perioperative staining did not correlate (p > 0.5).
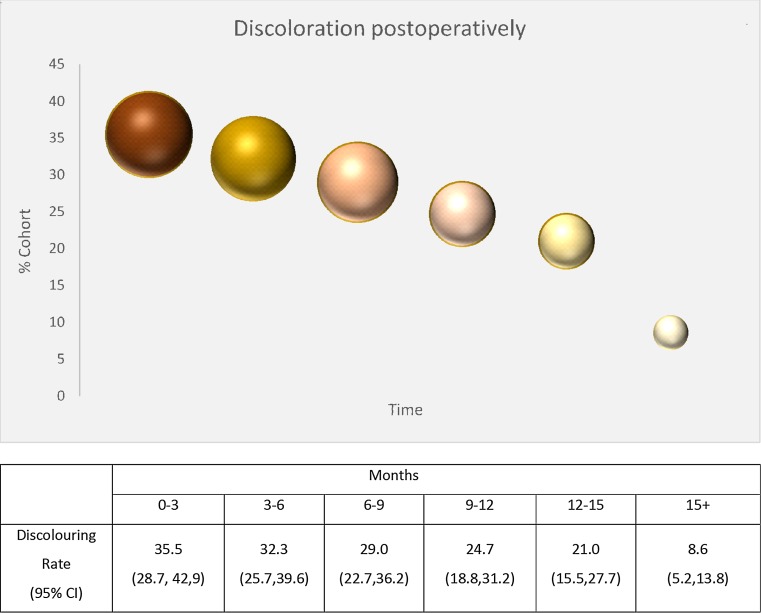
Discoloration was present in 35.5 % of patients postoperatively (0–3 months) and faded progressively in size and color over time to 21 % of patients after a year. Staining remained present in 8.6 % 15 months after the operation, but much smaller and paler. Discoloration for the entire cohort and the BCS group are essentially the same (within 95 %CI). Additionally, the curve demonstrated below in Fig. 2 is identical for both.
Fig. 2.
Discoloration in the follow-up cohort; size and the colour of the spheres represent the medians of the discoloured surface in cm2 in the discoloured proportion of the cohort and the fading, respectively
Finally, all the patients who had discoloration and were checked with the probe transcutaneously presented magnetic activity (positive prognostic value 100 %); however, no correlation of the transcutaneous counts was found with size, intensity, or duration of the skin discoloration.
Meta-analysis
Seven studies were included (ref. 10-15 and our study) with a total of 1118 cases performed and 2300 nodes retrieved. No difference was observed in the detection rates per case in any of the studies (fixed OR 1.10; 0.67, 1.79, p = 0.71) between SPIO and conventional methods (Fig. 3).
Fig. 3.

Forest plot comparing detection rates per patient
However, moderate heterogeneity was present (I2 = 48 %) among the studies as far as nodal detection rate was concerned. Three studies (10,12,15) demonstrated higher detection rate per node with SPIO (Table 5). Subsequently, the random OR was 1.84 (1.37,2.47), resulting in significant difference in favor of SPIO (p < 0.0001) compared to the conventional method, as seen in Fig. 4.
Fig. 4.

Forest plot comparing detection rates per node
As to malignancy rates per case and per node, two studies [10, 12] did not present complete relevant data. The detection rates in cases with a positive SNB were comparable for both methods (fixed OR:1.33; 0.63, 2.81, p = 0.45) (Fig. 5), as well as detection rates per malignant nodes (fixed OR:1.55; 0.86, 2.79, p = 0.14) (Fig. 6).
Fig. 5.
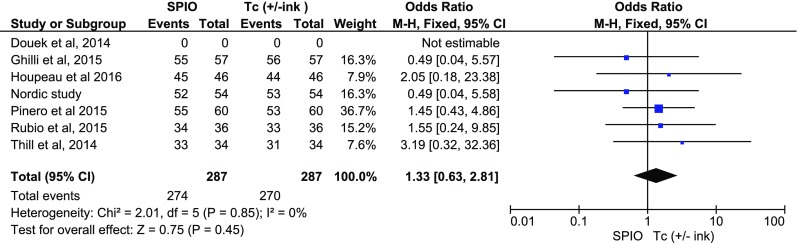
Forest plot comparing detection rates per patient in the presence of malignancy
Fig. 6.
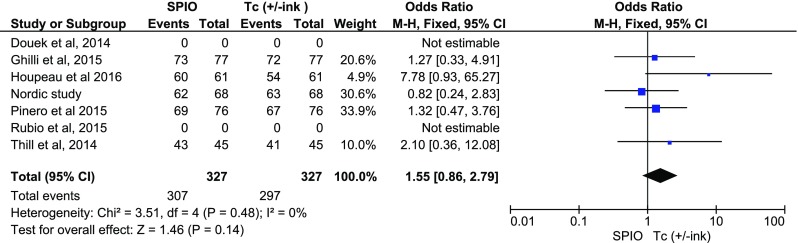
Forest plot comparing detection rates per node in the presence of malignancy
Concordance and reverse concordance rates were recalculated for all included studies according to the definitions (standard+ and Sienna +/standard+) and (standard+ and SPIO +/SPIO+) with 95 %CI. No substantial differences were noted (Table 7).
Table 7.
Concordance and reverse concordance rates per study are presented as linears with 95 %CI
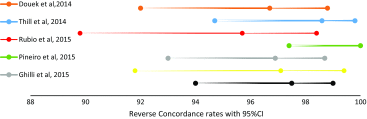
| Study (Ref.) | Concordance (95 % CI) | Reverse concordance (95 % CI) |
|---|---|---|
| Douek et al. [10] | 96.0 (91.2, 98,4) | 96.7 (92, 98.8) |
| Thill et al. [11] | 99.3 (95.7, 99.9) | 98.6 (94.7, 99.8) |
| Rubio et al. [12] | 98.2 (93.1, 99.7) | 95.7 (89.7, 98.4) |
| Pineiro et al. [13] | 99.4 (96.4, 99.9) | 100 (97.4,100) |
| Ghilli et al. [14] | 95.9 (91.8, 98.1) | 96.9 (93, 98.7) |
| Hopeau et al. [15] | 99.0 (93.9, 100) | 97.1 (91.8, 99.4) |
| Nordic study | 98.0 (94.6, 99.4) | 97.5 (94, 99) |

| ||
| ||
Subsequent inverse variance analysis was conducted using “risk difference” defined as |Concordance-Reverse concordance|, depicting similar rates per case among studies (p = 0.82) (Fig. 6). The comparison of concordance rates per node however revealed heterogeneity among the studies (I2 = 54 %), as well as that more SNs are detected with SPIO (p = 0.0003) (Fig. 7). The same comparisons were conducted in the presence of malignancy, without any evidence that imply a difference per case (p = 0.73, Fig. 8) or per node (p = 0.10, Figs. 9–10).
Fig. 7.
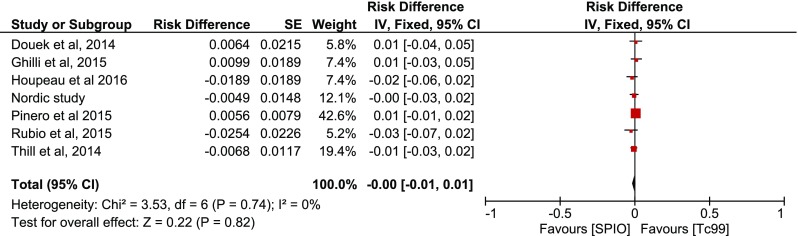
Forest plot comparing concordance versus reverse concordance per patient
Fig. 8.

Forest plot comparing concordance versus reverse concordance per node
Fig. 9.
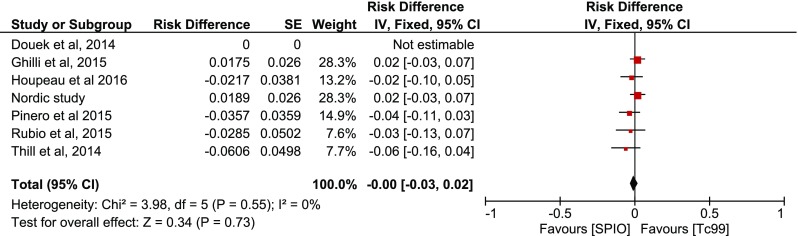
Forest plot comparing concordance versus reverse concordance per patient in the presence of malignancy
Fig. 10.
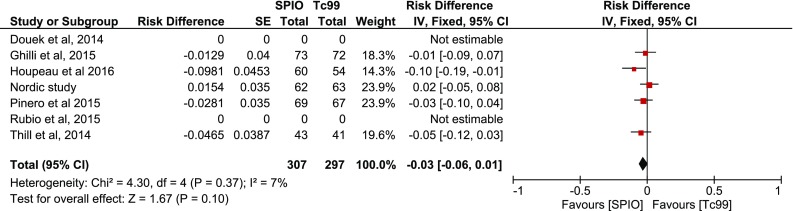
Forest plot comparing concordance versus reverse concordance per node in the presence of malignancy
Discussion
This multicenter study, as well as the meta-analysis performed, confirms that SPIO can be used as a safe alternative to the conventional technique. The use of Tc ± blue dye has been the standard of care in almost 20 years and statistical superiority of any new method is hard to demonstrate mostly in terms of discrepancy in numbers of patients. However, the motivation for the development of new techniques has risen from the drawbacks of the conventional methods. The use of SPIO has been reported before [18]. The current study verifies that detection rates do not differ and the presence of metastases does not affect the effectivity of the method. Constantly high concordance rates between methods also suggest that SPIO identify the “right” SN. One more conclusion from the meta-analysis is the higher nodal rate for SPIO, possibly resulting in the retrieval of more SNs, a finding not confirmed in our study. However, the clinical significance and utility of that remain to be defined.
The current study confirms findings from other groups [13–15] that it can be easier to use without the restrictions in the availability, use, manipulation, and disposal of the isotope [19, 20].
The brownish coloring of the SN when SPIO is used has been commented [10, 12–16] as an aid intraoperatively. We made the same observation, but it was difficult to quantify due to the presence of ink. The utility of an optic tracer is of value, as shown for the blue dye [21]. However, it is a complementary property in the presence of isotope localization [22]. Still, it seems that SPIO may offer the same, with none of the blue dye disadvantages [23–27].
Advantages of the study include the adherence to a distinct protocol and feedback that the technique is easily adopted among surgeons experienced with the SN technique, a conclusion reached by other groups [15, 28]. A weakness of the study is that the simultaneous use of both methods made it difficult to disentangle detection by either method and to define overlapping between the two.
Another observation of our group in agreement with Thill et al. [11] was that lower transcutaneous signals were found in patients with higher BMI which is shown in our results, but, despite the difference between transcutaneous and overall detection rates for SPIO (p < 0.05), it is of no consequence after the skin incision is made. This means that a low transcutaneous signal should not discourage the surgeon.
If SPIO would have been the only option, some nodes might have been more difficult to find, since the probe is a little bigger than the gamma probe, and the operating field was sometimes covered by the probe. This was a common finding in all studies. That remark has been addressed by a slimmer version of the probe. The distortion of the ferromagnetic signal by metallic instruments has also been addressed by the removal of metallic instruments when the probe is to be used, or by the use of plastic instruments [15].
Discoloration in the form of a brown-grayish tattoo has been briefly mentioned previously [12–16]. Rubio et al. [12], reported discoloration in 19 %, which faded progressively after 6 months, comparable to the effect produced by blue dye. Piñero et al. [13] report the pigmentation, whereas Ghilli et al. [14] report an incidence of 40 % at 6 months which is transient in 91 % of pigmented cases. Similar observations regarding staining were made with the use of another type of SPIO, ferucarbotran, in which a skin stain that lasted 2 months and resolved spontaneously thereafter was described [18]. Recent experimental data comparing three different types of SPIO on a porcine model noted skin discoloration as a common fact, but no follow-up was performed [29]. The absence of data motivated follow-up and quantification of this effect. Postoperatively, it was present in 35.5 % of patients and faded slowly over time. In 8.6 %, a pale discoloration was present after 15 months. In most cases, it would shrink and fade; in some others it faded by spreading, like a subcutaneous hematoma. Therefore, it is something that should be kept in mind when informing a patient.
The discoloration found after Sienna + injections implies that the substance remains for a long time in the breast. In a small pilot study [30], our group showed that it remains for at least up to 3 weeks in the SN, and can easily be measured after that time with higher counts transcutaneously as well as in situ and ex vivo. In our trial, this was confirmed since magnetic counts were present in the tissue up to 515 days after the operation. Therefore, injection of Sienna+ could be administered prior to the operation, thus facilitating planning and logistics. Moreover, it implies that a deeper peritumoral injection with subsequent excision of that area, such as that proposed by Ghilli et al. [14], may result in smaller or no discoloration.
Other methods such as the use of near-infrared (NIR) imaging with indocyanine green (ICG) have presented interest. However, disadvantages in the method include the complexity of the procedure and the longer learning curve; the high complexity and cost of the infrared camera; the higher mean (3.41) of nodes retrieved, possibly implying bigger morbidity; the skin pigmentation over a period of time; and the impaired detection in cases of high body mass index [31, 32].
One theoretical disadvantage of SPIOs is the inability to obtain a simple and cheap preoperative localization scan, which is of importance for localization of SN in melanoma patients. However, it is not necessary to perform a preoperative scintigraphy for the detection of SN in patients with breast cancer (5), and most centers have abandoned it. Besides, SPIOs can identify SN in axillary MRI in patients with breast cancer [9] and SPIO injection as a contrast material in MR sentinel lymphography and as a tracer in SN biopsy using an integrated method [33, 34], with excellent imaging quality, in contrast to the poor spatial resolution of scintigraphy. This may be a promising perspective in cases where preoperative localization will still be needed such as in melanomas of the trunk and head and neck, and recurrent breast cancer, where the lymphatic drainage is sometimes distorted.
Upon the completion of this trial, a group from USA analyzed the data from the first five trials [35]. In the current trial, we present two more studies with additional features and comprehensively investigate skin discoloration. The fact that similar statistical conclusions are reached despite minor differences in methodology strengthens the quality of clinical information on this topic.
In conclusion, current research has shown non-inferiority for SPIO over conventional techniques for SN detection. This new method has none of the disadvantages of the standard technique and is promising as a safe and effective alternative in the absence of nuclear medicine facilities.
The SentiMag detection system and Sienna + vials were provided by Sysmex Europe during the study.
Compliance with ethical standards
Conflict of interest
None.
References
- 1.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari A, Rovera F, Dionigi P, Limonta G, Marelli M, Besana Ciani I, Bianchi V, Vanoli C, Dionigi R. Sentinel lymph node biopsy as the new standard of care in the surgical treatment for breast cancer. Expert Rev Anticancer Ther. 2006;6:1503–1515. doi: 10.1586/14737140.6.10.1503. [DOI] [PubMed] [Google Scholar]
- 3.Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, Benson AB, III, Bosserman LD, Burstein HJ, Cody H, III, Hayman J, Perkins CL, Podoloff DA, Giuliano AE, American Society of Clinical Oncology Clinical Practice Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2014;32:1365–1383. doi: 10.1200/JCO.2013.54.1177. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann M, von Minckwitz G, Mamounas EP, Cameron D, Carey LA, Cristofanilli M, Denkert C, Eiermann W, Gnant M, Harris JR, Karn T, Liedtke C, Mauri D, Rouzier R, Ruckhaeberle E, Semiglazov V, Symmans WF, Tutt A, Pusztai L. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19:1508–1516. doi: 10.1245/s10434-011-2108-2. [DOI] [PubMed] [Google Scholar]
- 5.Straver ME, Meijnen P, van Tienhoven G, et al. Sentinel node identification rate and nodal involvement in the EORTC 10981-22023 AMAROS trial. Ann Surg Oncol. 2010;17:1854–1861. doi: 10.1245/s10434-010-0945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed M, Purushotham AD, Douek M. Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol. 2014;15:e351–e362. doi: 10.1016/S1470-2045(13)70590-4. [DOI] [PubMed] [Google Scholar]
- 7.Masannat Y, Shenoy H, Speirs V, Hanby A, Horgan K. Properties and characteristics of the dyes injected to assist axillary sentinel node localization in breast surgery. Eur J Surg Oncol. 2006;32:381–384. doi: 10.1016/j.ejso.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 8.White V, Harvey JR, Griffith CD, Youssef M, Carr M. Sentinel lymph node biopsy in early breast cancer surgery–working with the risks of vital blue dye to reap the benefits. Eur J Surg Oncol. 2011;37:101–108. doi: 10.1016/j.ejso.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Motomura K, Ishitobi M, Komoike Y, Koyama H, Noguchi A, Sumino H, Kumatani Y, Inaji H, Horinouchi T, Nakanishi K. SPIO-enhanced magnetic resonance imaging for the detection of metastases in sentinel nodes localized by computed tomography lymphography in patients with breast cancer. Ann Surg Oncol. 2011;18:3422–3429. doi: 10.1245/s10434-011-1710-7. [DOI] [PubMed] [Google Scholar]
- 10.Douek M, Klaase J, Monypenny I, Kothari A, Zechmeister K, Brown D, Wyld L, Drew P, Garmo H, Agbaje O, Pankhurst Q, Anninga B, Grootendorst M, Ten Haken B, Hall-Craggs MA, Purushotham A, Pinder S, SentiMAG Trialists Group Sentinel node biopsy using a magnetic tracer versus standard technique: the SentiMAG Multicentre Trial. Ann Surg Oncol. 2014;21:1237–1245. doi: 10.1245/s10434-013-3379-6. [DOI] [PubMed] [Google Scholar]
- 11.Thill M, Kurylcio A, Welter R, van Haasteren V, Grosse B, Berclaz G, Polkowski W, Hauser N. The Central-European SentiMag study: sentinel lymph node biopsy with superparamagnetic iron oxide (SPIO) vs. radioisotope. Breast. 2014;23:175–179. doi: 10.1016/j.breast.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Rubio IT, Diaz-Botero S, Esgueva A, Rodriguez R, Cortadellas T, Cordoba O, Espinosa-Bravo M. The superparamagnetic iron oxide is equivalent to the Tc99 radiotracer method for identifying the sentinel lymph node in breast cancer. Eur J Surg Oncol. 2015;41:46–51. doi: 10.1016/j.ejso.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Piñero-Madrona A, Torro-Richart JA, de Leon-Carrillo JM, de Castro-Parga G, Navarro-Cecilia J, Domingez-Cunchillos F, Roman-Santamarıa JM, Fuster-Diana C, Pardo-Garcıa R, on behalf of the “Grupo de Estudios Senologicos de la Sociedad Española de Patologia Mamaria (SESPM). (2015). Superparamagnetic iron oxide as a tracer for sentinel node biopsy in breast cancer: A comparative non-inferiority study. Eur J Surg Oncol 41: 991–997. doi: 10.1016/j.ejso.2015.04.017 [DOI] [PubMed]
- 14.Ghilli M, Carretta E, Di Filippo F, Battaglia C, Fustaino L, Galanou I, Di Filippo S, Rucci P, Fantini MP, Roncella M. The superparamagnetic iron oxide tracer: a valid alternative in sentinel node biopsy for breast cancer treatment. European Journal of Cancer Care. 2015 doi: 10.1111/ecc.12385. [DOI] [PubMed] [Google Scholar]
- 15.Houpeau JL, Chauvet MP, Guillemin F, Bendavid-Athias C, Charitansky H, Kramar A, Giard S. Sentinel lymph node identification using superparamagnetic iron oxide particles versus radioisotope: the French Sentimag feasibility trial. J Surg Oncol. 2016 doi: 10.1002/jso.24164. [DOI] [PubMed] [Google Scholar]
- 16.Coufal O, Fait V, Lžičařová E, Chrenko V, Žaloudík J. SentiMag–the magnetic detection system of sentinel lymph nodes in breast cancer. Rozhl Chir. 2015;94:283–288. [PubMed] [Google Scholar]
- 17.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological Index for Non-randomized Studies (MINORS): development and validation of a new instrument. ANJ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 18.Shiozawa M, Lefor AT, Hozumi Y, Kurihara K, Sata N, Yasuda Y, Kusakabe M. Sentinel lymph node biopsy in patients with breast cancer using superparamagnetic iron oxide and a magnetometer. Breast Cancer. 2013;20:223–229. doi: 10.1007/s12282-011-0327-9. [DOI] [PubMed] [Google Scholar]
- 19.Pouw JJ, Ahmed M, Anninga B, Schuurman K, Pinder SE, Van Hemelrijck M, Pankhurst QA, Douek M, Ten Haken B. Comparison of three magnetic nanoparticle tracers for sentinel lymph node biopsy in an in vivo porcine model. Int J Nanomedicine. 2015;10:1235–1243. doi: 10.2147/IJN.S76962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rescigno J, Zampell JC, Axelrod D. Patterns of axillary surgical care for breast cancer in the era of sentinel lymph node biopsy. Ann Surg Oncol. 2009;16:687–696. doi: 10.1245/s10434-008-0195-5. [DOI] [PubMed] [Google Scholar]
- 21.Leong SP, Shen ZZ, Liu TJ, et al. Is breast cancer the same disease in Asian and Western countries? World J Surg. 2010;34:2308–2324. doi: 10.1007/s00268-010-0683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ang CH, Tan MY, Teo C, Seah DW, Chen JC, Chan MY, Tan EY. Blue dye is sufficient for sentinel lymph node biopsy in breast cancer. Br J Surg. 2014;101:383–389. doi: 10.1002/bjs.9390. [DOI] [PubMed] [Google Scholar]
- 23.OʼReilly EA, Prichard RS, Al Azawi D, Aucharaz N, Kelly G, Evoy D, Geraghty J, Rothwell J, OʼDoherty A, Quinn C, Skehan SJ, McDermott EW. The value of isosulfan blue dye in addition to isotope scanning in the identification of the sentinel lymph node in breast cancer patients with a positive lymphoscintigraphy: a randomized controlled trial (ISRCTN98849733) Ann Surg. 2015;262:243–248. doi: 10.1097/SLA.0000000000001213. [DOI] [PubMed] [Google Scholar]
- 24.Cimmino VM, Brown AC, Szocik JF, Pass HA, Moline S, De SK, Domino EF. Allergic reactions to isosulfan blue during sentinel node biopsy–a common event. Surgery. 2001;130:439–442. doi: 10.1067/msy.2001.116407. [DOI] [PubMed] [Google Scholar]
- 25.Raut CP, Daley MD, Hunt KK, Akins J, Ross MI, Singletary SE, Marshall GD, Jr, Meric-Bernstam F, Babiera G, Feig BW, Ames FC, Kuerer HM. Anaphylactoid reactions to isosulfan blue dye during breast cancer lymphatic mapping in patients given preoperative prophylaxis. J Clin Oncol. 2004;22(3):567–568. doi: 10.1200/JCO.2004.99.276. [DOI] [PubMed] [Google Scholar]
- 26.Govaert GA, Oostenbroek RJ, Plaisier PW. Prolonged skin staining after intradermal use of patent blue in sentinel lymph node biopsy for breast cancer. Eur J Surg Oncol. 2005;31:373–375. doi: 10.1016/j.ejso.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Olliver JR, Wild CP, Sahay P, Dexter S, Hardie LJ. Chromoendoscopy with methylene blue and associated DNA damage in Barrett’s oesophagus. Lancet. 2003;362(9381):373–374. doi: 10.1016/S0140-6736(03)14026-3. [DOI] [PubMed] [Google Scholar]
- 28.Anninga B, Ahmed M, Van Hemelrijck M, Pouw J, Westbroek D, Pinder S, Ten Haken B, Pankhurst Q, Douek M. Magnetic sentinel lymph node biopsy and localization properties of a magnetic tracer in an in vivo porcine model. Breast Cancer Res Treat. 2013;141:33–42. doi: 10.1007/s10549-013-2657-0. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed M, Anninga B, Pouw JJ, Vreeman S, Peek M, Van Hemelrijck M, Ten Haken B, Pankhurst Q, Douek M. Optimising magnetic sentinel lymph node biopsy in an in vivo porcine model. Nanomedicine. 2015;11:993–1002. doi: 10.1016/j.nano.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 30.Karakatsanis A, Olofsson H, Bergkvist L, Abdsaleh S, Sund M, Wärnberg F. How to avoid unnecessary SLNB in patients with DCIS. The Breast. 2015;24:S133–S134. doi: 10.1016/S0960-9776(15)70341-3. [DOI] [Google Scholar]
- 31.Sugie T, Sawada T, Tagaya N, Kinoshita T, Yamagami K, Suwa H, Ikeda T, Yoshimura K, Niimi M, Shimizu A, Toi M. Comparison of the indocyanine green fluorescence and blue dye methods in detection of sentinel lymph nodes in early-stage breast cancer. Ann Surg Oncol. 2013;20:2213–2218. doi: 10.1245/s10434-013-2890-0. [DOI] [PubMed] [Google Scholar]
- 32.Aoyama K, Kamio T, Ohchi T, Nishizawa M, Kameoka S. Sentinel lymph node biopsy for breast cancer patients using fluorescence navigation with indocyanine green. World J Surg Oncol. 2011;9:157. doi: 10.1186/1477-7819-9-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiozawa M, Kobayashi S, Sato Y, Maeshima H, Hozumi Y, Lefor AT, Kurihara K, Sata N, Yasuda Y. Magnetic resonance lymphography of sentinel lymph nodes in patients with breast cancer using superparamagnetic iron oxide: a feasibility study. Breast Cancer. 2014;21:394–401. doi: 10.1007/s12282-012-0401-y. [DOI] [PubMed] [Google Scholar]
- 34.Pouw JJ, Grootendorst MR, Bezooijen R, Klazen CA, De Bruin WI, Klaase JM, Hall-Craggs MA, Douek M, Ten Haken B. (2015) Pre-operative sentinel lymph node localization in breast cancer with superparamagnetic iron oxide MRI: the SentiMAG Multicentre Trial imaging subprotocol [DOI] [PMC free article] [PubMed]
- 35.Teshome M, Wei C, Hunt KK, Thompson A, Rodriguez K, Mittendorf EA. Use of a magnetic tracer for sentinel lymph node detection in early-stage breast cancer patients: a meta-analysis. Ann Surg Oncol. 2016 doi: 10.1245/s10434-016-5135-1. [DOI] [PubMed] [Google Scholar]