Abstract
Cells of the continuous murine hemopoietic cell line FDC-P1 expressing macrophage-colony-stimulating factor (M-CSF) receptors following retroviral insertion of murine c-fms cDNA proliferated clonally when stimulated by granulocyte/macrophage (GM)-CSF, multipotential CSF, or M-CSF. However, M-CSF combined with either GM-CSF or multi-CSF, even at low CSF concentrations, strongly inhibited colony formation, with loss of clonogenicity in affected cells accompanied by increased macrophage differentiation. Stimulation by these CSF combinations did not induce short-term changes in CSF receptor expression or internalization. FDC-P1 cells expressing another inserted tyrosine kinase receptor, basic fibroblast growth factor receptor, did not exhibit suppression when GM-CSF was combined with fibroblast growth factor. This phenomenon of synergistic suppression may have relevance for the future clinical use of combinations of CSFs, because a potentially similar suppression is also observable with some normal macrophage progenitor cells.
Full text
PDF
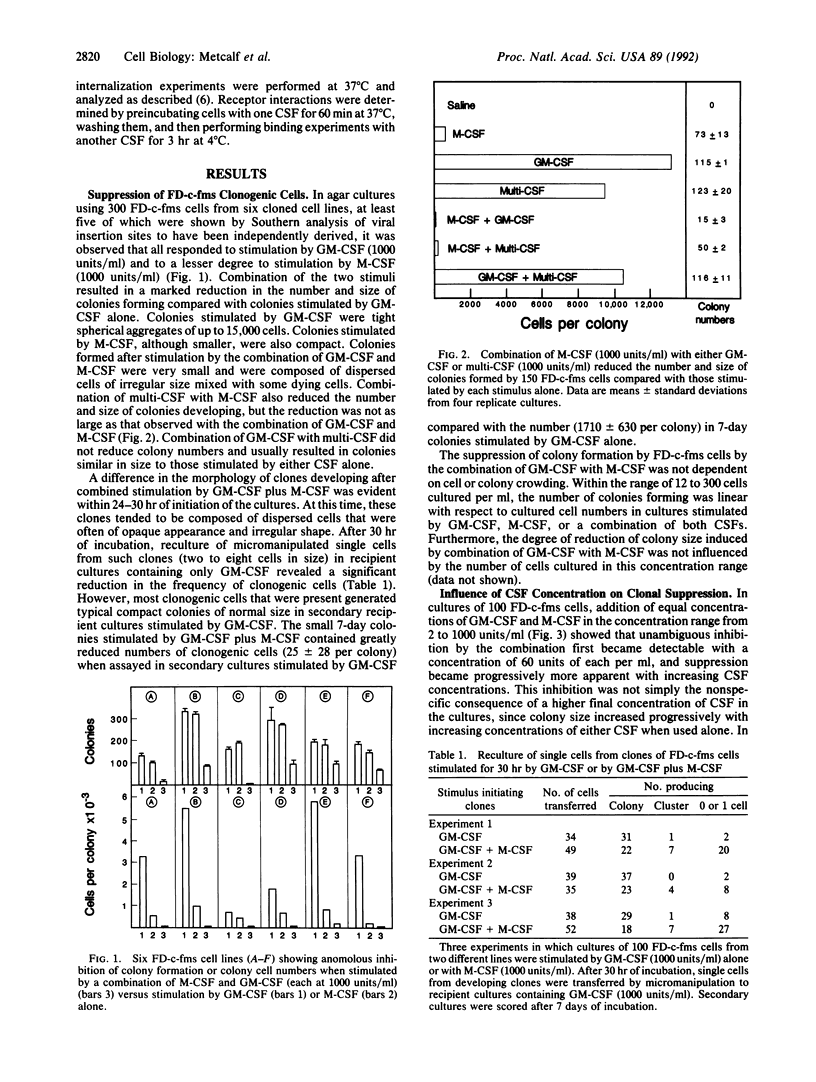
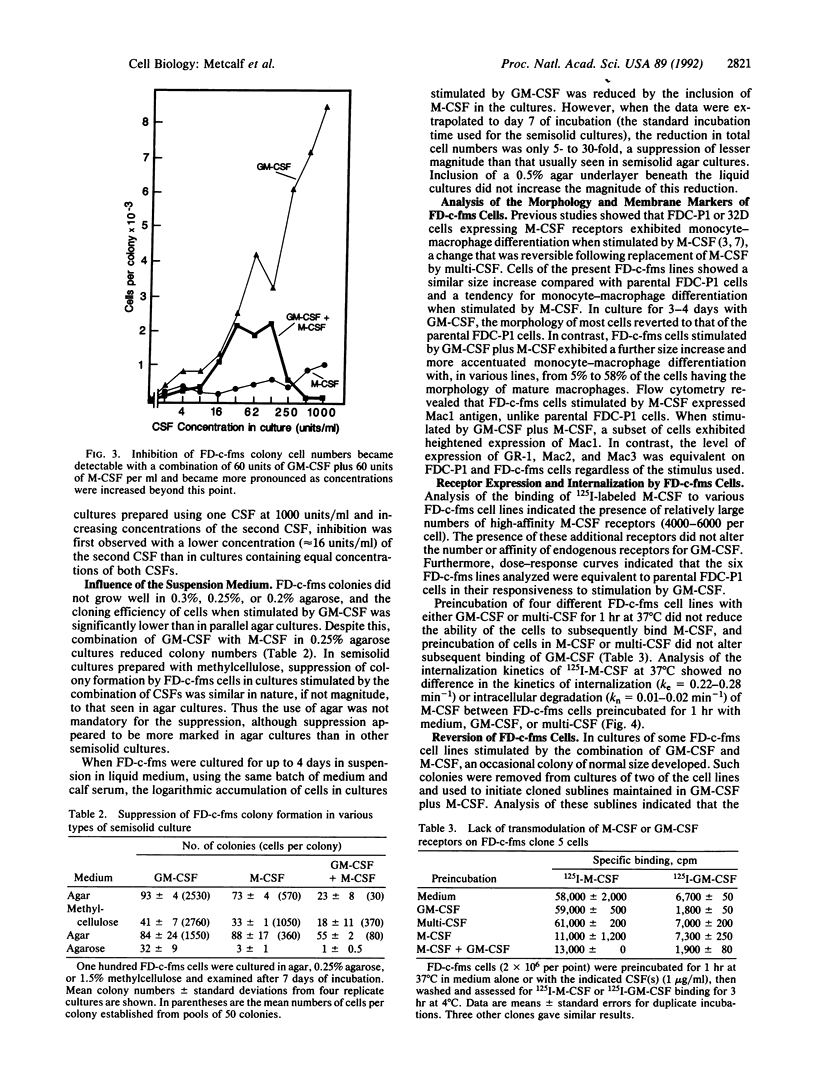
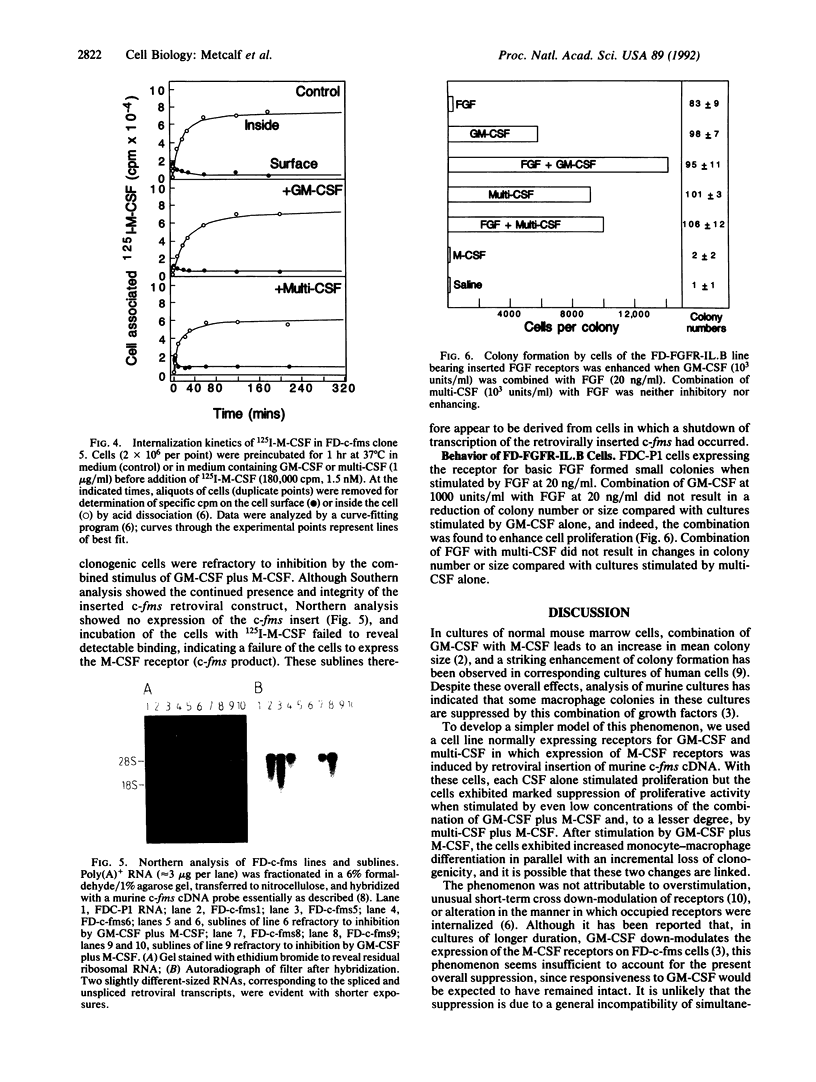

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Begley C. G., Metcalf D., Nicola N. A. Purified colony stimulating factors (G-CSF and GM-CSF) induce differentiation in human HL60 leukemic cells with suppression of clonogenicity. Int J Cancer. 1987 Jan 15;39(1):99–105. doi: 10.1002/ijc.2910390118. [DOI] [PubMed] [Google Scholar]
- Bernard O., Li M., Reid H. H. Expression of two different forms of fibroblast growth factor receptor 1 in different mouse tissues and cell lines. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7625–7629. doi: 10.1073/pnas.88.17.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caracciolo D., Shirsat N., Wong G. G., Lange B., Clark S., Rovera G. Recombinant human macrophage colony-stimulating factor (M-CSF) requires subliminal concentrations of granulocyte/macrophage (GM)-CSF for optimal stimulation of human macrophage colony formation in vitro. J Exp Med. 1987 Dec 1;166(6):1851–1860. doi: 10.1084/jem.166.6.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliniak B. C., Rohrschneider L. R. Expression of the M-CSF receptor is controlled posttranscriptionally by the dominant actions of GM-CSF or multi-CSF. Cell. 1990 Nov 30;63(5):1073–1083. doi: 10.1016/0092-8674(90)90510-l. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Kelso A. GM-CSF expression is preferential to multi-CSF (IL-3) expression in murine T lymphocyte clones. Growth Factors. 1989;1(4):287–298. doi: 10.3109/08977198909000253. [DOI] [PubMed] [Google Scholar]
- Maekawa T., Metcalf D., Gearing D. P. Enhanced suppression of human myeloid leukemic cell lines by combinations of IL-6, LIF, GM-CSF and G-CSF. Int J Cancer. 1990 Feb 15;45(2):353–358. doi: 10.1002/ijc.2910450224. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Control of granulocytes and macrophages: molecular, cellular, and clinical aspects. Science. 1991 Oct 25;254(5031):529–533. doi: 10.1126/science.1948028. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A., Gearing D. P., Gough N. M. Low-affinity placenta-derived receptors for human granulocyte-macrophage colony-stimulating factor can deliver a proliferative signal to murine hemopoietic cells. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4670–4674. doi: 10.1073/pnas.87.12.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. The Florey Lecture, 1991. The colony-stimulating factors: discovery to clinical use. Philos Trans R Soc Lond B Biol Sci. 1991 Jul 29;333(1266):147–173. doi: 10.1098/rstb.1991.0065. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Subunit promiscuity among hemopoietic growth factor receptors. Cell. 1991 Oct 4;67(1):1–4. doi: 10.1016/0092-8674(91)90564-f. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Peterson L., Hilton D. J., Metcalf D. Cellular processing of murine colony-stimulating factor (Multi-CSF, GM-CSF, G-CSF) receptors by normal hemopoietic cells and cell lines. Growth Factors. 1988;1(1):41–49. doi: 10.3109/08977198809000245. [DOI] [PubMed] [Google Scholar]
- Pierce J. H., Di Marco E., Cox G. W., Lombardi D., Ruggiero M., Varesio L., Wang L. M., Choudhury G. G., Sakaguchi A. Y., Di Fiore P. P. Macrophage-colony-stimulating factor (CSF-1) induces proliferation, chemotaxis, and reversible monocytic differentiation in myeloid progenitor cells transfected with the human c-fms/CSF-1 receptor cDNA. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5613–5617. doi: 10.1073/pnas.87.15.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L. R., Metcalf D. Induction of macrophage colony-stimulating factor-dependent growth and differentiation after introduction of the murine c-fms gene into FDC-P1 cells. Mol Cell Biol. 1989 Nov;9(11):5081–5092. doi: 10.1128/mcb.9.11.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Walker F., Nicola N. A., Metcalf D., Burgess A. W. Hierarchical down-modulation of hemopoietic growth factor receptors. Cell. 1985 Nov;43(1):269–276. doi: 10.1016/0092-8674(85)90032-7. [DOI] [PubMed] [Google Scholar]