Abstract
Aims
Atrial fibrillation (AF) is associated with an increased risk of stroke, which is currently estimated by clinical characteristics. The cardiac biomarkers N-terminal fragment B-type natriuretic peptide (NT-proBNP) and cardiac troponin high-sensitivity (cTn-hs) are independently associated with risk of stroke in AF. Our objective was to develop and validate a new biomarker-based risk score to improve prognostication of stroke in patients with AF.
Methods and results
A new risk score was developed and internally validated in 14 701 patients with AF and biomarkers levels determined at baseline, median follow-up of 1.9 years. Biomarkers and clinical variables significantly contributing to predicting stroke or systemic embolism were assessed by Cox-regression and each variable obtained a weight proportional to the model coefficients. External validation was performed in 1400 patients with AF, median follow-up of 3.4 years. The most important predictors were prior stroke/transient ischaemic attack, NT-proBNP, cTn-hs, and age, which were included in the ABC ( A ge, B iomarkers, C linical history) stroke risk score. The ABC-stroke score was well calibrated and yielded higher c-indices than the widely used CHA 2 DS 2 -VASc score in both the derivation cohort (0.68 vs. 0.62, P < 0.001) and the external validation cohort (0.66 vs. 0.58, P < 0.001). Moreover, the ABC-stroke score consistently provided higher c-indices in several important subgroups.
Conclusion
A novel biomarker-based risk score for predicting stroke in AF was successfully developed and internally validated in a large cohort of patients with AF and further externally validated in an independent AF cohort. The ABC-stroke score performed better than the presently used clinically based risk score and may provide improved decision support in AF.
ClinicalTrials. gov identifier
Keywords: Atrial fibrillation, Biomarkers, Natriuretic peptides, Risk score, Stroke, Troponin
Introduction
Atrial fibrillation (AF) is a common and treatable risk factor for stroke and systemic embolism. 1 , 2 The AF population is heterogeneous with a variable risk of stroke. 3 Development of risk stratification schemes in AF patients emerged in the 1990s and have thereafter been reconstructed and refined continuously. 4–7 Current guidelines recommend a risk-based approach to decisions on anticoagulation treatment in AF based on the CHA 2 DS 2 -VASc score [which assigns 1 point each for a history of congestive heart failure, hypertension, diabetes mellitus, vascular disease, age 65–74 years, and sex category (female gender), and 2 points for age ≥75 years and, prior stroke/transient ischaemic attack (TIA)]. 5 The CHA 2 DS 2 -VASc and other currently used risk scores are all based solely on clinical variables. Recently, it was demonstrated that biomarkers reflecting cardiac and renal dysfunction as well as inflammation and oxidative stress are related to the risk of stroke and other outcomes in patients with AF. 8 We demonstrated that cardiac troponin measured with high-sensitivity assays (cTn-hs), indicating myocardial injury, and N-terminal fragment B-type natriuretic peptide (NT-proBNP), indicating myocyte stress, contained more prognostic information than most clinical characteristics in patients with AF. 9–12 Based on these experiences, we aimed to improve risk stratification of stroke in AF by developing and validating a risk score that included the prognostically most important biomarkers and clinical characteristics. The project was performed in accordance with the recent TRIPOD statement. 13 The development cohort was 14 701 patients with AF with biomarkers measured at entry in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial, and the validation cohort was 1400 participants with AF and biomarkers measured at entry in the STabilization of Atherosclerotic plaque By Initiation of darapLadIb TherapY (STABILITY) trial. 14–17
Methods
Study populations
The details of the ARISTOTLE trial (ClinicalTrials.gov identifier: NCT00412984) have been published previously. 14 , 15 Briefly, ARISTOTLE was a double-blind, double-dummy, randomized clinical trial that enrolled 18 201 patients between December 2006 and April 2010. Patients included had paroxysmal, persistent or permanent AF, or atrial flutter, and one or more of the following risk factors: age ≥75 years, prior stroke, TIA, or systemic embolus, heart failure, diabetes mellitus, or hypertension requiring pharmacological treatment. Participants were randomized to warfarin ( n = 9081) or apixaban ( n = 9120). The primary endpoint was stroke or systemic embolism. The median length of follow-up was 1.9 years for the subset of patients with cardiac biomarkers available at randomization ( n = 14 701) after exclusion of 45 (0.3%) patients with missing variables. A total of 1056 deaths occurred during follow-up. The median time in therapeutic range (TTR) in the warfarin-treated group was 66.1 calculated by the method of Rosendaal. 14
The external validation cohort consisted of 1400 participants with AF or atrial flutter (689 on oral anticoagulation) and biomarkers available at entry in the STABILITY trial. The details of the STABILITY trial (ClinicalTrials.gov identifier: NCT00799903) have been published previously. 16 , 17 Briefly, STABILITY was a double-blind, international, multicentre, event-driven trial that randomly assigned 15 828 patients with stable coronary heart disease to receive either once-daily darapladib or placebo between December 2008 and April 2010. Patients included had stable coronary heart disease and at least one of the following additional cardiovascular risk factors: age ≥60 years, diabetes, high-density lipoprotein cholesterol level of <1.03 mmol/L, smoker of five or more cigarettes per day, moderate renal dysfunction (≥30 and ≤59 mL/min), or polyvascular arterial disease. The primary endpoint was a composite of cardiovascular death, myocardial infarction, or stroke, and median follow-up was 3.4 years in this external validation cohort.
Ethics committee approval was obtained for all investigational sites, and all patients gave written informed consent.
Endpoints and outcome assessment
In both trials, all outcomes were adjudicated by the same international team of adjudicators blinded to treatment assignment. Stroke was defined as the sudden onset of a focal neurological deficit in a location consistent with the territory of a major cerebral artery and categorized as ischaemic, haemorrhagic, or unspecified. Haemorrhagic transformation of ischaemic stroke was not considered to be haemorrhagic stroke. In the ARISTOTLE trial, systemic embolism was also adjudicated and defined as an acute vascular occlusion of an extremity or organ, documented by means of imaging, surgery, or autopsy.
Biochemical methods
In both trials, patients provided blood samples at study entry. Plasma was frozen in aliquots and stored until analysed centrally at the Uppsala Clinical Research Center (UCR) laboratory, Uppsala, Sweden. Plasma cardiac troponin-I levels (cTnI-hs) were determined with high-sensitivity sandwich immunoassays on the ARCHITECT i1000SR (Abbott Diagnostics) according to the instructions of the manufacturer, and NT-proBNP and troponin-T (cTnT-hs) levels in plasma were determined with high-sensitivity sandwich immunoassays on the Cobas ® Analytics e601 Immunoanalyzers (Roche Diagnostics, Germany) according to the instructions of the manufacturer. The limit of detection (LoD) and analytical range for these assays have been described previously. 10–12
Statistical analyses
Derivation of the prediction model
All biomarkers were log-transformed and values below the reporting limit were set to half the limit. As a first step, a model including all candidate predictors (listed in Table 1 ) was fitted. Separate models were derived for inclusion of cTnI-hs and cTnT-hs. Possible non-linearities were evaluated by transforming the continuous variables using restricted cubic splines, each with four knots placed at the respective 5th, 35th, 65th, and 95th sample percentiles. To allow for different prediction models for subjects with and without a prior stroke, bivariate interactions between each variable and prior stroke were included. The global tests of any non-linearity or interaction were not statistically significant, wherefore the full prediction model included only main effects and linear terms. For a more parsimonious and clinically useful model, we approximated the full model by using a fast backward algorithm on an ordinary least squares model in which the estimated linear predictor from the full Cox model was the outcome and all candidate variables were entered in exactly the same manner as in the full Cox model. 18 Thus, in the first step R2 = 1.0 by design and by removing variables in a stepwise manner, the full model could be approximated to an arbitrary level. The final model approximated 96.5% of the full model while only including the four variables: age, cTnI-hs (or cTnT-hs), NT-proBNP, and prior stroke/TIA. The strong correlation between age and creatinine clearance entailed that one of these biomarkers could be excluded without an effect on the outcome. Age was included as it is a readily available variable, and a required determinant for estimating creatinine clearance. As a sensitivity analysis, we fitted a competing risks model with stroke/systemic embolism as the event of interest and death of any cause as a competing risk.
Table 1.
Demographics and baseline characteristics in the derivation and external validation cohorts
| Variable |
Derivation
( n = 14 701) |
External validation
( n = 1400) |
|---|---|---|
| Age (years) | 70.0 (19.0–97.0) | 69.0 (37.0–88.0) |
| Gender (female) | 35.7% (5255) | 14.4% (201) |
| Current smoker | 8.1% (1188) | 11.8% (165) |
| Permanent or persistent AF | 84.8% (12 473) | 36.6% (512) |
| Heart failure | 31.0% (4555) | 31.2% (437) |
| Hypertension | 87.5% (12 868) | 75.4% (1055) |
| Diabetes | 24.7% (3632) | 39.4% (552) |
| Prior stroke/TIA | 18.8% (2770) | 14.4% (201) |
| Vascular disease | 24.8% (3649) | 58.3% (816) |
| Prior myocardial infarction | 12.8% (1884) | 54.1% (758) |
| Peripheral arterial disease | 4.9% (718) | 9.3% (130) |
| Troponin I high-sensitivity (ng/L) | 5.4 (<2.0–11 230.0) | NA |
| Troponin T high-sensitivity (ng/L) | 10.9 (<5.0–1580.0) | 12.2 (<5.0–156.0) |
| NT-proBNP (ng/L) | 713.0 (<5.0–31 309.0) | 399.0 (<5.0–25 770.0) |
| Renal function, eGFR (mL/min/m 2 ) | 74.0 (18.3–345.0) | 67.9 (24.9–118.4) |
AF, atrial fibrillation; TIA, transient ischaemic attack; NT-proBNP, N-terminal fragment B-type natriuretic peptide; eGFR, estimated glomerular filtration rate.
Model validation
The model was internally validated using 300 bootstrap samples. Within each bootstrap sample, we refitted the model and compared the apparent performance in the bootstrap sample with the test performance (applying the refitted model to the original data). The optimism was quantified as the mean difference of these performance estimates. To reduce the optimism of new predictions, we applied uniform shrinkage to the regression parameters by calculating the linear predictor for all subjects in the original sample using the estimated regression coefficients from the models fitted within each bootstrap sample followed by using the observed outcomes in the original sample, the slope for the linear predictor estimated using a Cox-regression model. The average of all slopes determined the shrinkage. External validation was conducted in 1400 patients from the STABILTY trial using the cTnT-hs model as cTnI-hs was not measured in the STABILITY trial.
Discrimination was assessed by Harrell's c-index and graphically presented by plotting Kaplan–Meier curves for the predefined risk classes. Calibration was assessed graphically by comparing observed event rates with the predicted risk at 1, 2, and 3 years.
The final prediction model was presented as a nomogram and its discriminative ability was compared with the presently used CHA 2 DS 2 -VASc score using 1000 bootstrap samples. ABC-stroke risk classes were defined as low (<1%), medium (1–2%), and high (>2%) 1-year risk of stroke or systemic embolism according to established stroke risk categories.
To illustrate the comparison with the CHA 2 DS 2 -VASc score for risk stratification, the ABC-stroke risk classes were also based on treatment decision values for stroke risk adjusted for antithrombotic treatment according to 0–0.3%, 0.3–1%, 1–2%, and >2% 1-year risk of stroke or systemic embolism.
The final model was also evaluated for consistency in various subgroups, e.g. in warfarin-treated patients with low TTR, in whom anticoagulation is sub-therapeutic. The analyses followed the framework for derivation and validation of prediction models proposed by Harrell, Steyerberg, and Steyerberg and Vergouwe. 18–20 The external validation followed the principles and methods described by Royston and Altman and the reporting followed the recently published TRIPOD statement (protocol checklist, Supplementary material online, Figure G ). 13 , 21 All analyses were performed using R version 3.1 using the packages rms and Hmisc. 18
Results
Baseline demographics and cardiac biomarkers in the derivation cohort
A total of 14 701 patients in the ARISTOTLE trial had plasma samples available for biomarker measurements at entry. Baseline demographics and biomarker levels are presented in Table 1 . The median age was 70 (range 19–97) years and 35.7% were women. Hypertension was the most common CHA 2 DS 2 -VASc risk factor (87.5%), followed by age >65 years (69.9%), female sex (35.7%), heart failure (31.0%), diabetes mellitus (24.7%), prior stroke or TIA (18.8%), and vascular disease (24.8%). Cardiac troponin-I high-sensitivity was detectable in 93.6% of patients and elevated in 9.2%, cTnT-hs was detectable in 93.5% and elevated in 34.4%, and 75% had elevated NT-proBNP levels.
Development of a new biomarker-based risk score in atrial fibrillation in the derivation cohort
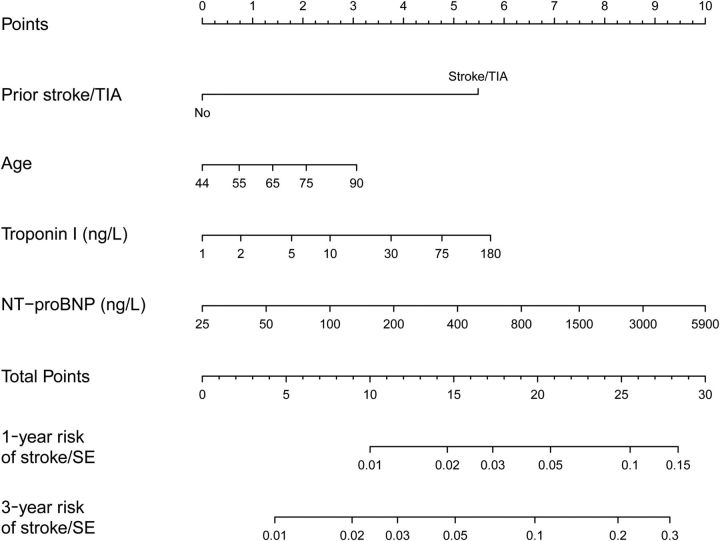
The development of the new risk score and selection of the prognostically most important clinical variables and biomarkers was based on 27929 person-years of follow-up and of 391 stroke or systemic embolism events in the ARISTOTLE cohort. The most important predictors of stroke were prior stroke or TIA, NT-proBNP, cTnI-hs (or cTnT-hs), and age ( Supplementary material online, Figure A ). The remaining clinical variables or biomarkers did not add significant information to the risk score. The final score was named ABC-stroke score based on inclusion of A ge, B iomarkers (cTnI-hs and NT-proBNP), and C linical history (prior stroke/TIA) ( Figure 1 ).
Figure 1.
Nomogram for the new biomarker-based risk score. For each predictor, read the points assigned on the 0–10 scale at the top and then sum these points. Find the number on the ‘Total Points’ scale and then read the corresponding predictions of 1- and 3-year risk of stroke or systemic embolism below it. Continuous variables are represented from the 1st to the 99th percentiles. The prediction model is preferably used as a web-based calculator or app. Application of the nomogram is exemplified in Supplementary material online, Figures E and F .
Internal validation of the ABC-stroke score and comparison with CHA 2 DS 2 -VASc
The ABC-stroke score that included cTnI-hs yielded a c-index of 0.68 for stroke or systemic embolism ( Table 2 ). The internal bootstrap validation indicated only modest over-fitting (optimism-corrected c-index of 0.67). The ABC-stroke score that included cTnT-hs provided similar results. The sensitivity analysis using a competing risks model showed similar results ( Supplementary material online, Figure B ) with a c-index of 0.67.
Table 2.
C-indices (95% confidence interval) for the ABC-stroke and CHA 2 DS 2 -VASc scores in the derivation ( n = 14 701) and the validation cohort ( n = 1400)
| Full cohort | No prior stroke | TTR <65% | |
|---|---|---|---|
| Derivation cohort | |||
| ABC-stroke (troponin I) | 0.68 (0.65, 0.71) | 0.66 (0.62, 0.69) | 0.67 (0.62, 0.71) |
| ABC-stroke (troponin T) | 0.67 (0.65, 0.70) | 0.65 (0.61, 0.68) | 0.67 (0.63, 0.71) |
| CHA 2 DS 2 -VASc | 0.62 (0.60, 0.65) | 0.59 (0.55, 0.63) | 0.62 (0.57, 0.66) |
| Validation cohort | |||
| ABC-stroke (troponin T) | 0.66 (0.58, 0.74) | 0.63 (0.54, 0.72) | NA |
| CHA 2 DS 2 -VASc | 0.58 (0.49, 0.67) | 0.50 (0.40, 0.60) | NA |
TTR, time in therapeutic range (International normalized ratio 2.0–3.0); ABC-stroke, A ge, B iomarkers (cardiac troponin and NT-proBNP), C linical history (prior stroke/transient ischaemic attack); CHA 2 DS 2 -VASc, assigns 1 point each for Congestive heart failure, Hypertension, Diabetes mellitus, Vascular disease, Age 65–74 years, and Gender category (female gender), and 2 points for Age ≥75 years and, prior Stroke/transient ischaemic attack.
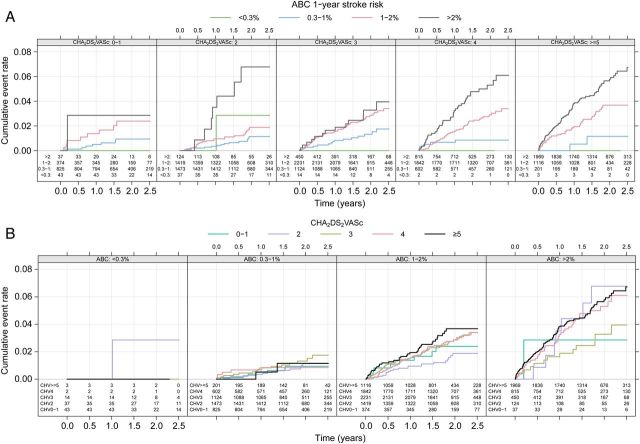
For comparison, the widely used CHA 2 DS 2 -VASc score achieved a c-index of 0.62 in this cohort ( P < 0.001). The ABC-stroke score that included cTnI-hs consistently achieved higher c-indices when compared with the CHA 2 DS 2 -VASc score in subgroups without prior stroke in order to assess the score performance in the primary prevention setting (0.66 vs. 0.59, P < 0.001). It also achieved well in patients with a low time (<65%) in therapeutic range (TTR) (0.67 vs. 0.62, P = 0.014) ( Table 2 ). Also with cTnT-hs, the ABC-stroke score (nomogram in Supplementary material online, Figure C ) consistently achieved higher c-indices ( P < 0.001) when compared with the CHA 2 DS 2 -VASc score with c-index of 0.67 in the total cohort, c-index of 0.65 ( P = 0.006) in the subgroup without prior stroke, and c-index of 0.67 ( P = 0.005) in patients with low TTR ( Table 2 ). Within both low- and high-risk CHA 2 DS 2 -VASc score cohorts, the ABC-stroke risk score was well calibrated and further stratified patients into subgroups with observed 1-year event rates close to the 1-year predicted by the ABC-stroke score ( Figure 2 and Supplementary material online, Table ).
Figure 2.
( A ) Kaplan–Meier estimated cumulative event rate by four ABC-stroke risk classes for the CHA 2 DS 2 -VASc score (panel): 0–1, 2, 3, 4, and ≥5 points. ( B ) Kaplan–Meier estimated cumulative event rate by the CHA 2 DS 2 -VASc score: 0–1, 2, 3, 4, and ≥5 points for the three ABC-stroke risk classes (panel): 0–0.3%, 0.3–1%, 1–2, and >2%.
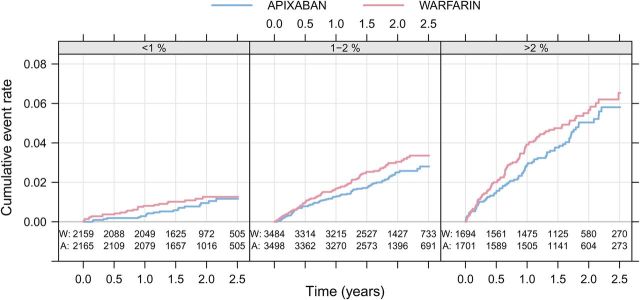
There was no significant interaction between the ABC-stroke score and the relative reduction of stroke with apixaban when compared with warfarin. The absolute difference in the rate of stroke or systemic embolism was larger in patients with a predicted annual risk >1% in the ABC-stroke score ( Figure 3 ).
Figure 3.
Kaplan–Meier estimated cumulative event rate by randomized treatment (colour) for the three ABC-stroke risk classes (panel): low (<1%), medium (1–2%), and high (>2%).
External validation of the ABC-stroke score and comparison with CHA 2 DS 2 -VASc
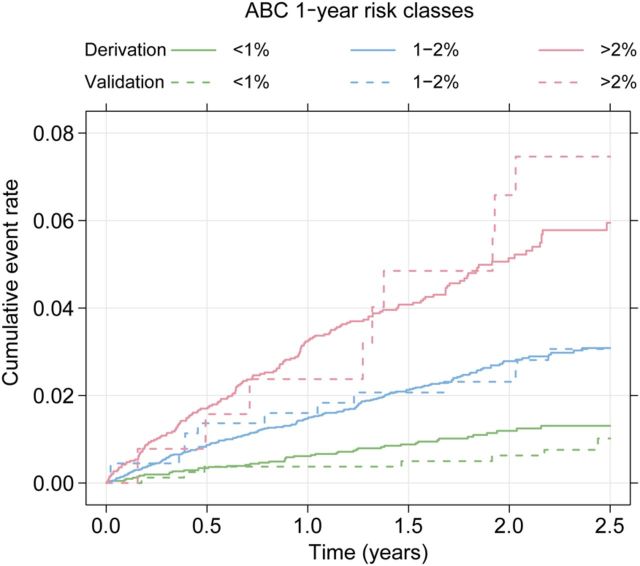
Baseline demographics and biomarker levels in the external validation cohort are presented in Table 1 . In the external validation, based on 4751 person-years of follow-up and 48 adjudicated stroke events, ABC-stroke score achieved a c-index of 0.66 in comparison with 0.58 for the CHA 2 DS 2 -VASc score ( P < 0.001). The incidence rates (events per 100 person-years) were similar in the derivation and external validation data within each predefined risk class: 0.56 vs. 0.56 (low), 1.37 vs. 1.29 (medium), and 2.63 vs. 3.22 (high) ( Table 3 ). The relative hazard ratios between the risk classes in the derivation and validation cohorts were similar ( Table 3 ). Kaplan–Meier curves within risk classes for both the derivation and validation data illustrate that the ABC-stroke score was well calibrated with a good discriminative ability in different cohorts of patients with AF ( Figure 4 and calibration plot in Supplementary material online, Figure D ).
Table 3.
Event rates and hazard ratios between ABC-stroke risk classes for the derivation and the validation cohorts
| Risk class | n | Events | Incidence rate a | Hazard ratio |
|---|---|---|---|---|
| Derivation cohort | ||||
| Low (<1%) | 4170 | 46 | 0.56 (0.41, 0.74) | 1.00 (ref) |
| Medium (1–2%) | 7154 | 187 | 1.37 (1.18, 1.58) | 2.45 (1.78, 3.38) |
| High (>2%) | 3377 | 158 | 2.63 (2.24, 3.08) | 4.67 (3.36, 6.48) |
| Validation cohort | ||||
| Low (<1%) | 820 | 16 | 0.56 (0.32, 0.90) | 1.00 (ref) |
| Medium (1–2%) | 448 | 19 | 1.29 (0.78, 2.02) | 2.34 (1.20, 4.55) |
| High (>2%) | 132 | 13 | 3.22 (1.71, 5.50) | 5.80 (2.79, 12.1) |
a Per 100 person-years.
Figure 4.
Cumulative risk of stroke by predicted 1-year ABC-stroke risk group (green <1%, blue 1–2%, and red >2%) for the derivation (solid lines, n = 14 701) and the external validation (dashed lines, n = 1400) data.
Discussion
The ABC-stroke score is a novel biomarker-based risk score for predicting stroke or systemic embolism and was developed in a very large cohort of patients with AF treated with oral anticoagulation. The score included two biomarkers—NT-proBNP and cardiac troponin-hs—and two pieces of directly available clinical information—age and prior stroke. The ABC-stroke score consistently predicted stroke or systemic embolism with a significantly higher accuracy than the guideline-recommended CHA 2 DS 2 -VASc risk model when validated internally using bootstrap samples and multiple subgroups. The ABC-stroke score was also found well calibrated and with consistent superiority over the clinical risk score in the external validation in a smaller cohort of patients with AF. The new biomarker-based ABC-stroke score was developed and validated in accordance with the recent TRIPOD statement and should therefore be acceptable for implementation in clinical care.
The ABC-stroke score is simple, only encompassing four variables ( A ge, B iomarkers (troponin and NT-proBNP), and prior stroke or TIA as C linical history). These four variables provided most of the prognostic information in multivariable models from the Cox analyses. When including the two biomarkers in the risk score model previously identified clinical risk factors, such as hypertension, diabetes mellitus, congestive heart failure, and other cardiovascular diseases or gender, no longer carried any important incremental prognostic information. The biomarkers appear to add important information concerning subclinical cardiovascular dysfunction and were better associated with vascular vulnerability than disease diagnosis. 22 This might be explained by more information is gained by biomarkers being more sensitive indicators of myocardial stress and dysfunction. 23–25 In contrast to other scores, using categorical irreversible risk factors, the proposed ABC-stroke score also contains continuous risk variables. The ABC-stroke score is dynamic with the opportunity to increase or decrease and would thereby be expected to allow monitoring of the patient's clinical condition and changes in risk of future events in either direction. 26 A dynamic risk score may facilitate its use as a decision support tool for selection of treatment in different clinical settings, e.g. in relation to the profiles of new treatments such as dabigatran, rivaroxaban, and apixaban, when compared with warfarin. 14 , 23 , 27 Risk stratification with the ABC-stroke score indicated that the largest gain in stroke or systemic embolism prevention with apixaban when compared with warfarin was obtained in high-risk patients.
The ABC-stroke score includes two biomarkers, NT-proBNP and cardiac troponin-hs, both of which are readily available in most parts of the world. Even without a thorough understanding of the exact mechanisms, information on cardiac biomarker levels substantially improves the risk prediction in patients with AF. Other biomarkers, e.g. markers of inflammation (C-reactive protein, interleukin-6), oxidative stress (GDF-15), and coagulation (e.g. D-dimer), have in some studies shown associations with stroke in AF in addition to clinical risk factors. 8 , 28 However, these biomarkers were not included in the present evaluation, as they have not displayed independent associations with stroke in the presence of NT-proBNP and cardiac troponin, or are cumbersome to measure and assess in clinical practice. 8 , 28 Furthermore, in our sensitivity analyses (not shown), adding these biomarkers to the construction model did not add important prognostic information to the presented model.
The ABC-stroke score was constructed using established statistical methods for development of the clinical prediction models and adhered to the recently developed TRIPOD statement. 13 The model construction was based on an extensive clinical trial with standardized recording of clinical characteristics and careful long-term follow-up of centrally adjudicated outcome events. The large number of events and the relatively simple model appears to have prevented over-fitting as shown by the internal bootstrap validation and the successful external validation in an independent cohort of patients with AF. The new ABC-risk score seemed robust, since it provided similar information when based on two different troponin assays. Although the ABC-stroke risk score yielded a modest c-index of 0.68, it consistently outperformed the current guidelines-recommended stroke risk score. It was also well calibrated as predicted event rates were associated with the observed event rates both in the derivation and validation cohorts. Evaluation of the score was also assessed in patients with ineffective and without anticoagulant treatment. Warfarin-treated patients with AF and a TTR below 58–65% derive little or no net benefit from oral anticoagulation. 29 , 30 The consistent performance of the ABC-stroke score was strengthened by the consistent results in the subgroup of warfarin-treated patients with TTR <65% during follow-up in the derivation cohort. The robustness of the score was further confirmed by the similar results in the cohort of patients without oral anticoagulation in the smaller external validation cohort. As prior stroke was the strongest risk factor in the score, it was important that the ABC-stroke score's performance was verified also in the subgroup of patients without prior stroke, indicating its usefulness also in the primary prevention setting. Thus, in all subgroups of both the derivation and validation cohort, the ABC-stroke score consistently achieved better prediction than the currently used CHA 2 DS 2 -VASc, suggesting that this risk score may be an important step forward as decision support concerning stroke prevention in AF.
Treatment of AF, as well as other cardiovascular diseases, is moving towards more individualized patient care in which biomarkers have an important role in increasing the understanding of pathophysiology, improving risk stratification, and personalizing treatment. 8 , 27 In this aspect, the use of the proposed ABC-stroke score seems appealing because of its simplicity and the possibility to apply across different healthcare settings. The implementation of the algorithm used in the score will either be based on the nomogram or, preferably, based on an electronic tool integrated into electronic patient records or an online tool.
Conclusions
A novel risk score for predicting stroke or systemic embolism using two biomarkers (NT-proBNP and cTn-hs) and information on age and prior stroke/TIA, named ABC-stroke score ( A ge, B iomarkers, C linical history), was developed in a large cohort and validated in a smaller cohort of patients with AF. The ABC-stroke risk score consistently performed better than presently used clinically based risk scores in terms of risk prediction and risk stratification, and may provide improved decision support in patients with AF.
Supplementary material
Supplementary material is available at European Heart Journal online .
Authors’ contributions
J.L. performed statistical analysis. All authors had full access to the data and were involved in study design, data analyses, and interpretation. Lead authors (Z.H. and L.W.) and members of the Executive (J.H.A., M.H., E.M.H., C.B.G., L.W.), Clinical Events (C.H. and R.D.L.), and Biomarker Substudy (L.W., C.B.G., A.S., E.M.H., and M.H.) committees designed the ARISTOTLE trial and supervised the conduct, as previously published. Members of the Executive (H.D.W. and L.W.) and Executive Operations (C.H. and R.A.H.S.) committees designed the STABILITY trial and supervised the conduct, as previously published. The first draft of the manuscript was written by Z.H., which thereafter was revised by all co-authors until agreement to submit was reached.
Funding
This work was supported by a grant from The Swedish Foundation for Strategic Research. The ARISTOTLE trial was funded by Bristol-Myers Squibb, Co., Princeton, NJ, USA and Pfizer Inc., New York, NY, USA, and coordinated by the Duke Clinical Research Institute, Durham, NC, USA and Uppsala Clinical Research Center (UCR), Uppsala, Sweden. The STABILITY trial was sponsored by GlaxoSmithKline, Philadelphia, PA, USA. Funding to pay the Open Access publication charges for this article was provided by Uppsala Clinical Research Center (UCR).
Conflict of interest: Z.H.: institutional research grants from Boehringer Ingelheim and Bristol-Myers Squibb/Pfizer; lecture fees from Boehringer Ingelheim. J.L.: nothing to report. J.H.A.: institutional research grants from Bristol-Myers Squibb, Merck-Schering Plough, and Regado Biosciences; consulting fee/honoraria from Bristol-Myers Squibb, Pfizer, Merck-Schering Plough, AstraZeneca, Boehringer Ingelheim, Ortho-McNeil-Janssen, Polymedix, Regado Biosciences, and Bayer. M.H.: employee of Bristol-Myers Squibb. C.H.: institutional research grants from AstraZeneca, Merck, GlaxoSmithKline, Roche, and Bristol-Myers Squibb; advisory board fees and honoraria from AstraZeneca. E.M.H.: consulting fees, travel support, and adjudication committee membership for Bristol-Myers Squibb, Daiichi Sankyo, Merck, Ortho-McNeil, Johnson & Johnson, and Pfizer; lecture fees from Boehringer Ingelheim. R.D.L.: grants and personal fees from Bristol-Myers Squibb; grants from GlaxoSmithKline; personal fees from Bayer, Boehringer Ingleheim, and Pfizer. J.O.: institutional grant, consulting and lecture fees from Boehringer Ingelheim; consulting and lecture fees from Bayer, Bristol-Myers Squibb, and Pfizer. A.S.: institutional research grants from AstraZeneca, Boehringer Ingelheim, and Bristol-Myers Squibb. R.A.H.S.: grants and non-financial support from GlaxoSmithKline. H.D.W.: grants and personal fees from GlaxoSmithKline and AstraZeneca; grants from Sanofi-Aventis, Eli Lilly, National Institute of Health, Merck Sharp & Dohme, and Daiichi Sankyo Pharma Development. C.B.G.: institutional research grants from Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Medtronic Foundation, Merck & Co., Pfizer, Sanofi-Aventis, Takeda, and The Medicines Company; consulting fees from Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Hoffmann-La Roche, Novartis Pharmaceutical Company, Lilly, Pfizer, Sanofi-Aventis, Takeda, The Medicines Company, and AstraZeneca. L.W.: research grants from Bristol-Myers Squibb, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck & Co., and Pfizer Inc.; consulting fee/honoraria from Abbott, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Merck & Co., and Regado Biosciences.
Supplementary Material
Acknowledgements
Ebba Bergman, PhD, and Martina Tillberg, MSc, at Uppsala Clinical Research Center, Uppsala, Sweden, provided editorial assistance, by funds from Bristol-Myers Squibb and Pfizer Inc.
References
- 1. Hart RG, Pearce LA, Aguilar MI . Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation . Ann Intern Med 2007. ; 146 : 857 – 867 . [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE . Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study . JAMA 2001. ; 285 : 2370 – 2375 . [DOI] [PubMed] [Google Scholar]
- 3. Wolf PA, Abbott RD, Kannel WB . Atrial fibrillation as an independent risk factor for stroke: the Framingham Study . Stroke 1991. ; 22 : 983 – 988 . [DOI] [PubMed] [Google Scholar]
- 4. AFI . Patients with nonvalvular atrial fibrillation at low risk of stroke during treatment with aspirin: Stroke Prevention in Atrial Fibrillation III Study. The SPAF III Writing Committee for the Stroke Prevention in Atrial Fibrillation Investigators . JAMA 1998. ; 279 : 1273 – 1277 . [PubMed] [Google Scholar]
- 5. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH . Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) . Eur Heart J 2010. ; 31 : 2369 – 2429 . [DOI] [PubMed] [Google Scholar]
- 6. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ . Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation . JAMA 2001. ; 285 : 2864 – 2870 . [DOI] [PubMed] [Google Scholar]
- 7. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ . Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation . Chest 2010. ; 137 : 263 – 272 . [DOI] [PubMed] [Google Scholar]
- 8. Hijazi Z, Oldgren J, Siegbahn A, Granger CB, Wallentin L . Biomarkers in atrial fibrillation: a clinical review . Eur Heart J 2013. ; 34 : 1475 – 1480 . [DOI] [PubMed] [Google Scholar]
- 9. Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Ezekowitz MD, Hohnloser SH, Reilly PA, Vinereanu D, Siegbahn A, Yusuf S, Wallentin L . Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long-term Anticoagulation Therapy (RE-LY) substudy . Circulation 2012. ; 125 : 1605 – 1616 . [DOI] [PubMed] [Google Scholar]
- 10. Hijazi Z, Siegbahn A, Andersson U, Granger CB, Alexander JH, Atar D, Gersh BJ, Mohan P, Harjola VP, Horowitz J, Husted S, Hylek EM, Lopes RD, McMurray JJ, Wallentin L , ARISTOTLE Investigators . High-sensitivity troponin I for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial . Circulation 2014. ; 129 : 625 – 634 . [DOI] [PubMed] [Google Scholar]
- 11. Hijazi Z, Wallentin L, Siegbahn A, Andersson U, Alexander JH, Atar D, Gersh BJ, Hanna M, Harjola VP, Horowitz JD, Husted S, Hylek EM, Lopes RD, McMurray JJ, Granger CB , ARISTOTLE Investigators . High-sensitivity troponin T and risk stratification in patients with atrial fibrillation during treatment with apixaban or warfarin . J Am Coll Cardiol 2014. ; 63 : 52 – 61 . [DOI] [PubMed] [Google Scholar]
- 12. Hijazi Z, Wallentin L, Siegbahn A, Andersson U, Christersson C, Ezekowitz J, Gersh BJ, Hanna M, Hohnloser S, Horowitz J, Huber K, Hylek EM, Lopes RD, McMurray JJ, Granger CB . N-terminal pro-B-type natriuretic peptide for risk assessment in patients with atrial fibrillation: insights from the ARISTOTLE Trial (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation) . J Am Coll Cardiol 2013. ; 61 : 2274 – 2284 . [DOI] [PubMed] [Google Scholar]
- 13. Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS . Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): explanation and elaboration . Ann Intern Med 2015. ; 162 : W1 – W73 . [DOI] [PubMed] [Google Scholar]
- 14. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L . Apixaban versus warfarin in patients with atrial fibrillation . N Engl J Med 2011. ; 365 : 981 – 992 . [DOI] [PubMed] [Google Scholar]
- 15. Lopes RD, Alexander JH, Al-Khatib SM, Ansell J, Diaz R, Easton JD, Gersh BJ, Granger CB, Hanna M, Horowitz J, Hylek EM, McMurray JJ, Verheugt FW, Wallentin L . Apixaban for reduction in stroke and other ThromboemboLic events in atrial fibrillation (ARISTOTLE) trial: design and rationale . Am Heart J 2010. ; 159 : 331 – 339 . [DOI] [PubMed] [Google Scholar]
- 16. STABILITY Investigators White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, Budaj A, Harrington RA, Steg PG, Ardissino D, Armstrong PW, Avezum A, Aylward PE, Bryce A, Chen H, Chen MF, Corbalan R, Dalby AJ, Danchin N, De Winter RJ, Denchev S, Diaz R, Elisaf M, Flather MD, Goudev AR, Granger CB, Grinfeld L, Hochman JS, Husted S, Kim HS, Koenig W, Linhart A, Lonn E, Lopez-Sendon J, Manolis AJ, Mohler ER, III, Nicolau JC, Pais P, Parkhomenko A, Pedersen TR, Pella D, Ramos-Corrales MA, Ruda M, Sereg M, Siddique S, Sinnaeve P, Smith P, Sritara P, Swart HP, Sy RG, Teramoto T, Tse HF, Watson D, Weaver WD, Weiss R, Viigimaa M, Vinereanu D, Zhu J, Cannon CP, Wallentin L . Darapladib for preventing ischemic events in stable coronary heart disease . N Engl J Med 2014. ; 370 : 1702 – 1711 . [DOI] [PubMed] [Google Scholar]
- 17. White H, Held C, Stewart R, Watson D, Harrington R, Budaj A, Steg PG, Cannon CP, Krug-Gourley S, Wittes J, Trivedi T, Tarka E, Wallentin L . Study design and rationale for the clinical outcomes of the STABILITY Trial (STabilization of Atherosclerotic plaque By Initiation of darapLadIb TherapY) comparing darapladib versus placebo in patients with coronary heart disease . Am Heart J 2010. ; 160 : 655 – 661 . [DOI] [PubMed] [Google Scholar]
- 18. Harrell FE . Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis . New York, NY: : Springer; ; 2015. . [Google Scholar]
- 19. Steyerberg EW . Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating . New York, NY: : Springer; ; 2009. . [Google Scholar]
- 20. Steyerberg EW, Vergouwe Y . Towards better clinical prediction models: seven steps for development and an ABCD for validation . Eur Heart J 2014. ; 35 : 1925 – 1931 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Royston P, Altman DG . External validation of a Cox prognostic model: principles and methods . BMC Med Res Methodol 2013. ; 13 : 33 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhalla MA, Chiang A, Epshteyn VA, Kazanegra R, Bhalla V, Clopton P, Krishnaswamy P, Morrison LK, Chiu A, Gardetto N, Mudaliar S, Edelman SV, Henry RR, Maisel AS . Prognostic role of B-type natriuretic peptide levels in patients with type 2 diabetes mellitus . J Am Coll Cardiol 2004. ; 44 : 1047 – 1052 . [DOI] [PubMed] [Google Scholar]
- 23. Eckman MH, Singer DE, Rosand J, Greenberg SM . Moving the tipping point: the decision to anticoagulate patients with atrial fibrillation . Circ Cardiovasc Qual Outcomes 2011. ; 4 : 14 – 21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Friberg L, Rosenqvist M, Lip GY . Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study . Eur Heart J 2012. ; 33 : 1500 – 1510 . [DOI] [PubMed] [Google Scholar]
- 25. Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, Gutnikov SA, Edwards P, Mant D, Sackley CM, Farmer A, Sandercock PA, Dennis MS, Warlow CP, Bamford JM, Anslow P , Oxford Vascular Study . Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study) . Lancet 2004. ; 363 : 1925 – 1933 . [DOI] [PubMed] [Google Scholar]
- 26. Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Ezekowitz MD, Hohnloser SH, Reilly PA, Siegbahn A, Yusuf S, Wallentin L . Importance of persistent elevation of cardiac biomarkers in atrial fibrillation: a RE-LY substudy . Heart 2014. ; 100 : 1193 – 1200 . [DOI] [PubMed] [Google Scholar]
- 27. Kirchhof P, Breithardt G, Aliot E, Al Khatib S, Apostolakis S, Auricchio A, Bailleul C, Bax J, Benninger G, Blomstrom-Lundqvist C, Boersma L, Boriani G, Brandes A, Brown H, Brueckmann M, Calkins H, Casadei B, Clemens A, Crijns H, Derwand R, Dobrev D, Ezekowitz M, Fetsch T, Gerth A, Gillis A, Gulizia M, Hack G, Haegeli L, Hatem S, Georg Hausler K, Heidbuchel H, Hernandez-Brichis J, Jais P, Kappenberger L, Kautzner J, Kim S, Kuck KH, Lane D, Leute A, Lewalter T, Meyer R, Mont L, Moses G, Mueller M, Munzel F, Nabauer M, Nielsen JC, Oeff M, Oto A, Pieske B, Pisters R, Potpara T, Rasmussen L, Ravens U, Reiffel J, Richard-Lordereau I, Schafer H, Schotten U, Stegink W, Stein K, Steinbeck G, Szumowski L, Tavazzi L, Themistoclakis S, Thomitzek K, Van Gelder IC, von Stritzky B, Vincent A, Werring D, Willems S, Lip GY, Camm AJ . Personalized management of atrial fibrillation: proceedings from the fourth Atrial Fibrillation competence NETwork/European Heart Rhythm Association consensus conference . Europace 2013. ; 15 : 1540 – 1556 . [DOI] [PubMed] [Google Scholar]
- 28. Wallentin L, Hijazi Z, Andersson U, Alexander JH, De Caterina R, Hanna M, Horowitz JD, Hylek EM, Lopes RD, Asberg S, Granger CB, Siegbahn A , ARISTOTLE Investigators . Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial . Circulation 2014. ; 130 : 1847 – 1858 . [DOI] [PubMed] [Google Scholar]
- 29. Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, Healey JS, Yusuf S , ACTIVE W Investigators . Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range . Circulation 2008. ; 118 : 2029 – 2037 . [DOI] [PubMed] [Google Scholar]
- 30. Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, Selby JV, Singer DE . Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation . N Engl J Med 2003. ; 349 : 1019 – 1026 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.