Abstract
Background
A high prevalence of neutropenia has been reported in several ethnic groups amongst whom many healthy individuals with low neutrophil counts undergo unnecessary investigations. This study aims to ascertain the prevalence of neutropenia (NP) in a large cohort of children from North African, Middle Eastern, and Asian countries residing in the United Arab Emirates.
Methods
Neutrophil counts of 26,542 children (one day to six years of age) from 86 countries were analyzed. The subjects were enrolled in the Well-Child-Care program of Ambulatory Health Services of Emirate of Abu Dhabi, United Arab Emirates. NP was defined as a neutrophil count <1.5 × 109/L and severe NP <0.5 × 109/L.
Results
The neutrophil counts reached a nadir in the fourth week of life and changed slightly from the age of six-months to six-years. The frequency of NP was (from West-to-East): North African Arabs 15.4 %, Green Crescent Arabs 9.8 %, Peninsular Arabs 10.9 %, Iranians 3.1 %, Afghanis 2.5 %, Pakistanis 5.6 %, Indians 10.2 %, and Filipinos 7.3 %. The frequency of severe NP in North African Arabs (Sudanese) was 2.8 %, Green Crescent and Peninsular Arabs ≤1 %, Indians 1.5 %, and Filipinos 1.8 %. In 12,703 Emirati children, the frequency of NP was 10.6 % similar to their adult counterparts.
Conclusion
The prevalence of childhood NP varied considerably by geoethnicity. Measures to prevent the inappropriate investigations of healthy children with benign neutropenia are proposed.
Electronic supplementary material
The online version of this article (doi:10.1186/s12878-016-0054-8) contains supplementary material, which is available to authorized users.
Keywords: Public health, Ethnicity, Monocyte count, Malaria hypothesis
Background
Neutropenia (NP) is common amongst several ethnic groups from Africa and Asia [1–8]. A NP frequency of up to 30 % from Africa has been reported [1]. Among African Americans, its prevalence is 4.4 % [3]. In United Arab Emirates (UAE), 10.7 % of the native population has absolute neutrophil counts less than 1.5×109/L [7]. The evidence suggests the inheritance of NP is autosomal dominant or co-dominant in people of Sudanese origin and among natives of Arabia [6, 7]. Molecular studies in some people of African ancestry, on the other hand, have shown a strong association between familial NP and the null Duffy genotype (Fy-/Fy-) and no association with the heterozygote (Fy-/Fy+) and wild-homozygote (Fy+/Fy+) genotypes, suggesting an autosomal recessive inheritance [9].
The benign nature of ethnic NP is based on reports of the absence of recurrent infections in such individuals [1–8]. However, many healthy individuals with low neutrophil counts often undergo unnecessary investigations to exclude pathologic NP. In addition, benign neutropenia often changes medical management such as delaying administration of myelosuppressants, premature stopping of drug therapy, postponing elective surgery and preventing recruitment into clinical trials [10–17]. This study ascertained the prevalence of NP in a large cohort of infants and children from North African, Middle Eastern and Asian countries who reside in UAE.
Methods
Study setting and population
This study was conducted in the Emirate of Abu Dhabi, UAE. The country’s population is eight million, of which 15 % are Emiratis (ethnically Arab) and the remaining 85 % are temporary foreign workers from numerous countries including the Indian subcontinent, the Middle East and North Africa. The study cohort comprised 26,542 infants and children. Their ages ranged from 1 day to 6 years. These children were registered in the Well-Child Care Program at Ambulatory Health Services (AHS) funded by the Health Authority of Abu Dhabi. The blood samples were obtained at the treating physician’s discretion between April 2008 and December 2013 at three hospitals (64 %), 26 outpatient AHS centers (19 %) or unidentified sites (17 %). Only one sample per child was used in the analysis. Written consent was not obtained as all blood counts were performed as part of the standard care.
Complete blood count
The blood samples were collected in BD Vacutainer® spray-coated K2EDTA tubes. The samples were mixed by inversion, transported at 2-8 °C, and tested as soon as they arrived at the laboratory. Blood cell counts were determined using the Cell-Dyne Ruby analyzers (Abbott Laboratories, Illinois, USA). The laboratories run daily internal quality controls before running patient samples and participate in External Quality Assurance program through the College of American Pathologists Proficiency Testing.
Definition of neutropenia and estimation of gene frequency
NP was considered mild, moderate and severe if the count was <1.5 × 109/L, <1.0 × 109/L and <0.5 × 109/L, respectively. As the neutrophil count normally oscillates, some children with NP occasionally have >1.5 × 109/L neutrophils. In a cross-sectional study, this fraction contributes to undetected (hidden) NP [7].
We hypothesized that severe NP in Emiratis was caused by a homozygote genotype and milder NP by a heterozygote genotype, i.e., inherited at a single locus with two alleles (q and p = 1-q). The native population of UAE is tribal, nearly half of the marriages are arranged between close cousins, and the mean coefficient of inbreeding in the population (F) is 0.022 [18–20]. Therefore, the frequency of the NP allele (q) in a large sample of Emiratis was determined using the Hardy-Weinberg equation adjusted for inbreeding. The frequency of homozygotes was q2(1-F) + qF and heterozygotes 2pq(1-F) [21].
Statistical analysis
As neutrophil counts did not follow a normal distribution (Shapiro-Wilk test p < 0.001), the non-parametric two-sample Wilcoxon rank-sum (Mann–Whitney) test was used to compare values between two categories, and Kruskal-Wallis rank test for three or more categories. The Spearman’s correlation test was used to analyze the relation between neutrophil and monocyte counts. For all analyses, two-tailed p-value of < 0.05 defined statistical significance. Other standard descriptive and statistical methods were used. One subject was removed because of an impossible value.
Ethics approval
The study was approved by the Institutional Review Board of College of Medicine and Health Sciences – UAE University (#13/14).
Results
The enrolled children represented 86 nationalities, of which 25,435 (96 %) were from 32 countries in North Africa, the Middle East and Asia. The largest group (12,073) was that of Emirati nationals.
Figure 1 shows the neutrophil counts by age (26,542 children from 86 nationalities). The counts decreased during the first week of life, reached a nadir in the fourth week and changed slightly between six-months and six-years. In the first month, the median (±SD) count (×109/L) was lower (p ≤ 0.05) in 2,856 males (6.4 ± 4.9) than in 3,551 females (7.3 ± 5.6), but later in life the differences were insignificant (Table 1). The median, 10th and 90th percentile neutrophil count for eight Arab population is shown in Additional file 1: Figure S1.
Fig. 1.
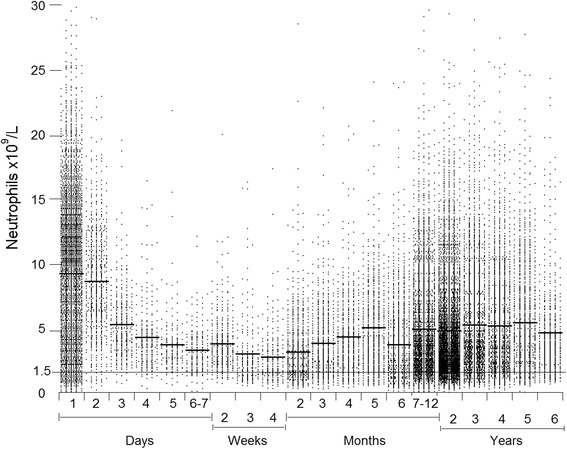
Neutrophil counts of the 26,542 children. Horizontal bars are means
Table 1.
Median and 2.5th percentile neutrophil counts of children from North Africa, Middle East and Asia
| Neutrophils × 109/L | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Days | Weeks | Months | Years | ||||||
| 1 | 2–7 | 2–4 | 2–12 | 2 | 3 | 4 | 5–6 | ||
| All | Median | 8.7 | 5.1 | 2.7 | 3.3 | 3.4 | 3.7 | 3.8 | 3.8 |
| 2.5th | 0.9 | 1.3 | 0.8 | 0.7 | 0.8 | 0.9 | 0.9 | 1.0 | |
| (3,360) | (1,429) | (615) | (6,997) | (5,846) | (3,097) | (2,463) | (2,470) | ||
| Females | Median | 9.7 | 5.6 | 3.1 | 3.3 | 3.4 | 3.9 | 3.8 | 3.8 |
| 2.5th | 1.1 | 1.4 | 0.8 | 0.7 | 0.9 | 0.9 | 1.0 | 0.9 | |
| (1,511) | (647) | (274) | (3,109) | (2,748) | (1,454) | (1,172) | (1,207) | ||
| Males | Median | 8.0 | 4.7 | 2.5 | 3.3 | 3.4 | 3.7 | 3.8 | 3.7 |
| 2.5th | 0.9 | 1.1 | 0.8 | 0.7 | 0.8 | 0.9 | 0.9 | 1.0 | |
| (1,849) | (782) | (341) | (3,888) | (3,098) | (1,643) | (1,291) | (1,263) | ||
| Outpatient | Median | - | - | - | 2.5 | 2.7 | 3.2 | 3.4 | 3.6 |
| 2.5th | 0.5 | 0.7 | 0.8 | 0.9 | 0.9 | ||||
| (1,544) | (2,905) | (1,812) | (1,673) | (2,021) | |||||
| Inpatient | Median | 8.7 | 5.1 | 2.8 | 3.6 | 4.8 | 4.9 | 4.9 | 5.0 |
| 2.5th | 0.9 | 1.3 | 0.8 | 0.8 | 1.0 | 1.0 | 1.2 | 1.1 | |
| (3,360) | (1,425) | (591) | (5,453) | (2,941) | (1,285) | (790) | (449) | ||
Values in parenthesis are number of children (only for n ≥50)
Figure 2 shows the prevalence of NP by nationality in six-month to six-year-old children. Analysis of the neutrophil counts by geoethnicity is shown in Table 2. NP was most common amongst African Arabs (14.7 to 20.2 %); in these populations, the 2.5th percentile count for 2-year-old children was 0.7×109/L and for 5 to 6 year-old children, 1.1×109/L. The frequency of NP in North Africans was 15.4 %, Peninsular Arabs 10.9 %, Indians 10.2 %, Green Crescent Arabs 9.8 %, Filipinos 7.3 %, Pakistanis 5.6 %, Iranians 3.1 %, and Afghanis 2.5 %. The prevalence of NP in Iranians and Afghanis was similar to that reported in Europeans [22–24]. The prevalence of severe NP in Sudanese was 2.8 %, Filipinos 1.8 %, Indians 1.5 %, and Middle Easterners ≤1 % (Fig. 2). The frequency of NP among eight Arab populations was not significantly different (see supplemental material).
Fig. 2.
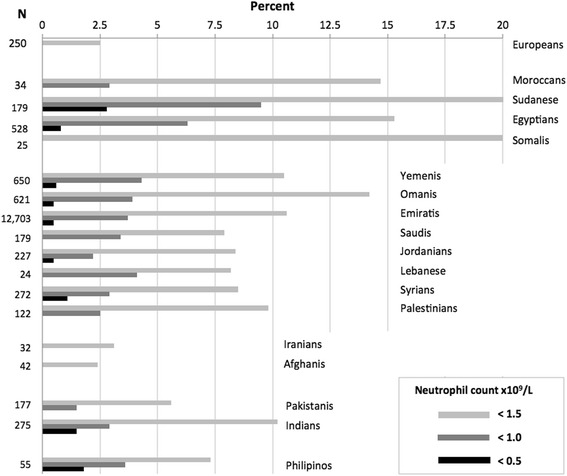
Prevalence of neutropenia in 0.5 to 6 year-old children by nationality. Data for Europeans are from reference [2]
Table 2.
Median and 2.5th percentile neutrophil counts of children by geo-ethnicity
| Neutrophils × 109/L | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Days | Weeks | Months | Years | ||||||
| 1 | 2–7 | 2–4 | 2–12 | 2 | 3 | 4 | 5–6 | ||
| North Africans | Median | 5.8 | 4.6 | - | 2.7 | 2.6 | 3.0 | 3.1 | 3.8 |
| 2.5th | 0.9 | 0.9 | 0.5 | 0.7 | 0.7 | 0.6 | 0.9 | ||
| (171) | (84) | (252) | (363) | (132) | (104) | (108) | |||
| Peninsular Arabs | Median | 9.0 | 5.3 | 2.7 | 3.4 | 3.6 | 3.8 | 3.8 | 3.5 |
| 2.5th | 1.0 | 1.3 | 0.8 | 0.7 | 0.8 | 1.0 | 1.0 | 1.0 | |
| (2,585) | (969) | (529) | (5,929) | (4,782) | (2,187) | (1,399) | (776) | ||
| 2 – 6 | |||||||||
| Green Crescent Arabs | Median | 7.2 | 5.6 | - | 3.2 | 3.3 | |||
| 2.5th | 0.7 | 1.4 | 0.9 | 1.0 | |||||
| (178) | (95) | (239) | (880) | ||||||
| Iranians & Afghanis | Median | - | - | - | - | 3.9 | |||
| 2.5th | 1.4 | ||||||||
| (57) | |||||||||
| Indian Subcontinent | Median | 7.5 | 5.1 | - | 3.2 | 3.2 | |||
| 2.5th | 0.9 | 1.9 | 0.7 | 1.1 | |||||
| (180) | (98) | (211) | (510) | ||||||
| Filipinos | Median | - | - | - | - | 2.8 | |||
| 2.5th | 1.4 | ||||||||
| (51) | |||||||||
Values in parenthesis are number of children (only for n ≥50)
North Africans (1,243): Egyptians (771), Sudanese (278), Moroccan (67), Somalis (44), Mauritanians (39), Tunisians (20), Ethiopians (10), Algerians (7), Eritreans (4), Libyans (2)
Peninsular Arabs (21,883): Emiratis (19,545), Yemenis (1,058), Omanis (952), Saudis (292), Qataris (18), Bahrainis (7), Bedouins (6), Kuwaitis (5)
Green Crescent Arabs (1,119): Syrians (448), Jordanians (365), Palestinians (222), Lebanese (51), Iraqis (33)
Indian Subcontinent (1,042): Indians (490), Pakistanis (448), Bangladeshis (82), Sri Lankans (16), Nepalese (6)
We attempted to confirm the previously reported correlation between the monocyte and the neutrophil counts [7]. We found that the Spearman’s correlation coefficients between neutrophil and monocyte counts in the four largest ethnic groups were as follows: Egyptians, 0.46; Sudanese, 0.51; Emiratis, 0.44; and Indians, 0.47.
In the 12,703 Emirati children (aged six-months to six-years), the prevalence of NP (Fig. 2) was 10.6 %, similar to that of healthy adult Emiratis (10.7 %) [7]. The prevalence of severe NP of the children in this study was 0.53 %. For this sub-group we estimated frequency of NP allele which was 6.3 %, while the frequency of phenotype-derived heterozygosity was 11.6 %. The latter value was 1.51 % higher than the milder NP (10.7 % – 0.53 % = 10.07 %) observed in the studied population. This 1.51 % discrepancy in the frequency is explained by hidden NP.
Discussion
This study confirms that NP is common in people from North Africa, the Middle East and South Asia (Fig. 2 and Table 2). The neutrophil counts were highest at birth, decreased in the first seven days of life, and reached a nadir in the fourth week of life. Thereafter, the counts increased until 6 months of age and changed a little from six months to six years (Fig. 1). This pattern is similar to that in European children [22–24]. The median neutrophil count was lower in male neonates than female neonates (Table 1). In one study of adult Africans, males had lower neutrophil counts than females [16].
People from North African countries have the highest frequency of NP, ranging from 14.7 to 20.7 % (Fig. 2). Benign NP was first reported from Africa and from countries to which Africans have migrated [2, 3, 25]. A high prevalence of NP was found in the Sudanese who migrated to the Middle East, and amongst Yemenis and Ethiopian Jews who might have acquired the trait from neighboring African populations [6, 8]. This study shows that 10.5 % of Yemenis and 20 % children from the neighboring Somalia have NP. The frequency of NP in Peninsular Arabs ranged from 7.9 to 14.2 %, and Green Crescent Middle East residents from 8.2 to 9.8 % (Fig. 2). The Duffy negative blood group, strongly associated with benign NP in societies of African ancestry, is also common among Arab populations [26]. This finding is consistent with the historical mixing of Arabs with native African populations suggesting a common origin. In the geographically more distant Iranians and Afghanis, the frequency of NP is considerably lower (3.1 and 2.5 %, respectively) being similar to Europeans (<2.5 %) [23]. Furthermore, Iranians, Afghanis and Europeans share common pre-historic origins. In the Indian subcontinent, frequency of NP increased from 5.6 % in Pakistanis (closer to Afghanis) to 10.2 % in Indians.
This geoethnic distribution of frequencies raises a possibility that Afro-Arab and Indian NP have independent origins (involving the same or different genetic mutations). This hypothesis is supported by the absence of associations with the Duffy negative genotype (a biomarker for African NP) among Indians [9, 27]. High prevalence of NP is also found in Filipinos (Fig. 2); historically, this nation has received migrants from the Indian subcontinent and does not have the Duffy negative genotype [28].
Considerable number of children have neutrophil counts <0.5 ×109/L (2.8 % of Sudanese, 1.8 % of Filipinos and 1.5 % of Egyptians and Indians). A different genetic mutation could account for this severe NP phenotype. On the other side, in pedigree analyses in earlier study, consanguineous parents of offspring with severe NP have milder phenotype, suggesting a co-dominant inheritance [7]. Severe NP, thus, could represent a homozygote genotype and milder NP (nearly always >0.8 ×109/L) a heterozygote genotype. This mode of inheritance is supported by our estimated frequency of presumed heterozygote (2pq, milder plus hidden phenotype) derived from frequency of severe NP (presumed homozygote, q2) in 12,703 Emiratis. In over dozen kinship groups (data not shown), the frequency of both phenotypes correlates (r = 0.468), providing additional support that one mutation is a cause of benign NP. However, other genes and polymorphisms are known to impact neutrophil production and more than one gene could be involved in benign NP [29]. A possible effect of environmental factors that could affect gene expression could not be excluded.
In studied populations, two observations support the benign nature of NP. The prevalence of NP in 12,703 Emirati children (10.6 %) is similar to 10.7 % in 1,032 healthy adult Emiratis [7]. This unchanged frequency, over an estimated18-year-period, is an epidemiological evidence of its benign nature. The positive correlation between the monocyte and neutrophil counts in four large ethnic groups (Egyptians, Sudanese, Emiratis and Indians) supports benign rather than secondary NP, in which monocytosis is a more common finding [30–32]. In general, our findings agree with earlier reports of a high frequency of benign NP in children from Sudan and Jordan [4, 6].
The “malaria hypothesis” proposed as an explanation of high prevalence of benign NP is based on (i) a long history of endemic malaria in populations which have high prevalence of NP and (ii) its associated monocytopenia which is linked with a less intense phagocyte-mediated inflammation [7, 33–35]. The finding of low prevalence of NP among Iranians and Afghanis (whose ancestral population recently migrated from the north to this region) and a high prevalence among people from Indian subcontinent and Philippines (in whom NP has not been previously reported) supports the hypothesis (Fig. 2). In addition, the low monocyte count we found in four ethnic groups with NP (correlation coefficients, 0.44 to 0.51) supports the assertion of the benign nature of the observed NP and, indirectly, the “malaria hypothesis.”
An important clinical implication of this study is that our suggested cutoff for NP (2.5th percentile) in African Arabs should be 0.9 × 109/L, in Middle Easterners 1.0 × 109/L, and in Indians 1.1 × 109/L (Table 2). In healthy adult Emiratis and adult Africans, this threshold should be 1.0 × 109/L and 0.9 × 109/L, respectively [7, 16]. These numbers are considerable lower than the commonly used NP cutoff of 1.5 × 109/L. Consequently, healthy individuals from these regions with neutrophil counts <1.5 × 109/L often undergo unnecessary investigations and inappropriate treatment [15, 17]. This problem could be addressed in several ways: (i) issuing ‘benign NP health cards’ to identify subjects with NP; (ii) creating a national electronic registry for NP; (iii) the screening for NP by adding leucocyte differential count to the screening tests for hemoglobinopathies already performed in many countries, and (iv) developing ethnic/nation-specific neutrophil reference values. In addition, general guidelines for investigating apparently healthy neutropenic subjects could improve the quality of health care delivery and lower health care costs. In conclusion, at least 137 million of the consolidated 1.8 billion children and adults of the 16 countries represented in this study (Additional file 1: Table S1) could benefit from higher awareness of physicians about benign neutropenia, preventing investigation and management pitfalls in neutropenic patients.
Acknowledgment
Authors thank Mukesh M. Agarwal for the useful comments.
Authors’ contributions
SD designed the study, performed research, interpreted data, and wrote the manuscript. HN performed research, interpreted data, and revised the manuscript. LAAM, SAH, OAJ, and AKS performed research and interpreted data. All authors read and approved the manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional file
Neutrophil count distribution of 14,796 children from eight Arab populations. Table S1. Estimated number of children and adults with neutropenia in 16 North African, Middle Eastern and Asian countries. (DOCX 255 kb)
References
- 1.Haddy TB, Rana SR, Castro O. Benign ethnic neutropenia: What is a normal absolute neutrophil count? J Lab Clin Med. 1999;133:15–22. doi: 10.1053/lc.1999.v133.a94931. [DOI] [PubMed] [Google Scholar]
- 2.Howells DP. Neutropenia in people of African origin. Lancet. 1971;2:1318–9. doi: 10.1016/S0140-6736(71)90636-2. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh MM, Everhart JE, Byrd-Holt DD, Tisdale JF, Rodgers GP. Prevalence of neutropenia in the U.S. population: Age, sex, smoking status, and ethnic differences. Ann Intern Med. 2007;146:486–92. doi: 10.7326/0003-4819-146-7-200704030-00004. [DOI] [PubMed] [Google Scholar]
- 4.Jumean HG, Sudah FI. Chronic benign idiopathic neutropenia in Jordanians. Acta haemat. 1983;69:59–60. doi: 10.1159/000206841. [DOI] [PubMed] [Google Scholar]
- 5.Kaab SA, Fadhli SA, Burhamah M, Al Jafar H, Khamis A. Lymphocyte subsets in healthy adult Kuwaiti Arabs with relative benign ethnic neutropenia. Immunology Letter. 2004;91:49–53. doi: 10.1016/j.imlet.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Shoenfeld Y, Alkan ML, Asaly A, Carmell Y, Katz M. Benign familial leucopenia and neutropenia in different ethnic groups. Eur J Haematol. 1988;41:273–7. doi: 10.1111/j.1600-0609.1988.tb01192.x. [DOI] [PubMed] [Google Scholar]
- 7.Denic S, Showqi S, Klein C, Takala M, Nagelkerke N, Agarwal MM. Prevalence, phenotype and inheritance of benign neutropenia in Arabs. BMC Blood Disord. 2009;9:3. doi: 10.1186/1471-2326-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weingarten MA, Pottick-Schwartz EA, Brauner A. The epidemiology of benign leucopenia in Yemenite Jews. Isr J Med Sci. 1993;29:297–9. [PubMed] [Google Scholar]
- 9.Grann VR, Ziv E, Joseph CK, Neugut AI, Wei Y, Jacobson JS, Horwitz MS, Bowman N, Beckmann K, Hershman DL. Duffy (Fy), DARC, and neutropenia among women from the United States, Europe and the Caribbean. Br J Haemat. 2008;143:288–293. doi: 10.1111/j.1365-2141.2008.07335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly DL, Kreyenbuhl J, Dixon L, et al. Clozapine underutilization and discontinuation in African Americans due to leucopenia. Schizophr Bull. 2007;33:1221–4. doi: 10.1093/schbul/sbl068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuno E, Rothbard AB. Racial disparities in antipsychotic prescription patterns for patients with schizophrenia. Am J Psychiatry. 2002;159:567–72. doi: 10.1176/appi.ajp.159.4.567. [DOI] [PubMed] [Google Scholar]
- 12.Smith K, Wray L, Klein-Cabral M, Schuchter L, Fox K, Glick J, DeMichele A. Ethnic disparities in adjuvant chemotherapy for breast cancer are not caused by excess toxicity in black patients. Clin Breast Cancer. 2005;6:260–6. doi: 10.3816/CBC.2005.n.029. [DOI] [PubMed] [Google Scholar]
- 13.Hershman D, Weinberg M, Rosner Z, et al. Ethnic neutropenia and treatment delay in African American women undergoing chemotherapy for early-stage breast cancer. J Natl Cancer Inst. 2003;15:1545–8. doi: 10.1093/jnci/djg073. [DOI] [PubMed] [Google Scholar]
- 14.Melia MT, Muir AJ, McCone J, Shiffman ML, King JW, Herrine SK, Galler GW, Bloomer JR, Nunes FA, Brown KA, Mullen KD, Ravendhran N, Ghalib RH, Boparai N, Jiang R, Noviello S, Brass CA, Albrecht JK, McHutchison JG, Sulkowski MS, IDEAL Study Team Racial differences in hepatitis C treatment eligibility. Hepatology. 2011;54:70–8. doi: 10.1002/hep.24358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Rooijen CR, Slieker WA, Simsek S. Benign ethnic neutropenia; an unrecognized cause of leukopenia in negroid patients. Ned Tijdschr Geneeskd. 2012;156:A4708. [PubMed] [Google Scholar]
- 16.Eller LA, Eller MA, Ouma B, Kataaha P, Kyabaggu D, Tumusiime R, Wandege J, Sanya R, Sateren WB, Wabwire-Mangen F, Kibuuka H, Robb ML, Michael NL, de Souza MS. Reference intervals in healthy adult Ugandan blood donors and their impact on conducting international vaccine trials. Plos One. 2008;3:e3919. doi: 10.1371/journal.pone.0003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denic S, Nicholls MG. A call for screening for benign neutropenia in Arab populations. Saudi Medical Journal. 2011;32:738–9. [PubMed] [Google Scholar]
- 18.Heard-Bey F. The tribal society of the UAE and its traditional economy. In: Al Abed I, Hellyer P, editors. United Arab Emirates: A new perspective. London: Trident Press; 2001. pp. 98–116. [Google Scholar]
- 19.Al-Gazali IL, Bener A, Abdulrazzaq MY, Micallef R, Al Khayat AI, Gaber T. Consanguineous marriages in the United Arab Emirates. J Biosoc Sci. 1997;29:491–7. doi: 10.1017/S0021932097004914. [DOI] [PubMed] [Google Scholar]
- 20.Denic S. Aden B, Nagelkerke N, Al Essa A. Beta-thalassemia in Abu Dhabi: Consanguinity and tribal stratification are major factors explaining the high prevalence of disease. Hemoglobin. 2013. Early Online: 1–8. [DOI] [PubMed]
- 21.Gillespie JH. Population Genetics: A Concise Guide Baltimore. Baltimore: The John Hopkins Univ Press; 1998. [Google Scholar]
- 22.Segel GB, Halterman JS. Neutropenia in pediatric practice. Pediatr Rev. 2008;29(1):12–23. doi: 10.1542/pir.29-1-12. [DOI] [PubMed] [Google Scholar]
- 23.Aldrimer M, Ridefelt P, Rödöö P, Niklasson F, Gustafsson J, Hellberg D. Population-based pediatric reference intervals for hematology, iron and transferrin. Scand J Clin Lab Invest. 2013;73:253–61. doi: 10.3109/00365513.2013.769625. [DOI] [PubMed] [Google Scholar]
- 24.Ahsan S, Noether J. The Harriet Lane. Philadelphia: Elsevier Mosby; 2011. Hematology, Chapter 14; pp. 322–353. [Google Scholar]
- 25.Zezulka AV, Gill JS, Beevers DG. 'Neutropenia' in black west Indians. Postgrad Med J. 1987;63:257–61. doi: 10.1136/pgmj.63.738.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandler SG, Kravitz C, Sharon R, Hermoni D, Ezekiel E, Cohen T. The Duffy blood group system in Israeli Jews and Arabs. Vox Sanguinis. 1979;37:41–6. doi: 10.1111/j.1423-0410.1979.tb02267.x. [DOI] [PubMed] [Google Scholar]
- 27.Anita C, Sujata M, Yogesh Kumar J, Aparup D. Natural Selection Mediated Association of the Duffy (FY) Gene Polymorphisms with Plasmodium vivax Malaria in India. Plos One. 2012;7:e45219. doi: 10.1371/journal.pone.0045219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng CT, Tsai CH, Lee HH, Lin CL, Wang NM, Chang JG. Molecular analysis of Duffy, Yt and Colton blood groups in Taiwanese, Filipinos and Thais. Kaohsiung J Med Sci. 2000;16:63–7. [PubMed] [Google Scholar]
- 29.Reiner AP, Lettre G, Nalls MA, Ganesh SK, Mathias R, et al. Genome-Wide Association Study of White Blood Cell Count in 16,388 African Americans: the Continental Origins and Genetic Epidemiology Network (COGENT) PLoS Genet. 2011;7(6):e1002108. doi: 10.1371/journal.pgen.1002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thobakgale FC, Ndung’u T. Neutrophil counts in persons of African origin. Curr Opin Hematol. 2014;21:50–7. doi: 10.1097/MOH.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 31.Bux J, Kissel K, Nowak K, Spengel U, Mueller-Eckhardt C. Autoimmune neutropenia: clinical and laboratory studies in 143 patients. Ann Hematol. 1991;63:249–52. doi: 10.1007/BF01698373. [DOI] [PubMed] [Google Scholar]
- 32.Coşkun O, Avci IY, Sener K, Yaman H, Ogur R, Bodur H, Eyigün CP. Relative lymphopenia and monocytosis may be considered as a surrogate marker of pandemic influenza a (H1N1) J Clin Virol. 2010;47:388–9. doi: 10.1016/j.jcv.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Chung BH, Chan GC, Lee T, Kwok JS, Chiang AK, Ho HK, Ha SY, Lau YL. Chronic benign neutropenia among Chinese children. Hong Kong Med J. 2004;10:231–6. [PubMed] [Google Scholar]
- 34.Nathan C. Neutrophils and immunity: challenges and opportunities. Nature Rev Imm. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen PH, Day N, Pram TD, Ferguson DJ, White NJ. Intraleucocytic malaria pigment and prognosis in severe malaria. Trans R Soc Trop Med Hyg. 1995;89:200–4. doi: 10.1016/0035-9203(95)90496-4. [DOI] [PubMed] [Google Scholar]


