Abstract
Background
Comorbid diabetes is common in heart failure and associated with increased hospitalization and mortality. Nonetheless, the association between glycemic control and outcomes among patients with heart failure and diabetes remains poorly characterized, particularly among low income and minority patients.
Methods
We performed a retrospective cohort study of outpatients with heart failure and diabetes in the New York City Health and Hospitals Corporation, the largest municipal health care system in the United States. Cox proportional hazard models were used to measure the association between HbA1c levels and outcomes of all-cause hospitalization, heart failure hospitalization, and mortality.
Results
Of 4723 patients with heart failure and diabetes, 42.6 % were black, 30.5 % were Hispanic/Latino, 31.4 % were Medicaid beneficiaries and 22.9 % were uninsured. As compared to patients with an HbA1c of 8.0–8.9 %, patients with an HbA1c of <6.5, 6.5–6.9, 7.0–7.9, and ≥9.0 % had an adjusted hazard ratio (aHR) (95 % CI) for all-cause hospitalization of 1.03 (0.90–1.17), 1.05 (0.91–1.22), 1.03 (0.90–1.17), and 1.13 (1.00–1.28), respectively. An HbA1c ≥ 9.0 % was also associated with an increased risk of heart failure hospitalization (aHR 1.33; 95 % CI 1.11–1.59) and a non-significant increased risk in mortality (aHR 1.20; 95 % CI 0.99–1.45) when compared to HbA1c of 8.0–8.9 %.
Conclusions
Among a cohort of primarily minority and low income patients with heart failure and diabetes, an increased risk of hospitalization was observed only for an HbA1c greater than 9 %.
Keywords: Heart failure, Diabetes, hbA1c
Background
Heart failure and diabetes are two chronic diseases that commonly coexist. Among patients with heart failure, the prevalence of diabetes is as high as 40 % [1–3] and is increasing [4]. The high rate of diabetes in heart failure holds clinical significance as diabetes has been associated with worse outcomes in heart failure. For instance, in a meta-analysis of 17 studies, patients with heart failure and diabetes were found to have a 28 % increase in mortality and a 36 % increase in hospitalizations as compared to patients with heart failure but no diabetes [5]. The relationship between heart failure and diabetes may be magnified among high-risk populations such as racial minorities, individuals of low socioeconomic status, and the uninsured [6–10].
Despite the increased risk of poor outcomes among patients with heart failure and diabetes, the optimal hemoglobin A1c (HbA1c) level for patients with heart failure and diabetes remains uncertain. Due to lack of existing data, heart failure guidelines do not specifically address the intensity of glycemic control among individuals with heart failure [11], and the American Diabetes Association (ADA) guidelines do not provide a specific treatment target [12]. Although elevated levels of HbA1c have been associated with increased risk for heart failure related hospitalizations [13–16], intervention trials in patients with Type 2 diabetes have failed to show benefit with tight glycemic control and some studies have even suggested harm [17–19]. Additionally, tight glycemic control could predispose to volume retention in heart failure by decreasing the osmotic diuretic effect of glucosemia [20]. Given the potential side effects of both tight and loose glycemic control among heart failure patients, moderate glycemic control would seem appropriate. Indeed, a study of primarily male patients from Veterans Affairs (VA) medical centers demonstrated a U-shaped relationship between HbA1c and mortality, with the lowest risk observed for patients with an HbA1c between 7.1 and 7.8 % [21]. Conversely, two studies from a single academic advanced heart failure clinic found low [22, 23] HbA1c values to be associated with increased risk of mortality, while a high HbA1c was associated with mortality for heart failure patients enrolled in the CHARM study [24]. Notably, these prior studies focused on select patient populations such as those who were fully insured or who agreed to participate in a clinical trial [21–24]. As a result, the optimal HbA1c target for heart failure patients with diabetes remains uncertain, especially for members of racial and ethnic minorities not well represented in previous studies [21–24].
The purpose of this study was to evaluate the association of HbA1c with all-cause hospitalization among patients with heart failure and diabetes in a hospital system serving a diverse, urban, and primarily low-income population. We hypothesized that hospitalization rates would vary with level of HbA1c with a U-shaped relationship, such that moderate glycemic control would be associated with reduced all-cause hospitalization, heart failure hospitalization, and mortality.
Methods
We performed a non-concurrent cohort study of patients in the New York City Health and Hospitals Corporation (HHC), the largest municipal health care system in the United States. The corporation serves approximately 1.4 million people, of whom nearly 500,000 are uninsured, and includes 11 hospitals, six diagnostic and treatment centers, four long term care centers, and one hundred community health centers [25]. The primary data source for the study was the HHC data warehouse which contains data from the electronic health records (EHRs) from each of the hospitals and includes demographic information, clinical measures, problem lists, laboratory tests and results, and medication orders. Patient data from HHC was matched to two New York State data registries to obtain outcomes data: the New York State Vital Statistics and the New York Statewide Planning and Research Cooperative System (SPARCS). Vital statistics maintains records of deaths in the state, while SPARCS contains all acute care admissions for non-federal hospitals in the state.
We included patients with heart failure and diabetes who had an outpatient clinic visit in HHC between 1/1/2007 and 12/31/2010. Diagnoses were based on International Classification of Diseases, Clinical Modification (ICD-9-CM) codes of 428 and 250, respectively, in the problem list [3, 26]. Additional inclusion criteria were age 18 years and older and measurement of an HbA1c either on or up to 90 days prior to the clinic visit. For patients with multiple encounters, we only included the first visit for which inclusion criteria were met.
The primary outcome was all-cause hospitalization. Secondary outcomes were heart failure hospitalization and mortality. Heart failure hospitalization was based on a principal diagnosis of heart failure using standard ICD-9-CM codes [3, 27].
The primary exposure was HbA1c, which was categorized into the following clinically relevant categories: <6.5, 6.5–6.9, 7.0–7.9, 8.0–8.9, and ≥9.0 %. Other patient characteristics included age, gender, race, ethnicity, primary payer, prior utilization, comorbidities, diabetes severity, systolic blood pressure, pulse, creatinine, hemoglobin, active medications, prior utilization, and HHC facility of the clinic visit. Characteristics were assessed at time of clinic visit with the exception of laboratory values which, if unavailable at time of clinical visit, could be included if assessed up to 90 days prior to the clinic visit. In the data warehouse, race was self-reported as a single category and included responses of “Black or African American”, “Hispanic Black”, and “Hispanic or Latino”. From this information, we created a race variable that was categorized as black or other and an ethnicity variable which was categorized as Hispanic/Latino and other. Comorbidities were based on standard algorithms [28]. Diabetes severity was assessed using the diabetes complication severity index, a validated index of complications of diabetes that has been associated with hospitalization and mortality [29]. Medication utilization was based on an active prescription and included the following medication classes: loop diuretic, angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB), beta blocker, insulin, metformin, and sulfonylurea. Prior utilization included hospitalization in the prior 90 days and number of hospitalizations, emergency department visits, and clinic visits in the prior year.
Follow up time was defined from time of clinic visit. Patients were followed to the time of death or December 31, 2010; patients who were unable to be accurately matched to mortality data (n = 117) were censored on their last date of service.
Statistical analysis
Differences in frequency of outcomes among all HbA1c groups were compared using Pearson chi-squared tests. We plotted Kaplan-Meier curves for hospitalization; curves were compared using the log-rank test. Cox proportional hazard models were developed to determine the relative association of HbA1c category with the primary outcome variable, all-cause hospitalization. The proportionality assumption was evaluated graphically using the -log(−log(survival function)) plot and statistically using the Schoenfeld residuals. We developed adjusted Cox models incorporating baseline patient characteristics as well as shared-frailty to account for clustering within each HHC site [30]. Covariates included in the adjusted model were age, sex, race, ethnicity, insurance, comorbidities, blood pressure, heart rate, creatinine, hemoglobin, medications, and prior utilization. Similar models were developed for the secondary outcomes. We performed a sensitivity analysis in which we developed similar models with the primary independent variable of HbA1c categorized as <6.5, 6.5–6.9, 7.0–7.9, 8.0–8.9, 9.0–9.9, 10.0–11.9, and ≥12.0 %.
Statistical significance was pre-specified with an alpha level of 0.05 (two-tailed). Statistical analyses were performed using Stata 13 (StataCorp, College Station, TX).
This study was approved by the New York University School of Medicine Institutional Review Board.
Results
Of the 4723 patients with heart failure and diabetes included in the study, the median age was 64 years, 42.6 % were black, and 30.5 % were Hispanic or Latino. At the time of clinic visit, 31.4 % of patients were Medicaid beneficiaries and 22.9 % of patients had no insurance.
The mean HbA1c (SD) for the cohort was 8.2 (2.4). The distribution of HbA1c categories of <6.5, 6.5–6.9, 7.0–7.9, 8.0–8.9, and ≥9.0 % was: 21.8, 12.7, 22.6, 15.0, and 28.0 %, respectively. Patients with high HbA1c were younger and had high rates of Medicaid insurance when compared to patients with low HbA1c (Table 1). Patients in the lowest HbA1c group were most likely to be black and had the highest prevalence of kidney disease. Patients with higher HbA1c had higher rates of insulin use, while metformin was used most frequently among patients with HbA1c of 6.5–6.9 %. HbA1c appeared to be inversely correlated with number of outpatient encounters, as patients in the lowest two HbA1c groups had the highest average number of clinic visits. (Table 1).
Table 1.
Baseline characteristics of 4723 patients with heart failure and diabetes, by hemoglobin A1c (HbA1c) category
| HbA1c range | |||||
|---|---|---|---|---|---|
| <6.5 | 6.5–6.9 | 7–7.9 | 8–8.9 | ≥9 | |
| (n = 1028) | (n = 600) | (n = 1065) | (n = 706) | (n = 1324) | |
| Age, years | 67.1 (12.9) | 66.3 (12.4) | 65.4 (12.0) | 63.7 (11.7) | 60.9 (11.5) |
| Female | 53.6 | 52.0 | 55.6 | 52.3 | 49.2 |
| Black Race | 47.2 | 41.7 | 41.2 | 39.7 | 42.0 |
| Hispanic/Latino Ethnicity | 28.7 | 28.8 | 29.8 | 32.2 | 32.2 |
| Insurance | |||||
| Medicaid | 28.2 | 31.2 | 30.6 | 33.3 | 33.5 |
| Medicare | 30.5 | 27.0 | 25.7 | 24.1 | 18.7 |
| Private | 4.1 | 4.5 | 3.4 | 4.7 | 4.6 |
| Self-Pay | 18.0 | 18.8 | 22.4 | 24.9 | 27.7 |
| Other | 19.3 | 18.5 | 17.9 | 13.0 | 15.4 |
| Diabetes Severity Index | 3.5 (1.7) | 3.3 (1.7) | 3.3 (1.6) | 3.5 (1.7) | 3.4 (1.7) |
| Comorbid Conditions | |||||
| Myocardial Infarction | 10.8 | 13.0 | 11.8 | 11.2 | 12.2 |
| Peripheral Vascular Disease | 14.7 | 11.7 | 13.3 | 15.0 | 11.7 |
| Cerebrovascular Disease | 17.8 | 15.5 | 16.9 | 13.2 | 10.7 |
| Dementia | 2.2 | 2.3 | 2.0 | 1.4 | 1.1 |
| Chronic Pulmonary Disease | 23.8 | 26.3 | 27.2 | 24.5 | 25.8 |
| Rheumatic Disease | 2.4 | 1.8 | 2.0 | 1.1 | 1.4 |
| Liver Disease | 7.5 | 5.2 | 4.0 | 3.7 | 4.8 |
| Renal Disease | 30.3 | 19.7 | 18.6 | 21.1 | 21.4 |
| Malignancy or Tumor | 8.6 | 10.0 | 6.4 | 6.5 | 5.8 |
| Heart Rate, beats/min | 74.8 (13.1) | 75.2 (12.9) | 76.3 (13.2) | 77.6 (14.1) | 78.2 (13.8) |
| Systolic Blood Pressure, mmHG | 133.7 (22.4) | 135.3 (23.0) | 134.6 (22.2) | 135.6 (23.4) | 134.7 (22.9) |
| Creatinine, mg/dl | 1.9 (1.8) | 1.5 (1.2) | 1.5 (1.2) | 1.5 (1.3) | 1.5 (1.3) |
| Hemoglobin, g/dl | 11.6 (2.0) | 12.0 (1.9) | 12.0 (1.9) | 12.1 (1.9) | 12.2 (2.0) |
| ACE inhibitor or ARB | 55.1 | 58.2 | 58.8 | 56.7 | 59.9 |
| Beta-Blocker | 36.7 | 34.8 | 37.5 | 35.6 | 34.5 |
| Loop Diuretic | 57.9 | 63.0 | 62.4 | 62.3 | 57.4 |
| Insulin | 21.7 | 25.2 | 35.8 | 50.9 | 62.3 |
| Metformin | 14.1 | 22.2 | 16.4 | 18.8 | 19.5 |
| Sulfonylurea | 9.0 | 11.7 | 12.4 | 9.8 | 10.6 |
| Prior Utilization | |||||
| Hospitalization in Prior 90 Days | 54.3 | 45.3 | 52.8 | 53.8 | 58.9 |
| Hospitalizations, Prior Year | 1.5 (2.0) | 1.2 (1.8) | 1.4 (1.9) | 1.4 (1.7) | 1.5 (2.0) |
| ED Visits, Prior Year | 0.6 (1.3) | 0.5 (1.1) | 0.6 (1.7) | 0.5 (1.3) | 0.6 (1.3) |
| Clinic Visits, Prior Year | 6.9 (12.8) | 7.3 (15.3) | 6.6 (15.0) | 5.3 (12.6) | 5.5 (12.2) |
Values are percentages or mean (standard deviation)
For the primary outcome of all-cause hospitalization, patients were followed for a median (25th,75th percentile) of 173 (46,452) days. During follow up, 67.0 % of patients were hospitalized for any cause. Overall rates of hospitalization were similar across HbA1c groups (Table 2). Similarly, we found no difference in the Kaplan Meier curves between HbA1c groups (see Fig. 1). In unadjusted analysis, an HbA1c ≥9 % was associated with increased risk of hospitalization, while an HbA1c < 6.5 % was associated with risk of hospitalization that did not reach statistical significance (Table 3). After adjusting for covariates, the lowest four categories of HbA1c (<6.5, 6.5–6.9, 7.0–7.9, and 8.0–8.9 %) were associated with similar hazard for hospitalization (Table 3). Only patients with an HbA1c ≥ 9.0 % had a significant increased risk for hospitalization (adjusted HR 1.13; 95 % CI 1.00–1.28) when compared to patients with HbA1c 8.0–8.9 % (Table 3).
Table 2.
Frequency of outcome events of hospitalization, mortality, and heart failure hospitalization during follow up, by HbA1c category
| HbA1c range | p | |||||
|---|---|---|---|---|---|---|
| <6.5 (n = 1028) | 6.5–6.9 (n = 600) | 7–7.9 (n = 1065) | 8–8.9 (n = 706) | ≥9 (n = 1324) | ||
| Hospitalization | 68.6 | 64.7 | 65.2 | 66.2 | 68.9 | 0.16 |
| Heart Failure Hospitalization | 26.6 | 27.5 | 28.9 | 28.8 | 35.3 | <0.001 |
| Mortality | 30.5 | 25.5 | 24.1 | 23.7 | 26.8 | <0.01 |
Fig. 1.
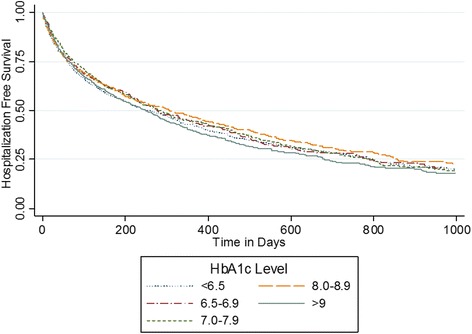
Kaplan Meier curves for hospitalization free survival by HbA1c category among 4723 patients with heart failure and diabetes. p = 0.10 for differences among curves using the log-rank test
Table 3.
Hazard ratio for outcomes of hospitalization, mortality, and heart failure hospitalization by HbA1c category among 4723 patients with heart failure and diabetes. Adjusted for age, sex, race, ethnicity, insurance, comorbidities, blood pressure, heart rate, creatinine, hemoglobin, medications, and prior utilization
| Hospitalization | Heart failure hospitalization | Mortality | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| HbA1c category | ||||||
| <6.5 | 1.12 (0.99–1.26) | 1.03 (0.90–1.17) | 0.95 (0.79–1.14) | 0.92 (0.75–1.13) | 1.32 (1.10–1.60) | 1.16 (0.94–1.42) |
| 6.5–6.9 | 1.06 (0.93–1.22) | 1.05 (0.91–1.22) | 0.97 (0.79–1.20) | 1.03 (0.83–1.29) | 1.09 (0.87–1.35) | 1.11 (0.88–1.41) |
| 7–7.9 | 1.04 (0.93–1.18) | 1.03 (0.90–1.17) | 1.07 (0.89–1.28) | 1.12 (0.93–1.36) | 1.03 (0.85–1.25) | 1.08 (0.88–1.33) |
| 8–8.9 | 1 [ref] | 1 [ref] | 1 [ref] | 1 [ref] | 1 [ref] | 1 [ref] |
| ≥9 | 1.15 (1.03–1.29) | 1.13 (1.00–1.28) | 1.31 (1.11–1.55) | 1.33 (1.11–1.59) | 1.13 (0.94–1.36) | 1.20 (0.99–1.45) |
The rate of heart failure hospitalizations was 35.3 % for patients with HbA1c ≥ 9.0 % and 26.6–28.9 % for patients in categories with lower HbA1c (Table 2). After adjustment for covariates, HbA1c ≥ 9.0 % was associated with a hazard ratio for heart failure hospitalization of 1.33 (95 % CI 1.11–1.59) when compared to HbA1c 8.0–8.9 %. Other categories of HbA1c did not significantly differ in hazard ratio for heart failure hospitalization when compared to the reference (Table 3).
The median (25th,75th percentile) follow up for mortality was 658 (289,1114) days. Over 30 % of patients with HbA1c < 6.5 % died during the period while mortality rates for higher HbA1c categories ranged from 23.7 to 26.8 % (Table 2). In unadjusted analysis, HbA1c < 6.5 % was associated with a 32 % (95 % CI 10–60 %) increase in hazard of death as compared to the reference group of HbA1c 8.0–8.9 %. However, this association was attenuated and was no longer significant after adjusting for covariates (adjusted HR 1.16; 95 % CI 0.94–1.42; Table 3). Similarly, an elevated HbA1c ≥ 9 % was associated with increased mortality in multivariable analysis that did not reach statistical significance (adjusted HR 1.20; 95 % CI 0.99–1.45; Table 3). Indeed, in multivariate adjustment, no level of HbA1c was statistically associated with increased mortality.
In sensitivity analysis in which we categorized higher HbA1c values into 9.0–9.9 %, 10.0–11.9 %, and ≥12 %, we found that hospitalization risk was similar for each of these individual categories as when grouped together (Appendix). An HbA1c over 12 % was associated with a significant increase in mortality as compared to HbA1c 8.0–8.9 %, with an adjusted HR 1.33 (95 % CI 1.03–1.73). We found no significant association between HbA1c of 9.0–9.9 % or 10.0–11.9 % and mortality (Appendix).
Discussion
In this cohort of patients with heart failure and diabetes from a diverse, urban, and primarily low-income hospital system, we found no difference in risk of hospitalization or mortality for HbA1c levels up to 8.9 %. Hospitalization risk only increased at the highest HbA1c level; an HbA1c of 9.0 % or higher was associated with a 13 % increase in the relative hazard of all-cause hospitalization and a 33 % increase in the relative hazard of heart failure hospitalization when compared to lower HbA1c values. Furthermore, we found the association between HbA1c and mortality to be significant only for HbA1c levels above 12 %. These findings suggest that, in this patient population, glycemic control is not a strong predictor of outcomes in heart failure and diabetes and risk of hospitalization begins to increase only when HbA1c ≥ 9 %.
Consistent with our initial hypothesis, that both high and low levels of HbA1c would be associated poor outcomes, we found that an HbA1c < 6.5 % was associated with increased risk of mortality and a nonsignificant increase in hospitalization in unadjusted analysis. However, a low HbA1c was also associated with a number of factors associated with poor outcomes, including increased age, cerebrovascular disease, and creatinine. After adjusting for such confounders, a low HbA1c was no longer associated with mortality or hospitalization. Some studies have found that HbA1c may have a U- or J-shaped relationship to outcomes [21, 31, 32], suggesting there may also be a low threshold, below which risk increases [32]. In this population of patients with heart failure and diabetes, any risk associated with low HbA1c was explained by other factors. These findings do not support a low threshold effect in patients with heart failure and diabetes.
In our study, only levels of HbA1c of 9 % or higher were associated with hospitalization and mortality. Conversely, observational studies in the general population have suggested that mortality risk increases when the HbA1c rises above 7 % [33, 34], the level recommended by the ADA for most patients with diabetes [12]. Results of four prior studies of the association between HbA1c and health outcomes among patients with heart failure and diabetes have been mixed. Two studies of patients with advanced heart failure from the same single center suggested that tighter glycemic control was associated with increased mortality [22, 23]. Conversely, among a subgroup of patients with heart failure and diabetes enrolled in the CHARM study, Gerstein and colleagues found that a 1 % increase in HbA1c was associated with an increased hazard ratio of 1.13 for heart failure hospitalization and 1.11 for mortality [24]. Finally, Aguilar and colleagues studied 5815 male patients in the VA and found that an HbA1c of 7.1–7.8 % was associated with a hazard ratio for mortality of 1.31–1.45 as compared to both higher and lower values. However, the authors found no significant association between HbA1c and hospitalization [21].
While the studies by Gerstein [24] and Aguilar [21] found higher HbA1c was associated with increased risk of mortality, we found no statistical association between HbA1c and mortality in our primary analysis. The difference between our study and the prior studies is that we included a real-world patient population with an even gender mix and high rates of patients who were uninsured and of racial/ethnic minorities; studies by Gerstein and Aguilar included more selective subjects who were enrolled in a clinical trial or were primarily male and insured through the VA, respectively. Nonetheless, we do not believe findings in our study can be fully explained by racial or ethnic mix. Although both black and Hispanic patients are known to have higher levels of HbA1c as compared to white patients [35–37], HbA1c has been shown to have a similar relationship for clinical outcomes among different racial groups [36, 38] and the same target HbA1c has been recommended for all patients with diabetes, regardless of race [12].
Our findings should be interpreted in the context of study limitations. First, inclusion was based on diagnostic coding algorithms and may be subject to misclassification despite using validated diagnostic codes [39, 40]. Second, although the HHC database contains extensive data on vital signs, inpatient and outpatient claims, medication utilization and laboratory data, it may lack data on several potentially important variables, including ejection fraction and functional status. As a result, we were unable to determine of HbA1c had a differential association with outcomes for patients with heart failure with reduced versus preserved ejection fraction or patients with type 1 versus type 2 diabetes. Third, as we were relied on real world EHR data, some of our data may be subject to misclassification, such as self-reported race field. Fourth, the data relied on the EHR within a single hospital system so we may have missed medication prescriptions and clinical encounters that occurred at another facility. Fifth, as we relied on a state registry for hospitalization information, we may have missed some hospitalizations that occurred out of state. Sixth, the median follow up for mortality was 658 days. Nonetheless, as the mortality rate was over 25 % in this study, the null finding of association between HbA1c and mortality was probably not fully attributable to low event rate.
Our study had a number of unique strengths. We included patients from a large public health system which serves many uninsured and minority patients in real world practice settings. Such patients are frequently underrepresented in clinical trials [41]. Additionally, we linked our EHR data to state registry data to obtain complete hospitalization follow up. This is notable as a large number heart failure related hospitalizations are hospitals other than where care is primarily obtained [42].
Conclusions
We found the associated risk of hospitalization increases for HbA1c ≥ 9 %, and not lower HbA1c values, among patients with heart failure and diabetes. The ADA currently recommends targeting an HbA1c < 7 % for most patients with diabetes but suggests that “less stringent A1c goals (such as < 8 %) may be appropriate” for subpopulations such as those with limited life expectancy or significant comorbidity [12]. In support of less stringent glycemic target for patients with heart failure and diabetes, our data suggest that there may be a weak threshold effect of HbA1c above 9 % in this population. Nonetheless, randomized control trials are needed to determine the recommended HbA1c for patients with heart failure and diabetes, who are at high risk of hospitalization and mortality.
Ethics approval and consent to participate
This study was approved by the NYU School of Medicine institutional review board, which granted a waiver of consent.
Consent for publication
Not applicable.
Availability of data and materials
Data will not be shared due to restrictions related to patient confidentiality.
Acknowledgements
We thank Yan Rosentsveyg, Frank Chimkin, and Lisa Elkind at the New York City Health and Hospitals Corporation for their assistance with obtaining the data used in this study and Margaret Paul, MS for assistance with data cleaning.
Funding
This work was supported by the National Center for Advancing Translational Sciences (NCATS) grant KL2 TR000053 and the Agency for Healthcare Research and Quality (AHRQ) K08 HS23683.
Abbreviations
- HbA1c
hemoglobin A1c
- ADA
American Diabetes Association
- VA
Veterans Affairs
- HHC
New York City Health and Hospitals Corporation
- ICD-9-CM
International Classification of Diseases, Clinical Modification
- ACE
angiotensin converting enzyme
- ARB
angiotensin receptor blocker
- HR
hazard ratio
- CI
confidence interval
- EHR
electronic health record
Appendix
Table 4.
Hazard ratio for outcomes using further stratification of HbA1c vlues. Adjusted for age, sex, race, ethnicity, insurance, comorbidities, blood pressure, heart rate, creatinine, hemoglobin, medications, and prior utilization
| Hospitalization | Heart failure hospitalization | Mortality | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| HbA1c Category | ||||||
| <6.5 | 1.12 (0.99–1.26) | 1.03 (0.90–1.17) | 0.95 (0.79–1.14) | 0.92 (0.75–1.13) | 1.32 (1.10–1.60) | 1.16 (0.94–1.42) |
| 6.5–6.9 | 1.06 (0.93–1.22) | 1.05 (0.91–1.22) | 0.97 (0.79–1.20) | 1.03 (0.83–1.29) | 1.09 (0.87–1.35) | 1.11 (0.88–1.41) |
| 7–7.9 | 1.04 (0.93–1.18) | 1.03 (0.90–1.17) | 1.07 (0.89–1.28) | 1.12 (0.93–1.36) | 1.03 (0.85–1.25) | 1.08 (0.88–1.33) |
| 8–8.9 | 1 [ref] | 1 [ref] | 1 [ref] | 1 [ref] | 1 [ref] | 1 [ref] |
| 9–9.9 | 1.14 (0.99–1.32) | 1.10 (0.94–1.28) | 1.23 (0.99–1.52) | 1.22 (0.98–1.53) | 1.14 (0.90–1.44) | 1.15 (0.90–1.48) |
| 10–11.9 | 1.15 (1.00–1.33) | 1.17 (1.01–1.36) | 1.27 (1.03–1.57) | 1.39 (1.12–1.73) | 1.07 (0.85–1.34) | 1.16 (0.91–1.48) |
| ≥12 | 1.16 (0.99–1.36) | 1.11 (0.94–1.31) | 1.47 (1.18–1.82) | 1.38 (1.09–1.74) | 1.22 (0.95–1.56) | 1.33 (1.03–1.73) |
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SB led the study conception and design, participated in analysis of data, and drafted the manuscript. HP performed the statistical analysis. SK participated in conception and design and revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
References
- 1.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149(2):209–16. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg BH, Abraham WT, Albert NM, Chiswell K, Clare R, Stough WG, et al. Influence of diabetes on characteristics and outcomes in patients hospitalized with heart failure: a report from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Am Heart J. 2007;154(2):277. doi: 10.1016/j.ahj.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure-associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61(12):1259–67. doi: 10.1016/j.jacc.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.From AM, Leibson CL, Bursi F, Redfield MM, Weston SA, Jacobsen SJ, et al. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006;119(7):591–9. doi: 10.1016/j.amjmed.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Kamalesh M, Cleophas TJ. Heart failure due to systolic dysfunction and mortality in diabetes: pooled analysis of 39,505 subjects. J Card Fail. 2009;15(4):305–9. doi: 10.1016/j.cardfail.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168(19):2138–45. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foraker RE, Rose KM, Suchindran CM, Chang PP, McNeill AM, Rosamond WD. Socioeconomic status, Medicaid coverage, clinical comorbidity, and rehospitalization or death after an incident heart failure hospitalization: Atherosclerosis Risk in Communities cohort (1987 to 2004) Circ Heart Fail. 2011;4(3):308–16. doi: 10.1161/CIRCHEARTFAILURE.110.959031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Link CL, McKinlay JB. Disparities in the prevalence of diabetes: is it race/ethnicity or socioeconomic status? Results from the Boston Area Community Health (BACH) survey. Ethn Dis. 2009;19(3):288–92. [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins JM, Vaccarino V, Zhang H, Kasl SV. Socioeconomic status and type 2 diabetes in African American and non-Hispanic white women and men: evidence from the Third National Health and Nutrition Examination Survey. Am J Public Health. 2001;91(1):76–83. doi: 10.2105/AJPH.91.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winkleby MA, Kraemer HC, Ahn DK, Varady AN. Ethnic and socioeconomic differences in cardiovascular disease risk factors: findings for women from the Third National Health and Nutrition Examination Survey, 1988–1994. JAMA. 1998;280(4):356–62. doi: 10.1001/jama.280.4.356. [DOI] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 12.American DA. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 13.Matsushita K, Blecker S, Pazin-Filho A, Bertoni A, Chang PP, Coresh J, et al. The association of hemoglobin a1c with incident heart failure among people without diabetes: the atherosclerosis risk in communities study. Diabetes. 2010;59(8):2020–6. doi: 10.2337/db10-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pazin-Filho A, Kottgen A, Bertoni AG, Russell SD, Selvin E, Rosamond WD, et al. HbA 1c as a risk factor for heart failure in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia. 2008;51(12):2197–204. doi: 10.1007/s00125-008-1164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero SP, Garcia-Egido A, Escobar MA, Andrey JL, Corzo R, Perez V, et al. Impact of new-onset diabetes mellitus and glycemic control on the prognosis of heart failure patients: a propensity-matched study in the community. Int J Cardiol. 2012;167:1206. doi: 10.1016/j.ijcard.2012.03.134. [DOI] [PubMed] [Google Scholar]
- 16.van Melle JP, Bot M, de Jonge P, de Boer RA, van Veldhuisen DJ, Whooley MA. Diabetes, glycemic control, and new-onset heart failure in patients with stable coronary artery disease: data from the heart and soul study. Diabetes Care. 2010;33(9):2084–9. doi: 10.2337/dc10-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 18.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 20.Rose BD, Rennke HG. Renal pathophysiology - the essentials Philadelphia: Lippincott Williams & Wilkins. 1994. [Google Scholar]
- 21.Aguilar D, Bozkurt B, Ramasubbu K, Deswal A. Relationship of hemoglobin A1C and mortality in heart failure patients with diabetes. J Am Coll Cardiol. 2009;54(5):422–8. doi: 10.1016/j.jacc.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eshaghian S, Horwich TB, Fonarow GC. An unexpected inverse relationship between HbA1c levels and mortality in patients with diabetes and advanced systolic heart failure. Am Heart J. 2006;151(1):91. doi: 10.1016/j.ahj.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Tomova GS, Nimbal V, Horwich TB. Relation between hemoglobin a(1c) and outcomes in heart failure patients with and without diabetes mellitus. Am J Cardiol. 2012;109(12):1767–73. doi: 10.1016/j.amjcard.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerstein HC, Swedberg K, Carlsson J, McMurray JJ, Michelson EL, Olofsson B, et al. The hemoglobin A1c level as a progressive risk factor for cardiovascular death, hospitalization for heart failure, or death in patients with chronic heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Arch Intern Med. 2008;168(15):1699–704. doi: 10.1001/archinte.168.15.1699. [DOI] [PubMed] [Google Scholar]
- 25.Capponi L, Trinh-Shevrin C, Cronstein BN, Hochman JS. A public-private partnership: the New York University-Health and Hospitals Corporation Clinical and Translational Science Institute. Clin Transl Sci. 2012;5(3):223–5. doi: 10.1111/j.1752-8062.2012.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blecker S, Herbert R, Brancati FL. Comorbid diabetes and end-of-life expenditures among medicare beneficiaries with heart failure. J Card Fail. 2012;18(1):41–6. doi: 10.1016/j.cardfail.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonow RO, Ganiats TG, Beam CT, Blake K, Casey DE, Jr, Goodlin SJ, et al. ACCF/AHA/AMA-PCPI 2011 performance measures for adults with heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures and the American Medical Association-Physician Consortium for Performance Improvement. Circulation. 2012;125(19):2382–401. doi: 10.1161/CIR.0b013e3182507bec. [DOI] [PubMed] [Google Scholar]
- 28.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 29.Young BA, Lin E, Von Korff M, Simon G, Ciechanowski P, Ludman EJ, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14(1):15–23. [PMC free article] [PubMed] [Google Scholar]
- 30.McGilchrist CA, Aisbett CW. Regression with frailty in survival analysis. Biometrics. 1991;47(2):461–6. doi: 10.2307/2532138. [DOI] [PubMed] [Google Scholar]
- 31.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362(9):800–11. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nichols GA, Joshua-Gotlib S, Parasuraman S. Glycemic control and risk of cardiovascular disease hospitalization and all-cause mortality. J Am Coll Cardiol. 2013;62(2):121–7. doi: 10.1016/j.jacc.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 33.Zoungas S, Chalmers J, Ninomiya T, Li Q, Cooper ME, Colagiuri S, et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia. 2012;55(3):636–43. doi: 10.1007/s00125-011-2404-1. [DOI] [PubMed] [Google Scholar]
- 34.Eeg-Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Svensson AM, Gudbjornsdottir S, et al. New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR) J Intern Med. 2010;268(5):471–82. doi: 10.1111/j.1365-2796.2010.02265.x. [DOI] [PubMed] [Google Scholar]
- 35.Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30(10):2453–7. doi: 10.2337/dc06-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selvin E, Rawlings AM, Bergenstal RM, Coresh J, Brancati FL. No racial differences in the association of glycated hemoglobin with kidney disease and cardiovascular outcomes. Diabetes Care. 2013;36(10):2995–3001. doi: 10.2337/dc12-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolffenbuttel BH, Herman WH, Gross JL, Dharmalingam M, Jiang HH, Hardin DS. Ethnic differences in glycemic markers in patients with type 2 diabetes. Diabetes Care. 2013;36(10):2931–6. doi: 10.2337/dc12-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsugawa Y, Mukamal KJ, Davis RB, Taylor WC, Wee CC. Should the hemoglobin A1c diagnostic cutoff differ between blacks and whites? A cross-sectional study. Ann Intern Med. 2012;157(3):153–9. doi: 10.7326/0003-4819-157-3-201208070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43(5):480–5. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 40.Goff DC, Jr, Pandey DK, Chan FA, Ortiz C, Nichaman MZ. Congestive heart failure in the United States: is there more than meets the I(CD code)? The Corpus Christi Heart Project. Arch Intern Med. 2000;160(2):197–202. doi: 10.1001/archinte.160.2.197. [DOI] [PubMed] [Google Scholar]
- 41.Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. 2002;162(15):1682–8. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 42.Nasir K, Lin Z, Bueno H, Normand SL, Drye EE, Keenan PS, et al. Is same-hospital readmission rate a good surrogate for all-hospital readmission rate? Med Care. 2010;48(5):477–81. doi: 10.1097/MLR.0b013e3181d5fb24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will not be shared due to restrictions related to patient confidentiality.


