Abstract
Background
In order to better understand respiratory syncytial virus (RSV) epidemiology and burden in tropical Africa, optimal case definitions for detection of RSV cases need to be identified.
Methods
We used data collected between September 2009 - August 2013 from children aged <5 years hospitalized with acute respiratory Illness at Siaya County Referral Hospital. We evaluated the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of individual signs, symptoms and standard respiratory disease case definitions (severe acute respiratory illness [SARI]; hospitalized influenza-like illness [hILI]; integrated management of childhood illness [IMCI] pneumonia) to detect laboratory-confirmed RSV infection. We also evaluated an alternative case definition of cough or difficulty breathing plus hypoxia, in-drawing, or wheeze.
Results
Among 4714 children hospitalized with ARI, 3810 (81 %) were tested for RSV; and 470 (12 %) were positive. Among individual signs and symptoms, cough alone had the highest sensitivity to detect laboratory-confirmed RSV [96 %, 95 % CI (95–98)]. Hypoxia, wheezing, stridor, nasal flaring and chest wall in-drawing had sensitivities ranging from 8 to 31 %, but had specificities >75 %. Of the standard respiratory case definitions, SARI had the highest sensitivity [83 %, 95 % CI (79–86)] whereas IMCI severe pneumonia had the highest specificity [91 %, 95 % CI (90–92)]. The alternative case definition (cough or difficulty breathing plus hypoxia, in-drawing, or wheeze) had a sensitivity of [55 %, 95 % CI (50–59)] and a specificity of [60 %, 95 % CI (59–62)]. The PPV for all case definitions and individual signs/symptoms ranged from 11 to 20 % while the negative predictive values were >87 %. When we stratified by age <1 year and 1- < 5 years, difficulty breathing, severe pneumonia and the alternative case definition were more sensitive in children aged <1 year [70 % vs. 54 %, p < 0.01], [19 % vs. 11 %, p = 0.01] and [66 % vs. 43 %, p < 0.01] respectively, while non-severe pneumonia was more sensitive [14 % vs. 26 %, p < 0.01] among children aged 1- < 5 years.
Conclusion
The sensitivity and specificity of different commonly used case definitions for detecting laboratory-confirmed RSV cases varied widely, while the positive predictive value was consistently low. Optimal choice of case definition will depend upon study context and research objectives.
Keywords: Respiratory syncytial virus, Case definitions, Sensitivity and specificity
Background
Respiratory syncytial virus (RSV) is a leading viral cause of lower respiratory tract infection in infants and young children, and an important cause of neonatal death in Kenya [1–9] and worldwide [10–12]. Testing for RSV is not routinely performed in resource-poor settings, and the utility of clinical case definitions for measuring RSV disease in these contexts is not well described. In Kenya, case definitions designed for other purposes, such as for influenza surveillance, have been used to estimate RSV burden [7, 9]. However, the most sensitive and or specific clinical case definitions for detection of RSV among patients with respiratory illness remain underexplored. In addition, with candidate RSV vaccines in development [2, 13], studies are needed to fully assess RSV disease burden and standardized RSV case definitions will be needed to evaluate vaccine efficacy and impact. An understanding of the performance of different clinical case definitions for identifying RSV infection can guide research and surveillance methodology.
We used data collected between September 2009 - August 2013 from children aged <5 years hospitalized with any respiratory illness at Siaya County Referral Hospital. We evaluated the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of individual respiratory signs, symptoms and case definitions used for respiratory disease surveillance, including severe acute respiratory infection (SARI), Integrated Management of Childhood Illness (IMCI) non-severe, severe and very severe pneumonia and a hospitalized influenza like illness (hILI) case definition to detect laboratory-confirmed RSV infections.
Methods
Study site and population
The Kenya Medical Research Institute, in partnership with the U.S Centers for Disease Control and Prevention (KEMRI/CDC), has conducted surveillance for hospitalized acute respiratory illness (ARI) at Siaya County Referral Hospital (SCRH) since August 2009. SCRH is an inpatient facility with a bed capacity of 200, located in Karemo Division, Siaya County, in Western Kenya. The population is culturally homogenous and almost entirely rural [14]. The area is endemic for malaria [15] with a high under five mortality ratio (94 deaths per 1000 live births in 2012, KEMRI/CDC unpublished data) and a high HIV prevalence, estimated at 25.3 % among women aged ≥15 years in Siaya County [16]. Antiretroviral treatment is recommended for all HIV-infected children <10 years, and coverage among children in Siaya is estimated to be 43 % [16].
Data collection and management
Hospitalized acute respiratory infection (ARI) was broadly defined as hospitalization with cough or difficulty breathing or chest pain with an onset within the last 14 days. All children meeting the ARI case definition and whose parents/guardians provided consent were enrolled into the surveillance by trained study staff (nurses and clinical officers). Demographic, clinical, and physical examination data were collected using a structured questionnaire. Axillary temperatures were measured. Data processing and management procedures for this surveillance system have been described previously [17, 18].
Specimen collection and laboratory
Nasopharyngeal (NP) and oropharyngeal (OP) swabs were collected from enrolled patients using flexible mini-tip flocked swabs, then combined into a single vial containing viral transport media. The samples were transported at 4 °C and once received in the laboratory were stored at −80 °C until testing. The cold chain was maintained throughout the testing procedure. Nucleic acids were extracted from the combined NP/OP swab specimens, using a MagMax viral RNA kit and Kingfisher mL instrument (Life Technologies, New York, NY). The extracted nucleic acids were tested in a 1-step real-time reverse-transcription polymerase chain reaction (RT-PCR) assay with pathogen specific primers and probes, using the AgPath-ID One-step RT-PCR kit (Applied Biosystems, Foster City, CA) for RSV and other viruses. RSV was detected using a fluorescence labelled hydrolysis/Taqman probe at a final concentration of 0.1 μM, and the assay was considered positive for RSV when exponential fluorescence curves crossed the assigned threshold at a threshold cycle value of < 40.0 [9].
Data analysis
We analysed data on children aged <5 years hospitalized with ARI at SCRH from September 2009 through August 2013. Age specific cut-offs were used to define tachypnea: respiratory rate ≥60 breaths per minute in children <2 months, ≥50 breaths per minute in children 2–11 months, ≥40 breaths per minute for children aged 1- < 5 years [19]. Hypoxia was defined as oxygen saturation <90 % on room air [19].
Standard World Health Organization case definitions were used to classify ARI cases that met the criteria for severe acute respiratory infection (SARI, based on 2013 definition [20]), and very severe, severe and non-severe pneumonia according to the Integrated Management of Childhood Illness (IMCI) guidelines [19]. We also applied an influenza-like illness case definition (hILI, normally used in outpatient settings) to hospitalized patients [20], (Table 1). We further developed an alternative case definition by including all individual signs and symptoms in a logistic regression model. We forced the inclusion of cough and difficulty breathing into the model and used stepwise selection to identify important covariates. The resulting case definition was defined as cough or difficulty breathing plus any of hypoxia, chest in-drawing or wheezing.
Table 1.
Case definitions
| Case definition | All age groups |
|---|---|
| SARI | An Acute Respiratory Infection with: • History of fever or Measured fever of ≥38 °C • And cough • With an onset within the last 10 days • And requires hospitalization |
| IMCI non-severe pneumoniaa | Cough or difficulty breathing with fast breathing. - Fast breathing • <2 months ≥ 60 breaths/minute • 2- > 12 months- ≥ 50breaths/min • 12–59 months- ≥ 40 breaths/min |
| IMCI severe pneumoniab | Cough or difficulty breathing with chest in-drawing when calm |
| IMCI very severe pneumonia | Cough or difficulty breathing with any one of: - Stridor when calm - Not able to breastfeed/drink - Convulsions - Lethargy - Unconscious - Vomit everything |
| Hospitalized ILI | A hospitalized Acute Respiratory Infection with: • Measured fever of ≥38 °C • And cough • With an onset within the last 10 days |
aExcludes those with chest in-drawing and those with danger signs (stridor, unable to drink/breastfeed, convulsions, lethargy, unconsciousness and vomit everything)
bExcludes those with danger signs
For each respiratory sign, symptom or case definition, we calculated the risk ratio (RR) and 95 % confidence interval to evaluate their association with laboratory-confirmed RSV, and then evaluated the sensitivity, specificity, PPV and NPV for detection of laboratory-confirmed RSV infections. Case definition performance characteristics were calculated for all children aged <5 years, then stratified into two age groups (Infants aged <1 year and children aged 1- < 5 years). We also stratified our results by RSV peak and low season; RSV peak season was defined as the months with average RSV prevalence above 10 %, these were April, May, June and July. Cumulative sensitivities and specificities of the SARI, non-severe, severe and very severe IMCI pneumonia, and hILI case definitions were calculated for increasing symptom durations (days 1 to 14 after first symptom onset). For the case definitions, symptom duration was defined as the number of days from the earliest onset of cough, difficulty breathing, or fever to specimen collection.
We described demographic characteristics and laboratory outcomes of patients using proportions and means. Proportions were compared across groups using z-test for proportions whereas t-tests were used to compare means of continuous variables. Associations between categorical variables were assessed using chi square test of independence. Tests with p-value < 0.05 were considered statistically significant. Analysis was performed using SAS (version 9.2, Cary, NC).
Results
During September 2009 − August 2013, a total of 4714 children aged <5 years were hospitalized with an ARI. Of these, 3878 (82.3 %) had NP/OP swabs collected; reasons for non-collection (n = 836) included refusal (n = 464, 55.5 %), death before sample collection (n = 156, 18.7 %), transfer to other facilities before sample collection (n = 30, 3.6 %), too ill to have sample collected (n = 22, 2.6 %), and other (n = 164, 19.6 %). Among the 3878 with swabs collected, 3810 (98.3 %) had RSV test results available; the remaining 68 (1.7 %) were not tested in the laboratory because the vials were either open or the transport media volume did not meet the required threshold. RSV was detected in 470 (12.3 %) of the children tested.
The mean age and oxygen saturation was similar between those tested and untested for RSV, (mean age 1.4 years vs. 1.4 years, p = 0.99) and (mean oxygen saturation 93.7 vs. 93.4, p = 0.26) respectively. Of those tested for RSV, 54.0 % were males compared to 58.0 % of the untested (p = 0.03). Approximately 3 % (103/3810) of those tested for RSV died compared to 19.6 % (177/904) of ARI cases not tested for RSV (p < 0.01), (Table 2).
Table 2.
Demographic and clinical characteristics of patients tested and not tested for RSV
| Tested N = 3810 | Untested N = 904 | p-value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Patient characteristics | |||
| Age | (n = 3810) | (n = 904) | |
| < 1 years | 1756(46.1) | 418(46.2) | 0.94 |
| 1- < 5 years | 2054(53.9) | 486(53.8) | |
| Mean age (years)a | 1.4(1.1) | 1.4(1.1) | 0.99 |
| Gender | (n = 3810) | (n = 904) | |
| Male | 2056(54.0) | 524(58.0) | 0.03 |
| Illness Severity | |||
| Death | (n = 3810) | (n = 904) | |
| Discharged dead | 103(2.7) | 177(19.6) | <0.01 |
| Oxygen saturation | (n = 3796) | (n = 900) | |
| < 90 % | 628(16.5) | 157(17.4) | 0.52 |
| Mean Oxygen saturationa | 93.7(6.8) | 93.4(7.6) | 0.26 |
aMean(sd) for the continuous variables
Slightly more than half 238 (50.6 %) of RSV-positive cases were aged <1 year, (Table 3) and more than three-quarters of all cases were aged <2 years, 360 (76.6 %). Cough, difficulty breathing, night sweats, hypoxia, wheezing, stridor, nasal flaring and chest wall in-drawing were significantly associated with having a laboratory-confirmed RSV infection, (Table 3). The symptom/sign most strongly associated with RSV was cough (RR, 2.56; 95 % CI, 1.60-4.10), (Table 3). The presence of fever (measured or reported) was not associated with RSV infection.
Table 3.
Demographic characteristics, signs, symptoms and case definitions associated with laboratory confirmed RSV infections
| RSV + ve | RSV -ve | Crude RR | p-value | |||
|---|---|---|---|---|---|---|
| n = 470 | n = 3340 | (95 % CI) | ||||
| Demographic characteristics | n | % | n | % | ||
| Age group | ||||||
| < 1 year | 238 | 50.6 | 1518 | 45.4 | Ref | |
| 1- < 5 years | 232 | 49.4 | 1822 | 54.6 | 0.83(0.70,0.99) | 0.03 |
| Sex | ||||||
| Male | 254 | 54.0 | 1802 | 54.0 | Ref | |
| Female | 216 | 46.0 | 1538 | 46.0 | 1.00(0.84,1.18) | 0.97 |
| HIV status | ||||||
| Positive | 24 | 5.1 | 152 | 4.6 | Ref | |
| Negative | 240 | 51.1 | 1728 | 51.7 | 0.89(0.61,1.32) | 0.57 |
| Unknown | 206 | 43.8 | 1460 | 43.7 | 0.91(0.61,1.34) | 0.63 |
| Sign, symptoms and case definitions | ||||||
| Any fever | 424 | 90.2 | 2984 | 89.3 | 1.09(0.82,1.45) | 0.56 |
| Measured fever ≥38 °C | 126 | 26.8 | 797 | 23.9 | 1.15(0.95,1.39) | 0.16 |
| Reported fever | 418 | 88.9 | 2957 | 88.5 | 1.04(0.79,1.36) | 0.8 |
| Cough | 453 | 96.4 | 3023 | 90.5 | 2.56(1.60,4.10) | <0.01 |
| Difficulty breathing | 291 | 61.9 | 1715 | 51.3 | 1.46(1.23,1.74) | <0.01 |
| Chest pain | 1 | 0.2 | 23 | 0.7 | 0.34(0.05,2.30) | 0.27 |
| Night sweats | 337 | 71.7 | 2224 | 66.6 | 1.24(1.02,1.49) | 0.03 |
| Tachypneaa | 288 | 61.3 | 1948 | 58.3 | 1.11(0.94,1.33) | 0.22 |
| Hypoxiab | 121 | 26.2 | 507 | 15.6 | 1.76(1.46,2.12) | <0.01 |
| Wheeze | 125 | 26.6 | 569 | 17.0 | 1.63(1.35,1.96) | <0.01 |
| Stridor | 36 | 7.7 | 142 | 4.3 | 1.69(1.25,2.30) | <0.01 |
| Nasal flaring | 146 | 31.1 | 739 | 22.1 | 1.49(1.24,1.78) | <0.01 |
| Chest In-drawing | 145 | 30.9 | 699 | 20.9 | 1.57(1.31,1.88) | <0.01 |
| SARI | 389 | 82.8 | 2579 | 77.2 | 1.36(1.09,1.71) | 0.01 |
| ILI | 112 | 23.8 | 678 | 20.3 | 1.20(0.98,1.46) | 0.08 |
| IMCI Non-severe pneumonia | 95 | 20.2 | 777 | 23.3 | 0.85(0.69,1.06) | 0.14 |
| IMCI severe pneumonia | 71 | 15.1 | 310 | 9.3 | 1.60(1.27,2.01) | <0.01 |
| IMCI very severe pneumonia | 236 | 50.2 | 1719 | 51.5 | 0.96(0.81,1.13) | 0.61 |
| Cough or difficulty breathing plus hypoxia, chest in-drawing or wheeze | 257 | 54.7 | 1316 | 39.4 | 1.72(1.15,2.03) | <0.01 |
aDefined as respiratory rate ≥60 breaths per minute in kids <2 months, ≥50 breaths per minute in kids 2–11 months, ≥40 breaths per minute for kids 1- < 5 years
bDefined as oxygen saturation <90 % on room air. 14/3810 missing oxygen saturation
Among the respiratory signs and symptoms examined, cough alone had the highest sensitivity for laboratory confirmed RSV (96.4 %, 95 % CI 94.7-98.1), followed by night sweats, difficulty breathing and tachypnea; (71.7 %, 95 % CI 67.6-75.8), (61.9 %, 95 % CI 57.5-66.3) and (61.3 %, 95 % CI 56.9-65.7) respectively. The other individual signs and symptoms evaluated had sensitivities that ranged from 8–31 %, (Table 4). The presence of hypoxia, wheezing, stridor, nasal flaring and chest wall in-drawing all had specificities >77 % for laboratory confirmed RSV (Table 4).
Table 4.
Sensitivity, specificity, PPV and NPV of signs, symptoms and case definitions
| Age group | Case definition | RSV positive n = 470 | RSV negative n = 3340 | Sensitivity %(95 % CI) | Specificity %(95 % CI) | PPV %(95 % CI) | NPV %(95 % CI) | ||
|---|---|---|---|---|---|---|---|---|---|
| All under 5 | n | % | n | % | |||||
| Cough | 453 | 96.4 | 3023 | 90.5 | 96.4(94.7,98.1) | 9.5(8.5,10.5) | 13.0(11.9,14.2) | 94.9(92.6,97.3) | |
| Difficulty breathing | 291 | 61.9 | 1715 | 51.3 | 61.9(57.5,66.3) | 48.7(47.0,50.4) | 14.5(13.0,16.1) | 90.1(88.7,91.5) | |
| Night sweats | 337 | 71.7 | 2224 | 66.6 | 71.7(67.6,75.8) | 33.4(31.8,35.0) | 13.2(11.9,14.5) | 89.4(87.6,91.1) | |
| Tachypnea | 288 | 61.3 | 1948 | 58.3 | 61.3(56.9,65.7) | 41.7(40.0,43.4) | 12.9(11.5,14.3) | 88.4(86.9,90.0) | |
| Hypoxia | 121 | 25.9 | 507 | 15.2 | 25.9(21.9,29.8) | 84.8(83.5,86.0) | 19.3(16.2,22.4) | 89.1(88.0,90.1) | |
| Wheeze | 125 | 26.6 | 569 | 17.0 | 26.6(22.6,30.6) | 83.0(81.7,84.2) | 18.0(15.2,20.9) | 88.9(87.8,90.0) | |
| Stridor | 36 | 7.7 | 142 | 4.3 | 7.7(5.3,10.1) | 95.8(95.1,96.4) | 20.2(14.3,26.1) | 88.1(87.0,89.1) | |
| Nasal flaring | 146 | 31.1 | 739 | 22.1 | 31.1(26.9,35.3) | 77.9(76.5,79.3) | 16.5(14.1,18.9) | 88.9(87.8,90.1) | |
| Chest In-drawing | 145 | 30.9 | 699 | 20.9 | 30.9(26.7,35.0) | 79.1(77.7,80.5) | 17.2(14.6,19.7) | 89.0(87.9,90.2) | |
| SARI | 389 | 82.8 | 2579 | 77.2 | 82.8(79.4,86.2) | 22.8(21.4,24.2) | 13.1(11.9,14.3) | 90.4(88.4,92.4) | |
| hILI | 112 | 23.8 | 678 | 20.3 | 23.8(20.0,27.7) | 79.7(78.3,81.1) | 14.2(11.7,16.6) | 88.2(87.0,89.3) | |
| IMCI Non-severe pneumonia | 95 | 20.2 | 777 | 23.3 | 20.2(16.6,23.8) | 76.7(75.3,78.2) | 10.9(8.8,13.0) | 87.2(86.0,88.4) | |
| IMCI severe pneumonia | 71 | 15.1 | 310 | 9.3 | 15.1(11.9,18.3) | 90.7(89.7,91.7) | 18.6(14.7,22.6) | 88.4(87.3,89.4) | |
| IMCI very severe pneumonia | 236 | 50.2 | 1719 | 51.5 | 50.2(45.7,54.7) | 48.5(46.8,50.2) | 12.1(10.6,13.5) | 87.4(85.9,88.9) | |
| Cough or difficulty breathing plus hypoxia, chest in-drawing or wheeze | 257 | 54.7 | 1316 | 39.4 | 54.7(50.2,59.2) | 60.1(58.9,62.3) | 16.3(14.5,18.2) | 90.5(89.3,91.7) | |
| Children < 1 years | n | 238 | 1518 | ||||||
| Cough | 232 | 97.5 | 1373 | 90.4 | 97.5(95.5,99.5) | 9.6(8.1,11.0) | 14.5(12.7,16.2) | 96.0(92.9,99.1) | |
| Difficulty breathing | 166 | 69.8 | 863 | 56.8 | 69.8(63.9,75.6)a | 43.2(40.7,45.6)a | 16.1(13.9,18.4) | 90.1(87.9,92.3) | |
| SARI | 189 | 79.4 | 1175 | 77.4 | 79.4(74.3,84.6) | 22.6(20.5,24.7) | 13.9(12.0,15.7) | 87.5(84.2,90.8) | |
| hILI | 52 | 21.9 | 307 | 20.2 | 21.9(16.6,27.1) | 79.8(77.8,81.8) | 14.5(10.8,18.1) | 86.7(84.9,89.5) | |
| IMCI Non-severe pneumonia | 34 | 14.3 | 312 | 20.6 | 14.3(9.8,18.7)a | 79.5(77.4,81.5)a | 9.8(6.7,13.0) | 85.5(83.7,87.4) | |
| IMCI severe pneumonia | 46 | 19.3 | 175 | 11.5 | 19.3(14.3,24.3)a | 88.5(86.9,90.1)a | 20.8(15.5,26.2) | 87.5(85.8,89.2) | |
| IMCI very severe pneumonia | 117 | 49.2 | 726 | 47.8 | 49.2(42.8,55.5) | 52.2(49.7,54.7) | 13.9(11.6,16.2) | 86.8(84.6,89.0) | |
| Cough or difficulty breathing plus hypoxia, chest in-drawing or wheeze | 157 | 66.0 | 698 | 46.0 | 66.0(60.0,72.0)a | 54.0(51.5,56.5)a | 18.4(15.8,21.0) | 91.0(89.1,92.9) | |
| Children 1- < 5 years | n | 232 | 1822 | ||||||
| Cough | 221 | 95.3 | 1650 | 90.6 | 95.3(92.5,98.0) | 9.4(8.1,10.8) | 11.8(10.4,13.3) | 94.0(90.6,97.4) | |
| Difficulty breathing | 125 | 53.9 | 852 | 46.8 | 53.9(47.5,60.3)a | 53.2(51.0,55.5)a | 12.8(10.7,14.9) | 90.0(88.3,91.9) | |
| SARI | 200 | 86.2 | 1404 | 77.1 | 86.2(81.8,90.6) | 22.9(21.0,24.9) | 12.5(10.9,14.1) | 92.9(90.5,95.3) | |
| hILI | 60 | 25.9 | 371 | 20.4 | 25.9(20.2,31.5) | 79.6(77.8,81.5) | 13.9(10.7,17.2) | 89.4(87.9,90.9) | |
| IMCI Non-severe pneumonia | 61 | 26.3 | 465 | 25.5 | 26.3(20.6,32.0)a | 74.5(72.5,76.5)a | 11.6(8.9,14.3) | 88.8(87.2,90.4) | |
| IMCI severe pneumonia | 25 | 10.8 | 135 | 7.4 | 10.8(6.8,14.8)a | 92.6(91.4,93.8)a | 15.6(10.0,21.3) | 89.1(87.7,90.5) | |
| IMCI very severe pneumonia | 119 | 51.3 | 993 | 54.5 | 51.3(44.9,57.7) | 45.5(43.2,47.8) | 10.7(8.9,12.5) | 88.0(85.9,90.1) | |
| Cough or difficulty breathing plus hypoxia, chest in-drawing or wheeze | 100 | 43.1 | 618 | 33.9 | 43.1(36.7,495)a | 66.1(63.9,68.3)a | 13.9(11.4,16.5) | 90.1(88.5,91.7) | |
aSensitivity and specificity significantly different between the two age groups
The standard respiratory case definition with the highest sensitivity for detecting RSV infection was SARI (82.8 %, 95 % CI 79.4-86.2), followed by IMCI very severe pneumonia (50.2 %, 95 % CI 45.7-54.7), (Table 4. Fig. 1). The sensitivities for IMCI non-severe pneumonia, IMCI severe pneumonia and hILI were lower; (20.2 %, 95 % CI 16.6-23.8), (15.1 %, 95 % CI 11.9-18.3) and (23.8 %, 95 % CI 20.0-27.7) respectively. The highest specificity was observed in IMCI severe pneumonia (90.7 %, 95 % CI 89.7-91.7), closely followed by hILI and IMCI non-severe pneumonia definitions; (79.7 %, 95 % CI 78.3-81.1) and (76.7 %, 95 % CI 75.3-78.2) respectively. SARI and IMCI very severe pneumonia had specificities of 22.8 % (95 % CI 21.4-24.2) and 48.5 % (95 % CI 46.8-50.2), respectively. The alternative case definition developed through logistic regression; cough or difficulty breathing plus hypoxia, chest in-drawing, or wheeze had a sensitivity and specificity of (54.7 %, 95 % CI 50.2-59.2) and (60.1 %, 95 % CI 58.9-62.3) respectively. We plotted a receiver operator characteristics chart for these case definitions, (Fig. 1). The PPV for all case definitions, signs and symptoms assessed ranged between 11–20 % whereas the negative predictive values were >87 %, (Table 4).
Fig. 1.
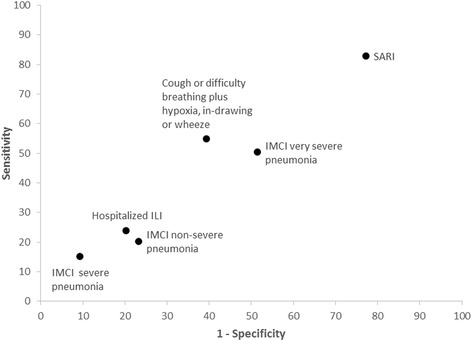
Receiver operator characteristic (ROC) chart for case definitions
When stratified by age group, SARI continued to be the most sensitive case definition (79.4 % sensitive in infants aged <1 year and 86.2 % sensitive in children aged 1- < 5 years), and IMCI severe pneumonia was the most specific (88.5 % in infants aged <1 year and 92.6 % in children aged 1- < 5 years). Comparing across age groups, IMCI severe pneumonia was significantly more sensitive (19.3 % vs. 10.8 %, p = 0.01) and less specific (88.5 % vs. 92.6 %, p < 0.01) among those aged <1 year compared to children aged 1- < 5 years (Table 4). Cough or difficulty breathing plus hypoxia, chest in-drawing or wheeze was also significantly more sensitive (66.0 % vs. 43.1 %, p < 0.01) and less specific (54.0 % vs. 66.1 %, p < 0.01) among the younger age group. Difficulty breathing was also significantly more sensitive (69.8 % vs. 53.9 %, p < 0.01) and less specific (43.2 % vs. 53.2 %, p < 0.01) among the younger age group. IMCI non-severe pneumonia was significantly less sensitive (14.3 % vs. 26.3 %, p < 0.01) and more specific (79.5 % vs. 74.5 %, p < 0.01) among infants aged <1 year compared to children aged 1- < 5 years, (Table 4). There was no significant difference in sensitivity, specificity and predictive values for the other case definitions between the <1 and 1- < 5 age groups. When we stratified by season, we observed significant difference in the PPVs for all signs, symptoms and case definitions evaluated, with significantly higher PPVs in the RSV peak season, (Table 5). There was no statistically significant difference in the sensitivities and specificities of the case definitions by number of days from symptom onset, (Figs. 2 and 3) except for SARI where specificity decreased from day 1 to 3 (46.2–25.4 %, p < 0.01) then stabilized, (Fig. 3).
Table 5.
PPVs and NPVs of signs, symptoms and case definitions by season
| RSV seasons | Case definition | PPV %(95 % CI) | NPV %(95 % CI) |
|---|---|---|---|
| Peak seasons | |||
| Cough | 22.6(20.3,24.9) | 91.4(86.1,96.8) | |
| Difficulty in breathing | 26.3(23.1,29.6) | 83.8(81.0,86.7) | |
| SARI | 22.8(20.3,25.3) | 83.2(78.9,87.5) | |
| hILI | 26.5(21.1,31.9) | 79.7(77.3,82.1) | |
| IMCI Non-severe pneumonia | 19.8(15.1,24.5) | 78.1(75.6,80.5) | |
| IMCI severe pneumonia | 35.4(27.6,43.2) | 80.2(77.9,82.4) | |
| IMCI very severe pneumonia | 19.9(16.9,22.8) | 76.6(73.3,79.9) | |
| Cough or difficulty breathing plus hypoxia, chest in-drawing or wheeze | 28.3(24.6,31.9) | 83.6(81.0,86.2) | |
| Low seasons | |||
| Cough | 7.7(6.6,8.8) | 96.5(94.1,98.9) | |
| Difficulty in breathing | 8.1(6.6,9.6) | 93.5(92.1,94.9) | |
| SARI | 7.8(6.6,9.0) | 94.2(92.2,96.2) | |
| hILI | 8.3(5.9,10.6) | 92.9(91.8,94.1) | |
| IMCI Non-severe pneumonia | 6.8(4.8,8.9) | 92.5(91.3,93.7) | |
| IMCI severe pneumonia | 8.4(4.9,12.0) | 92.8(91.7,93.9) | |
| IMCI very severe pneumonia | 7.6(6.1,9.1) | 92.9(91.5,94.3) | |
| Cough or difficulty breathing plus hypoxia, chest in-drawing or wheeze | 9.4(7.6,11.3) | 94.1(92.9,95.3) |
Fig. 2.
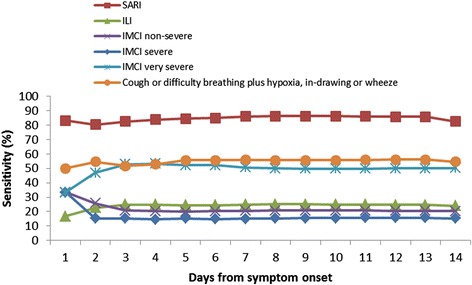
Cumulative sensitivity of case definitions to detect Laboratory confirmed RSV
Fig. 3.
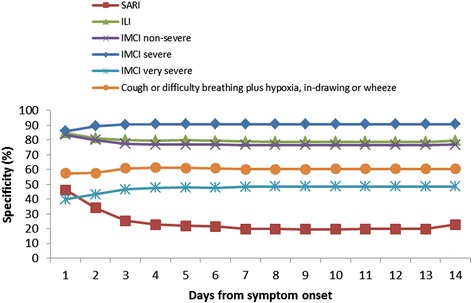
Cumulative specificity of case definitions to detect Laboratory confirmed RSV
Discussion
We observed wide variations in the sensitivity and specificity of the signs, symptoms and case definitions evaluated. SARI, a case definition requiring cough and either reported or measured fever and developed for global influenza surveillance was the most sensitive case definition for RSV detection with sensitivity of 83 %. The most specific case definition was IMCI severe pneumonia (cough or difficulty breathing plus chest in-drawing). Generally there was a trade-off between sensitivity and specificity. Cough or difficulty breathing plus hypoxia, chest in-drawing or wheeze provided the best balance; it was the only case definition for which both sensitivity and specificity exceeded 50 %. The predictive values were more consistent across case definitions, with all having relatively low PPV (11–20 %) and high NPV (87–90 %).
In determining the optimal case definition, consideration should be given to the context in which the case definition applies and the objectives for which it is being used. If the primary objective of the study is to estimate the burden of RSV disease then it is important to use a case definition offering high sensitivity. We found that cough alone had the highest sensitivity (96 %) to detect RSV. If the goal was to detect the maximum number of RSV cases then cough alone might be the best definition to use. However, using cough alone would also give very many false positives (PPV 13 %). Of the case definitions evaluated we found that the 2013 WHO SARI case definition was most sensitive for RSV detection. Nonetheless, the SARI case definition may miss up to 17 % of RSV cases. Much of what is known about RSV globally comes from surveillance systems that utilize case definitions intended for other diseases (e.g. influenza, pneumonia) that are not optimized for detection of RSV, the potential for underestimation should therefore be recognized in interpreting data on RSV burden, particularly if using a case definition that requires measured fever (e.g. hILI) or includes only pneumonia without danger signs (e.g. IMCI non-severe or severe pneumonia).
Sometimes maximum specificity is desired for RSV detection. If RSV vaccines currently under development are eventually introduced into routine immunization programs there will be a need to evaluate the impact and effectiveness of the vaccine. Given that RSV testing is not widely available in resource poor settings, a clinical case definition may be most practical for use in vaccine evaluations such as case–control vaccine effectiveness studies or analyses of trends in disease burden. For this purpose, a highly specific case definition is preferable [21, 22]. We found that the presence of stridor offered the highest specificity (96 %). However, it was present in < 8 % of cases, and therefore not easily embraceable as a case definition. Among the case definitions evaluated, we found that IMCI severe pneumonia was the most specific (91 %), while hILI and non-severe pneumonia case definitions also provided reasonable specificity (77–80 %). Measuring vaccine impact and effectiveness using the IMCI severe pneumonia case definitions may be more feasible than using less specific case definitions such as SARI. However, even if the most specific case definition were used, most cases identified may not be RSV-infected because all the case definitions were found to have poor PPV.
We observed positive predictive values (PPVs) ranging from 11–20 % for all signs, symptoms and case definitions, though the PPVs were significantly higher during periods of RSV circulation (cold months of the year in Western Kenya; April through July). A case definition with a high PPV is useful to investigators aiming to maximize the detection of laboratory confirmed cases among those tested. For example, studies focused on subtyping or sequencing for which the positive RSV isolate is essential may benefit from a case definition with optimal PPV. In this analysis, the case definition with the highest PPV for detection of laboratory confirmed RSV was IMCI severe pneumonia. However, even during peak RSV season, roughly one in three children with IMCI severe pneumonia will have a positive RSV test result.
Although the prevalence of RSV infection is generally higher among infants hospitalized with respiratory disease compared with older children hospitalized with respiratory disease [1], we did not observe significant differences in PPV when stratifying by age group. Difficulty breathing, severe pneumonia and the alternative definition were found to be more sensitive among the infants aged <1 year, while IMCI non-severe pneumonia was more sensitive among those aged 1- < 5 years. Older children with RSV are likely to be experiencing a reinfection rather than a primary infection, and therefore may present with milder illness [4]. These findings highlight that choice of case definition for detecting RSV cases should take into account the age group under surveillance.
We found that sensitivity, specificity and predictive values for the case definitions to detect RSV infections did not vary significantly with respect to duration from illness onset to sample collection, suggesting that these case definitions can be used within the 14-day period from symptom onset to detect RSV infection without concern about loss of sensitivity or specificity.
The presence of wheezing, hypoxia, stridor, nasal flaring and chest wall in-drawing were each found to be significantly associated with RSV infection. This finding is similar to findings from Rarieda and Kilifi districts in Kenya and Ballabgarh in northern India [1, 6, 9, 23] and suggest that some of the individual IMCI danger signs might be used to identify subsets of children more likely to have RSV virus infections. It should however be noted that there is some variation in the literature regarding the association of hypoxia with RSV infections. Hypoxia was found to be negatively associated with RSV infection in one study conducted in Kilifi District, Kenya [1], with another study reporting no significant association from the same site [6]. We found that neither measured nor reported fever were associated with RSV infection, however history of fever was a common symptom observed in nearly 90 % of children infected with RSV. Fever was a common symptom in many RSV infected patients in other studies as well [23–25] with one study [6] observing that fever was significantly more common among the RSV-infected children compared to children with respiratory disease that tested negative for RSV, RR = 1.54(95 % CI, 1.33–1.80). The high prevalence of malaria in our study site [14, 15] may impact the observed association between fever and RSV.
Interestingly, the performance of various case definitions for detecting RSV infection that we observed in this study differed substantially from the findings of a study in Ballabgarh, northern India, for which all hospitalized children aged <5 years were tested for RSV [23]. While we found the SARI case definition to be the most sensitive, and severe IMCI pneumonia to be most specific, that study reported SARI to have a low sensitivity and IMCI pneumonia or severe pneumonia (combined) to have a low specificity. Differences in epidemiologic contexts (e.g. malaria or HIV prevalence) may explain some of the variation in results. The precise definitions used for SARI and IMCI pneumonia also differed across the two studies. Furthermore, the study from India included all hospitalized children (regardless of clinical signs), while our study included only children hospitalized with cough or difficulty breathing or chest pain. Of note, the Indian study found that restricting to even a very broad respiratory case definition (acute onset cough or sore throat or shortness of breath or coryza or clinician judgement that illness due to infection) would fail to detect 13.5 % (10/74) of RSV cases; thus our analysis likely failed to include some children hospitalized with RSV infection.
In interpreting our results, the following additional limitations should be taken into consideration. This analysis was restricted to inpatients; the findings therefore may not be relevant for outpatient respiratory disease surveillance. Furthermore, because non-severe IMCI pneumonia is typically managed in the outpatient setting in Kenya, those children with non-severe pneumonia included in this analysis may be a somewhat biased group. In addition, a substantial number of severe cases were not swabbed and consequently excluded from this analysis; therefore these findings may not be representative of the most severe spectrum of cases with RSV infection. Lastly, RT-PCR was the only method used for RSV detection in our surveillance system; therefore sensitivities and specificities could vary in studies where other methods such as culture or serology are used.
Conclusion
The optimal case definition for use in detecting RSV varies depending upon context. Researchers must take into account their objectives, available resources, and the study population. For estimating the burden of RSV in a context similar to Western Kenya, the WHO SARI case definition could be used, particularly if it is already being implemented in a sentinel influenza surveillance system. However more specific case definitions may be required for evaluating future RSV vaccines in settings where diagnostic testing is not readily available. Additional research to assess the performance of various case definitions for detecting RSV infections across a broad range of epidemiologic contexts is needed.
Ethical considerations
This study was approved by the Ethical Review Committee of the Kenya Medical Research Institute (KEMRI SSC #1801) and the Institutional Review Board of CDC-Atlanta (CDC IRB #3308). Written informed consent was obtained from all caretakers/guardians of all minors prior to enrolment in the surveillance system. All patients admitted at the SCRH were given standard care treatment irrespective of their enrolment into the study or not.
Acknowledgments
We thank the KEMRI/CDC field, clinic and data room staff involved for their diligence in data collection and management and the laboratory staff for performing the RSV testing. We also thank the Kenya Ministry of Health medical staff and the study surveillance officers who helped collect the data used for this analysis and the study participants at Siaya County Referral Hospital. This manuscript is published with the permission of the Director of KEMRI.
Funding
This work was supported by funding from the U.S. Centers for Disease Control and Prevention.
Disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Abbreviations
- ARI
acute respiratory illness
- CDC
U.S Centers for Disease Control and Prevention
- hILI
hospitalized influenza like illness
- HIV
human immunodeficiency virus
- IMCI
integrated management of childhood illness
- KEMRI
Kenya Medical Research Institute
- NPV
negative predictive value
- PPV
positive predictive value
- RR
relative risk
- RSV
respiratory syncytial virus
- RT-PCR
real-time reverse-transcription polymerase chain reaction
- SARI
severe acute respiratory infection
- SAS
statistical analysis software
- SCRH
Siaya County Referral Hospital
- WHO
World Health Organisation
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BON, JAM, HNN, and JRV conceived the study, BON, JAM, HNN, LM, SK, RO, CM, NAO, GMB, MAK, DRF, and JRV contributed to study design and implementation, BON, JAM, and JRV analysed and interpreted the data. BON drafted the manuscript then all authors critically reviewed the manuscript for intellectual content and approved the final manuscript. All authors read and approved the final manuscript.
References
- 1.Nokes DJ, Ngama M, Bett A, Abwao J, Munywoki P, English M, Scott JA, Cane PA, Medley GF. Incidence and severity of respiratory syncytial virus pneumonia in rural Kenyan children identified through hospital surveillance. Clin Infect Dis. 2009;49(9):1341–9. doi: 10.1086/606055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nokes DJ, Okiro EA, Ngama M, Ochola R, White LJ, Scott PD, English M, Cane PA, Medley GF. Respiratory syncytial virus infection and disease in infants and young children observed from birth in Kilifi District, Kenya. Clin Infect Dis. 2008;46(1):50–7. doi: 10.1086/524019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nokes DJ, Okiro EA, Ngama M, White LJ, Ochola R, Scott PD, Cane PA, Medley GF. Respiratory syncytial virus epidemiology in a birth cohort from Kilifi district, Kenya: infection during the first year of life. J Infect Dis. 2004;190(10):1828–32. doi: 10.1086/425040. [DOI] [PubMed] [Google Scholar]
- 4.Ohuma EO, Okiro EA, Ochola R, Sande CJ, Cane PA, Medley GF, Bottomley C, Nokes DJ. The natural history of respiratory syncytial virus in a birth cohort: the influence of age and previous infection on reinfection and disease. Am J Epidemiol. 2012;176(9):794–802. doi: 10.1093/aje/kws257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okiro EA, Ngama M, Bett A, Cane PA, Medley GF, James Nokes D. Factors associated with increased risk of progression to respiratory syncytial virus-associated pneumonia in young Kenyan children. Trop Med Int Health. 2008;13(7):914–26. doi: 10.1111/j.1365-3156.2008.02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okiro EA, Ngama M, Bett A, Nokes DJ. The incidence and clinical burden of respiratory syncytial virus disease identified through hospital outpatient presentations in Kenyan children. PLoS ONE. 2012;7(12):e52520. doi: 10.1371/journal.pone.0052520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emukule GO, Khagayi S, McMorrow ML, Ochola R, Otieno N, Widdowson MA, Ochieng M, Feikin DR, Katz MA, Mott JA. The Burden of Influenza and RSV among Inpatients and Outpatients in Rural Western Kenya, 2009–2012. PLoS ONE. 2014;9(8):e105543. doi: 10.1371/journal.pone.0105543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feikin DR, Njenga MK, Bigogo G, Aura B, Aol G, Audi A, Jagero G, Muluare PO, Gikunju S, Nderitu L, et al. Viral and bacterial causes of severe acute respiratory illness among children aged less than 5 years in a high malaria prevalence area of western Kenya, 2007–2010. Pediatr Infect Dis J. 2013;32(1):e14–9. doi: 10.1097/INF.0b013e31826fd39b. [DOI] [PubMed] [Google Scholar]
- 9.Bigogo GM, Breiman RF, Feikin DR, Audi AO, Aura B, Cosmas L, Njenga MK, Fields BS, Omballa V, Njuguna H, et al. Epidemiology of respiratory syncytial virus infection in rural and urban Kenya. J Infect Dis. 2013;208(Suppl 3):S207–16. doi: 10.1093/infdis/jit489. [DOI] [PubMed] [Google Scholar]
- 10.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fry AM, Chittaganpitch M, Baggett HC, Peret TC, Dare RK, Sawatwong P, Thamthitiwat S, Areerat P, Sanasuttipun W, Fischer J, et al. The burden of hospitalized lower respiratory tract infection due to respiratory syncytial virus in rural Thailand. PLoS ONE. 2010;5(11):e15098. doi: 10.1371/journal.pone.0015098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venkatesh MP, Weisman LE. Prevention and treatment of respiratory syncytial virus infection in infants: an update. Expert Rev Vaccines. 2006;5(2):261–8. doi: 10.1586/14760584.5.2.261. [DOI] [PubMed] [Google Scholar]
- 14.Hamel MJ, Adazu K, Obor D, Sewe M, Vulule J, Williamson JM, Slutsker L, Feikin DR, Laserson KF. A reversal in reductions of child mortality in western Kenya, 2003–2009. Am J Trop Med Hyg. 2011;85(4):597–605. doi: 10.4269/ajtmh.2011.10-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odhiambo FO, Laserson KF, Sewe M, Hamel MJ, Feikin DR, Adazu K, Ogwang S, Obor D, Amek N, Bayoh N, et al. Profile: the KEMRI/CDC Health and Demographic Surveillance System--Western Kenya. Int J Epidemiol. 2012;41(4):977–87. doi: 10.1093/ije/dys108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National AIDS Control Council (NACC) Kenya HIV County Profiles. Kenya: Ministry of Health; 2014. [Google Scholar]
- 17.Feikin DR, Ope MO, Aura B, Fuller JA, Gikunju S, Vulule J, Ng’ang’a Z, Njenga MK, Breiman RF, Katz M. The population-based burden of influenza-associated hospitalization in rural western Kenya, 2007–2009. Bull World Health Organ. 2012;90(4):256–63A. doi: 10.2471/BLT.11.094326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray EL, Khagayi S, Ope M, Bigogo G, Ochola R, Muthoka P, Njenga K, Odhiambo F, Burton D, Laserson KF, et al. What are the most sensitive and specific sign and symptom combinations for influenza in patients hospitalized with acute respiratory illness? Results from western Kenya, January 2007-July 2010. Epidemiol Infect. 2012;141(1):212–22. doi: 10.1017/S095026881200043X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Handbook: IMCI integrated management of childhood illness. http://apps.who.int/iris/bitstream/10665/42939/1/9241546441.pdfWHO; 2005.
- 20.WHO. Global Epidemiological Surveillance Standards for Influenza. http://www.who.int/influenza/resources/documents/WHO_Epidemiological_Influenza_Surveillance_Standards_2014.pdfWHO; 2013.
- 21.Nichol KL, Mendelman P. Influence of clinical case definitions with differing levels of sensitivity and specificity on estimates of the relative and absolute health benefits of influenza vaccination among healthy working adults and implications for economic analyses. Virus Res. 2004;103(1–2):3–8. doi: 10.1016/j.virusres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Beyer WE. Heterogeneity of case definitions used in vaccine effectiveness studies--and its impact on meta-analysis. Vaccine. 2006;24(44–46):6602–4. doi: 10.1016/j.vaccine.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 23.Saha S, Pandey BG, Choudekar A, Krishnan A, Gerber SI, Rai SK, Singh P, Chadha M, Lal RB, Broor S. Evaluation of case definitions for estimation of respiratory syncytial virus associated hospitalizations among children in a rural community of northern India. J Glob Health. 2015;5(2):010419. doi: 10.7189/jogh.05.020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volling C, Hassan K, Mazzulli T, Green K, Al-Den A, Hunter P, Mangat R, Ng J, McGeer A. Respiratory syncytial virus infection-associated hospitalization in adults: a retrospective cohort study. BMC Infect Dis. 2014;13(1):665. doi: 10.1186/s12879-014-0665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tecu C, Mihai ME, Alexandrescu VI, Orăşeanu D, Zapucioiu C, Ivanciuc AE, Necula G, Lupulescu E, Chiriţă D, Piţigoi D. Single and multipathogen viral infections in hospitalized children with acute respiratory infections. Roum Arch Microbiol Immunol. 2013;72(4):242–9. [PubMed] [Google Scholar]


